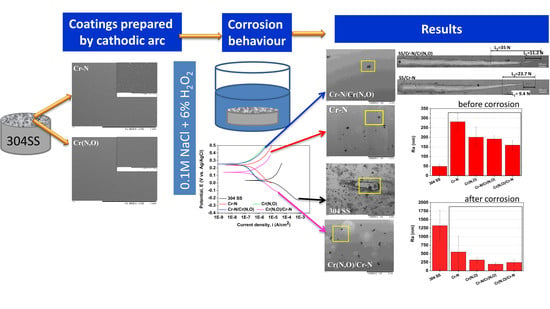
Enhancement of the Corrosion Resistance of 304 Stainless Steel by Cr–N and Cr(N,O) Coatings
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Surface Morphology and Elemental Composition before Corrosion Measurements
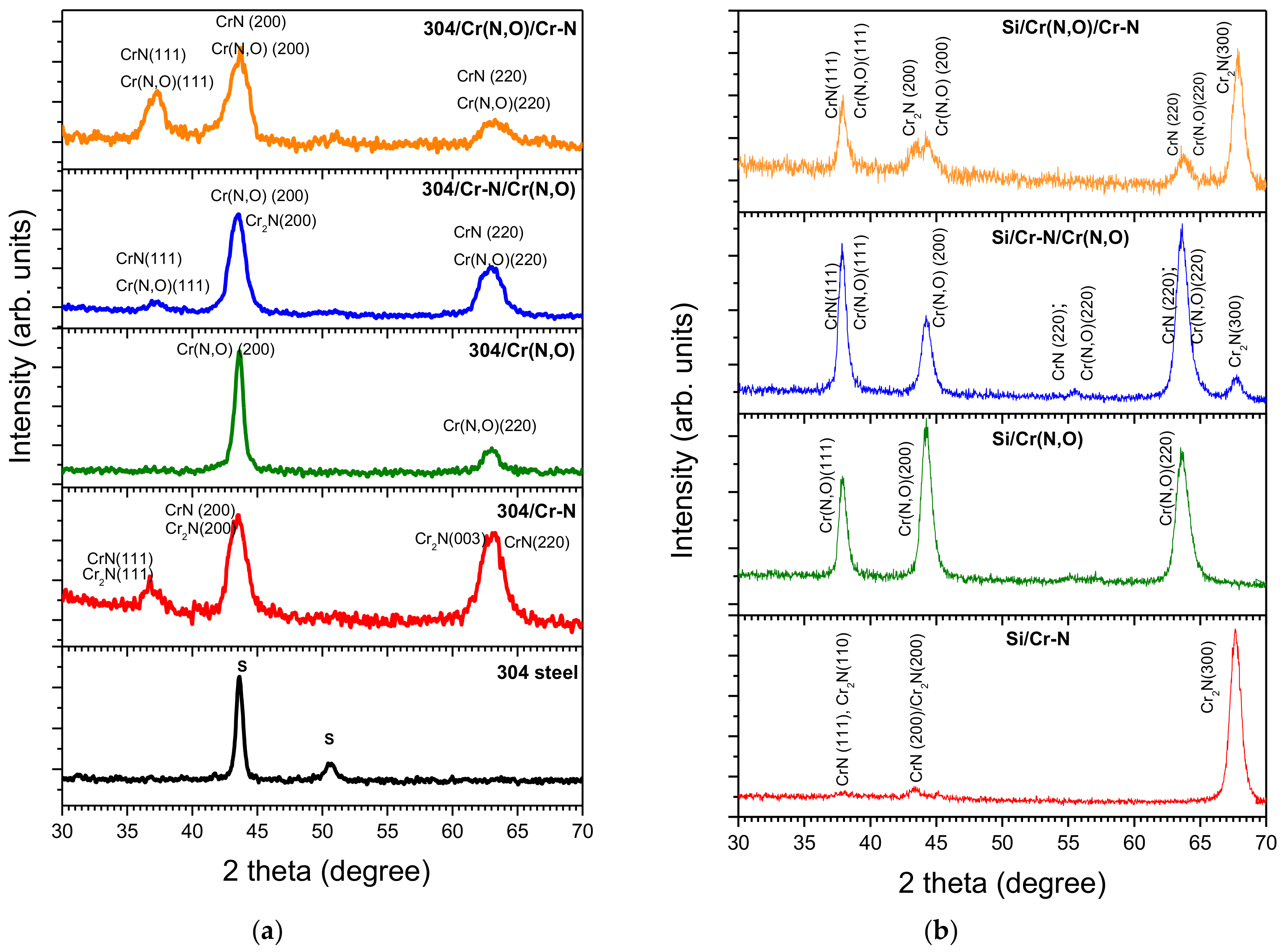
3.2. Phase Composition
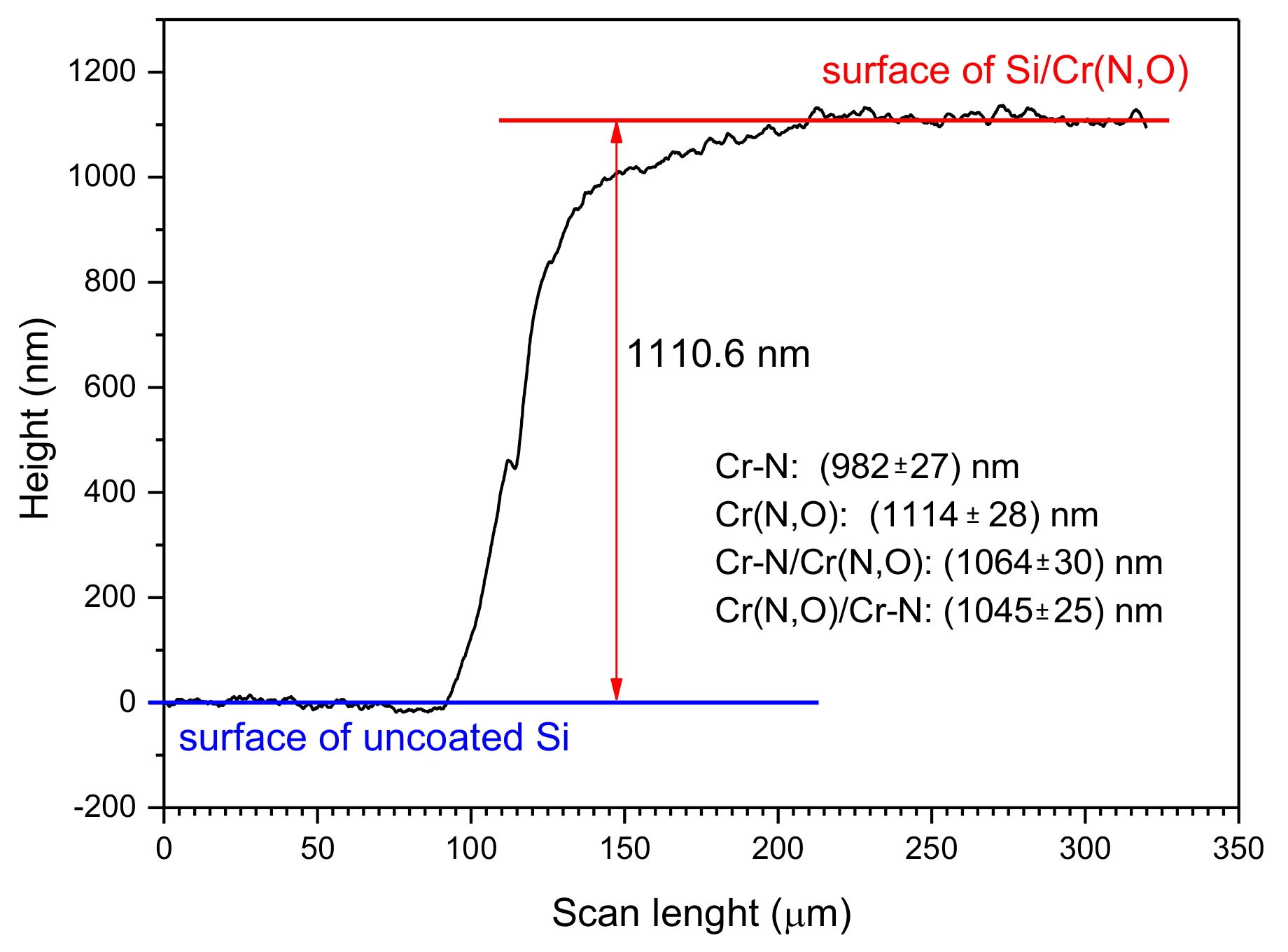
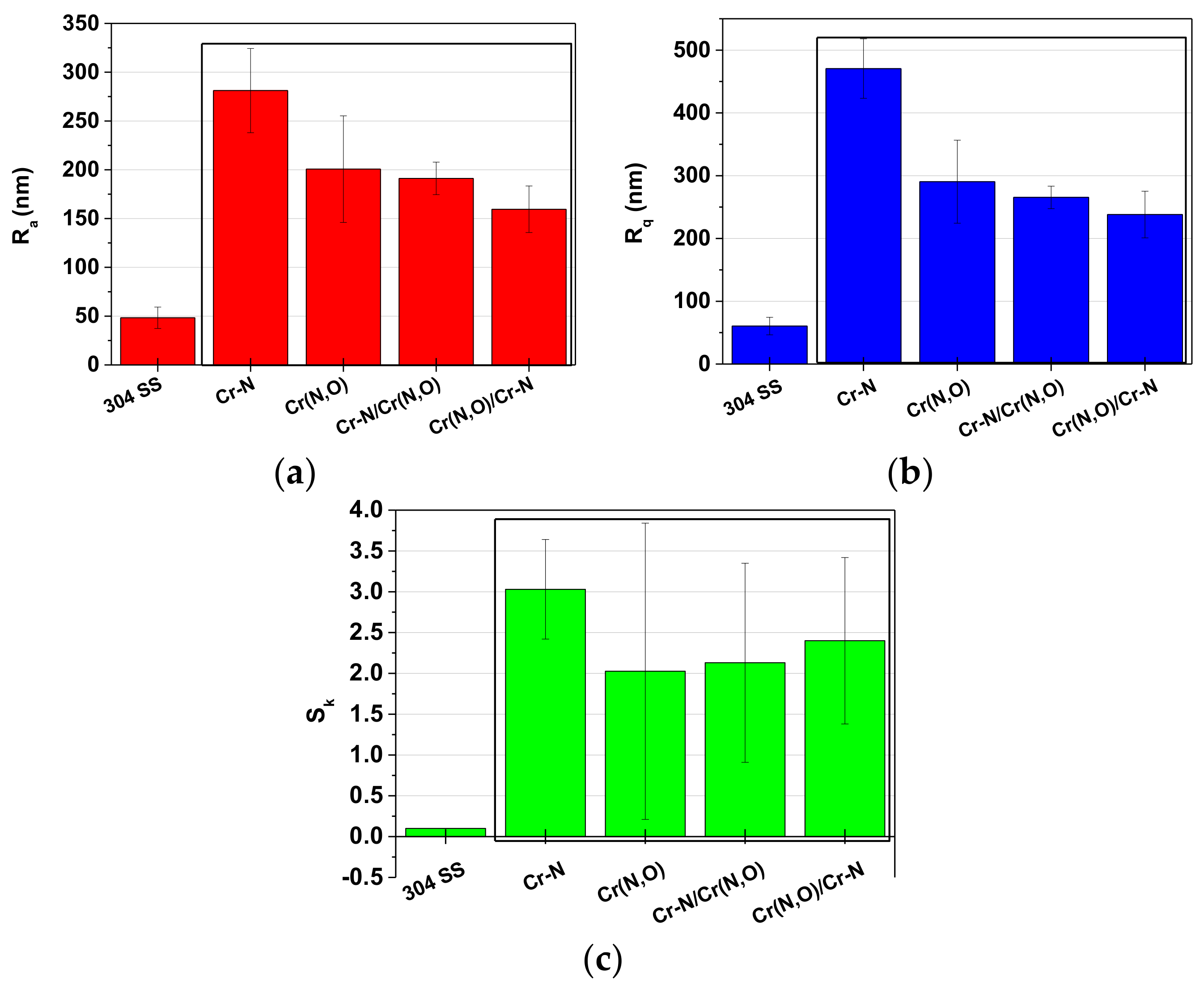
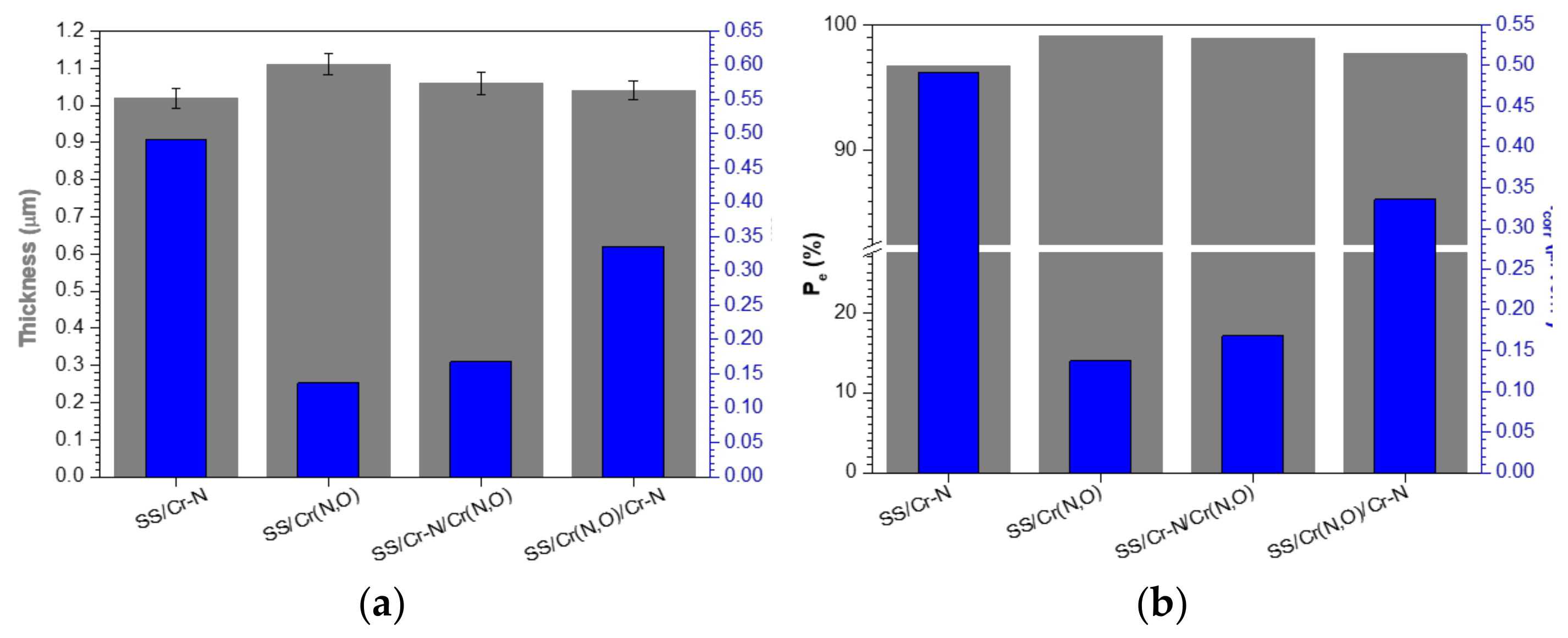
