Preparation and Corrosion Resistance of ETEO Modified Graphene Oxide/Epoxy Resin Coating
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
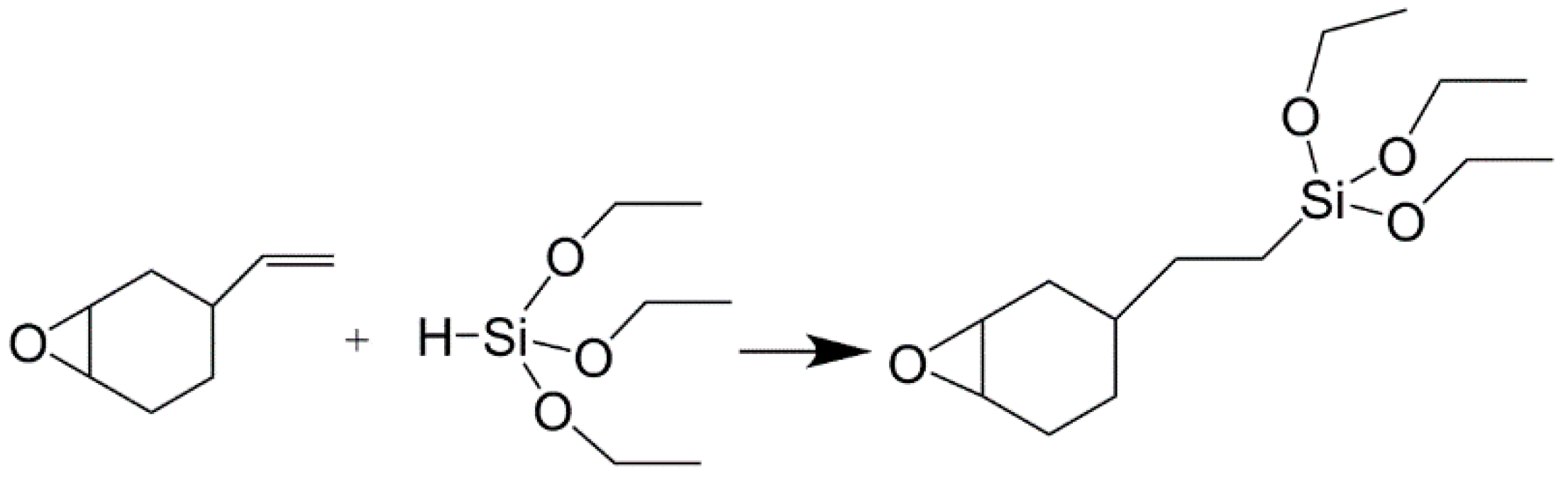
2.2. Preparation of 2-(3,4-Epoxycyclohexyl)Ethyl Triethoxysilane (ETEO)
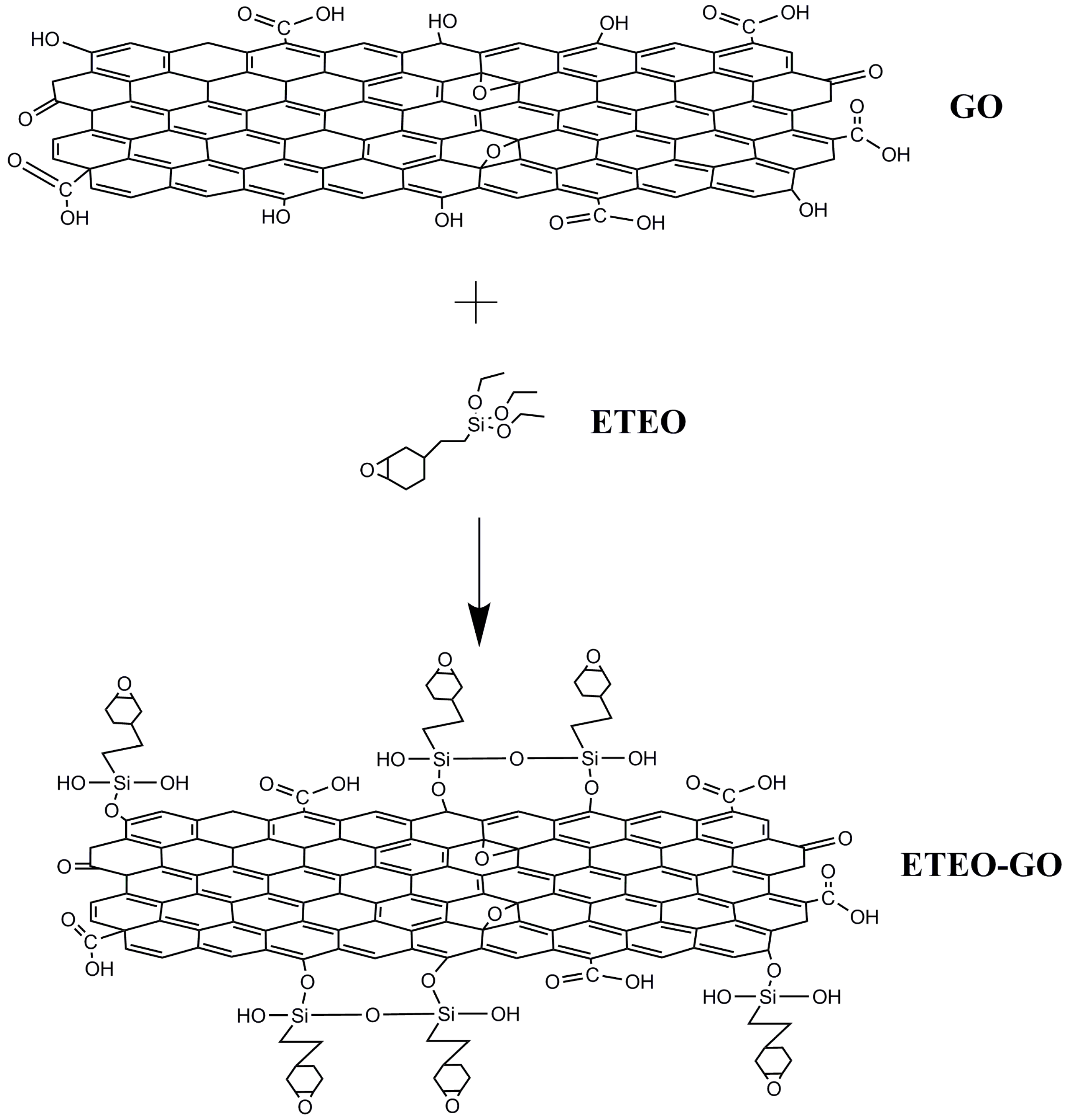
2.3. Preparation of ETEO-Modified GO (ETEO-GO)
2.4. Preparation of Coatings
2.5. Characterization
2.6. Mechanical Performance Test
2.7. Contact Angle Measurements
2.8. Coating Electrochemical Impedance Test
2.9. Salt Spray Test
3. Results and Discussion
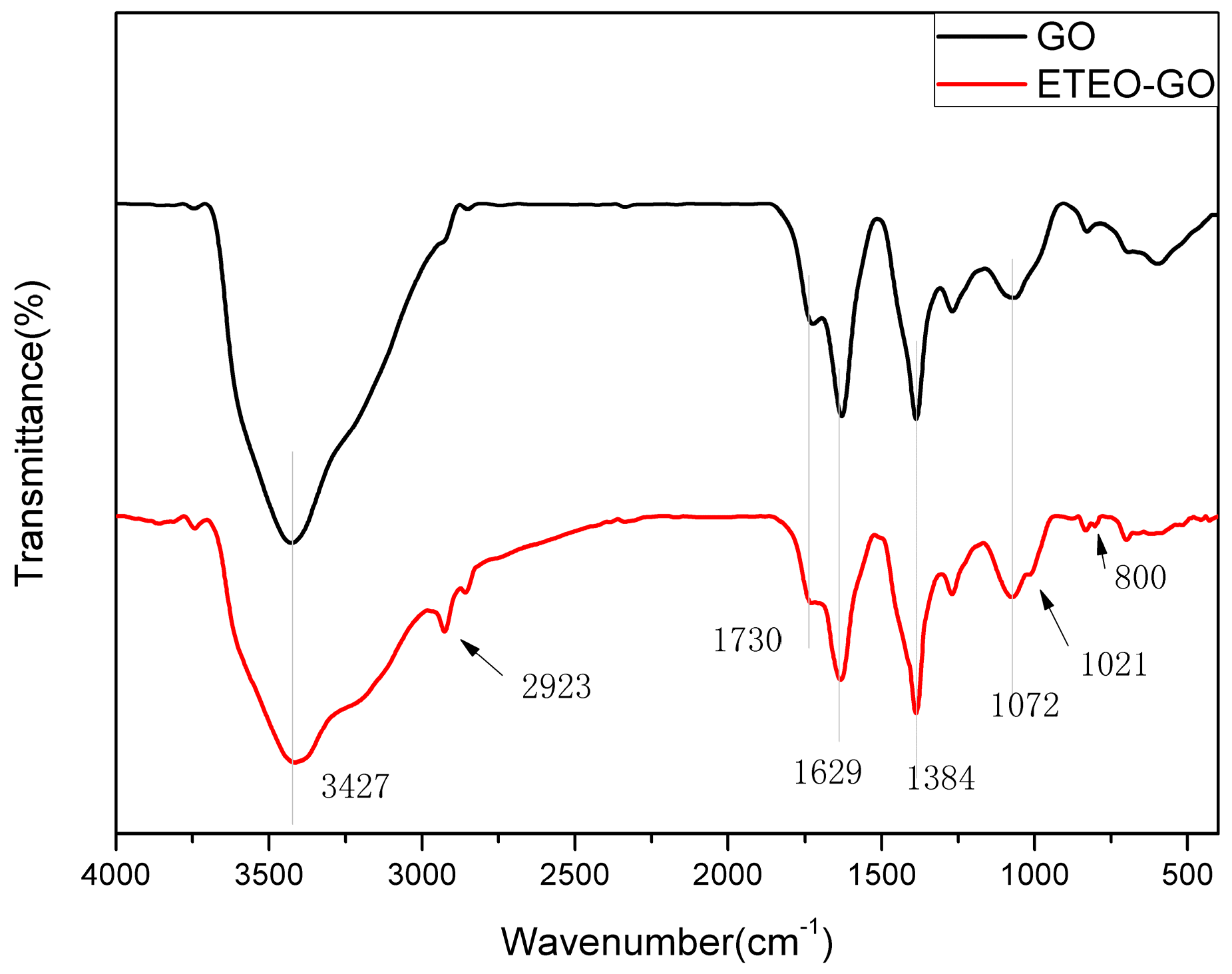
3.1. FTIR Analysis
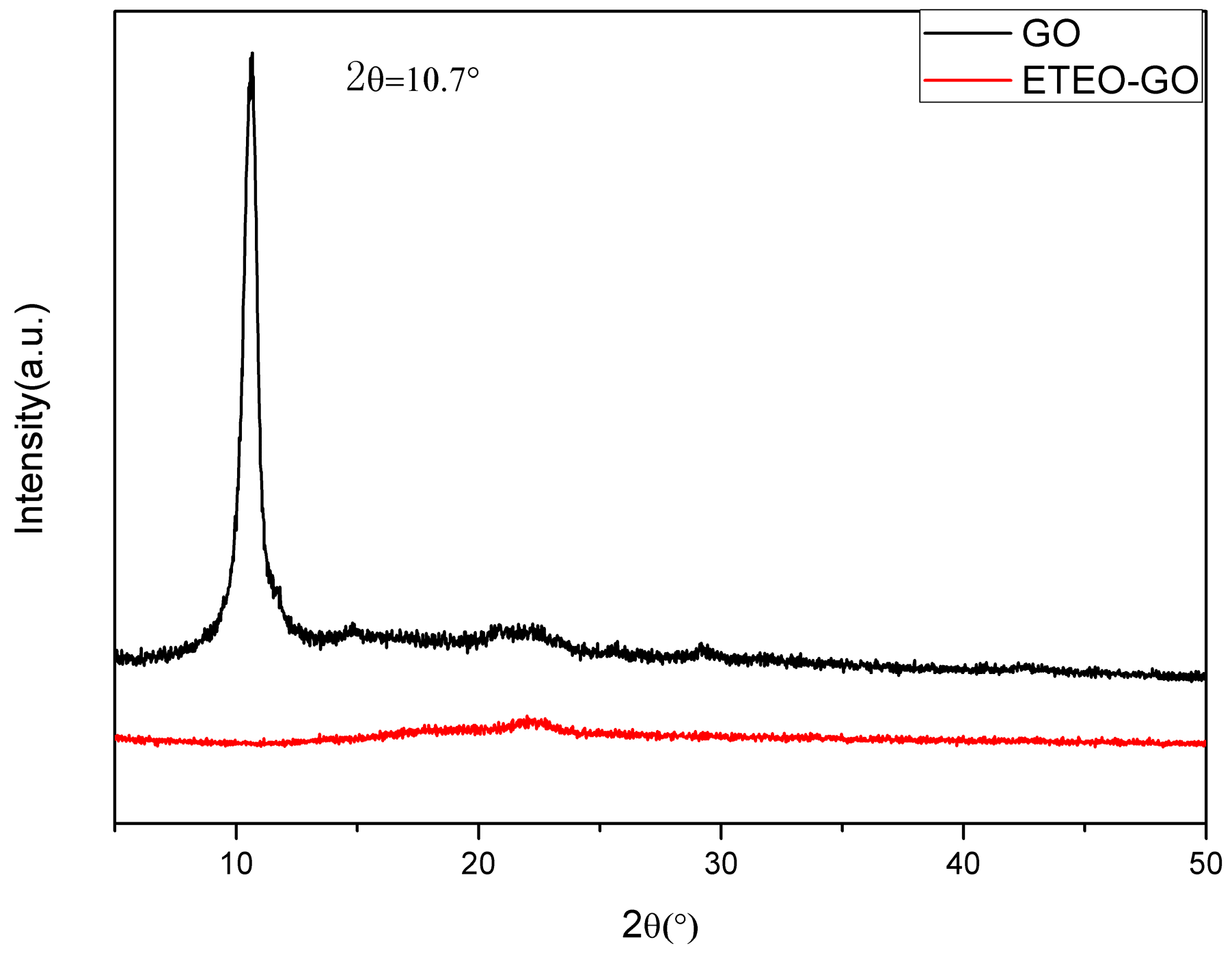
3.2. XRD Analysis
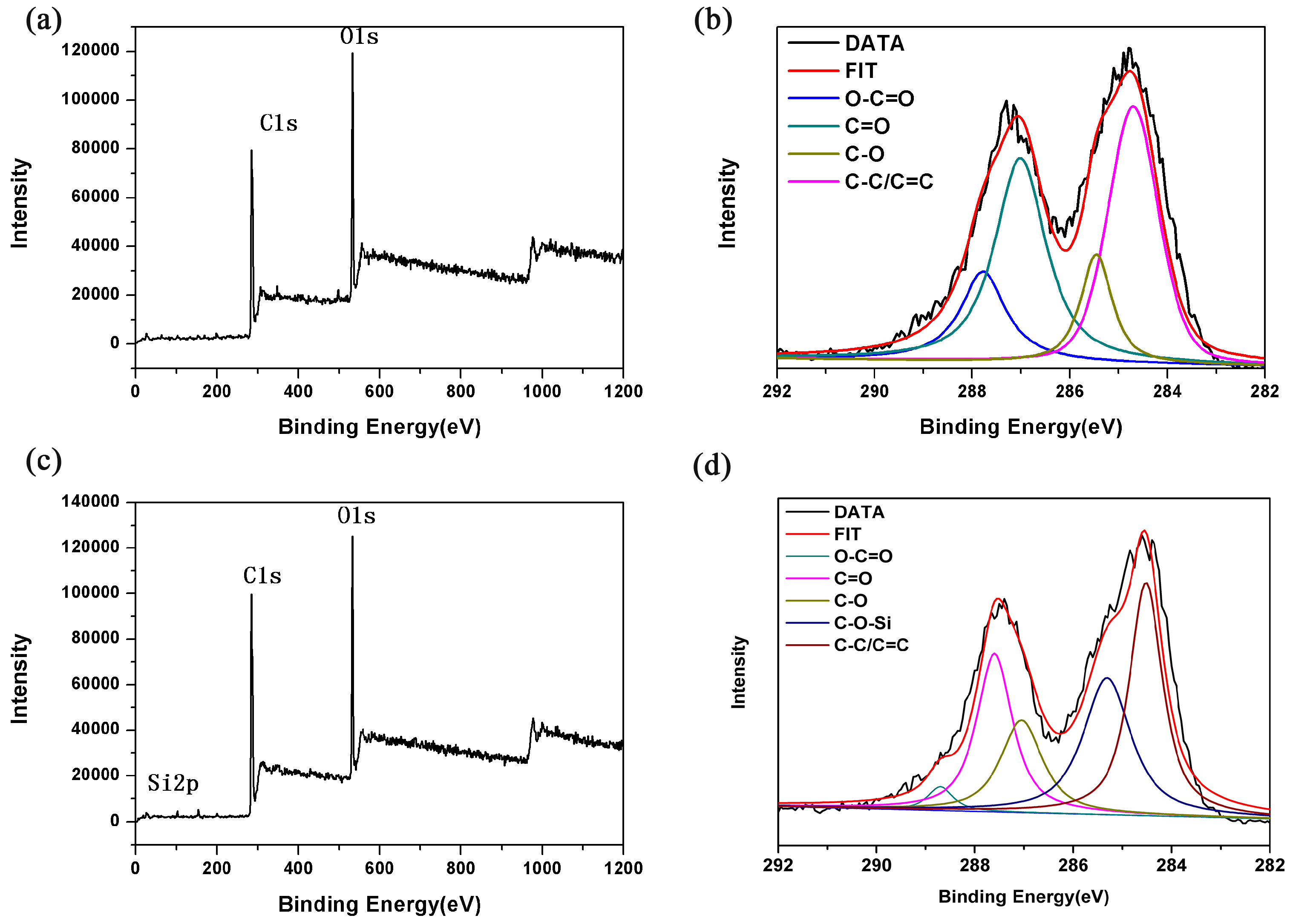
3.3. XPS Analysis
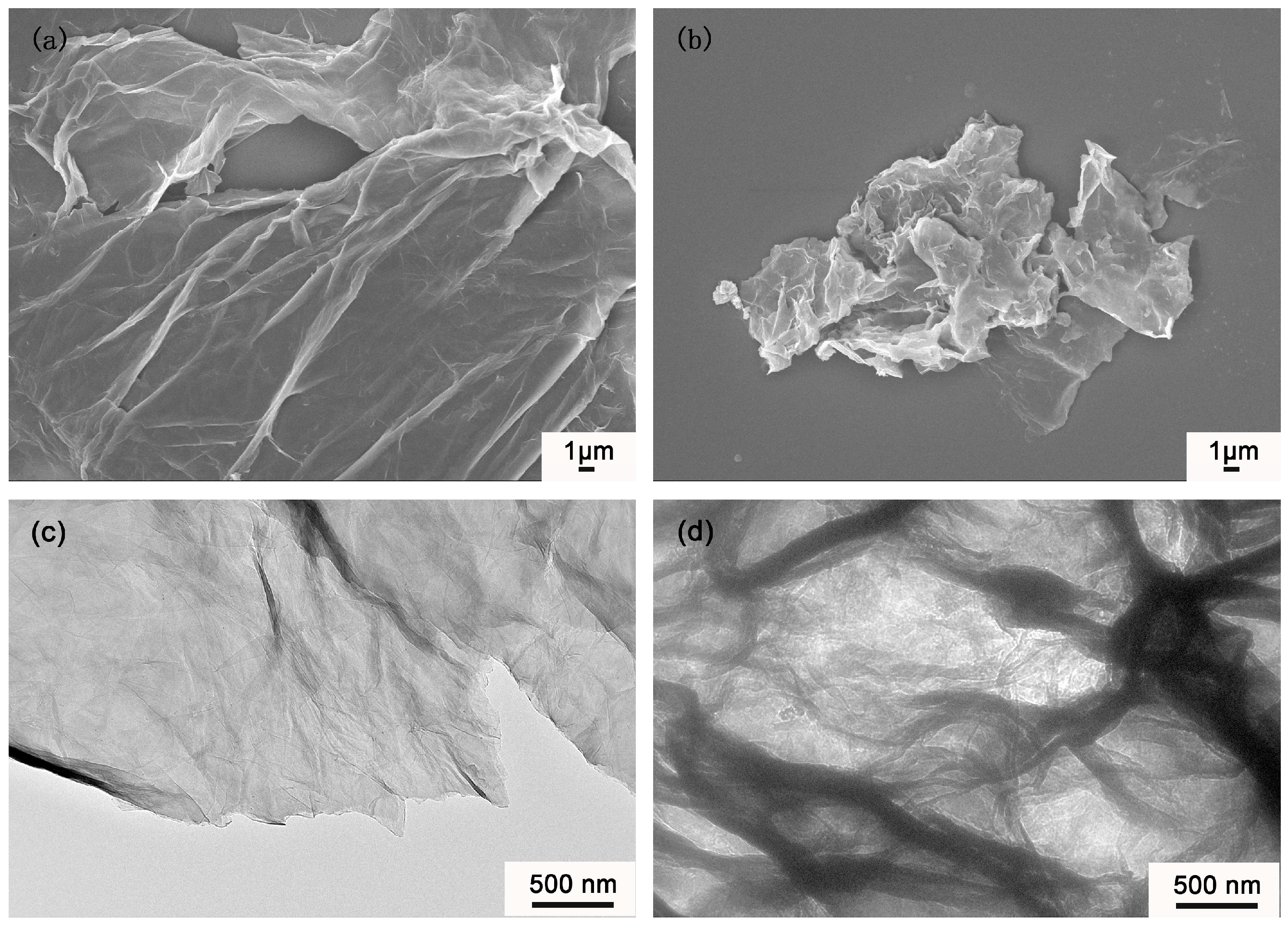
3.4. Morphology Analysis
3.5. Morphology Analysis of Coating Cross-Section
3.6. Mechanical Properties
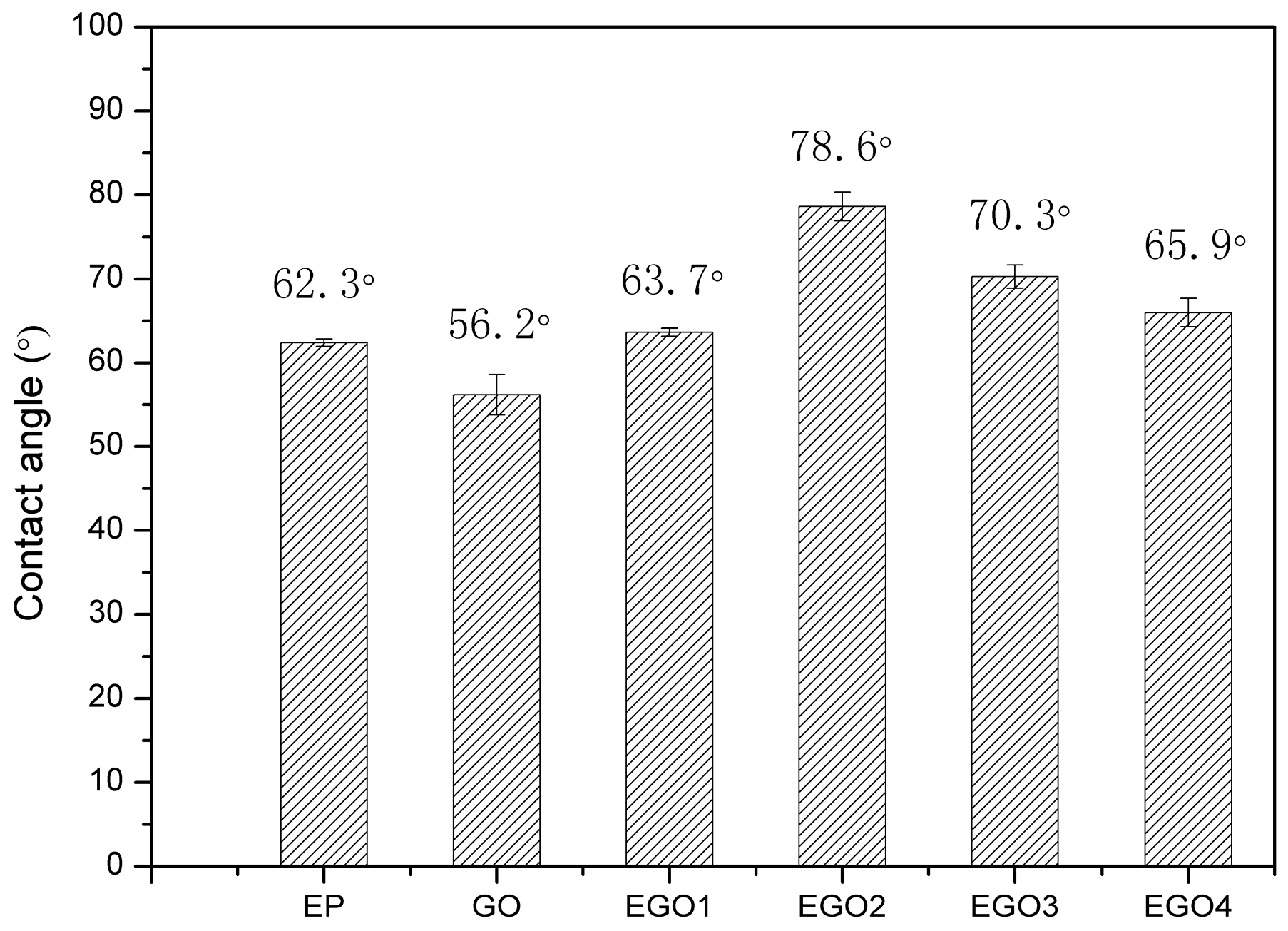
3.7. Wetting Performance
3.8. Electrochemical Impedance Spectroscopy
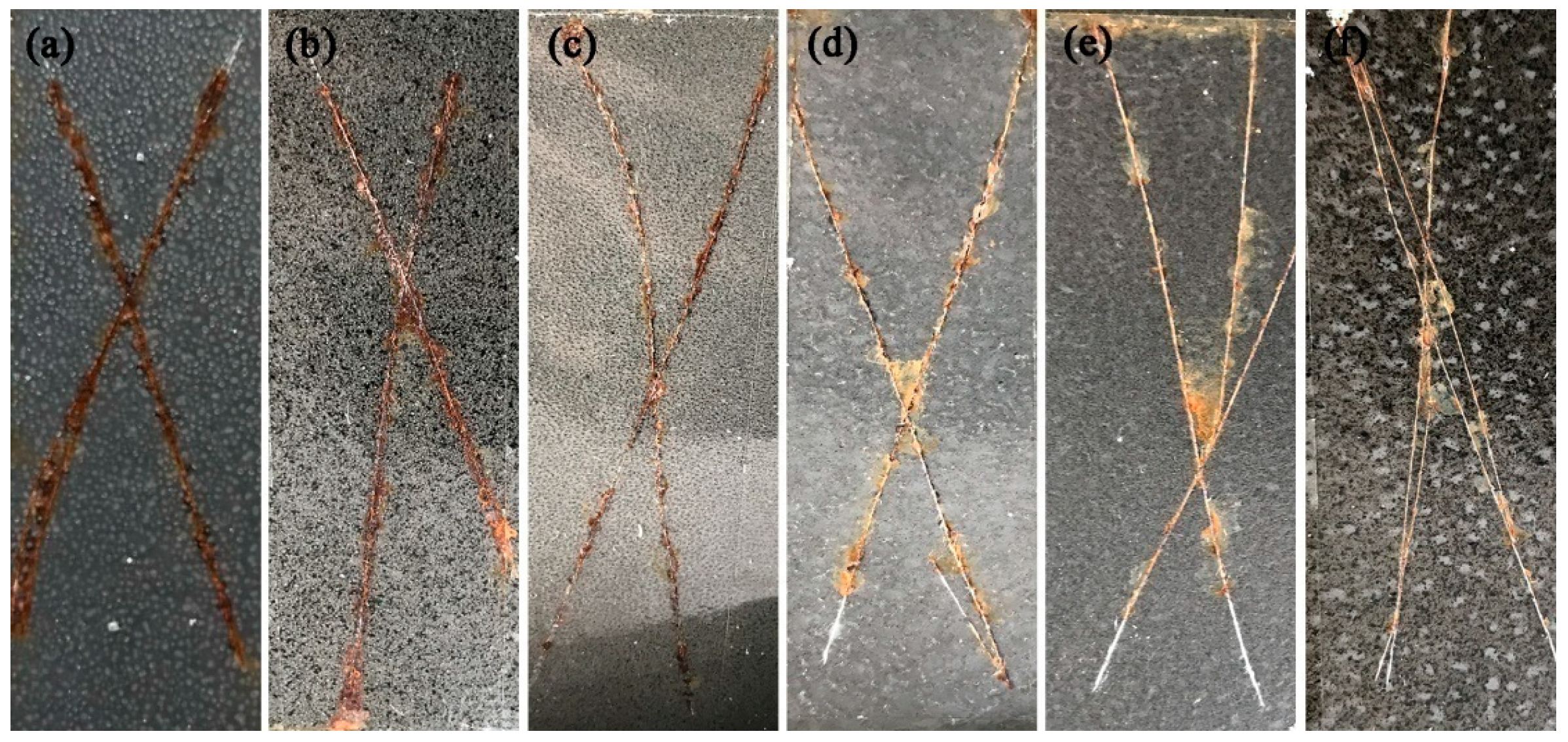
3.9. Salt Spray Test
3.10. Analysis of Corrosion Protection Mechanism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zheludkevich, M.L.; Tedim, J.; Ferreira, M.G.S. “Smart” coatings for active corrosion protection based on multi-functional micro and nanocontainers. Electrochim. Acta 2012, 82, 314–323. [Google Scholar] [CrossRef]
