Thickness-Dependent Bioelectrochemical and Energy Applications of Thickness-Controlled Meso-Macroporous Antimony-Doped Tin Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation for Viscosity Studies
2.2. Viscosity Measurements
2.3. Fabricating ATO Coatings
2.4. Characterizing the Coatings
2.5. Incorporation of Bacterial Reaction Centers into ATO Films
2.6. Cyclic Voltammetry and Photovoltaic Measurements
3. Results and Discussion
3.1. Studies of the Template System
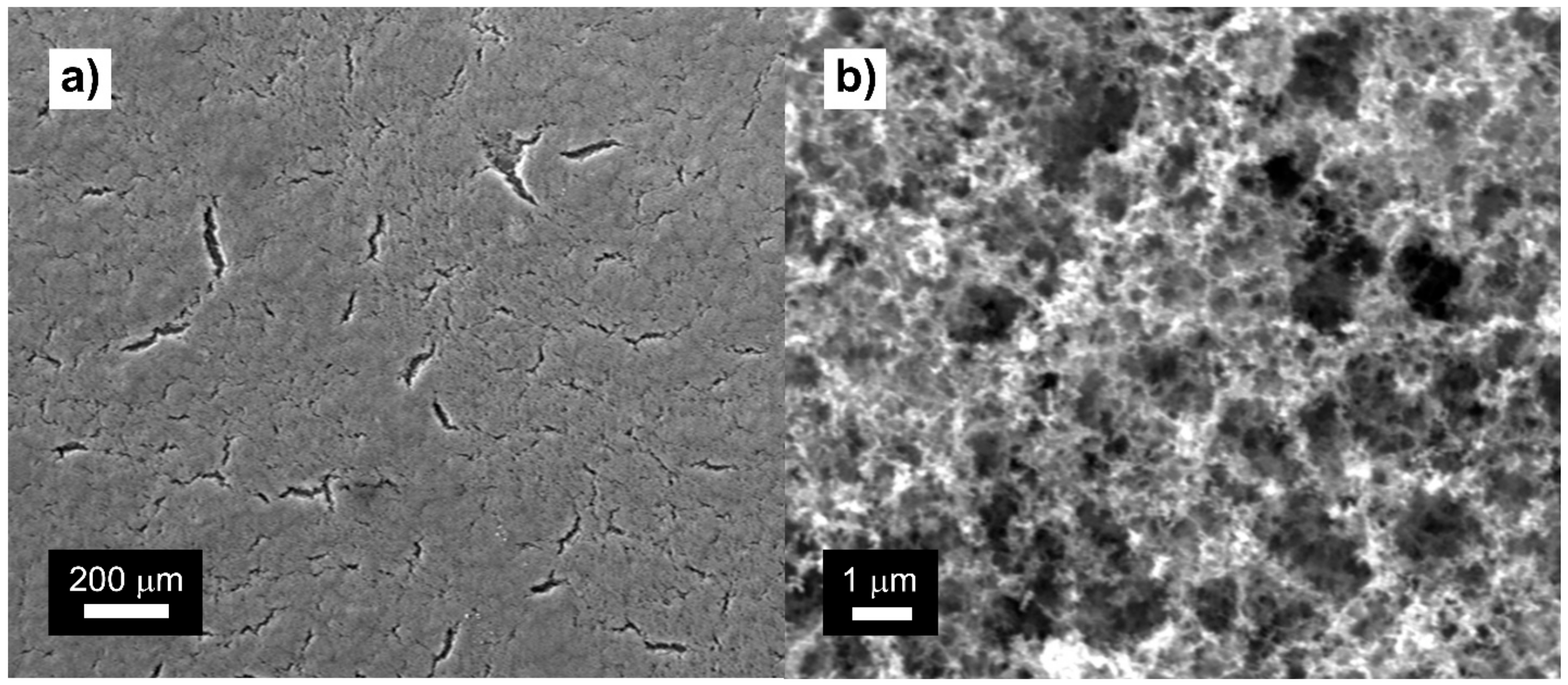
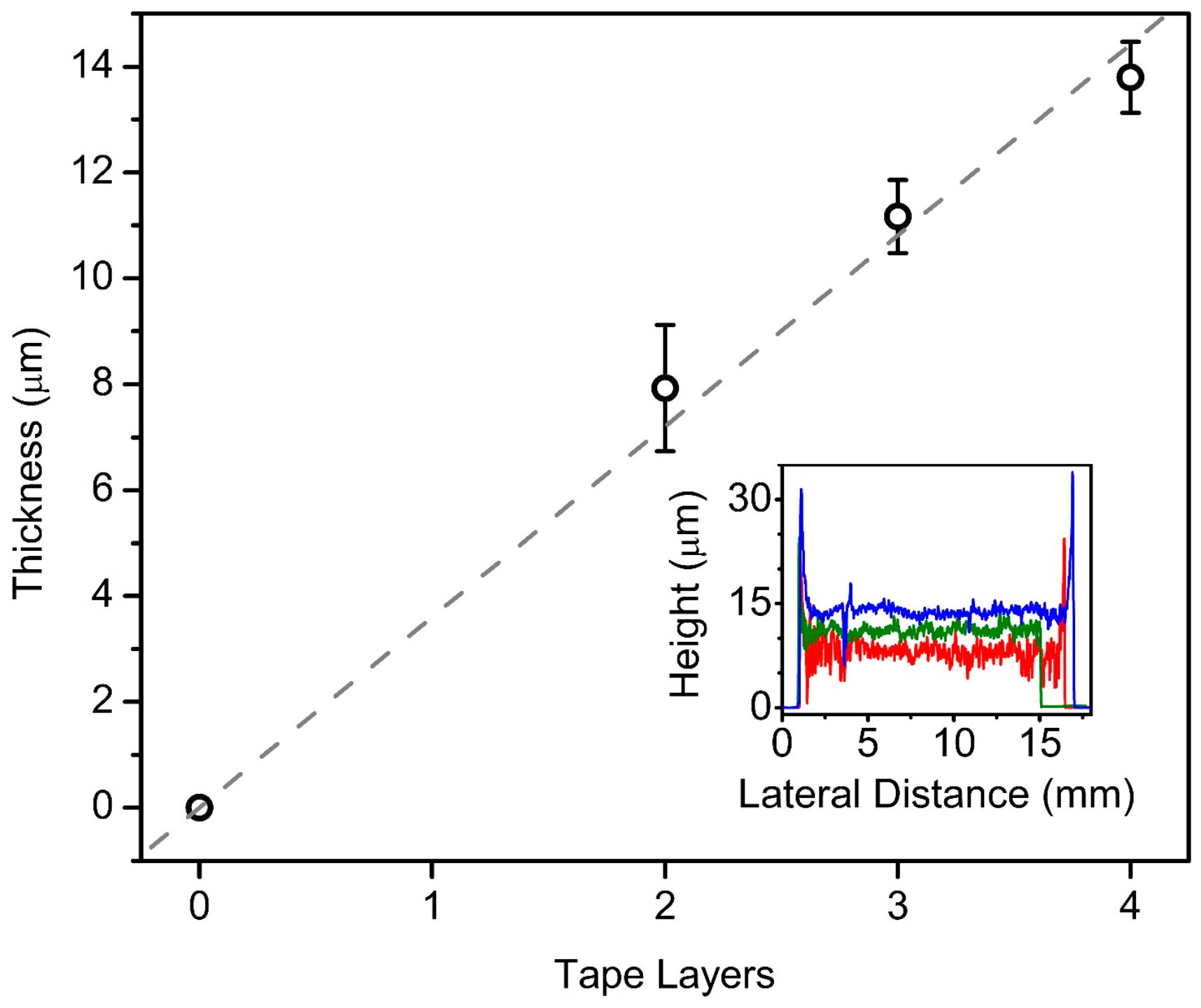
3.2. Fabrication and Characterization of Meso-Macroporous ATO Coatings with New Template System (CB7–PEG6–ATO)
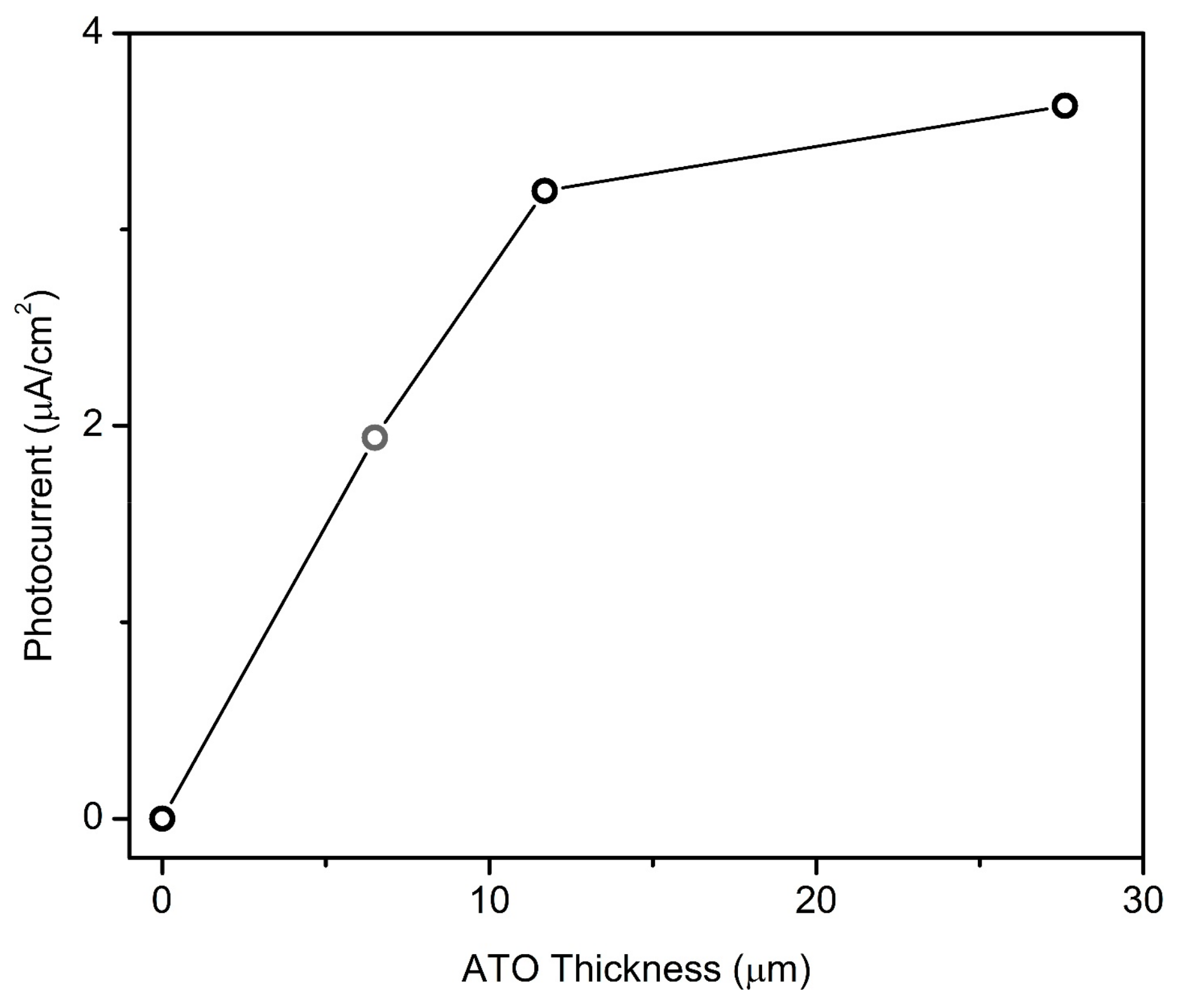
3.3. Biomacromolecule Modification and Electrochemical and Photovoltaic Studies
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carey, A.-M.; Zhang, H.; Mieritz, D.; Volosin, A.; Gardiner, A.T.; Cogdell, R.J.; Yan, H.; Seo, D.-K.; Lin, S.; Woodbury, N.W. Photocurrent generation by photosynthetic purple bacterial reaction centers interfaced with a porous antimony-doped tin oxide (ATO) electrode. ACS Appl. Mater. Interfaces 2016, 8, 25104–25110. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.; Lokupitiya, H.N.; Sarauli, D.; Labs, M.; Pribil, M.; Rathouský, J.; Kuhn, A.; Leister, D.; Stefik, M.; Fattakhova-Rohlfing, D. Nanostructured antimony-doped tin oxide layers with tunable pore architectures as versatile transparent current collectors for biophotovoltaics. Adv. Funct. Mater. 2016, 26, 6682–6692. [Google Scholar] [CrossRef]
- Jin, Q.; Li, Z.; Lin, K.; Wang, S.; Xu, R.; Wang, D. Synthesis of a hierarchically meso-macroporous TiO2 film based on UV light-induced in situ polymerization: Application to dye-sensitized solar cells. RSC Adv. 2014, 4, 44692–44699. [Google Scholar] [CrossRef]
