Experimental Evaluation of Polyester and Epoxy–Polyester Powder Coatings in Aggressive Media
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Coating Thickness Measurement
3.2. Chamber Testing Results
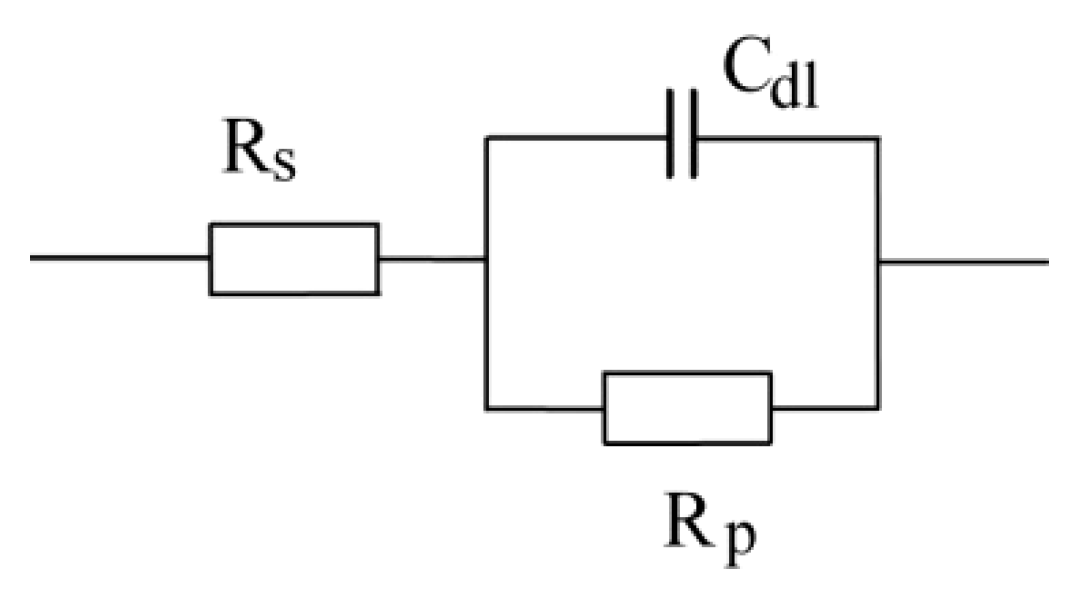
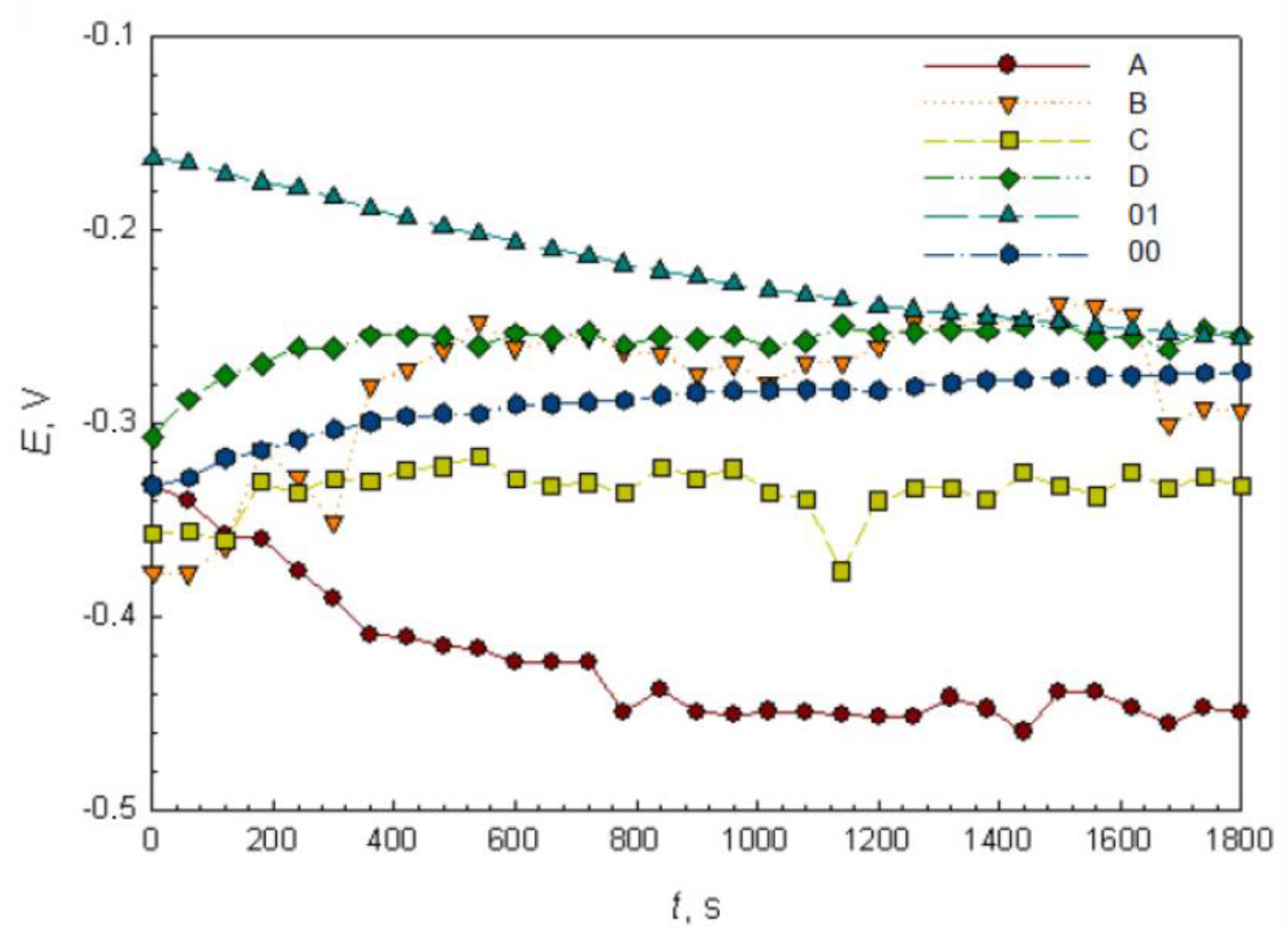
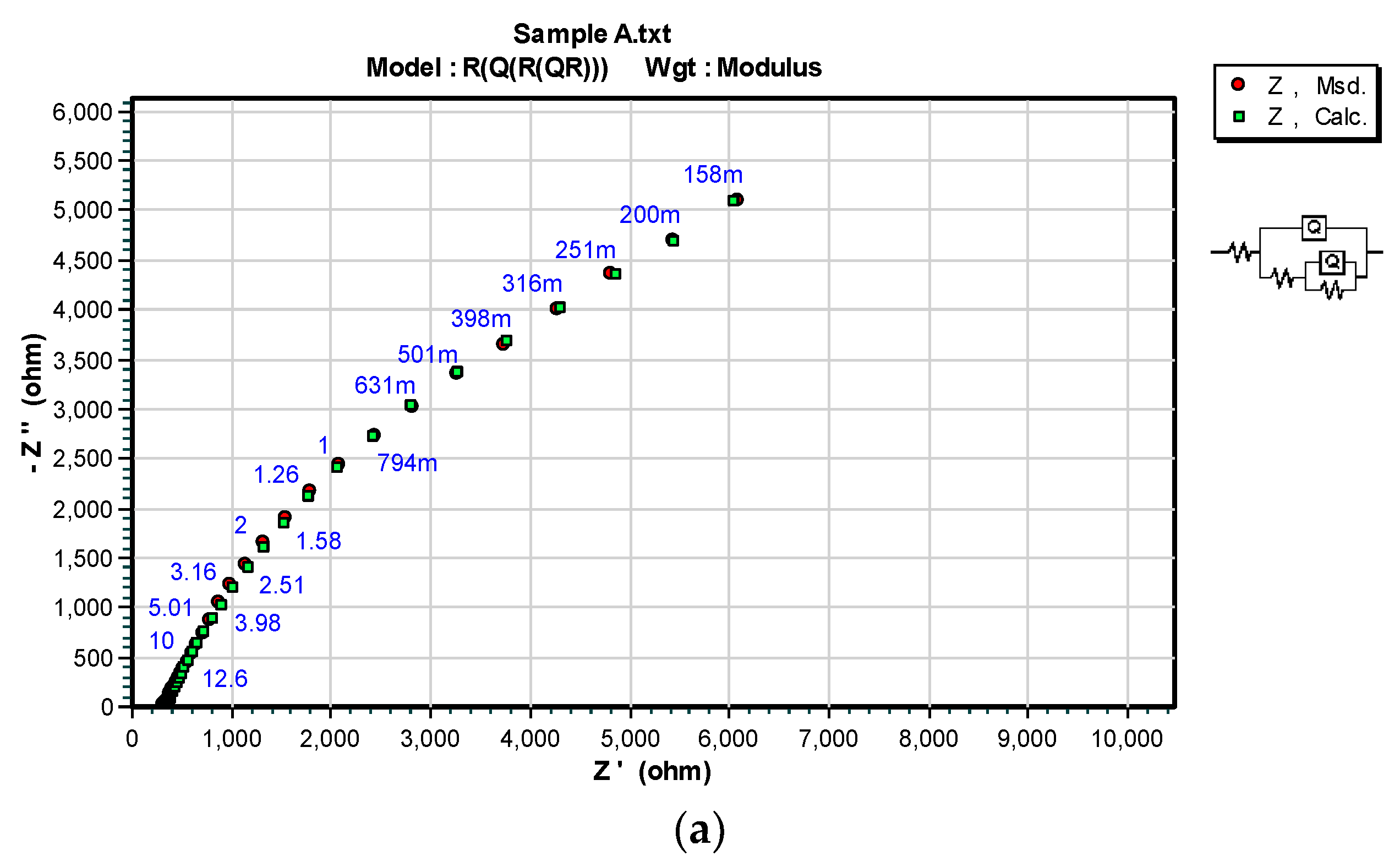
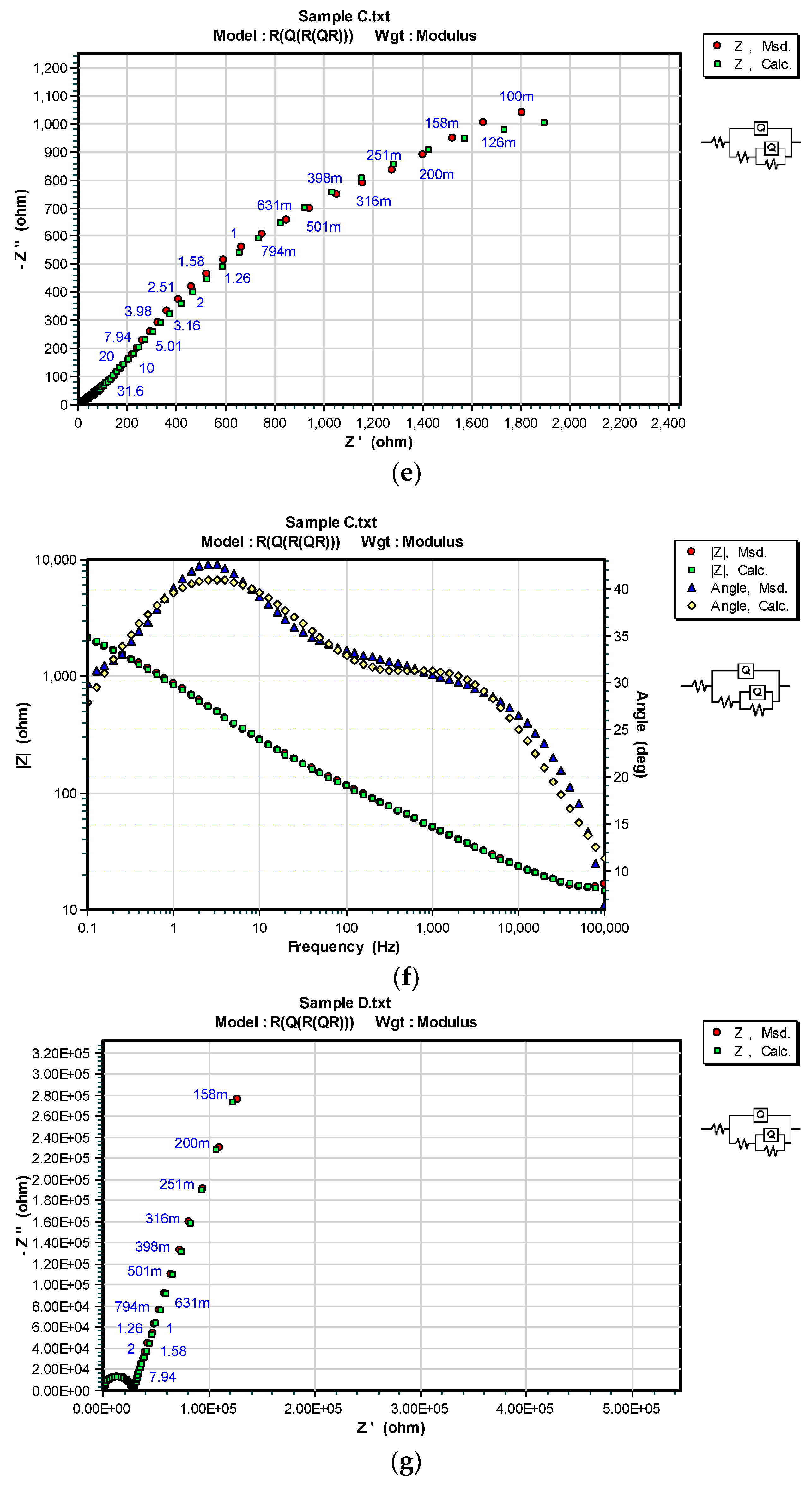
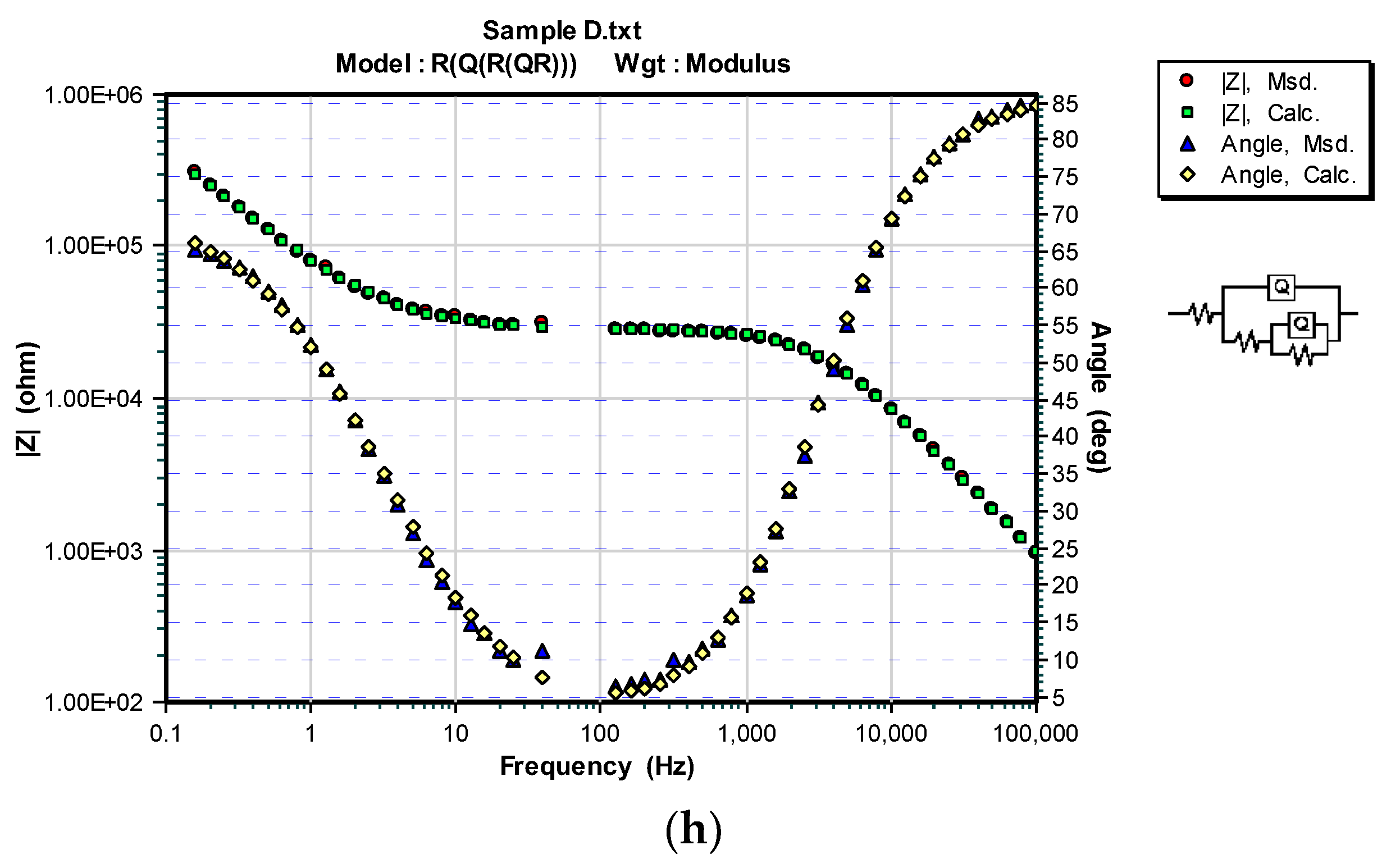
3.3. OCP and EIS Study