- Tallman, D.E.; Spinks, G.; Dominis, A.; Wallace, G.G. Electroactive conducting polymers for corrosion control. J. Solid State Electrochem. 2002, 6, 73–84. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Hubbard, J.B.; McFadden, G.B. Mathematical model for the cathodic blistering of organic coatings on steel immersed in electrolytes. J. Coat. Technol. 1991, 63, 43–52. [Google Scholar]
- Grundmeier, G.; Schmidt, W.; Stratmann, M. Corrosion protection by organic coatings: Electrochemical mechanism and novel methods of investigation. Electrochim. Acta 2000, 45, 2515–2533. [Google Scholar] [CrossRef]
- Babić, R.; Metikoš-Huković, M.; Radovčić, H. The study of coal tar epoxy protective coatings by impedance spectroscopy. Prog. Org. Coat. 1994, 23, 275–286. [Google Scholar] [CrossRef]
- Pascault, J.P.; Williams, R.J.J. Relationships between glass-transition temperature and conversion-analyses of limiting cases. Polym. Bull. 1990, 24, 115–121. [Google Scholar] [CrossRef]
- Chmielewska, D.; Sterzyński, T.; Dudziec, B. Epoxy compositions cured with aluminosilsesquioxanes: Thermomechanical properties. J. Appl. Polym. Sci. 2014, 131, 8444–8452. [Google Scholar] [CrossRef]
