Preparation and Photoluminescence of Tungsten Disulfide Monolayer
Abstract
1. Introduction
2. Materials and Methods
WS2 Layer Synthesis
3. Results and Discussion
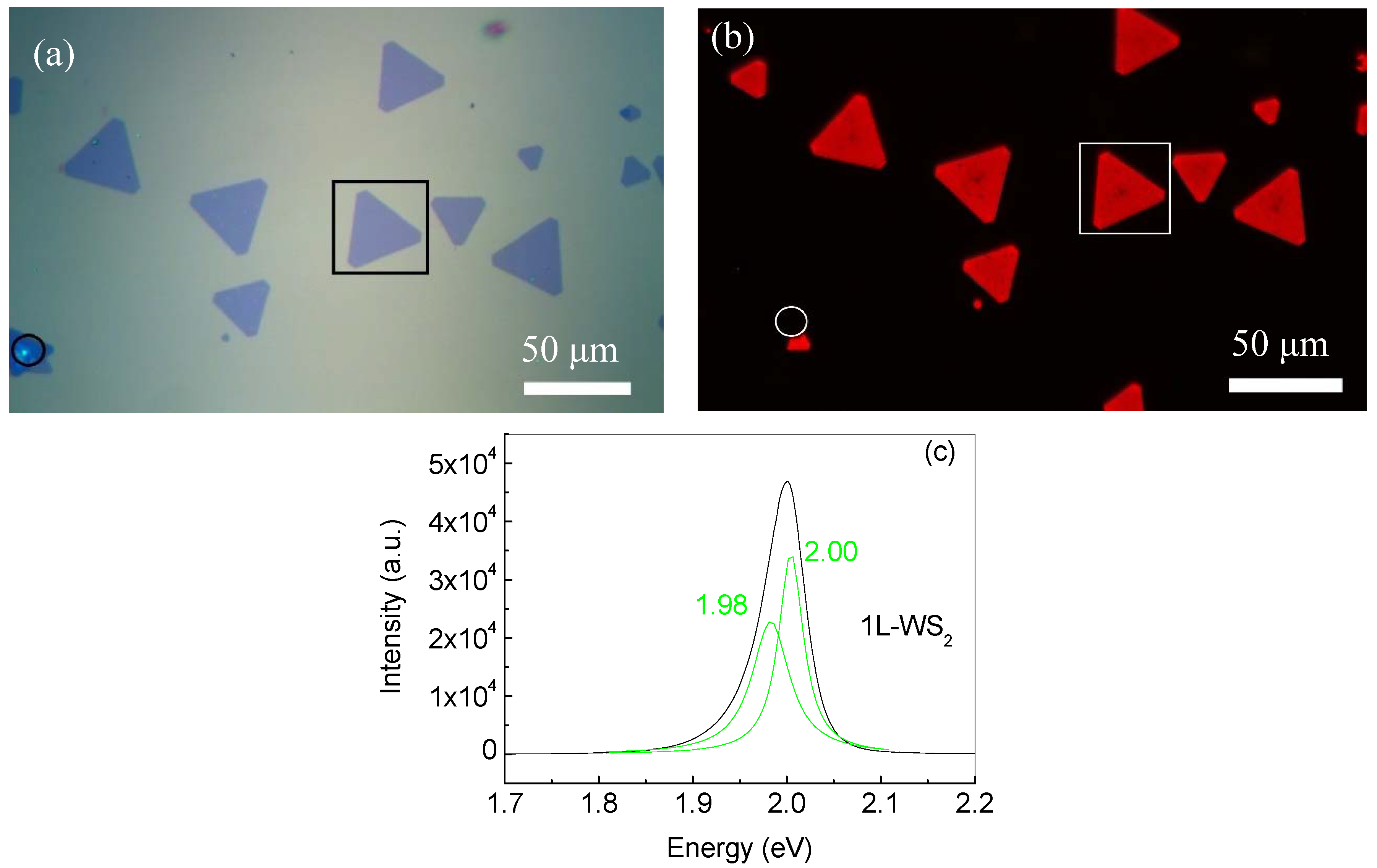
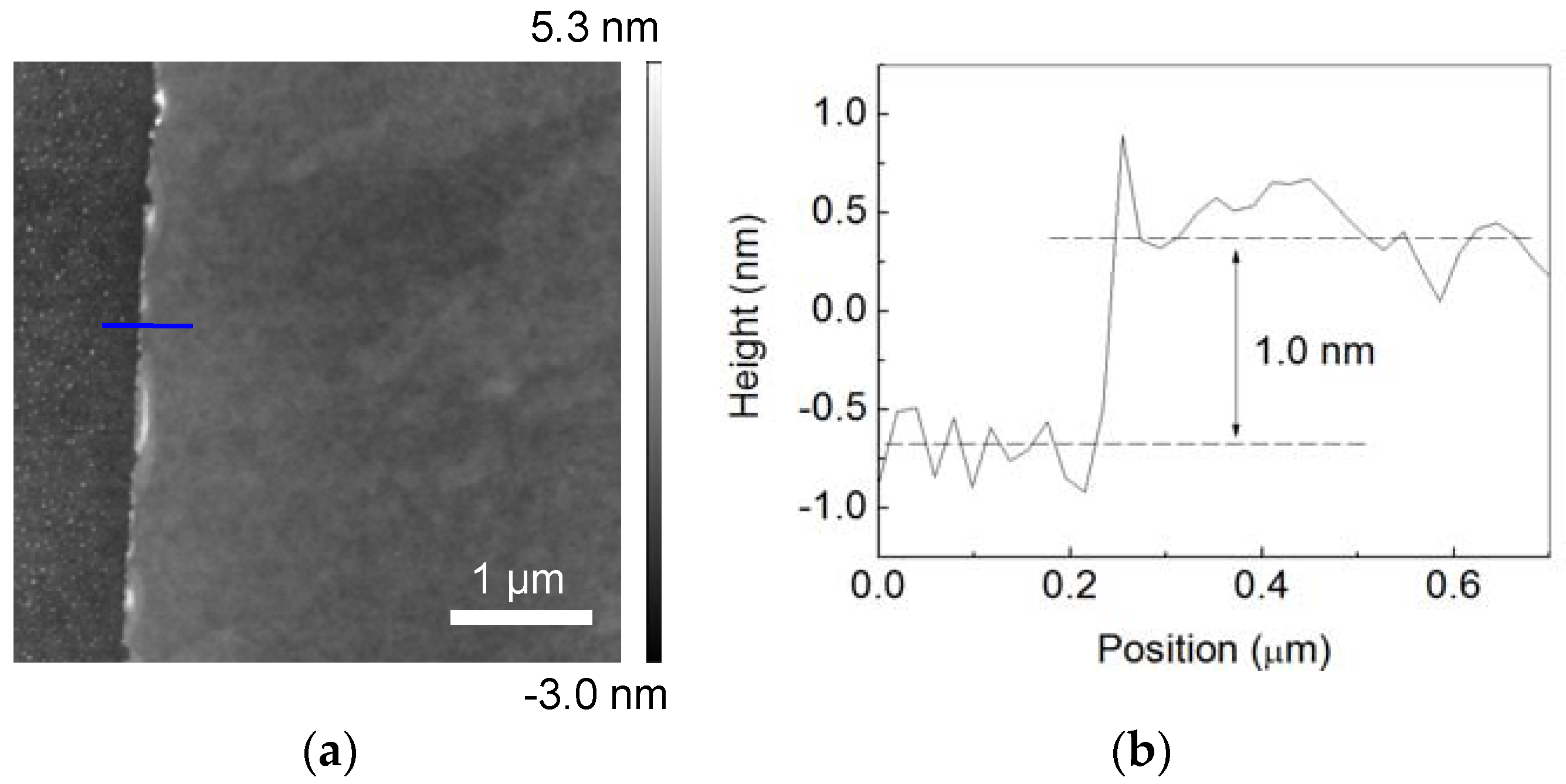
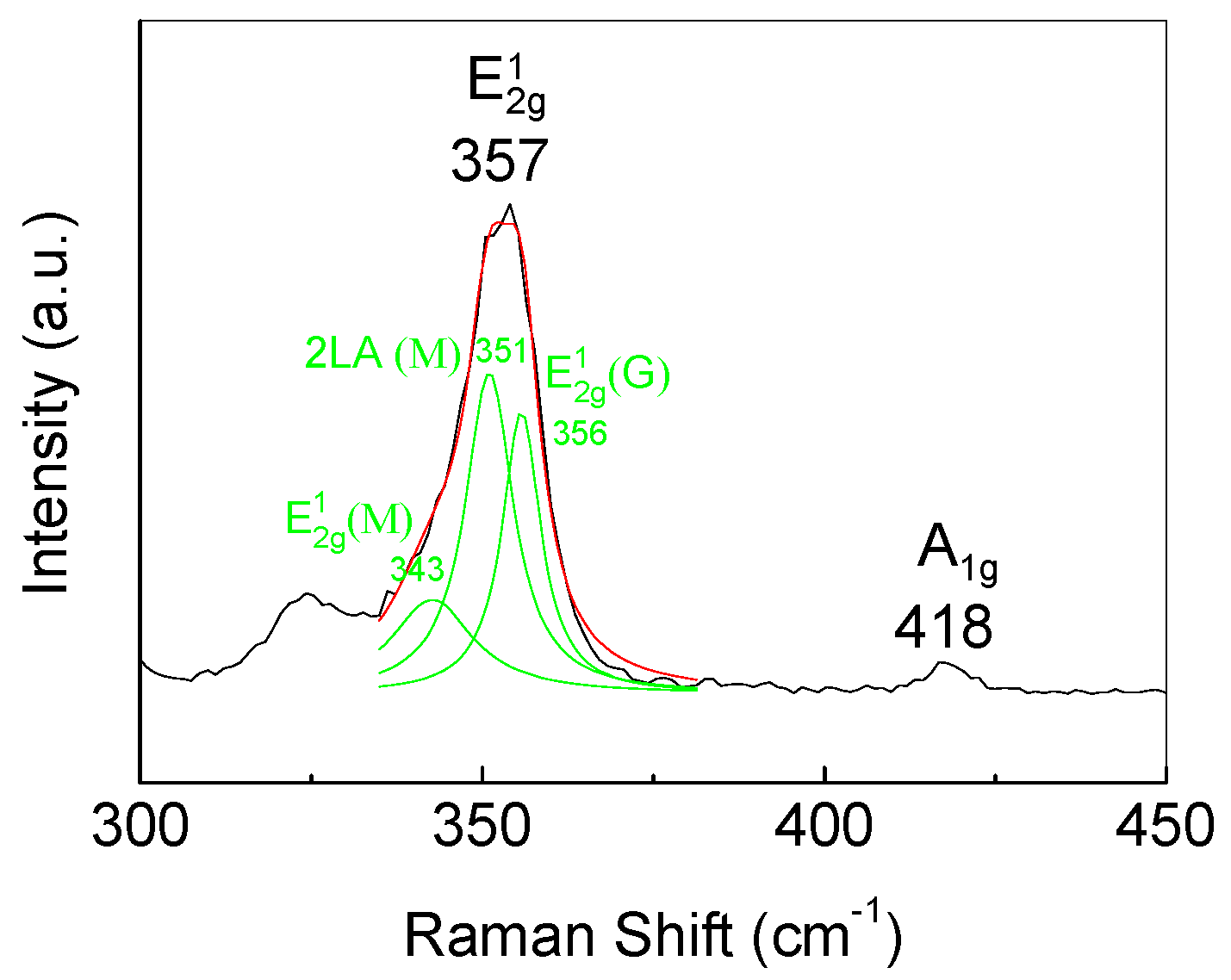
3.1. WS2 Monolayer Growth
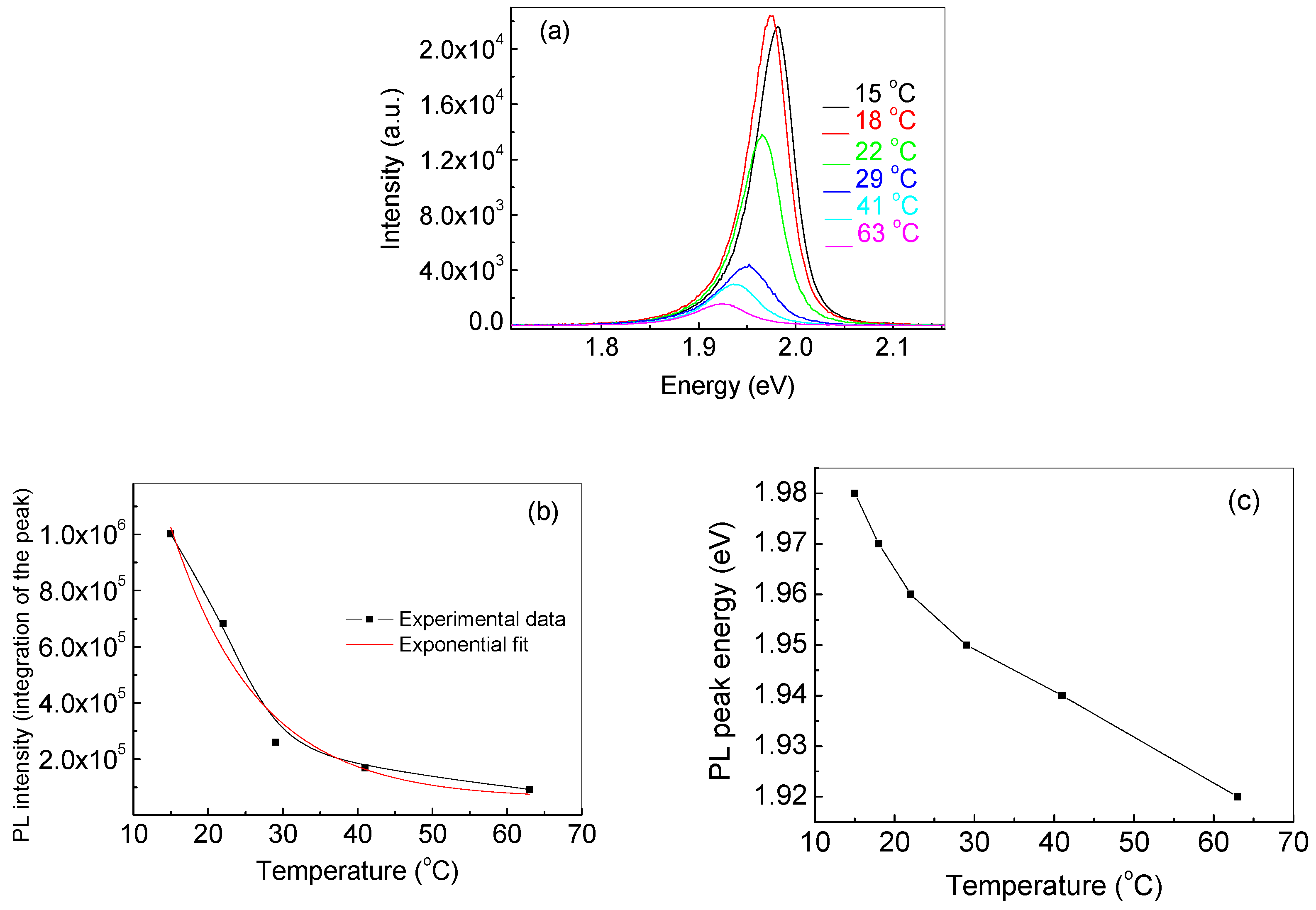
3.2. Temperature Dependence of the PL
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Zhang, Y.F.; Ji, Q.Q.; Ju, J.; Yuan, H.T.; Shi, J.P.; Gao, T.; Ma, D.L.; Liu, M.X.; Chen, Y.B.; et al. Controlled growth of high-quality monolayer WS2 layers on sapphire and imaging its grain boundary. ACS Nano 2013, 7, 8963–8971. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, H.R.; Perea-Lopez, N.; Elias, A.L.; Berkdemir, A.; Wang, B.; Lv, R.; Lopez-Urias, F.; Crespi, V.H.; Terrones, H.; Terrones, M. Extraordinary room-temperature photoluminescence in triangular WS2 monolayers. Nano Lett. 2013, 13, 3447–3454. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.J.; Chae, S.H.; Kim, H.; Park, J.C.; Park, J.H.; Han, G.H.; Lee, J.S.; Kim, S.M.; Oh, H.M.; Seok, J.; et al. Synthesis of centimeter-scale monolayer tungsten disulfide film on gold foils. ACS Nano 2015, 9, 5510–5519. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Yun, S.J.; Lee, Y.; Seo, C.; Han, G.H.; Kim, K.K.; Lee, Y.H.; Kim, J. Biexciton emission from edges and grain boundaries of triangular WS2 monolayers. ACS Nano 2016, 10, 2399–2405. [Google Scholar] [CrossRef] [PubMed]
- Gordo, V.O.; Balanta, M.A.G.; Gobato, Y.G.; Covre, F.S.; Galeti, H.V.A.; Iikawa, F.; Couto, O.D.D., Jr.; Qu, F.; Henini, M.; Hewak, D.W.; et al. Revealing the nature of low-temperature photoluminescence peaks by laser treatment in Van der Waals epitaxially grown WS2 monolayers. Nanoscale 2018, 10, 4807–4815. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, M.R.; Chuang, H.J.; McCreary, K.M.; Li, C.H.; Jonker, B.T. Electrical characterization of discrete defects and impact of defect density on photoluminescence in monolayer WS2. ACS Nano 2018, 12, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Liu, L.; Wang, Z.; Li, H.; Chen, L.; Pam, M.E.; Chen, W.; Yang, H.Y.; Zhang, W.; Shi, Y. Significant photoluminescence enhancement in WS2 monolayers through Na2S treatment. Nanoscale 2018, 10, 6105–6112. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Cong, C.X.; Peimyoo, N.; Chen, Y.; Shang, J.Z.; Zou, C.J.; Cao, B.C.; Wu, L.S.; Zhang, J.; Eginligil, M.; et al. Tunable excitonic emission of monolayer WS2 for the optical detection of DNA nucleobases. Nano Res. 2018, 11, 1744–1754. [Google Scholar] [CrossRef]
- Xu, D.Y.; Xu, P.T.; Zhu, Y.Z.; Peng, W.C.; Li, Y.; Zhang, G.L.; Zhang, F.B.; Mallouk, T.E.; Fan, X.B. High yield exfoliation of WS2 crystals into 1–2 layer semiconducting nanosheets and efficient photocatalytic hydrogen evolution from WS2/CdS nanorod composites. ACS Appl. Mater. Interfaces 2018, 10, 2810–2818. [Google Scholar] [CrossRef] [PubMed]
- Groven, B.; Heyne, M.; Mehta, A.N.; Bender, H.; Nuytten, T.; Meersschaut, J.; Conard, T.; Verdonck, P.; Van Elshocht, S.; Vandervorst, W.; et al. Plasma-enhanced atomic layer deposition of two-dimensional WS2 from WF6, H2 plasma, and H2S. Chem. Mater. 2017, 29, 2927–2938. [Google Scholar] [CrossRef]
- Sahu, R.; Radhakrishnan, D.; Vishal, B.; Negi, D.S.; Sil, A.; Narayana, C.; Datta, R. Substrate induced tuning of compressive strain and phonon modes in large area MoS2 and WS2 van der Waals epitaxial thin films. J. Cryst. Growth 2017, 470, 51–57. [Google Scholar] [CrossRef][Green Version]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.F.; Park, J.S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Kim, H.R.