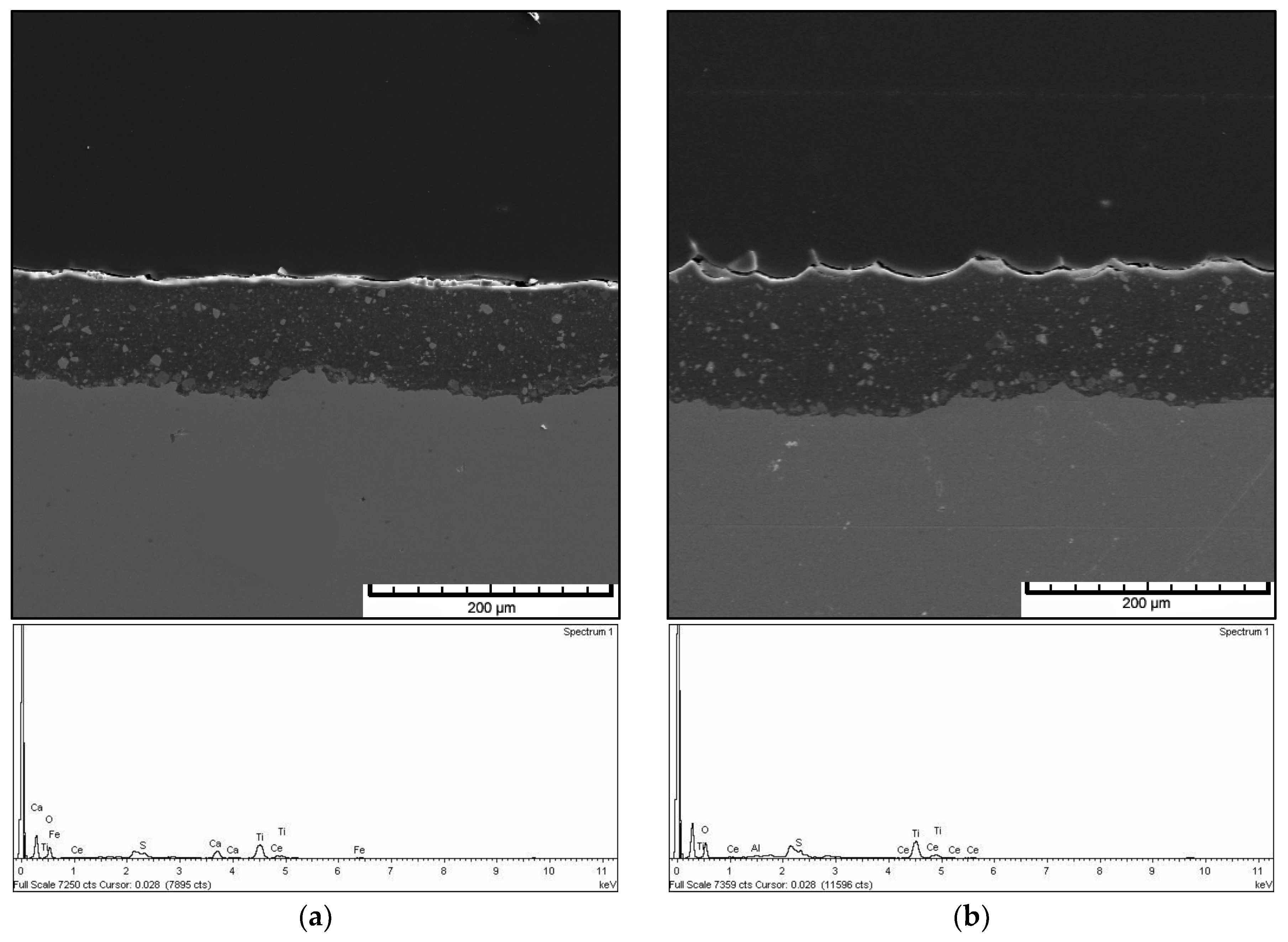
3.4. Morphology and EDX Spectra
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Khan, M.K.I.; Schutyser, M.A.I.; Schroën, K.; Boom, R.M. Electrostatic powder coating of foods–State of the art and opportunities. J. Food Eng. 2012, 111, 1–5. [Google Scholar] [CrossRef]
- Amefia, A.; Abu-Ali, J.; Barringer, S. Improved functionality of food additives with electrostatic coating. Innov. Food Sci. Emerg. Technol. 