POSS-Containing Polymethacrylates on Cellulose-Based Substrates: Immobilization and Ceramic Formation
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
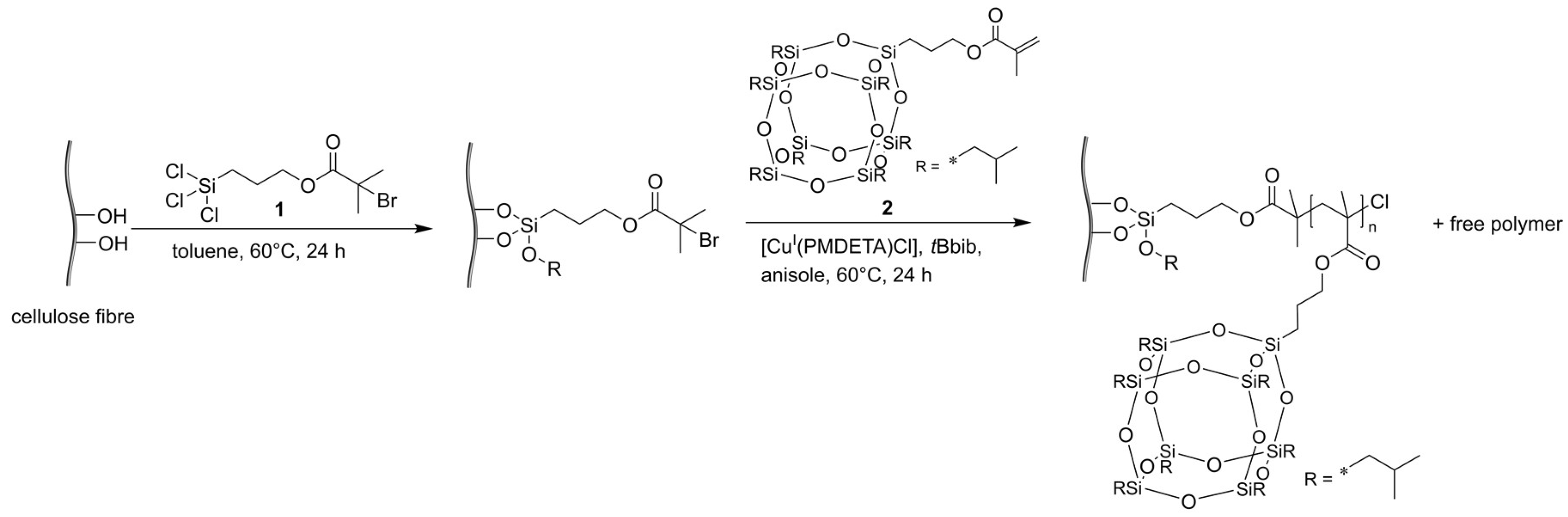
2.3. Paper Functionalization with ATRP Initiator
2.4. Surface-Initiated Polymerization of MAPOSS from Cellulose Substrate
3. Results and Discussion
3.1. Functionalization of Cellulose-Based Substrates with PMAPOSS
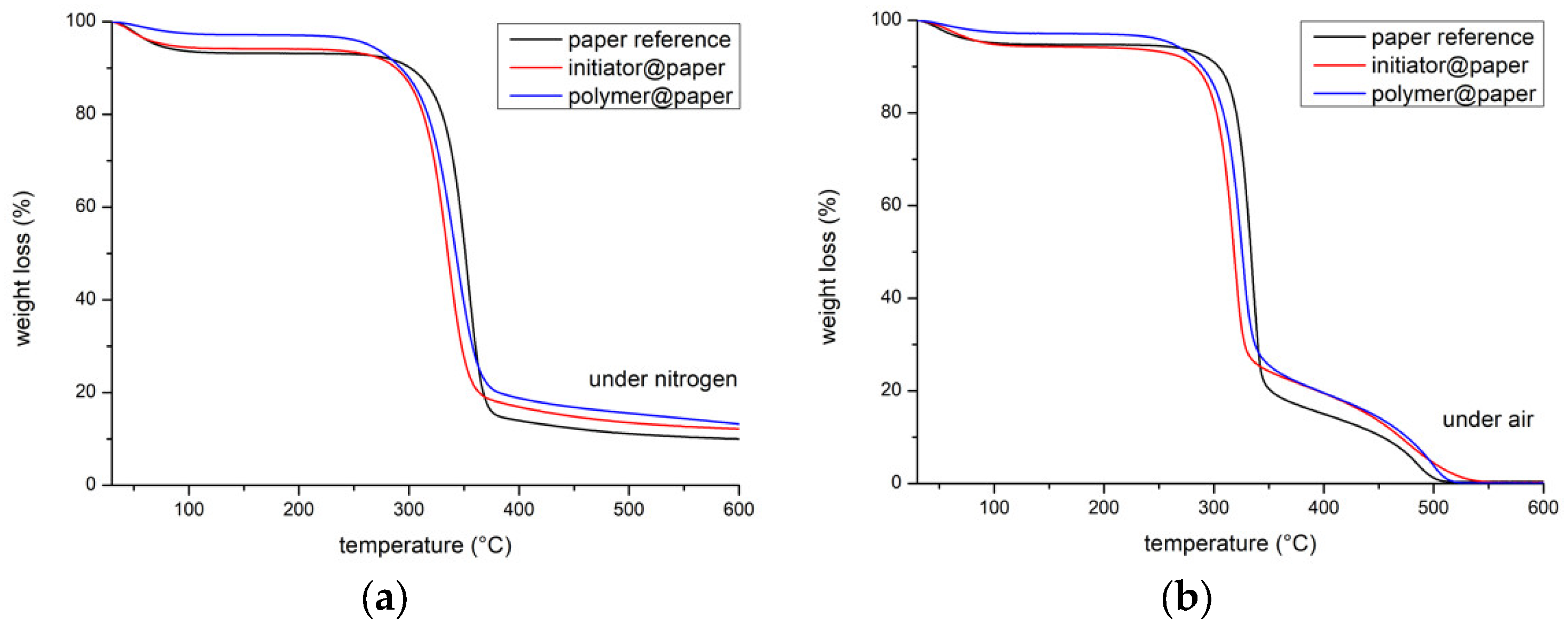
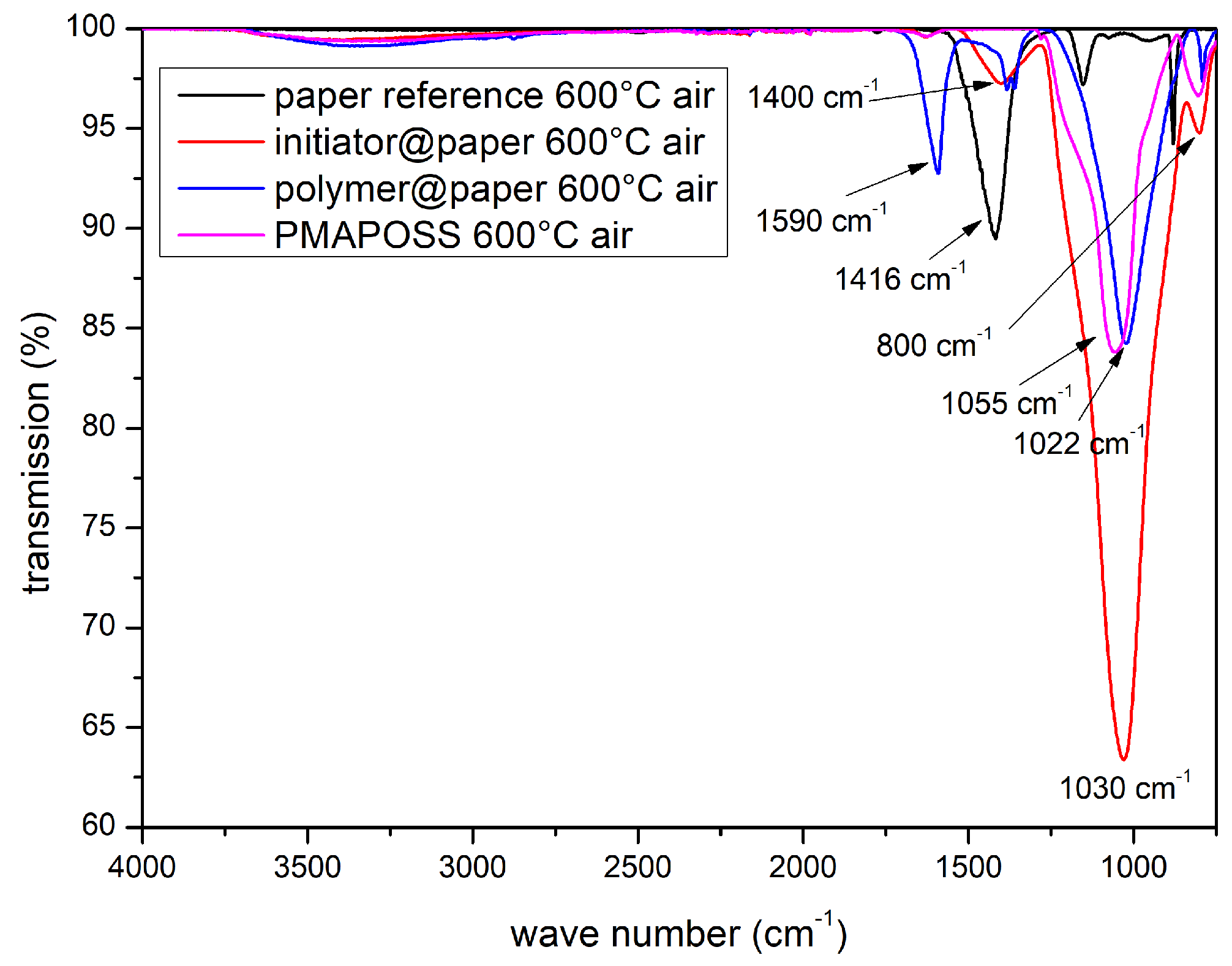
3.2. Ceramisation of the PMAPOSS-Modified Cellulose Substrates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Christodoulou, L.; Venables, J.D. Multifunctional material systems: The first generation. JOM 2003, 55, 39–45. [Google Scholar] [CrossRef]
- Dumanli, A.G.; Savin, T. Recent advances in the biomimicry of structural colours. Chem. Soc. Rev. 2016, 45, 6698–6724. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. Nanoscience, nanotechnology, and chemistry. Small 2005, 1, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.; Burns, A.; Kim, J.; Wiesner, U.; Hyeon, T. Designed fabrication of silica-based nanostructured particle systems for nanomedicine applications. Adv. Funct. Mater. 2008, 18, 3745–3758. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, L. Hollow micro/nanomaterials with multilevel interior structures. Adv. Mater. 2009, 21, 3621–3638. [Google Scholar] [CrossRef]
- Talapin, D.V.; Lee, J.S.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of colloidal nanocrystals for electronic and optoelectronic applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, C.G.; Vowinkel, S.; Hellmann, G.P.; Herdt, T.; Contiu, C.; Schneider, J.J.; Gallei, M. A polymer based and template-directed approach towards functional multidimensional microstructured organic/inorganic hybrid materials. J. Mater. Chem. C 2014, 2, 7960–7975. [Google Scholar] [CrossRef]