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.C.; Surwade, S.P.; Li, Z.T.; Liu, H.T. Photochemical oxidation of CVD-grown single layer graphene. Nanotechnology 2012, 23, 355703. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.C.; Zhou, F.; Li, Z.T.; Liu, H.T. Effect of precursor purity and flow rate on the CVD growth of hexagonal boron nitride. J. Alloys Compd. 2016, 688, 1006–1012. [Google Scholar] [CrossRef]
- Cong, C.X.; Shang, J.Z.; Wu, X.; Cao, B.C.; Peimyoo, N.; Qiu, C.; Sun, L.T.; Yu, T. Synthesis and optical properties of large-area single-crystalline 2D semiconductor WS2 monolayer from chemical vapor deposition. Adv. Opt. Mater. 2014, 2, 131–136. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Z.B.; Sun, D.M.; Huang, L.; Ma, L.P.; Yin, L.C.; Ma, T.; Zhang, Z.Y.; Ma, X.L.; Peng, L.M.; et al. Large-area synthesis of high-quality and uniform monolayer WS2 on reusable Au foils. Nat. Commun. 2015, 6, 8569. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, S.; Tang, D.; Zhao, W.; Xu, H.; Chu, L.; Bando, Y.; Golberg, D.; Eda, G. Halide-assisted atmospheric pressure growth of large WSe2 and WS2 monolayer crystals. Appl. Mater. Today 2015, 1, 60–66. [Google Scholar] [CrossRef]