2006, 7, 176–181. [Google Scholar] [CrossRef][Green Version]
- Shah, U.; Zhu, J.; Zhang, C.; Senior, J.N. Numerical investigation of coarse powder and air flow in an electrostatic powder coating process. Powder Technol. 2006, 164, 22–32. [Google Scholar] [CrossRef]
- Luo, Y.; Zhu, J.; Ma, Y.; Zhang, H. Dry coating, a novel coating technology for solid pharmaceutical dosage forms. Int. J. Pharm. 2008, 358, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Mafi, R.; Mirabedini, S.M.; Naderi, R.; Attar, M.M. Effect of curing characterization on the corrosion performance of polyester and polyester/epoxy powder coatings. Corros. Sci. 2008, 50, 3280–3286. [Google Scholar] [CrossRef]
- Meng, X.; Zhu, J.; Zhang, H. Influences of different powders on the characteristics of particle charging and deposition in powder coating processes. J. Electrostat. 2009, 67, 663–671. [Google Scholar] [CrossRef]
- Barletta, M.; Bellisario, D. Effects of IR pre-curing conditions on wear resistance of metal flake powder coatings. Prog. Org. Coat. 2011, 70, 273–286. [Google Scholar] [CrossRef]
- Mintsa, M.; Lecamp, L.; Bunel, C. A new UV-curable powder coating based on a α,ω-unsaturated copolyamide 6/11/12. Eur. Polym. J. 2009, 45, 2043–2052. [Google Scholar] [CrossRef]
- Bhadu, M.K.; Guin, A.K.; Singh, V.; Choudhary, S.K. Corrosion Study of Powder-Coated Galvanised Steel. ISRN Corros. 2013, 2013, 464710. [Google Scholar] [CrossRef]
- ISO 2808 Paints and Varnishes—Determination of Film Thickness; International Organization for Standardization: Geneva, Switzerland, 2007.
- ISO 2409 Paints and Varnishes—Cross-Cut Test; International Organization for Standardization: Geneva, Switzerland, 2013.