- Schüth, F.; Schmidt, W. Microporous and mesoporous materials. Adv. Mater. 2002, 14, 629–638. [Google Scholar] [CrossRef]
- Stein, A. Advances in microporous and mesoporous solids-highlights of recent progress. Adv. Mater. 2003, 15, 763–775. [Google Scholar] [CrossRef]
- Thomas, A.; Goettmann, F.; Antonietti, M. Hard templates for soft materials: Creating nanostructured organic materials. Chem. Mater. 2008, 20, 738–755. [Google Scholar] [CrossRef]
- Sahoo, J.K.; Tahir, M.N.; Yella, A.; Schladt, T.D.; Pfeiffer, S.; Nakhjavan, B.; Mugnaioli, E.; Kolb, U.; Tremel, W. From single molecules to nanoscopically structured materials: Self-Assembly of metal chalcogenide/metal oxide nanostructures based on the degree of pearson hardness. Chem. Mater. 2011, 23, 3534–3539. [Google Scholar] [CrossRef]
- Joshi, R.K.; Schneider, J.J. Assembly of one dimensional inorganic nanostructures into functional 2D and 3D architectures. Synthesis, arrangement and functionality. Chem. Soc. Rev. 2012, 41, 5285–5312. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Gallei, M.; Ionescu, E. Polymer–ceramic nanohybrid materials. In Organic-Inorganic Hybrid Nanomaterials; Kalia, S., Haldorai, Y., Eds.; Springer: Basel, Switzerland, 2015; Volume 267, pp. 143–185. [Google Scholar]
- Mera, G.; Gallei, M.; Bernard, S.; Ionescu, E. Ceramic nanocomposites from tailor-made preceramic polymers. Nanomaterials 2015, 5, 468–540. [Google Scholar] [CrossRef] [PubMed]
- Vowinkel, S.; Schäfer, C.G.; Cherkashinin, G.; Fasel, C.; Roth, F.; Liu, N.; Dietz, C.; Ionescu, E.; Gallei, M. 3D-ordered carbon materials by melt-shear organization for tailor-made hybrid core–shell polymer particle architectures. J. Mater. Chem. C 2016, 4, 3976–3986. [Google Scholar] [CrossRef]
- Gallei, M. Functional polymer opals and porous materials by shear-induced assembly of tailor-made particles. Macromol. Rapid Commun. 2018, 39, 1700648. [Google Scholar] [CrossRef]