3.3. Coating Thickness and Roughness
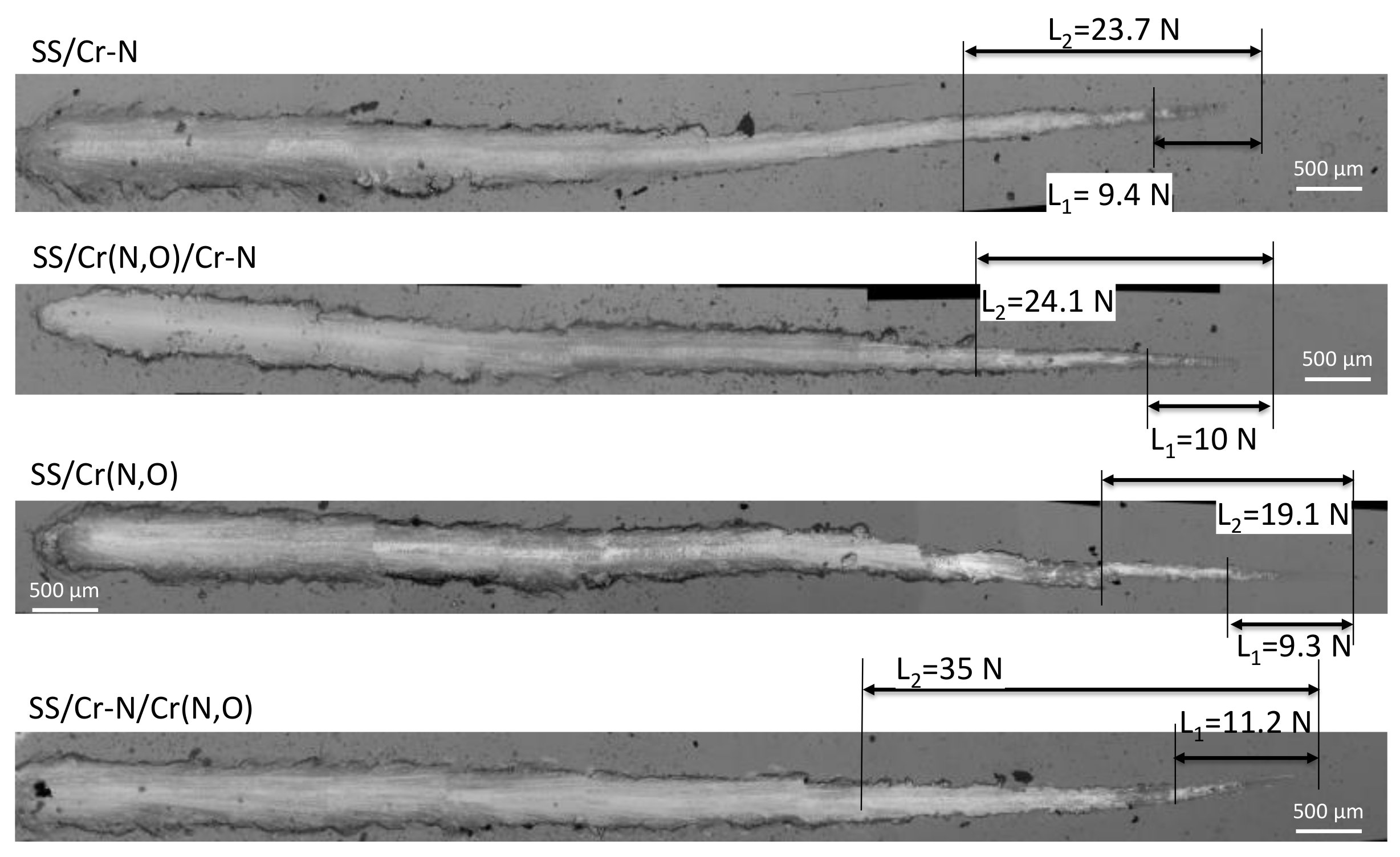
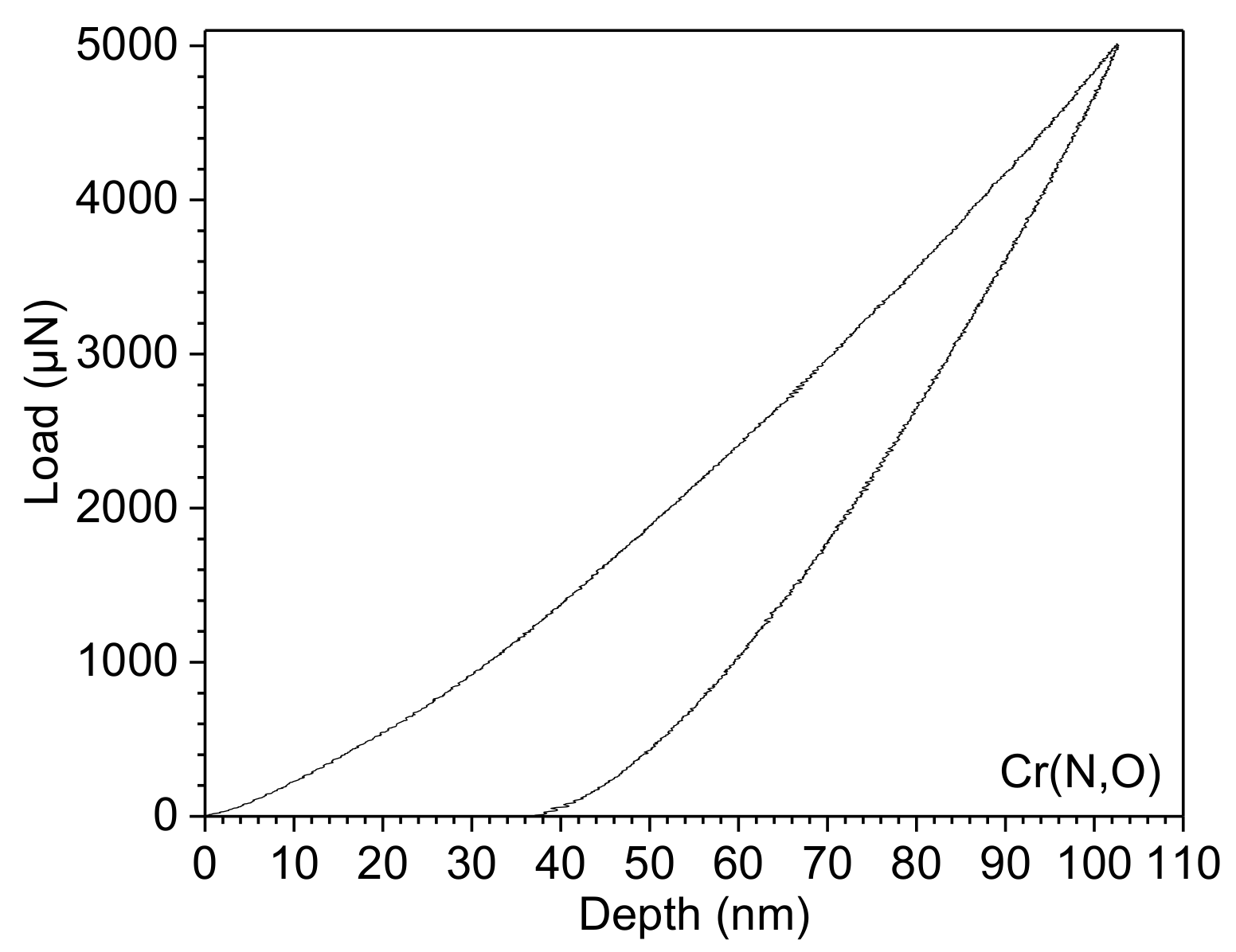
3.4. Coating Mechanical Properties
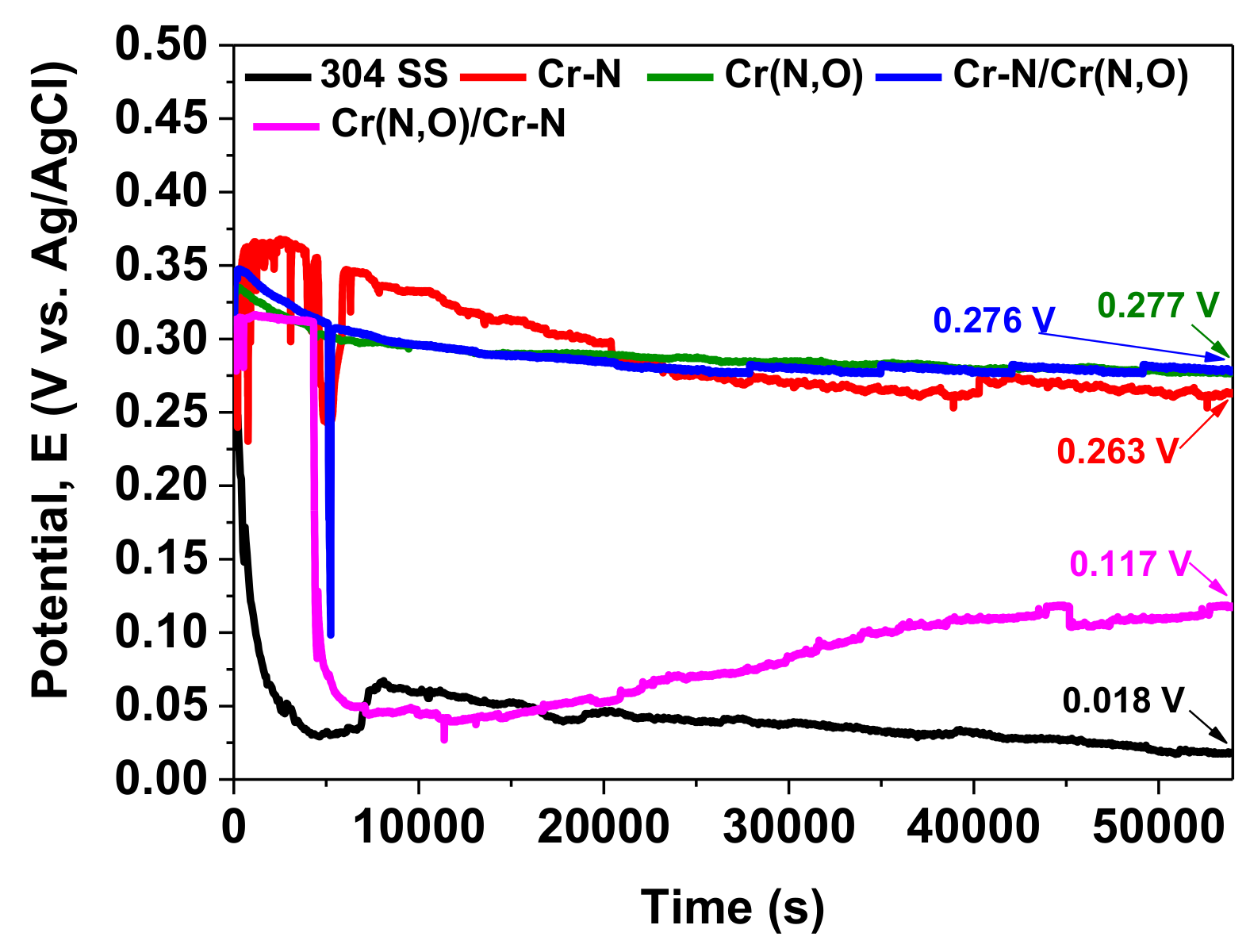
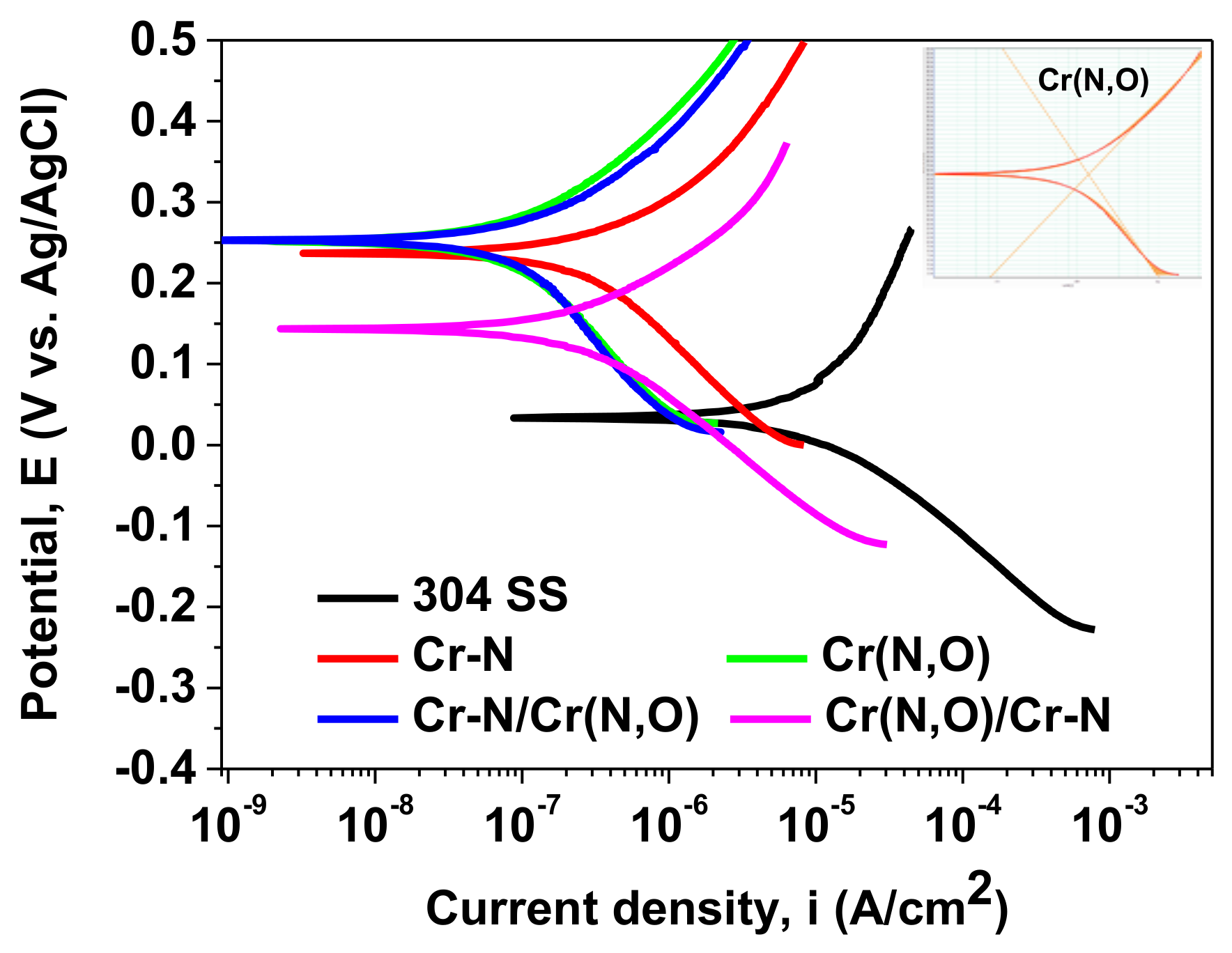
3.5. Corrosion Measurements
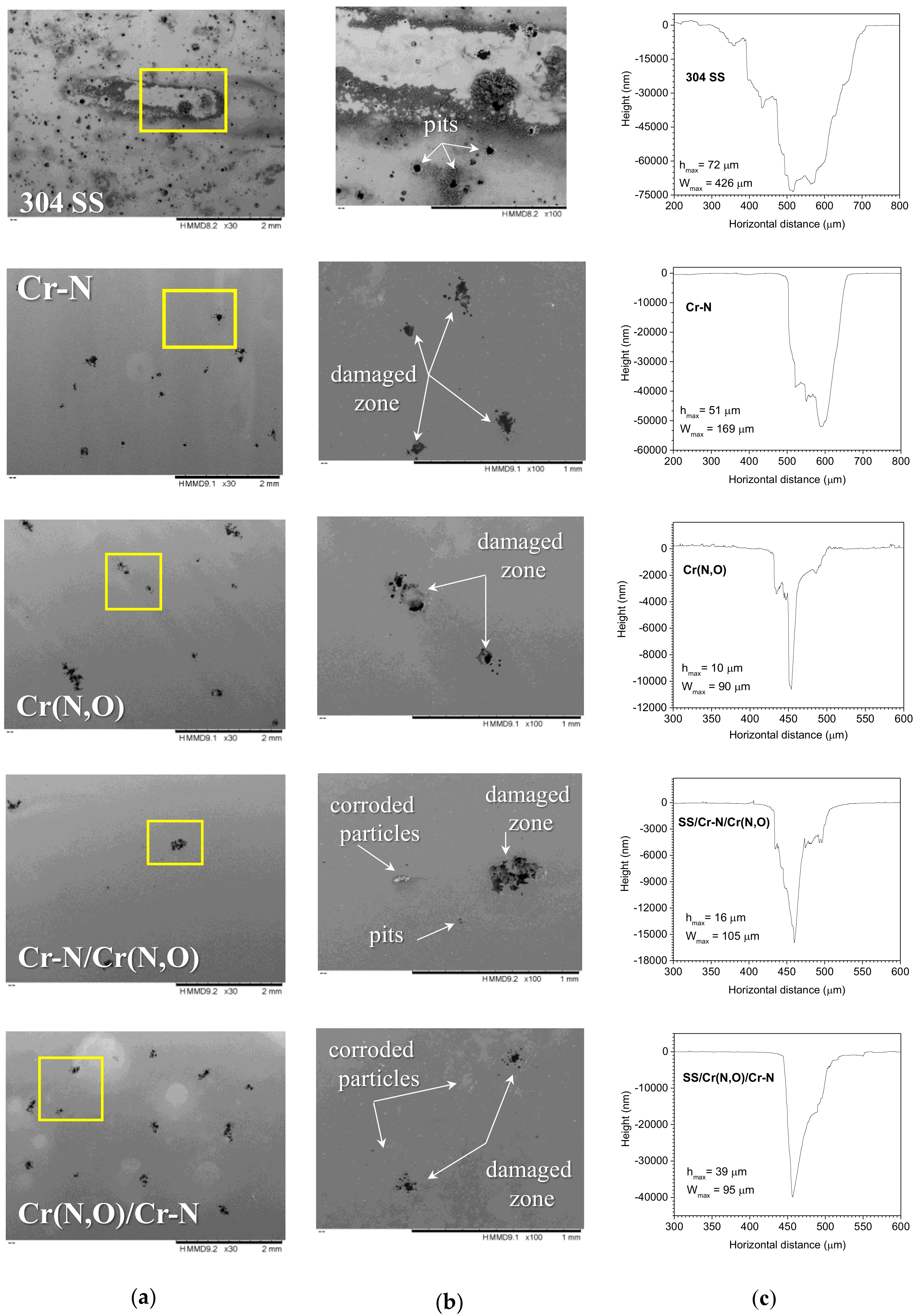
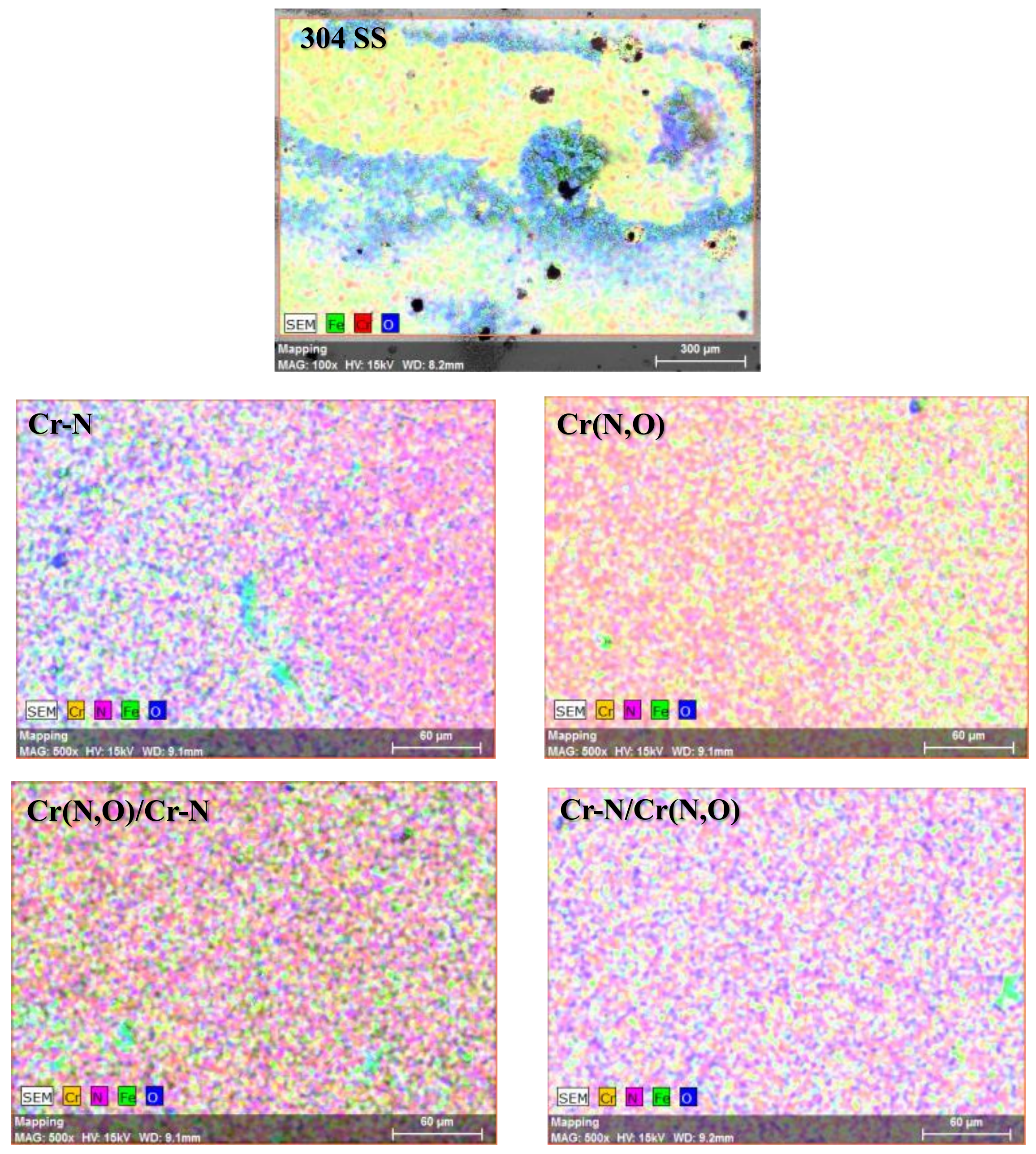
3.6. Characterisation of the Coatings after the Corrosion Tests
3.6.1. Coating Roughness
3.6.2. Surface Morphology and Elemental Composition
4. Conclusions
- The corrosion current densities of the coatings decreased by more than 30 times compared to the bare substrate.
- The Cr–N coating, as mono- or bilayer, had high porosity and lower protective performance.
- The protective efficiency of Cr(N,O) coating (99.1%) and porosity (0.007) were excellent when compared to other coatings, because this coating is denser, less porous, and more adherent to the substrate.
- Both bilayer coatings substantially improved the corrosion protection of 304 SS.
- The bilayer with Cr(N,O) on top possessed the best corrosion resistance behaviour, having the lowest current density corrosion and consequently the highest protective efficiency and the lowest porosity.
- The corrosion resistance can be ranked in the following order: Cr(N,O) > Cr–N/Cr(N,O) > Cr(N,O)/Cr–N > Cr–N.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baddoo, N.R. Stainless steel in construction: A review of research, applications, challenges and opportunities. J. Constr. Steel Res. 2008, 64, 1199–1206. [Google Scholar] [CrossRef]
- Disegi, J.A.; Eschbach, L. Stainless steel in bone surgery. Injury 2000, 31, D2–D6. [Google Scholar] [CrossRef]
- Handbook of Stainless Steel; Outokumpu: Helsinki, Finland, 2013.