- Arman, S.Y.; Ramezanzadeh, B.; Farghadani, S.; Mehdipour, M.; Rajabi, A. Application of the electrochemical noise to investigate the corrosion resistance of an epoxy zinc-rich coating loaded with lamellar aluminum and micaceous iron oxide particles. Corros. Sci. 2013, 77, 118–127. [Google Scholar] [CrossRef]
- Gharagozlou, M.; Ramezanzadeh, B.; Baradaran, Z. Synthesize and characterization of a novel anticorrosive cobalt ferrite nanoparticles dispersed in silica matrix (CoFe2O4-SiO2) to improve the corrosion protection performance of epoxy coating. Appl. Surf. Sci. 2016, 377, 86–98. [Google Scholar] [CrossRef]
- Shanker, A.K.; Cervantes, C.; Loza-Tavera, H.; Avudainayagam, S. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–753. [Google Scholar] [CrossRef]
- Hodgkin, J.H.; Simon, G.P.; Varley, R.J. Thermoplastic toughening of epoxy resins: A critical review. Polym. Adv. Technol. 1998, 9, 3–10. [Google Scholar] [CrossRef]
- Na, T.; Liu, X.; Jiang, H.; Zhao, L.; Zhao, C. Enhanced thermal conductivity of fluorinated epoxy resins by incorporating inorganic filler. React. Funct. Polym. 2018, 128, 84–90. [Google Scholar] [CrossRef]