- Mera, G.; Ionescu, E. Silicon-containing preceramic polymers. In Encyclopedia of Polymer Science and Technology, 3rd ed.; Mark, H.F., Ed.; John Wiley & Sons, Inc.: Hoboken, NY, USA, 2013. [Google Scholar] [CrossRef]
- Colombo, P.; Mera, G.; Riedel, R.; Sorarù, G.D. Polymer-derived ceramics: 40 years of research and innovation in advanced ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Mera, G.; Riedel, R. Organosilicon-based polymers as precursors for ceramics. In Polymer Derived Ceramics: From Nanostructure to Applications; Colombo, P., Ed.; DEStech Publications Inc.: Lancaster, PA, USA, 2010; pp. 51–89. [Google Scholar]
- Ionescu, E.; Kleebe, H.J.; Riedel, R. Silicon-containing polymer-derived ceramic nanocomposites (PDC-NCs): Preparative approaches and properties. Chem. Soc. Rev. 2012, 41, 5032–5052. [Google Scholar] [CrossRef]
- Riedel, R.; Mera, G.; Hauser, R.; Klonczynski, A. Silicon-based polymer-derived ceramics: Synthesis properties and applications-A review dedicated to Prof. Dr. Fritz Aldinger on the occasion of his 65th birthday. J. Ceram. Soc. Jpn. 2006, 114, 425–444. [Google Scholar] [CrossRef]
- Stabler, C.; Ionescu, E.; Graczyk-Zajac, M.; Gonzalo-Juan, I.; Riedel, R. Silicon oxycarbide glasses and glass-ceramics: “All-Rounder” materials for advanced structural and functional applications. J. Am. Ceram. Soc. 2018, 101, 4817–4856. [Google Scholar] [CrossRef]
- Abe, K.; Kotera, K.; Suzuki, K.; Citterio, D. Inkjet-printed paperfluidic immuno-chemical sensing device. Anal. Bioanal.Chem. 2010, 398, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Leung, V.; Shehata, A.A.M.; Filipe, C.D.M.; Pelton, R. Streaming potential sensing in paper-based microfluidic channels. Colloids Surf. A 2010, 364, 16–18. [Google Scholar] [CrossRef]
- Olsson, H.; Nyström, G.; Strømme, M.; Sjödin, M.; Nyholm, L. Cycling stability and self-protective properties of a paper-based polypyrrole energy storage device. Electrochem. Commun. 2011, 13, 869–871. [Google Scholar] [CrossRef]