- Seigo, I.; Keiji, I.; Ching-Ju, W.; Shoichiro, Y.; Tadashi, W. Dye-sensitized photocells with meso-macroporous TiO2 film electrodes. Bull. Chem. Soc. Jpn. 2000, 73, 2609–2614. [Google Scholar] [CrossRef]
- Tétreault, N.; Arsenault, É.; Heiniger, L.-P.; Soheilnia, N.; Brillet, J.; Moehl, T.; Zakeeruddin, S.; Ozin, G.A.; Grätzel, M. High-efficiency dye-sensitized solar cell with three-dimensional photoanode. Nano Lett. 2011, 11, 4579–4584. [Google Scholar] [CrossRef] [PubMed]
- Phadke, S.; Ho, J.; Birnie Iii, D.P. Emulsion templating to obtain dual-size-scale mesoporous titania coatings. Mater. Lett. 2009, 63, 2619–2621. [Google Scholar] [CrossRef]
- Phadke, S.; Du Pasquier, A.; Birnie, D.P. Enhanced electron transport through template-derived pore channels in dye-sensitized solar cells. J. Phys. Chem. C 2011, 115, 18342–18347. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Bae, S.-E.; Won, Y.S.; Huh, S. Simple preparation of lotus-root shaped meso-/macroporous TiO2 and their DSSC performances. J. Colloid Interface Sci. 2015, 448, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, M.; Peng, T.; Fan, K.; Lu, L.; Dai, K. Fabrication and properties of meso-macroporous electrodes screen-printed from mesoporous titania nanoparticles for dye-sensitized solar cells. Mater. Chem. Phys. 2009, 118, 477–483. [Google Scholar] [CrossRef]
- Schröder, M.; Sallard, S.; Böhm, M.; Einert, M.; Suchomski, C.; Smarsly, B.M.; Mutisya, S.; Bertino, M.F. An all low-temperature fabrication of macroporous, electrochemically addressable anatase thin films. Small 2014, 10, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T. Macroporous oxide platforms templated by non-close-packed spherical copolymer aggregates. Macromol. Rapid Commun 2013, 34, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Nursam, N.M.; Wang, X.; Caruso, R.A. Macro-/mesoporous titania thin films: Analysing the effect of pore architecture on photocatalytic activity using high-throughput screening. J. Mater. Chem. A 2015, 3, 24557–24567. [Google Scholar] [CrossRef]