- Stojanović, I.; Juraga, I.; Alar, V. Influence of Drying Temperature on Protective Properties of Waterborne and Solventborne Epoxy Coatings. Int. J. Electrochem. Sci. 2014, 9, 2507–2517. [Google Scholar]
- Nikravesh, B.; Ramezanzadeh, B.; Sarabi, A.A.; Kasiriha, S.M. Evaluation of the corrosion resistance of an epoxy-polyamide coating containing different ratios of micaceous iron oxide/Al pigments. Corros. Sci. 2011, 53, 1592–1603. [Google Scholar] [CrossRef]
- ISO 9227 Corrosion Tests in Artificial Atmospheres—Salt Spray Tests; International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 6270-2 Paints and Varnishes—Determination of Resistance to Humidity—Part 2: Condensation (In-Cabinet Exposure with Heated Water Reservoir); International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 4628 Paints and Varnishes—Evaluation of Degradation of Coatings—Designation of Quantity and Size of Defects, and of Intensity of Uniform Changes in Appearance; International Organization for Standardization: Geneva, Switzerland, 2016.
- Vilche, J.R.; Bucharsky, E.C.; Giudice, C.A. Application of EIS and SEM to evaluate the influence of pigment shape and content in ZRP formulations on the corrosion prevention of naval steel. Corros. Sci. 2002, 44, 1287–1309. [Google Scholar] [CrossRef]
- Bierwagen, G.; Tallman, D.; Li, J.; He, L.; Jeffcoate, C. EIS studies of coated metals in accelerated exposure. Prog. Org. Coat. 2003, 46, 148–157. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiong, J.; Tang, Y.; Zuo, Y. EIS study on failure process of two polyurethane composite coatings. Prog. Org. Coat. 2010, 69, 7–11. [Google Scholar] [CrossRef]
- Klüppel, I.; Schinkinger, B.; Grundmeier, G. In situ electrochemical studies of forming-induced defects of organic coatings on galvanised steel. Electrochim. Acta 2009, 54, 3553–3560. [Google Scholar] [CrossRef]
- Naderi, R.; Attar, M.M.; Moayed, M.H. EIS examination of mill scale on mild steel with polyester–epoxy powder coating. Prog. Org. Coat. 2004, 50, 162–165. [Google Scholar] [CrossRef]
- Jakovljević, S.; Alar, V.; Ivanković, A. Electrochemical Behaviour of PACVD TiN-Coated CoCrMo Medical Alloy. Metals 2017, 7, 231. [Google Scholar] [CrossRef]
- Alemdar, N.; Karagoz, B.; Erciyes, A.T.; Bicak, N. A method for polymethymetacrylate coating via self-curable unsaturated polyester primer on metal and glass surfaces. Prog. Org. Coat. 2007, 60, 69–74. [Google Scholar] [CrossRef]


| Sample | Chemical Type | Purpose | Gloss | Surface Appearance | Particle Size (µm) | Specific Weight (kg dm−3) |
|---|---|---|---|---|---|---|
| A | saturated carboxylated polyester resin and solid epoxy resin | for indoor use | semi-glossy | textured (rough) | < 100 | 1.6 |
| B | saturated carboxylated polyester resin cured with the β-hydroxyl-alkyl amide (HAA) | for outdoor use | glossy | river textured (rough) | 30–40 | 1.7 |
| C | saturated carboxylated polyester resin cured with the β-hydroxyl-alkyl amide (HAA) | for outdoor use | glossy | smooth | < 100 | 1.4-1.7 |