- Tobjörk, D.; Österbacka, R. Paper electronics. Adv. Mater. 2011, 23, 1935–1961. [Google Scholar] [CrossRef]
- Schöttner, S.; Schaffrath, H.J.; Gallei, M. Poly(2-hydroxyethyl methacrylate)-based amphiphilic block copolymers for high water flux membranes and ceramic templates. Macromolecules 2016, 49, 7286–7295. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, S.; You, B.; Wu, L. A facile method for the fabrication of superhydrophobic films with multiresponsive and reversibly tunable wettability. J. Mater. Chem. A 2013, 1, 3146–3154. [Google Scholar] [CrossRef]
- Janko, M.; Jocher, M.; Boehm, A.; Babel, L.; Bump, S.; Biesalski, M.; Meckel, T.; Stark, R.W. Cross-linking cellulosic fibers with photoreactive polymers: Visualization with confocal Raman and fluorescence microscopy. Biomacromolecules 2015, 16, 2179–2187. [Google Scholar] [CrossRef]
- Sieber, H.; Hoffmann, C.; Kaindl, A.; Greil, P. Biomorphic cellular ceramics. Adv. Eng. Mater. 2000, 2, 105–109. [Google Scholar] [CrossRef]
- Vyshnyakova, K.; Yushin, G.; Pereselentseva, L.; Gogotsi, Y. Formation of porous SiC ceramics by pyrolysis of wood impregnated with silica. Int. J. Appl. Ceram. Technol. 2006, 3, 485–490. [Google Scholar] [CrossRef]
- Ghanem, H.; Gerhard, H.; Popovska, N. Paper derived SiC–Si3N4 ceramics for high temperature applications. Ceram. Int. 2009, 35, 1021–1026. [Google Scholar] [CrossRef]
- Sieber, H.; Zollfrank, C.; Popovska, N.; Almeida, D.; Gerhard, H. Gas phase processing of porous, biomorphous TiC-ceramics. Key Eng. Mater. 2004, 264–268, 2227–2230. [Google Scholar] [CrossRef]
- Fan, T.; Li, X.; Ding, J.; Zhang, D.; Guo, Q. Synthesis of biomorphic Al2O3 based on natural plant templates and assembly of Ag nanoparticles controlled within the nanopores. Microporous Mesoporous Mater. 2008, 108, 204–212. [Google Scholar] [CrossRef]