- Liu, W.; Wang, A.; Tang, J.; Chen, S.-L.; Yuan, G.; Zhao, K.; Li, C.; Liu, X. Preparation and photocatalytic activity of hierarchically 3D ordered macro/mesoporous titania inverse opal films. Microporous Mesoporous Mater. 2015, 204, 143–148. [Google Scholar] [CrossRef]
- Xu, J.; Yang, B.; Wu, M.; Fu, Z.; Lv, Y.; Zhao, Y. Novel N–F–Co doped TiO2 inverse opal with a hierarchical meso-/macroporous structure: Synthesis, characterization, and photocatalysis. J. Phys. Chem. C 2010, 114, 15251–15259. [Google Scholar] [CrossRef]
- Liu, Y.; Peters, K.; Mandlmeier, B.; Müller, A.; Fominykh, K.; Rathousky, J.; Scheu, C.; Fattakhova-Rohlfing, D. Macroporous indium tin oxide electrode layers as conducting substrates for immobilization of bulky electroactive guests. Electrochim. Acta 2014, 140, 108–115. [Google Scholar] [CrossRef]
- Chandra, D.; Bekki, M.; Nakamura, M.; Sonezaki, S.; Ohji, T.; Kato, K.; Kimura, T. Dye-sensitized biosystem sensing using macroporous semiconducting metal oxide films. J. Mater. Chem. 2011, 21, 5738–5744. [Google Scholar] [CrossRef]
- Kwan, P.; Schmitt, D.; Volosin, A.M.; McIntosh, C.L.; Seo, D.-K.; Jones, A.K. Spectroelectrochemistry of cytochrome c and azurin immobilized in nanoporous antimony-doped tin oxide. Chem. Commun. 2011, 47, 12367–12369. [Google Scholar] [CrossRef] [PubMed]
- Topoglidis, E.; Astuti, Y.; Duriaux, F.; Grätzel, M.; Durrant, J.R. Direct electrochemistry and nitric oxide interaction of heme proteins adsorbed on nanocrystalline tin oxide electrodes. Langmuir 2003, 19, 6894–6900. [Google Scholar] [CrossRef]