| D | saturated carboxylated polyester resin and solid epoxy resin | for indoor use | semi-glossy | smooth | 30–40 | 1.65 |
| Testing | Sample | DFTmin (μm) | DFTmax (μm) | DFTmean (μm) | Blistering | Rusting | Cross-Cut Adhesion |
|---|---|---|---|---|---|---|---|
| Humidity chamber | AV 1 | 83.6 | 133 | 104.01 | 0 | 0 | 0 |
| AV 2 | 100 | 152 | 124.1 | 0 | 0 | 0 | |
| BV 1 | 64.5 | 114 | 87.98 | 0 | 0 | 5 | |
| BV 2 | 40.4 | 130 | 81.28 | 0 | 0 | 5 | |
| CV 1 | 96.7 | 116 | 107.28 | 0 | 0 | 0 | |
| CV 2 | 91.3 | 134 | 115.93 | 0 | 0 | 0 | |
| DV 1 | 80.8 | 106 | 94.74 | 0 | 0 | 0 | |
| DV 2 | 83.3 | 104 | 92.94 | 0 | 0 | 0 |
| Testing | Sample | DFTmin (μm) | DFTmax (μm) | DFTmean (μm) | Blistering | Rusting | Cross-Cut Adhesion |
|---|---|---|---|---|---|---|---|
| Salt spray chamber | AS 1 | 95 | 115 | 103.61 | 0 | 0 | 0 |
| AS 2 | 89.1 | 123 | 106.83 | 0 | 0 | 0 | |
| BS 1 | 76 | 150 | 107.73 | 0 | 0 | 0 | |
| BS 2 | 73.5 | 154 | 117.71 | 0 | 0 | 0 | |
| CS 1 | 97.7 | 119 | 107.95 | 0 | 0 | 0 | |
| CS 2 | 101 | 122 | 113 | 0 | 0 | 0 | |
| DS 1 | 82.7 | 103 | 91.81 | 0 | 0 | 0 | |
| DS 2 | 86.6 | 118 | 96.57 | 0 | 0 | 0 |
| Sample | A | B | C | D | 01 | 00 |
|---|---|---|---|---|---|---|
| DFTmean (µm) | 126.6 | 114.2 | 71.4 | 69.2 | – | – |
| Ecorr vs SCE (V) | −0.447 | −0.240 | −0.320 | −0.300 | −0.253 | −0.270 |
| Sample | DFTmean (µm) | Rs (Ω) | CPEcoat (F) | ncoat | Rcoat (Ω) | CPEdl (F) | ndl | Rp/Rct (Ω) |
|---|---|---|---|---|---|---|---|---|
| A | 126.6 | 150.7 | – | – | – | 4.975 × 10−11 | 0.96016 | 2.8963 × 106 |
| A/100 h | 331.2 | 7.665 × 10−5 | 0.7334 | 1.323 × 104 | 2.584 × 10−4 | 1 | 1.527 × 104 | |
| B | 114.2 | 113.5 | – | – | – | 6.549 × 10−1° | 0.94467 | 1.372 × 106 |
| B/100 h | 236.9 | 7.705 × 10−5 | 0.4012 | 2.856 × 102 | 1.100 × 10−4 | 0.6917 | 2.882 × 104 | |
| C | 71.4 | 467.8 | – | – | – | 6.452 × 10−11 | 0.96166 | 1.7562 × 106 |
| C/100 h | 121.6 | 1.064 × 10−4 | 0.5856 | 111.8 | 2.865 × 10−4 | 0.5569 | 4.252 × 103 | |
| D | 69.2 | 672.3 | – | – | – | 7.321 × 10−11 | 0.94090 | 1.8189 × 106 |
| D/100 h | 100 | 2.702 × 10−9 | 0.9620 | 2.809 × 104 | 3.462 × 10−6 | 0.7897 | 9.830 × 104 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojanović, I.; Šimunović, V.; Alar, V.; Kapor, F. Experimental Evaluation of Polyester and Epoxy–Polyester Powder Coatings in Aggressive Media. Coatings 2018, 8, 98. https://doi.org/10.3390/coatings8030098
Stojanović I, Šimunović V, Alar V, Kapor F. Experimental Evaluation of Polyester and Epoxy–Polyester Powder Coatings in Aggressive Media. Coatings. 2018; 8(3):98. https://doi.org/10.3390/coatings8030098
Chicago/Turabian StyleStojanović, Ivan, Vinko Šimunović, Vesna Alar, and Frankica Kapor. 2018. "Experimental Evaluation of Polyester and Epoxy–Polyester Powder Coatings in Aggressive Media" Coatings 8, no. 3: 98. https://doi.org/10.3390/coatings8030098
APA StyleStojanović, I., Šimunović, V., Alar, V., & Kapor, F. (2018). Experimental Evaluation of Polyester and Epoxy–Polyester Powder Coatings in Aggressive Media. Coatings, 8(3), 98. https://doi.org/10.3390/coatings8030098