- Zampieri, A.; Sieber, H.; Selvam, T.; Mabande, G.T.P.; Schwieger, W.; Scheffler, F.; Scheffler, M.; Greil, P. Biomorphic cellular SiSiC/zeolite ceramic composites: From rattan palm to bioinspired structured monoliths for catalysis and sorption. Adv. Mater. 2005, 17, 344–349. [Google Scholar] [CrossRef]
- Travitzky, N.; Windsheimer, H.; Fey, T.; Greil, P. Preceramic paper-derived ceramics. J. Am. Cer. Soc. 2008, 91, 3477–3492. [Google Scholar] [CrossRef]
- Gutbrod, B.; Haas, D.; Travitzky, N.; Greil, P. Preceramic paper derived alumina/zirconia ceramics. Adv. Eng. Mater. 2011, 13, 494–501. [Google Scholar] [CrossRef]
- Junkes, J.A.; Dermeik, B.; Gutbrod, B.; Hotza, D.; Greil, P.; Travitzky, N. Influence of coatings on microstructure and mechanical properties of preceramic paper-derived porous alumina substrates. J. Mater. Process. Technol. 2013, 213, 308–313. [Google Scholar] [CrossRef]
- Stares, S.L.; Kirilenko, A.; Fredel, M.C.; Greil, P.; Wondraczek, L.; Travitzky, N. Paper-derived bioactive glass tape. Adv. Eng. Mater. 2013, 15, 230–237. [Google Scholar] [CrossRef]
- Stares, S.L.; Fredel, M.C.; Greil, P.; Travitzky, N. Paper-derived hydroxyapatite. Ceram. Int. 2013, 39, 7179–7183. [Google Scholar] [CrossRef]
- Stares, S.L.; Fredel, M.C.; Greil, P.; Travitzky, N. Paper-derived β-TCP. Mater. Lett. 2013, 98, 161–163. [Google Scholar] [CrossRef]
- Scheid, D.; Cherkashinin, G.; Ionescu, E.; Gallei, M. Single-source magnetic nanorattles by using convenient emulsion polymerization protocols. Langmuir 2014, 30, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Rüttiger, C.; Mehlhase, S.; Vowinkel, S.; Cherkashinin, G.; Liu, N.; Dietz, C.; Stark, R.W.; Biesalski, M.; Gallei, M. Redox-mediated flux control in functional paper. Polymer 2016, 98, 429–436. [Google Scholar] [CrossRef]
- Vowinkel, S.; Malz, F.; Rode, K.; Gallei, M. Single-source macroporous hybrid materials by melt-shear organization of core–shell particles. J. Mater. Sci. 2017, 52, 11179–11190. [Google Scholar] [CrossRef]