- Topoglidis, E.; Cass, A.E.G.; O’Regan, B.; Durrant, J.R. Immobilisation and bioelectrochemistry of proteins on nanoporous TiO2 and ZnO films. J. Electroanal. Chem. 2001, 517, 20–27. [Google Scholar] [CrossRef]
- Topoglidis, E.; Lutz, T.; Durrant, J.R.; Palomares, E. Interfacial electron transfer on cytochrome-c sensitised conformally coated mesoporous TiO2 films. Bioelectrochemistry 2008, 74, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, K.; Liu, S.; Yao, Z.; Zhuang, S. Meso-macroporous Co3O4 electrode prepared by polystyrene spheres and carbowax templates for supercapacitors. J. Solid State Electrochem. 2011, 15, 587–592. [Google Scholar] [CrossRef]
- Moir, J.; Soheilnia, N.; O’Brien, P.; Jelle, A.; Grozea, C.M.; Faulkner, D.; Helander, M.G.; Ozin, G.A. Enhanced hematite water electrolysis using a 3D antimony-doped tin oxide electrode. ACS Nano 2013, 7, 4261–4274. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, E.; Soheilnia, N.; Ozin, G.A. Periodic macroporous nanocrystalline antimony-doped tin oxide electrode. ACS Nano 2011, 5, 2984–2988. [Google Scholar] [CrossRef] [PubMed]
- Frasca, S.; Molero Milan, A.; Guiet, A.; Goebel, C.; Pérez-Caballero, F.; Stiba, K.; Leimkühler, S.; Fischer, A.; Wollenberger, U. Bioelectrocatalysis at mesoporous antimony doped tin oxide electrodes—Electrochemical characterization and direct enzyme communication. Electrochim. Acta 2013, 110, 172–180. [Google Scholar] [CrossRef]
- Mieritz, D.; Li, X.; Volosin, A.; Liu, M.; Yan, H.; Walter, N.G.; Seo, D.-K. Tracking single DNA nanodevices in hierarchically meso-macroporous antimony-doped tin oxide demonstrates finite confinement. Langmuir 2017, 33, 6410–6418. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Wang, F.; Li, Z.; Chen, Z.; Zhang, H.; Jin, C. Honeycombed TiO2 films prepared by combining breath figure and sol gel methods. Surf. Coat. Technol. 2015, 276, 391–398. [Google Scholar] [CrossRef]
