A Rapid Surface-Enhanced Raman Scattering (SERS) Method for Pb2+ Detection Using L-Cysteine-Modified Ag-Coated Au Nanoparticles with Core–Shell Nanostructure
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
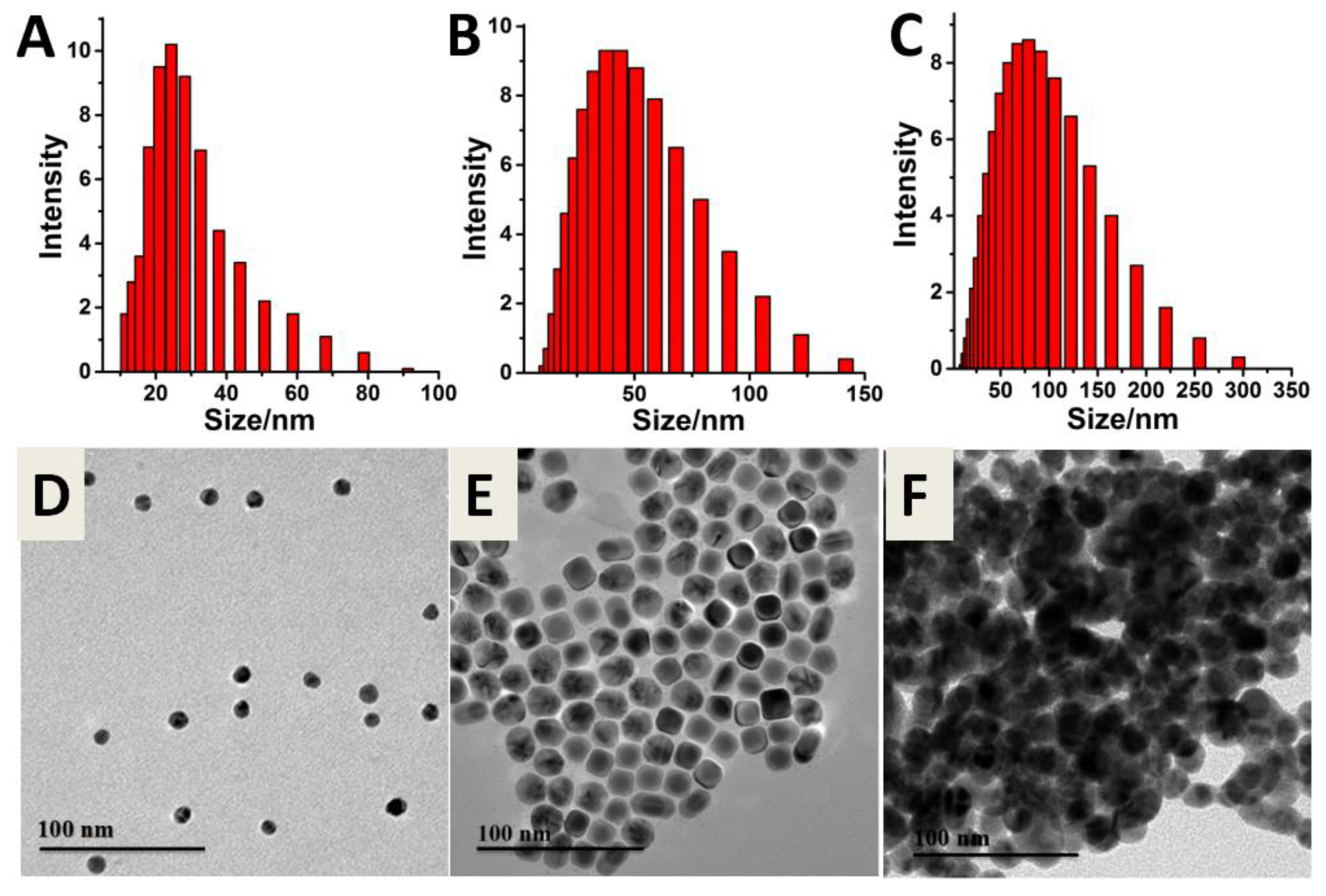