- von Irmer, J.; Vowinkel, S.; Scheid, D.; Schöttner, S.; Rüttiger, C.; Appold, M.; Gallei, M. Surface-initiated atom transfer radical polymerization of electrochemically responsive cobalt-methacrylates. Polymer 2017, 122, 303–311. [Google Scholar] [CrossRef]
- Vowinkel, S.; Boehm, A.; Schäfer, T.; Gutmann, T.; Ionescu, E.; Gallei, M. Preceramic core-shell particles for the preparation of hybrid colloidal crystal films by melt-shear organization and conversion into porous ceramics. Mater. Des. 2018, 160, 926–935. [Google Scholar] [CrossRef]
- Advincula, R.C. Surface initiated polymerization from nanoparticle surfaces. J. Disp. Sci. Technol. 2003, 24, 343–361. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Ataman, N.C.; Mocny, P.; Wang, J.; Moraes, J.; Klok, H.A. Surface-initiated controlled radical polymerization: State-of-the-art, opportunities, and challenges in surface and interface engineering with polymer brushes. Chem. Rev. 2017, 117, 1105–1318. [Google Scholar] [CrossRef] [PubMed]
- Siegwart, D.J.; Oh, J.K.; Matyjaszewski, K. ATRP in the design of functional materials for biomedical applications. Prog. Polym. Sci. 2012, 37, 18–37. [Google Scholar] [CrossRef]
- Mazurowski, M.; Sondergeld, K.; Elbert, J.; Kim, C.J.; Li, J.; Frielinghaus, H.; Gallei, M.; Stühn, B.; Rehahn, M. Polystyrene brushes on fully deuterated organic nanoparticles by surface-initiated nitroxide-mediated radical polymerization. Macromol. Chem. Phys. 2013, 214, 1094–1106. [Google Scholar] [CrossRef]
- Braunecker, W.A.; Matyjaszewski, K. Controlled/living radical polymerization: Features, developments, and perspectives. Prog. Polym. Sci. 2007, 32, 93–146. [Google Scholar] [CrossRef]
- Zeschky, J.; Höfner, T.; Arnold, C.; Weißmann, R.; Bahloul-Hourlier, D.; Scheffler, M.; Greil, P. Polysilsesquioxane derived ceramic foams with gradient porosity. Acta Mater. 2005, 53, 927–937. [Google Scholar] [CrossRef]