- Gedge, G. Structural uses of stainless steel—Buildings and civil engineering. J. Constr. Steel Res. 2008, 64, 1194–1198. [Google Scholar] [CrossRef]
- Pocaznoi, D.; Calmet, A.; Etcheverry, L.; Erable, B.; Bergel, A. Stainless steel is a promising electrode material for anodes of microbial fuel cells. Energy Environ. Sci. 2012, 5, 9645–9652. [Google Scholar] [CrossRef]
- Park, S.H.C.; Sato, Y.S.; Kokawa, H.; Okamoto, K.; Hirano, S.; Inagaki, M. Corrosion resistance of friction stir welded 304 stainless steel. Scr. Mater. 2004, 51, 101–105. [Google Scholar] [CrossRef]
- Arango, S.; Peláez-Vargas, A.; García, C. Coating and surface treatments on orthodontic metallic materials. Coatings 2013, 3, 1–15. [Google Scholar] [CrossRef]
- Hao, L.; Yoshida, H.; Itoi, T.; Lu, Y. Preparation of metal coatings on steel balls using mechanical coating technique and its process analysis. Coatings 2017, 7, 53. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Abdullah, A.M.; Younan, N.A. Corrosion behavior of superhydrophobic surfaces: A review. Arab. J. Chem. 2015, 8, 749–765. [Google Scholar] [CrossRef]
- Perillo, P.M. Corrosion behavior of coatings of titanium nitride and titanium-titanium nitride on steel substrates. Corrosion 2006, 62, 182–185. [Google Scholar] [CrossRef]
- Wicks, Z.W.; Jones, F.N.; Pappas, S.P.; Wicks, D.A. Corrosion protection by coatings. Org. Coat. 2007, 137–158. [Google Scholar] [CrossRef]
- Khelifa, F.; Habibi, Y.; Benard, F.; Dubois, P. Smart acrylic coatings containing silica particles for corrosion protection of aluminum and other metals. In Handbook of Smart Coatings for Materials Protection; Makhlouf, A.S.H., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 423–458. [Google Scholar] [CrossRef]
- Montemor, M.F. Functional and smart coatings for corrosion protection: A review of recent advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Fenker, M.; Balzer, M.; Kappl, H. Corrosion protection with hard coatings on steel: Past approaches and current research efforts. Surf. Coat. Technol. 2014, 257, 182–205. [Google Scholar] [CrossRef]
- Verran, J.; Packer, A.; Kelly, P.; Whitehead, K.A. Titanium-coating of stainless steel as an aid to improved cleanability. Int. J. Food Microbiol. 2010, 141, S134–S139. [Google Scholar] [CrossRef] [PubMed]
- Mackey, E.D.; Seacord, T.F. Guidelines for using stainless steel in the water and desalination industries. J. Am. Water Works Assoc. 2017, 109, E158–E169. [Google Scholar] [CrossRef]
- Theodore, N.D.; Holloway, B.C.; Manos, D.M.; Moore, R.; Hernandez, C.; Wang, T.; Dylla, H.F. Nitrogen-implanted silicon oxynitride: A coating for suppressing field emission from stainless steel used in high-voltage applications. IEEE Trans. Plasma Sci. 2006, 34, 1074–1079. [Google Scholar] [CrossRef]
- Zalnezhad, E.; Hamouda, A.M.S.; Faraji, G.; Shamshirband, S. TiO2 nanotube coating on stainless steel 304 for biomedical applications. Ceram. Int. 2015, 41, 2785–2793. [Google Scholar] [CrossRef]
- Shaigan, N.; Qu, W.; Ivey, D.G.; Chen, W. A review of recent progress in coatings, surface modifications and alloy developments for solid oxide fuel cell ferritic stainless steel interconnects. J. Power Sources 2010, 195, 1529–1542. [Google Scholar] [CrossRef]
- Pruncu, C.I.; Braic, M.; Dearn, K.D.; Farcau, C.; Watson, R.; Constantin, L.R.; Balaceanu, M.; Braic, V.; Vladescu, A. Corrosion and tribological performance of quasi-stoichiometric titanium containing carbo-nitride coatings. Arab. J. Chem. 2016, 10, 1015–1028. [Google Scholar] [CrossRef]
- Sperko, W.J. Rust on Stainless Steel; Sperko Engineering Services Inc.: Greensboro, NC, USA, 2014. [Google Scholar]
- Muslim, Z.R.; Abbas, A.A. The effect of pH and temperature on corrosion rate stainless steel 316L used as biomaterial. Int. J. Basic Appl. Sci. 2012, 4, 17–20. [Google Scholar]
- Truman, J.E. The influence of chloride content, pH and temperature of test solution on the occurrence of stress corrosion cracking with austenitic stainless steel. Corros. Sci. 1977, 17, 737–746. [Google Scholar] [CrossRef]
- Vitelaru, C.; Balaceanu, M.; Parau, A.; Luculescu, C.R.; Vladescu, A. Investigation of nanostructured TiSiC-Zr and TiSiC-Cr hard coatings for industrial applications. Surf. Coat. Technol. 2014, 251, 21–28. [Google Scholar] [CrossRef]
- Yun, J.S.; Hong, Y.S.; Kim, K.H.; Kwon, S.H.; Wang, Q.M. Characteristics of Ternary Cr-O-N Coatings Synthesized by Using an Arc Ion Plating Technique. J. Korean Phys. Soc. 2010, 57, 103. [Google Scholar]
- Chen, S.; Wu, B.H.; Xie, D.; Jiang, F.; Liu, J.; Sun, H.L.; Zhu, S.; Bai, B.; Leng, Y.X.; Huang, N.; et al. The adhesion and corrosion resistance of Ti–O films on CoCrMo alloy fabricated by high power pulsed magnetron sputtering (HPPMS). Surf. Coat. Technol. 2014, 252, 8–14. [Google Scholar] [CrossRef]
- Ryabchikov, A.I.; Ryabchikov, I.A.; Stepanov, I.B. Development of filtered DC metal plasma ion implantation and coating deposition methods based on high-frequency short-pulsed bias voltage application. Vacuum 2005, 78, 331–336. [Google Scholar] [CrossRef]
- Ryabchikov, A.I.; Ryabchikov, I.A.; Stepanov, I.B.; Usov, Y.P. High-frequency short-pulsed metal plasma-immersion ion implantation or deposition using filtered DC vacuum-arc plasma. Surf. Coat. Technol. 2007, 201, 6523–6525. [Google Scholar] [CrossRef]
- Ryabchikov, A.I.; Ryabchikov, I.A.; Stepanov, I.B.; Sivin, D.O. Recent advances in surface processing with the filtered DC vacuum-arc plasma. Vacuum 2005, 78, 445–449. [Google Scholar] [CrossRef]
- Ryabchikov, A.I.; Ryabchikov, I.A.; Stepanov, I.B.; Dektyarev, S.V. High current vacuum-arc ion source for ion implantation and coating deposition technologies. Rev. Sci. Instrum. 2006, 77, 03B516. [Google Scholar] [CrossRef]
- Stepanov, I.B.; Ryabchikov, A.I.; Nochovnaya, N.A.; Sharkeev, Y.P.; Shulepov, I.A.; Ryabchikov, I.A.; Sivin, D.O.; Fortuna, S.V. Vacuum arc filtered metal plasma application in hybrid technologies of ion-beam and plasma material processing. Surf. Coat. Technol. 2007, 201, 8596–8600. [Google Scholar] [CrossRef]
- Davis, J. Corrosion: Understanding the Basics; ASM International: Geauga County, OH, USA, 2000; p. 574. [Google Scholar]
- Estrada-Martínez, J.; Reyes-Gasga, J.; García-García, R.; Vargas-Becerril, N.; Zapata-Torres, M.G.; Gallardo-Rivas, N.V.; Mendoza-Martínez, A.M.; Paramo-García, U. Wettability modification of the AISI 304 and 316 stainless steel and glass surfaces by titanium oxide and titanium nitride coating. Surf. Coat. Technol. 2017, 330, 61–70. [Google Scholar] [CrossRef]
- Ali, F.; Kwak, J.S. The impact of number of interfaces on corrosion behavior of TiCrN films. J. Nanosci. Nanotechnol. 2017, 17, 4318–4321. [Google Scholar] [CrossRef]
- Adesina, A.Y.; Gasem, Z.M.; Madhan Kumar, A. Corrosion resistance behavior of single-layer cathodic arc PVD nitride-base coatings in 1M HCl and 3.5 pct NaCl Solutions. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2017, 48, 1321–1332. [Google Scholar] [CrossRef]