- Tao, Z.; Yang, S.; Ge, Z.; Chen, J.; Fan, L. Synthesis and properties of novel fluorinated epoxy resins based on 1,1-bis(4-glycidylesterphenyl)-1-(3′-trifluoromethylphenyl)-2,2,2-trifluoroethane. Eur. Polym. J. 2007, 43, 550–560. [Google Scholar] [CrossRef]
- Chikhi, N.; Fellahi, S.; Bakar, M. Modification of epoxy resin using reactive liquid (ATBN) rubber. Eur. Polym. J. 2002, 38, 251–264. [Google Scholar] [CrossRef]
- Chaudhary, S.; Surekha, P.; Kumar, D.; Rajagopal, C.; Roy, P.K. Amine-functionalized poly(styrene) microspheres as thermoplastic toughener for epoxy resin. Polym. Compos. 2015, 36, 174–183. [Google Scholar] [CrossRef]
- Conde, A.; Durán, A.; de Damborenea, J.J. Polymeric sol–gel coatings as protective layers of aluminium alloys. Prog. Org. Coat. 2003, 46, 288–296. [Google Scholar] [CrossRef]
- Dhoke, S.K.; Khanna, A.S. Effect of nano-Fe2O3 particles on the corrosion behavior of alkyd based waterborne coatings. Corros. Sci. 2009, 51, 6–20. [Google Scholar] [CrossRef]
- Dolatzadeh, F.; Moradian, S.; Jalili, M.M. Influence of various surface treated silica nanoparticles on the electrochemical properties of SiO2/polyurethane nanocoatings. Corros. Sci. 2011, 53, 4248–4257. [Google Scholar] [CrossRef]