- Qi, L.; Birnie Iii, D.P. Templated titania films with meso- and macroporosities. Mater. Lett. 2007, 61, 2191–2194. [Google Scholar] [CrossRef]
- Park, J.T.; Lee, C.S.; Kim, J.H. One-pot synthesis of hierarchical mesoporous SnO2 spheres using a graft copolymer: Enhanced photovoltaic and photocatalytic performance. RSC Adv. 2014, 4, 31452–31461. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Suhendi, A.; Kisakibaru, Y.; Ogi, T.; Okuyama, K. Generation of highly ordered porous antimony-doped tin oxide film by a simple coating method with colloidal template. IJCBS 2012, 6, 1142–1145. [Google Scholar]
- Aoki, Y.; Hatano, A.; Watanabe, H. Rheology of carbon black suspensions. I. Three types of viscoelastic behavior. Rheol. Acta 2003, 42, 209–216. [Google Scholar] [CrossRef]
- Aoki, Y. Rheological characterization of carbon black/polystyrene solution systems. J. Appl. Polym. Sci. 2008, 108, 2660–2666. [Google Scholar] [CrossRef]
- Dutta, P.K.; Levenberg, S.; Loskutov, A.; Jun, D.; Saer, R.; Beatty, J.T.; Lin, S.; Liu, Y.; Woodbury, N.W.; Yan, H. A DNA-directed light-harvesting/reaction center system. J. Am. Chem. Soc. 2014, 136, 16618–16625. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y. Rheology of carbon black suspensions. IV. Effect of suspending media on the sol–gel transition behavior. Rheol. Acta 2011, 50, 779–785. [Google Scholar] [CrossRef]
- Trappe, V.; Sandkühler, P. Colloidal gels—Low-density disordered solid-like states. Curr. Opin. Colloid Interface Sci. 2004, 8, 494–500. [Google Scholar] [CrossRef]