- Zhao, S.C.; Weng, J.X.; Jin, S.Z.; Lv, Y.F.; Ji, Z.G. Chemical vapor transport deposition of molybdenum disulfide layers using H2O vapor as the transport agent. Coatings 2018, 8, 78. [Google Scholar] [CrossRef]
- Ma, Y.D.; Dai, Y.; Guo, M.; Niu, C.W.; Lu, J.B.; Huang, B.B. Electronic and magnetic properties of perfect, vacancy-doped, and nonmetal adsorbed MoSe2, MoTe2 and WS2 monolayers. Phys. Chem. Chem. Phys. 2011, 13, 15546–15553. [Google Scholar] [CrossRef] [PubMed]
- Peimyoo, N.; Shang, J.Z.; Cong, C.X.; Shen, X.N.; Wu, X.Y.; Yeow, E.K.L.; Yu, T. Nonblinking, intense two-dimensional light emitter: Mono layer WS2 triangles. ACS Nano 2013, 7, 10985–10994. [Google Scholar] [CrossRef] [PubMed]
- Chow, P.K.; Jacobs-Gedrim, R.B.; Gao, J.; Lu, T.M.; Yu, B.; Terrones, H.; Koratkar, N. Defect-induced photoluminescence in monolayer semiconducting transition metal dichalcogenides. ACS Nano 2015, 9, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Stier, A.V.; McCreary, K.M.; Jonker, B.T.; Kono, J.; Crooker, S.A. Exciton diamagnetic shifts and valley Zeeman effects in monolayer WS2 and MoS2 to 65 Tesla. Nat. Commun. 2016, 7, 10643. [Google Scholar] [CrossRef] [PubMed]