- Kozhukharov, S.; Tsaneva, G.; Kozhukharov, V.; Gerwann, J.; Schem, M.; Schmidt, T.; Veith, M. Corrosion protection properties of composite hybrid coatings with involved nanoparticles of zirconia and ceria. J. Univ. Chem. Technol. Metall. 2008, 43, 73–80. [Google Scholar]
- Radhakrishnan, S.; Siju, C.R.; Mahanta, D.; Patil, S.; Madras, G. Conducting polyaniline–nano-TiO2 composites for smart corrosion resistant coatings. Electrochim. Acta 2009, 54, 1249–1254. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Attar, M.M. Studying the corrosion resistance and hydrolytic degradation of an epoxy coating containing zno nanoparticles. Mater. Chem. Phys. 2011, 130, 1208–1219. [Google Scholar] [CrossRef]
- Schem, M.; Schmidt, T.; Gerwann, J.; Wittmar, M.; Veith, M.; Thompson, G.E.; Molchan, I.S.; Hashimoto, T.; Skeldon, P.; Phani, A.R.; et al. CeO2-filled sol–gel coatings for corrosion protection of AA2024-T3 aluminium alloy. Corros. Sci. 2009, 51, 2304–2315. [Google Scholar] [CrossRef]
- Yu, H.J.; Wang, L.; Shi, Q.; Jiang, G.H.; Zhao, Z.R.; Dong, X.C. Study on nano-CaCO3 modified epoxy powder coatings. Prog. Org. Coat. 2006, 55, 296–300. [Google Scholar] [CrossRef]