- Margoliash, E.; Frohwirt, N. Spectrum of horse-heart cytochrome c. Biochem. J. 1959, 71, 570–572. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Volosin, A.M.; Schmitt, D.; Seo, D.K. Preparation and electrochemical properties of nanoporous transparent antimony-doped tin oxide (ATO) coatings. J. Mater. Chem. A 2013, 1, 699–706. [Google Scholar] [CrossRef]


| PEG Content | 0 wt % CB | 3 wt % CB | 5 wt % CB | 7 wt % CB |
|---|---|---|---|---|
| 6 wt % PEG | 8.65 cP | 26.3 cP | Non-Newtonian | Non-Newtonian |
| 10 wt % PEG | 21.6 cP | 58.5 cP | 108 cP | Non-Newtonian |
| 14 wt % PEG | 42.4 cP | 114 cP | 191 cP | Non-Newtonian |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mieritz, D.; Liang, R.; Zhang, H.; Carey, A.-M.; Chen, S.; Volosin, A.; Lin, S.; Woodbury, N.; Seo, D.-K. Thickness-Dependent Bioelectrochemical and Energy Applications of Thickness-Controlled Meso-Macroporous Antimony-Doped Tin Oxide. Coatings 2018, 8, 128. https://doi.org/10.3390/coatings8040128
Mieritz D, Liang R, Zhang H, Carey A-M, Chen S, Volosin A, Lin S, Woodbury N, Seo D-K. Thickness-Dependent Bioelectrochemical and Energy Applications of Thickness-Controlled Meso-Macroporous Antimony-Doped Tin Oxide. Coatings. 2018; 8(4):128. https://doi.org/10.3390/coatings8040128
Chicago/Turabian StyleMieritz, Daniel, Runli Liang, Haojie Zhang, Anne-Marie Carey, Shaojiang Chen, Alex Volosin, Su Lin, Neal Woodbury, and Dong-Kyun Seo. 2018. "Thickness-Dependent Bioelectrochemical and Energy Applications of Thickness-Controlled Meso-Macroporous Antimony-Doped Tin Oxide" Coatings 8, no. 4: 128. https://doi.org/10.3390/coatings8040128
APA StyleMieritz, D., Liang, R., Zhang, H., Carey, A.-M., Chen, S., Volosin, A., Lin, S., Woodbury, N., & Seo, D.-K. (2018). Thickness-Dependent Bioelectrochemical and Energy Applications of Thickness-Controlled Meso-Macroporous Antimony-Doped Tin Oxide. Coatings, 8(4), 128. https://doi.org/10.3390/coatings8040128