- Navinsek, B.; Seal, S. Transition metal nitride functional coatings. JOM 2001, 53, 51–54. [Google Scholar] [CrossRef]
- Mayrhofer, P.H.; Rachbauer, R.; Holec, D.; Rovere, F.; Schneider, J.M. Protective transition metal nitride coatings. In Comprehensive Materials Processing; Hashmi, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 4, pp. 355–388. ISBN 9780080965338. [Google Scholar]
- Braic, L.; Vasilantonakis, N.; Mihai, A.; Garcia, I.J.V.; Fearn, S.; Zou, B.; Alford, N.M.; Doiron, B.; Oulton, R.F.; Maier, S.A.; et al. Titanium oxynitride thin films with tunable double epsilon-near-zero behavior for nanophotonic applications. ACS Appl. Mater. Interfaces 2017, 9, 29857–29862. [Google Scholar] [CrossRef] [PubMed]
- Fenker, M.; Kappl, H.; Carvalho, P.; Vaz, F. Thermal stability, mechanical and corrosion behaviour of niobium-based coatings in the ternary system Nb–O–N. Thin Solid Films 2011, 519, 2457–2463. [Google Scholar] [CrossRef]
- Braic, M.; Balaceanu, M.; Vladescu, A.; Kiss, A.; Braic, V.; Epurescu, G.; Dinescu, G.; Moldovan, A.; Birjega, R.; Dinescu, M. Preparation and characterization of titanium oxy-nitride thin films. Appl. Surf. Sci. 2007, 253, 8210–8214. [Google Scholar] [CrossRef]
- Subramanian, B.; Muraleedharan, C.V.; Ananthakumar, R.; Jayachandran, M. A comparative study of titanium nitride (TiN), titanium oxy nitride (TiON) and titanium aluminum nitride (TiAlN), as surface coatings for bio implants. Surf. Coat. Technol. 2011, 205, 5014–5020. [Google Scholar] [CrossRef]
- Rtimi, S.; Baghriche, O.; Sanjines, R.; Pulgarin, C.; Bensimon, M.; Kiwi, J. TiON and TiON-Ag sputtered surfaces leading to bacterial inactivation under indoor actinic light. J. Photochem. Photobiol. A Chem. 2013, 256, 52–63. [Google Scholar] [CrossRef]
- Li, W.Z.; Yi, D.Q.; Li, Y.Q.; Liu, H.Q.; Sun, C. Effects of the constitution of CrON diffusion barrier on the oxidation resistance and interfacial fracture of duplex coating system. J. Alloys Compd. 2012, 518, 86–95. [Google Scholar] [CrossRef]
- Li, W.Z.; Yao, Y.; Wang, Q.M.; Bao, Z.B.; Gong, J.; Sun, C.; Jiang, X. Improvement of oxidation-resistance of NiCrAlY coatings by application of CrN or CrON interlayer. J. Mater. Res. 2011, 23, 341–352. [Google Scholar] [CrossRef]
- Warcholinski, B.; Gilewicz, A.; Lupicka, O.; Kuprin, A.S.; Tolmachova, G.N.; Ovcharenko, V.D.; Kolodiy, I.V.; Sawczak, M.; Kochmanska, A.E.; Kochmanski, P.; et al. Structure of CrON coatings formed in vacuum arc plasma fluxes. Surf. Coat. Technol. 2016, 309, 920–930. [Google Scholar] [CrossRef]
- Cubillos, G.I.; Bethencourt, M.; Olaya, J.J.; Alfonso, J.E.; Marco, J.F. The influence of deposition temperature on microstructure and corrosion resistance of ZrOxNy/ZrO2 coatings deposited using RF sputtering. Appl. Surf. Sci. 2014, 309, 181–187. [Google Scholar] [CrossRef]
- Tijani, J.O.; Mouele, M.E.S.; Tottito, T.C.; Fatoba, O.O.; Petrik, L.F. Degradation of 2-nitrophenol by dielectric barrier discharge system: The Influence of carbon doped TiO2 photocatalyst supported on stainless steel mesh. Plasma Chem. Plasma Process. 2017, 37, 1343–1373. [Google Scholar] [CrossRef]
- Ruden, A.; Restrepo-Parra, E.; Paladines, A.U.; Sequeda, F. Corrosion resistance of CrN thin films produced by dc magnetron sputtering. Appl. Surf. Sci. 2013, 270, 150–156. [Google Scholar] [CrossRef]
- Wang, D.; Hu, M.; Jiang, D.; Fu, Y.; Wang, Q.; Yang, J.; Sun, J.; Weng, L. The improved corrosion resistance of sputtered CrN thin films with Cr-ion bombardment layer by layer. Vacuum 2017, 143, 329–335. [Google Scholar] [CrossRef]
- Chen, Q.; Cao, Y.; Xie, Z.; Chen, T.; Wan, Y.; Wang, H.; Gao, X.; Chen, Y.; Zhou, Y.; Guo, Y. Tribocorrosion behaviors of CrN coating in 3.5 wt.% NaCl solution. Thin Solid Films 2017, 622, 41–47. [Google Scholar] [CrossRef]
- Shan, L.; Zhang, Y.R.; Wang, Y.X.; Li, J.L.; Jiang, X.; Chen, J.M. Corrosion and wear behaviors of PVD CrN and CrSiN coatings in seawater. Trans. Nonferrous Met. Soc. China 2016, 26, 175–184. [Google Scholar] [CrossRef]
- Ma, F.; Li, J.; Zeng, Z.; Gao, Y. Structural, mechanical and tribocorrosion behaviour in artificial seawater of CrN/AlN nano-multilayer coatings on F690 steel substrates. Appl. Surf. Sci. 2018, 428, 404–414. [Google Scholar] [CrossRef]
- Gulbiński, W.; Gilewicz, A.; Suszko, T.; Warcholinśki, B.; Kukliński, Z. Ti–Si–C sputter deposited thin film coatings. Surf. Coat. Technol. 2004, 180–181, 341–346. [Google Scholar] [CrossRef]
- Gilewicz, A.; Chmielewska, P.; Murzynski, D.; Dobruchowska, E.; Warcholinski, B. Corrosion resistance of CrN and CrCN/CrN coatings deposited using cathodic arc evaporation in Ringer's and Hank's solutions. Surf. Coat. Technol. 2016, 299, 7–14. [Google Scholar] [CrossRef]
- Dinu, M.; Hauffman, T.; Cordioli, C.; Vladescu, A.; Braic, M.; Hubin, A.; Cotrut, C.M. Protective performance of Zr and Cr based silico-oxynitrides used for dental applications by means of potentiodynamic polarization and odd random phase multisine electrochemical impedance spectroscopy. Corros. Sci. 2017, 115, 118–128. [Google Scholar] [CrossRef]
- Piedrahita, W.F.; Coy, L.E.; Amaya, C.; Llarena, I.; Caicedo, J.C.; Yate, L. Influence of the negative R.F. bias voltage on the structural, mechanical and electrical properties of Hf–C–N coatings. Surf. Coat. Technol. 2016, 286, 251–255. [Google Scholar] [CrossRef]
- BS EN 1071-3:2005 Advanced Technical Ceramics–Methods of Test for Ceramic Coatings; British Standards Institution: London, UK, 2005; p. 47.
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 6, 1564–1583. [Google Scholar] [CrossRef]
- Li, M.S.; Feng, C.J.; Wang, F.H. Effect of partial pressure of reactive gas on chromium nitride and chromium oxide deposited by arc ion plating. Trans. Nonferrous Met. Soc. China 2006, 16, s276–s279. [Google Scholar] [CrossRef]
- Münz, W.-D.; Smith, I.J.; Lewis, D.B.; Creasey, S. Droplet formation on steel substrates during cathodic steered arc metal ion etching. Vacuum 1997, 48, 473–481. [Google Scholar] [CrossRef]
- Massalski, T.B.; Okamoto, H.; Subramanian, P.R.; Kacprzak, L. Binary Alloy Phase Diagrams, 2nd ed.; ASM International: Geauga County, OH, USA, 1990. [Google Scholar]
- Ehrlich, A.; Kühn, M.; Richter, F.; Hoyer, W. Complex characterisation of vacuum arc-deposited chromium nitride thin films. Surf. Coat. Technol. 1995, 76–77, 280–286. [Google Scholar] [CrossRef]
- Minami, T.; Nishio, S.; Murata, Y. Periodic microstructures of Cr–O–N coatings deposited by arc ion plating. Surf. Coat. Technol. 2014, 254, 402–409. [Google Scholar] [CrossRef]
- Rebholz, C.; Ziegele, H.; Leyland, A.; Matthews, A. Structure, mechanical and tribological properties of nitrogen-containing chromium coatings prepared by reactive magnetron sputtering. Surf. Coat. Technol. 1999, 115, 222–229. [Google Scholar] [CrossRef]
- Anna, E.M.; McMurdie, H.F.; Ondik, H.M. Borides carbides and nitrides. In Phase Equilibria Diagrams; McHale, A.E., Ed.; The American Ceramic Society: Westerville, OH, USA, 1994; p. 415. [Google Scholar]