- Wan, Y.-J.; Tang, L.-C.; Gong, L.-X.; Yan, D.; Li, Y.-B.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q. Grafting of epoxy chains onto graphene oxide for epoxy composites with improved mechanical and thermal properties. Carbon 2014, 69, 467–480. [Google Scholar] [CrossRef]
- Yi, H.; Chen, C.; Zhong, F.; Xu, Z. Preparation of aluminum oxide-coated carbon nanotubes and the properties of composite epoxy coatings research. High Perform. Polym. 2014, 26, 255–264. [Google Scholar] [CrossRef]
- Kalenda, P.; Kalendová, A.; Štengl, V.; Antoš, P.; Šubrt, J.; Kváča, Z.; Bakardjieva, S. Properties of surface-treated mica in anticorrosive coatings. Prog. Org. Coat. 2004, 49, 137–145. [Google Scholar] [CrossRef]
- Dong, Y.; Li, S.; Zhou, Q. Self-healing capability of inhibitor-encapsulating polyvinyl alcohol/polyvinylidene fluoride coaxial nanofibers loaded in epoxy resin coatings. Prog. Org. Coat. 2018, 120, 49–57. [Google Scholar] [CrossRef]
- Spainhour, L.K.; Wootton, I.A. Corrosion process and abatement in reinforced concrete wrapped by fiber reinforced polymer. Cem. Concr. Compos. 2008, 30, 535–543. [Google Scholar] [CrossRef]