- Emmerling, S.G.; Langer, L.B.; Pihan, S.A.; Lellig, P.; Gutmann, J.S. Patterning of a surface immobilized atrp initiator with an inkjet printer. Macromolecules 2010, 43, 5033–5042. [Google Scholar] [CrossRef]
- Mazurowski, M.; Gallei, M.; Rehahn, M. Convenient quantification of accessible surface-attached ATRP initiators and RAFT chain transfer agents on cross-linked polystyrene nanoparticles. ACS Macro Lett. 2012, 1, 1362–1366. [Google Scholar] [CrossRef]
- Mazurowski, M.; Gallei, M.; Li, J.; Didzoleit, H.; Stühn, B.; Rehahn, M. Redox-responsive polymer brushes grafted from polystyrene nanoparticles by means of surface initiated atom transfer radical polymerization. Macromolecules 2012, 45, 8970–8981. [Google Scholar] [CrossRef]
- Drelich, J.; Chibowski, E.; Meng, D.D.; Terpilowski, K. Hydrophilic and superhydrophilic surfaces and materials. Soft Matter 2011, 7, 9804–9828. [Google Scholar] [CrossRef]
- Xin, B.; Hao, J. Reversibly switchable wettability. Chem. Soc. Rev. 2010, 39, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, T.; Kumari, B.; Zhao, L.; Breitzke, H.; Schöttner, S.; Rüttiger, C.; Gallei, M. Dynamic nuclear polarization signal amplification as a sensitive probe for specific functionalization of complex paper substrates. J. Phys. Chem. C 2017, 121, 3896–3903. [Google Scholar] [CrossRef]
- Launer, P.J.; Arkles, B. Infrared Analysis of Organosilicon Compounds: Spectra-structure Correlations; Gelest Inc.: Morrisville, PA, USA, 2013. [Google Scholar]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Zhou, C.; Fasel, C.; Ishikawa, R.; Gallei, M.; Ikuhara, Y.; Lauterbach, S.; Kleebe, H.J.; Riedel, R.; Ionescu, E. One-pot synthesis of a C/SiFeN(O)-based ceramic paper with in-situ generated hierarchical micro/nano-morphology. J. Eur. Ceram. Soc. 2017, 37, 5193–5203. [Google Scholar] [CrossRef]
- Pujar, V.V.; Cawley, J.D. Effect of stacking faults on the X-ray diffraction profiles of β-SiC powders. J. Am. Ceram. Soc. 1995, 78, 774–782. [Google Scholar] [CrossRef]