- Suzuki, K.; Endo, T.; Fukushima, T.; Sato, A.; Suzuki, T.; Nakayama, T.; Suematsu, H.; Niihara, K. Controlling oxygen content by varying oxygen partial pressure in chromium oxynitride thin films prepared by pulsed laser deposition. Mater. Trans. 2013, 54, 1140–1144. [Google Scholar] [CrossRef]
- Suzuki, K.; Endo, T.; Sato, A.; Suzuki, T.; Nakayama, T.; Suematsu, H.; Niihara, K. Epitaxial growth of chromium oxynitride thin films on magnesium oxide (100) substrates and their oxidation behavior. Mater. Trans. 2013, 54, 1957–1961. [Google Scholar] [CrossRef]
- Oh, U.C.; Je, J.H. Effects of strain energy on the preferred orientation of TiN thin films. J. Appl. Phys. 1993, 74, 1692–1696. [Google Scholar] [CrossRef]
- Pelleg, J.; Zevin, L.Z.Z.; Lungo, S.; Croitoru, N. Reactive-sputter-deposited TiN films on glass substrates. Thin Solid Films 1991, 197, 117–128. [Google Scholar] [CrossRef]
- Sebastiani, M.; Piccoli, M.; Bemporad, E. Effect of micro-droplets on the local residual stress field in CAE-PVD thin coatings. Surf. Coat. Technol. 2013, 215, 407–412. [Google Scholar] [CrossRef]
- Steyer, P.; Mege, A.; Pech, D.; Mendibide, C.; Fontaine, J.; Pierson, J.F.; Esnouf, C.; Goudeau, P. Influence of the nanostructuration of PVD hard TiN-based films on the durability of coated steel. Surf. Coat. Technol. 2008, 202, 2268–2277. [Google Scholar] [CrossRef]
- Musil, J.; Novák, P.; Čerstvý, R.; Soukup, Z. Tribological and mechanical properties of nanocrystalline-TiC/a-C nanocomposite thin films. J. Vac. Sci. Technol. A Vac. Surf. Film 2010, 28, 244–249. [Google Scholar] [CrossRef]
- Yate, L.; Martínez-de-Olcoz, L.; Esteve, J.; Lousa, A. Ultra low nanowear in novel chromium/amorphous chromium carbide nanocomposite films. Appl. Surf. Sci. 2017, 420, 707–713. [Google Scholar] [CrossRef]
- Castanho, J.M.; Vieira, M.T. Effect of ductile layers in mechanical behaviour of TiAlN thin coatings. J. Mater. Process. Technol. 2003, 143–144, 352–357. [Google Scholar] [CrossRef]
- Mohammadpour, E.; Jiang, Z.-T.; Altarawneh, M.; Mondinos, N.; Rahman, M.M.; Lim, H.N.; Huang, N.M.; Xie, Z.; Zhou, Z.; Dlugogorski, B.Z. Experimental and predicted mechanical properties of Cr1−xAlxN thin films, at high temperatures, incorporating in situ synchrotron radiation X-ray diffraction and computational modelling. RSC Adv. 2017, 7, 22094–22104. [Google Scholar] [CrossRef]
- Jagielski, J.; Khanna, A.S.; Kucinski, J.; Mishra, D.S.; Racolta, P.; Sioshansi, P.; Tobin, E.; Thereska, J.; Uglov, V.; Vilaithong, T.; et al. Effect of chromium nitride coating on the corrosion and wear resistance of stainless steel. Appl. Surf. Sci. 2000, 156, 47–64. [Google Scholar] [CrossRef]
- Han, S.; Lin, J.H.; Tsai, S.H.; Chung, S.C.; Wang, D.Y.; Lu, F.H.; Shih, H.C. Corrosion and tribological studies of chromium nitride coated on steel with an interlayer of electroplated chromium. Surf. Coat. Technol. 2000, 133–134, 460–465. [Google Scholar] [CrossRef]
- Han, S.; Lin, J.H.; Wang, D.Y.; Lu, F.-H.; Shih, H.C. Corrosion resistance of chromium nitride on low alloy steels by cathodic arc deposition. J. Vac. Sci. Technol. Part A Vac. Surf. Film 2001, 19, 1442–1446. [Google Scholar] [CrossRef]
- Yate, L.; Coy, L.E.; Aperador, W. Robust tribo-mechanical and hot corrosion resistance of ultra-refractory Ta–Hf–C ternary alloy films. Sci. Rep. 2017, 7, 3080. [Google Scholar] [CrossRef] [PubMed]
- Aperador, W.; Duque, J.; Delgado, E. Electrochemical and tribological and mechanical performances coatings multilayer type NbC/CrN. Int. J. Electrochem. Sci. 2016, 11, 6347–6355. [Google Scholar] [CrossRef]


| Composition | Element | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | C | Mn | Si | Cr | Ni | P | S | Co | Mo | Cu | |
| wt.% | 70.976 | 0.004 | 1.220 | 0.208 | 17.746 | 8.524 | 0.020 | 0.014 | 0.160 | 0.589 | 0.539 |
| at.% | 70.380 | 0.020 | 1.230 | 0.410 | 18.900 | 8.040 | 0.036 | 0.024 | 0.150 | 0.350 | 0.470 |
| Coating | Elemental Composition (at.%) | O/N | O/Cr | (O + N)/Cr | |||
|---|---|---|---|---|---|---|---|
| N | O | Cr | C | ||||
| Cr–N | 30.5 ± 1.2 | 2.6 ± 0.2 | 63.4 ± 2.5 | 3.5 ± 0.2 | 0.085 | 0.041 | 0.522 |
| Cr(N,O) | 35.6 ± 1.7 | 14.9 ± 0.8 | 46.8 ± 2.2 | 2.6 ± 0.1 | 0.419 | 0.318 | 1.079 |
| Coating | H (GPa) | ΔH (GPa) | Er (GPa) | ΔEr (GPa) | H/Er | H3/Er2 | H2/Er | L2 (N) |
|---|---|---|---|---|---|---|---|---|
| SS/Cr–N | 24.53 | ±1.17 | 227.12 | ±6.71 | 0.1080 | 0.286 | 2.649 | 23.7 |
| SS/Cr(N,O) | 21.43 | ±1.70 | 203.34 | ±7.95 | 0.1054 | 0.238 | 2.259 | 19.1 |
| SS/Cr(N,O)/Cr–N | 25.28 | ±1.56 | 233.57 | ±8.26 | 0.1082 | 0.296 | 2.736 | 24.1 |
| SS/Cr–N/Cr(N,O) | 22.75 | ±1.03 | 175.45 | ±5.31 | 0.1296 | 0.382 | 2.949 | 35 |
| Sample | EOC (mV) | Rp (kΩ) | Ecorr (mV) | icorr (µA/cm2) | P | Pe (%) |
|---|---|---|---|---|---|---|
| 304 SS | 18 | 2.109 | 34 | 14.689 | – | – |
| Cr–N | 263 | 86.136 | 236 | 0.492 | 0.016 | 96.7 |
| Cr(N,O) | 277 | 187.75 | 250 | 0.137 | 0.007 | 99.1 |
| Cr(N,O)/Cr–N | 117 | 85.599 | 143 | 0.336 | 0.020 | 97.7 |
| Cr–N/Cr(N,O) | 276 | 223.047 | 250 | 0.168 | 0.006 | 98.9 |
| Substrate/Coating (Image Zone) | Elemental Composition (at.%) | ||||
|---|---|---|---|---|---|
| N | O | Cr | C | Fe | |
| 304 SS (A) | – | 41.0 ± 2.8 | 9.5 ± 0.1 | – | 49.5 ± 3.2 |
| Cr–N (B) | 30.5 ± 1.2 | 2.6 ± 0.2 | 63.4 ± 2.5 | 3.5 ± 0.2 | – |
| Cr–N (A) | 22.7 ± 0.8 | 17.5 ± 0.1 | 56.8 ± 1.8 | – | 3.0 ± 0.1 |
| Cr(N,O) (B) | 35.6 ± 1.7 | 14.9 ± 0.8 | 46.8 ± 2.2 | 2.6 ± 0.1 | – |
| Cr(N,O) (A) | 32.4 ± 1.5 | 20.2 ± 0.3 | 46.2 ± 2.1 | – | 1.2 ± 0.1 |
| Cr–N/ Cr(N,O) (B) | 36.9 ± 1.7 | 13.5 ± 0.7 | 49.3 ± 2.2 | 2.2 ± 0.1 | – |
| Cr–N/ Cr(N,O) (A) | 35.8 ± 1.5 | 17.4 ± 0.8 | 46.6 ± 1.8 | – | 0.5 ± 0.1 |
| Cr(N,O)/Cr–N (B) | 31.5 ± 1.3 | 2.8 ± 0.2 | 61.5 ± 2.5 | 4.2 ± 0.2 | – |
| Cr(N,O)/Cr–N (A) | 31.1 ± 1.3 | 22.9 ± 1.3 | 44.1 ± 2.1 | – | 1.9 ± 0.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinu, M.; Mouele, E.S.M.; Parau, A.C.; Vladescu, A.; Petrik, L.F.; Braic, M. Enhancement of the Corrosion Resistance of 304 Stainless Steel by Cr–N and Cr(N,O) Coatings. Coatings 2018, 8, 132. https://doi.org/10.3390/coatings8040132
Dinu M, Mouele ESM, Parau AC, Vladescu A, Petrik LF, Braic M. Enhancement of the Corrosion Resistance of 304 Stainless Steel by Cr–N and Cr(N,O) Coatings. Coatings. 2018; 8(4):132. https://doi.org/10.3390/coatings8040132
Chicago/Turabian StyleDinu, Mihaela, Emile S. Massima Mouele, Anca C. Parau, Alina Vladescu, Leslie F. Petrik, and Mariana Braic. 2018. "Enhancement of the Corrosion Resistance of 304 Stainless Steel by Cr–N and Cr(N,O) Coatings" Coatings 8, no. 4: 132. https://doi.org/10.3390/coatings8040132
APA StyleDinu, M., Mouele, E. S. M., Parau, A. C., Vladescu, A., Petrik, L. F., & Braic, M. (2018). Enhancement of the Corrosion Resistance of 304 Stainless Steel by Cr–N and Cr(N,O) Coatings. Coatings, 8(4), 132. https://doi.org/10.3390/coatings8040132




.jpg)