- Shi, X.; Nguyen, T.A.; Suo, Z.; Liu, Y.; Avci, R. Effect of nanoparticles on the anticorrosion and mechanical properties of epoxy coating. Surf. Coat. Technol. 2009, 204, 237–245. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Liang, J.; H, Y.; Zhang, L.; Wang, Y.; Ma, Y.; Guo, T.; Chen, Y. Molecular-level dispersion of graphene into poly(vinyl alcohol) and effective reinforcement of their nanocomposites. Adv. Funct. Mater. 2009, 19, 2297–2302. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, R.; Young, R.J.; Deng, L.; Yang, F.; Hao, L.; Jiao, W.; Liu, W. Control of the functionality of graphene oxide for its application in epoxy nanocomposites. Polymer 2013, 54, 6437–6446. [Google Scholar] [CrossRef]
- Wang, Y.N.; Dai, X.Y.; Xu, T.L.; Qu, L.J.; Zhang, C.L. Preparation and anticorrosion properties of silane grafted nano-silica/epoxy composite coating. Chem. J. Chin. Univ. 2018, 39, 1564–1572. [Google Scholar]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- GB/T 6739-2006 Paints and Varnishes–Determination of Film Hardness by Pencil Test; Standardization Administration of China: Beijing, China, 2006. (In Chinese)
- ASTM D2794-93 Standard Test Method for Resistance of Organic Coatings to the Effects of Rapid Deformation (Impact); ASTM International: West Conshohocken, PA, USA, 2010.