- Roth, F.; Waleska, P.; Hess, C.; Ionescu, E.; Nicoloso, N. UV Raman spectroscopy of segregated carbon in silicon oxycarbides. J. Ceram. Soc. Jpn. 2016, 124, 1042–1045. [Google Scholar] [CrossRef]
- Rosenburg, F.; Ionescu, E.; Nicoloso, N.; Riedel, R. High-temperature Raman spectroscopy of nano-crystalline carbon in silicon oxycarbide. Materials 2018, 11, 93. [Google Scholar] [CrossRef] [PubMed]


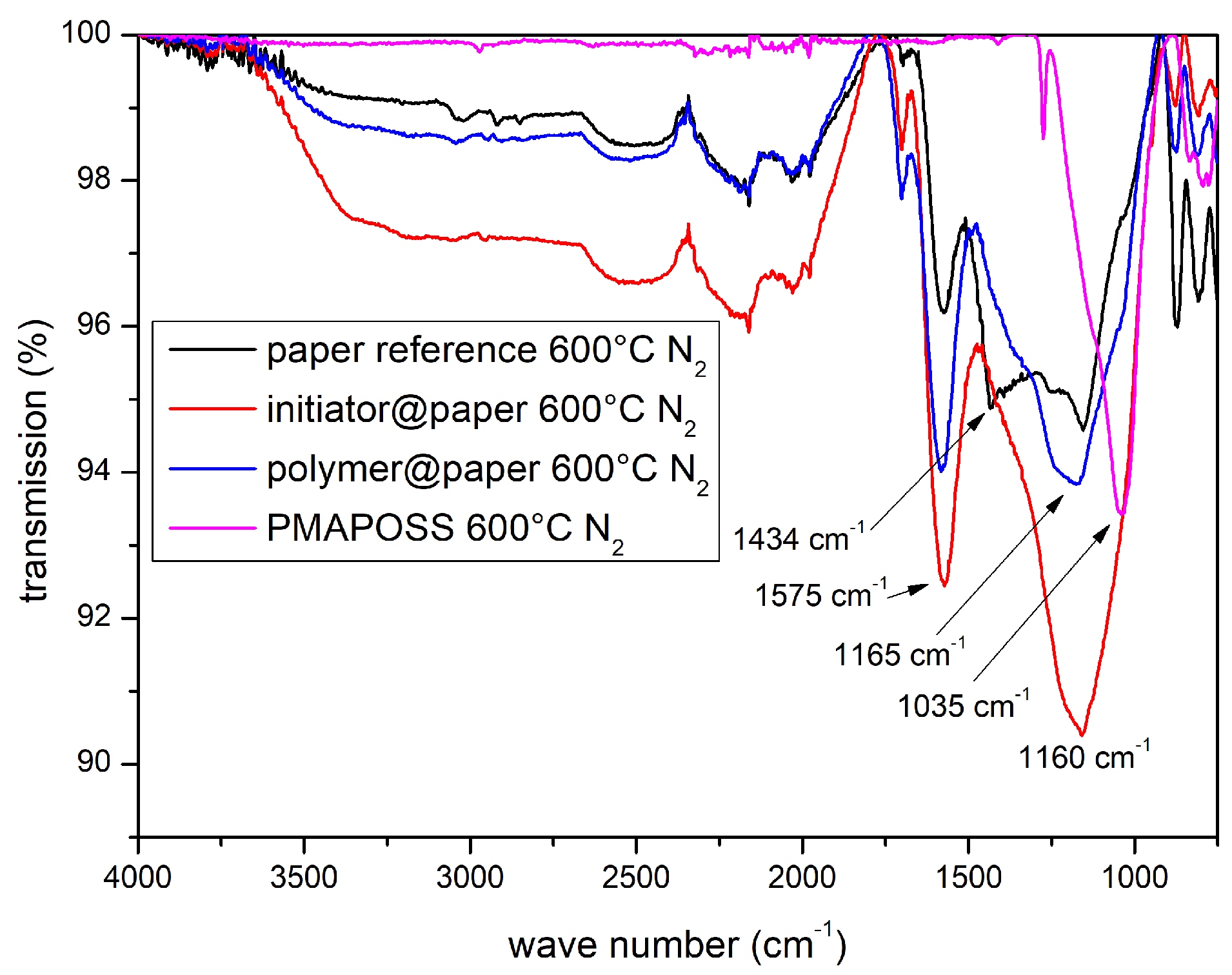
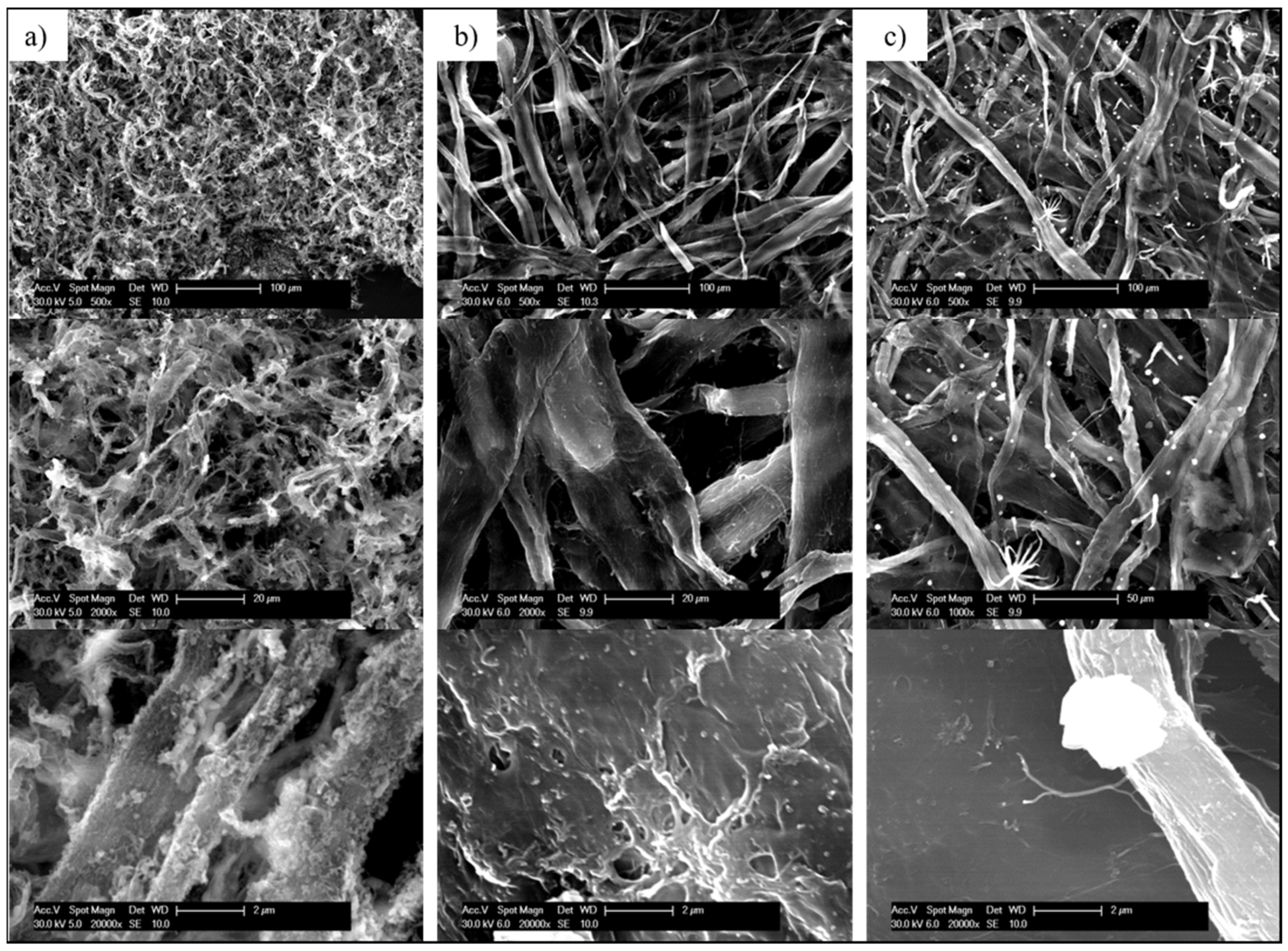
| Sample | CA/° |
|---|---|
| Cellulose Substrate | 5 ± 1 a |
| ATRP-Initiator@Cellulose | 121 ± 7 |
| PMAPOSS@Cellulose | 131 ± 3 |
| Sample | Residue (air) a | Residue (N2) b |
|---|---|---|
| Cellulose Substrate | 0.4 wt.% | 10.2 wt.% |
| ATRP-Initiator@Cellulose | 0.6 wt.% | 12.4 wt.% |
| PMAPOSS@Cellulose | 0.4 wt.% | 13.7 wt.% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rüttiger, C.; Vowinkel, S.; Herzog, N.; Hofmann, K.; Ionescu, E.; Gallei, M. POSS-Containing Polymethacrylates on Cellulose-Based Substrates: Immobilization and Ceramic Formation. Coatings 2018, 8, 446. https://doi.org/10.3390/coatings8120446
Rüttiger C, Vowinkel S, Herzog N, Hofmann K, Ionescu E, Gallei M. POSS-Containing Polymethacrylates on Cellulose-Based Substrates: Immobilization and Ceramic Formation. Coatings. 2018; 8(12):446. https://doi.org/10.3390/coatings8120446
Chicago/Turabian StyleRüttiger, Christian, Steffen Vowinkel, Nicole Herzog, Kathrin Hofmann, Emanuel Ionescu, and Markus Gallei. 2018. "POSS-Containing Polymethacrylates on Cellulose-Based Substrates: Immobilization and Ceramic Formation" Coatings 8, no. 12: 446. https://doi.org/10.3390/coatings8120446
APA StyleRüttiger, C., Vowinkel, S., Herzog, N., Hofmann, K., Ionescu, E., & Gallei, M. (2018). POSS-Containing Polymethacrylates on Cellulose-Based Substrates: Immobilization and Ceramic Formation. Coatings, 8(12), 446. https://doi.org/10.3390/coatings8120446