- Su, L.Q.; Yu, Y.F.; Cao, L.Y.; Zhang, Y. Effects of substrate type and material-substrate bonding on high-temperature behavior of monolayer WS2. Nano Res. 2015, 8, 2686–2697. [Google Scholar] [CrossRef]
- Motta, C.; Mandal, P.; Sanvito, S. Effects of molecular dipole orientation on the exciton binding energy of CH3NH3PbI3. Phys. Rev. B 2016, 94, 045202. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Y.; Huang, F.; Zhang, L.; Weng, J.; Zhao, S.; Ji, Z. Preparation and Photoluminescence of Tungsten Disulfide Monolayer. Coatings 2018, 8, 205. https://doi.org/10.3390/coatings8060205
Lv Y, Huang F, Zhang L, Weng J, Zhao S, Ji Z. Preparation and Photoluminescence of Tungsten Disulfide Monolayer. Coatings. 2018; 8(6):205. https://doi.org/10.3390/coatings8060205
Chicago/Turabian StyleLv, Yanfei, Feng Huang, Luxi Zhang, Jiaxin Weng, Shichao Zhao, and Zhenguo Ji. 2018. "Preparation and Photoluminescence of Tungsten Disulfide Monolayer" Coatings 8, no. 6: 205. https://doi.org/10.3390/coatings8060205
APA StyleLv, Y., Huang, F., Zhang, L., Weng, J., Zhao, S., & Ji, Z. (2018). Preparation and Photoluminescence of Tungsten Disulfide Monolayer. Coatings, 8(6), 205. https://doi.org/10.3390/coatings8060205