- GB/T 10125-1997 Corrosion Tests in Artificial Atmospheres–Salt Spray Tests; Standardization Administration of China: Beijing, China, 2006. (In Chinese)
- Ramezanzadeh, B.; Ahmadi, A.; Mahdavian, M. Enhancement of the corrosion protection performance and cathodic delamination resistance of epoxy coating through treatment of steel substrate by a novel nanometric sol-gel based silane composite film filled with functionalized graphene oxide nanosheets. Corros. Sci. 2016, 109, 182–205. [Google Scholar] [CrossRef]
- Ganjaee Sari, M.; Shamshiri, M.; Ramezanzadeh, B. Fabricating an epoxy composite coating with enhanced corrosion resistance through impregnation of functionalized graphene oxide-co-montmorillonite nanoplatelet. Corros. Sci. 2017, 129, 38–53. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, L.; Qian, H.; Li, X. Superhydrophobic surfaces for corrosion protection: A review of recent progresses and future directions. J. Coat. Technol. Res. 2016, 13, 11–29. [Google Scholar] [CrossRef]
- Kang, W.-S.; Rhee, K.Y.; Park, S.-J. Influence of surface energetics of graphene oxide on fracture toughness of epoxy nanocomposites. Compos. Part B Eng. 2017, 114, 175–183. [Google Scholar] [CrossRef]



| Sample | Epoxy Resin (g) | Curing Agent (g) | Filler (wt %) |
|---|---|---|---|
| Pure epoxy (EP) | 20 | 16 | – |
| GO | 20 | 16 | GO (0.7 wt %) |
| EGO1 | 20 | 16 | ETEO-GO (0.1 wt %) |
| EGO2 | 20 | 16 | ETEO-GO (0.4 wt %) |
| EGO3 | 20 | 16 | ETEO-GO (0.7 wt %) |
| EGO4 | 20 | 16 | ETEO-GO (1.0 wt %) |
| Sample | Hardness (H) |
|---|---|
| EP | 2 |
| GO | 3 |
| EGO1 | 2 |
| EGO2 | 3 |
| EGO3 | 4 |
| EGO4 | 4 |
| Sample | Height (cm) |
|---|---|
| EP | 10.2 ± 0.5 |
| GO | 38.7 ± 1.6 |
| EGO1 | 19.4 ± 0.4 |
| EGO2 | 22.4 ± 0.5 |
| EGO3 | ≥50 |
| EGO4 | 40.1 ± 0.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Dai, X.; Wang, Y.; Sun, G.; Li, P.; Qu, L.; Sui, Y.; Dou, Y. Preparation and Corrosion Resistance of ETEO Modified Graphene Oxide/Epoxy Resin Coating. Coatings 2019, 9, 46. https://doi.org/10.3390/coatings9010046
Zhang C, Dai X, Wang Y, Sun G, Li P, Qu L, Sui Y, Dou Y. Preparation and Corrosion Resistance of ETEO Modified Graphene Oxide/Epoxy Resin Coating. Coatings. 2019; 9(1):46. https://doi.org/10.3390/coatings9010046
Chicago/Turabian StyleZhang, Chunling, Xueyan Dai, Yingnan Wang, Guoen Sun, Peihong Li, Lijie Qu, Yanlong Sui, and Yanli Dou. 2019. "Preparation and Corrosion Resistance of ETEO Modified Graphene Oxide/Epoxy Resin Coating" Coatings 9, no. 1: 46. https://doi.org/10.3390/coatings9010046
APA StyleZhang, C., Dai, X., Wang, Y., Sun, G., Li, P., Qu, L., Sui, Y., & Dou, Y. (2019). Preparation and Corrosion Resistance of ETEO Modified Graphene Oxide/Epoxy Resin Coating. Coatings, 9(1), 46. https://doi.org/10.3390/coatings9010046