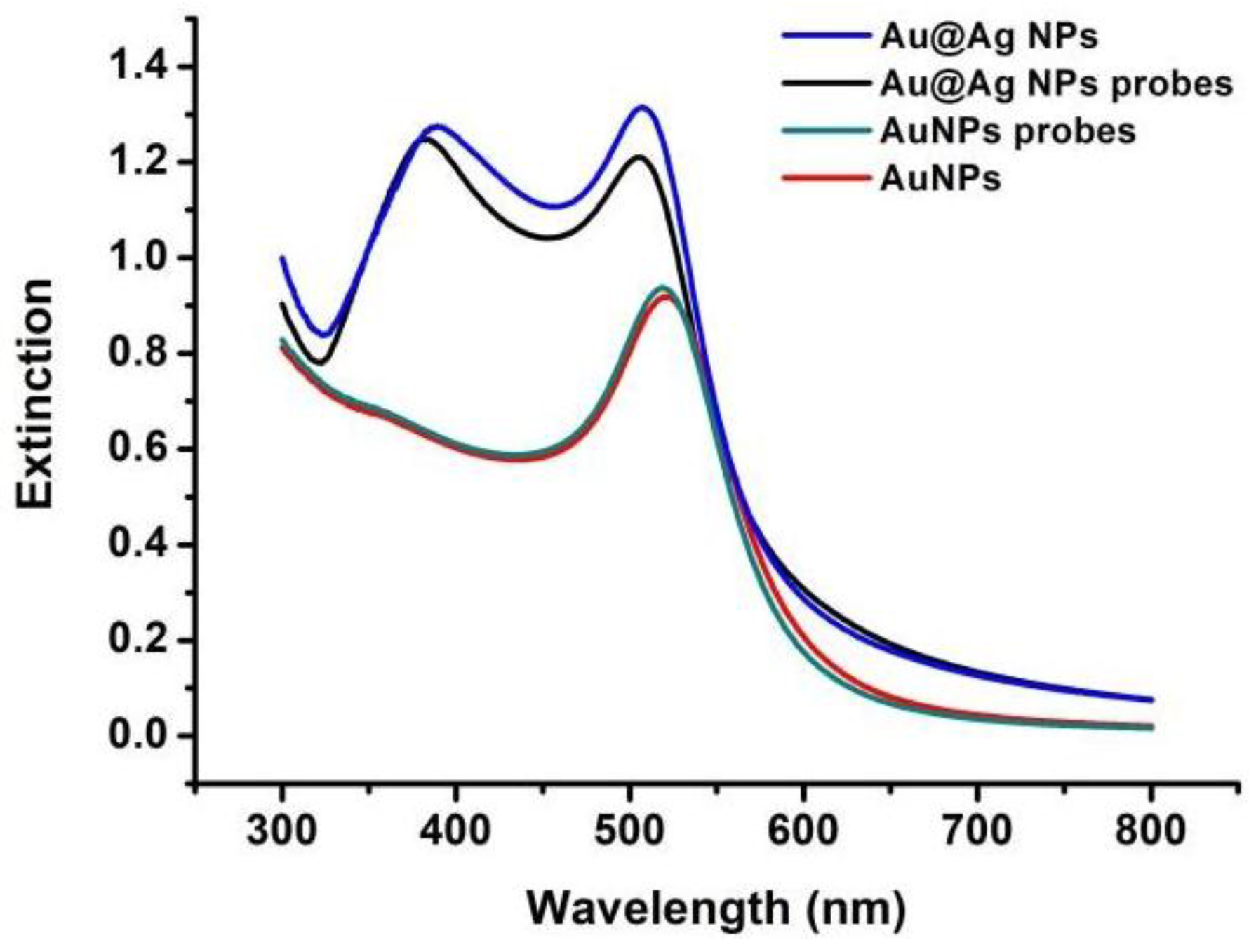
2.3. Synthesis of Au@Ag NPs
2.4. Synthesis of Au@Ag NPs Probes
2.5. SERS Assay for Pb2+ Detection
2.6. Specificity Analysis
2.7. Recovery of Pb2+ Spiked in Drink Water Samples
3. Results
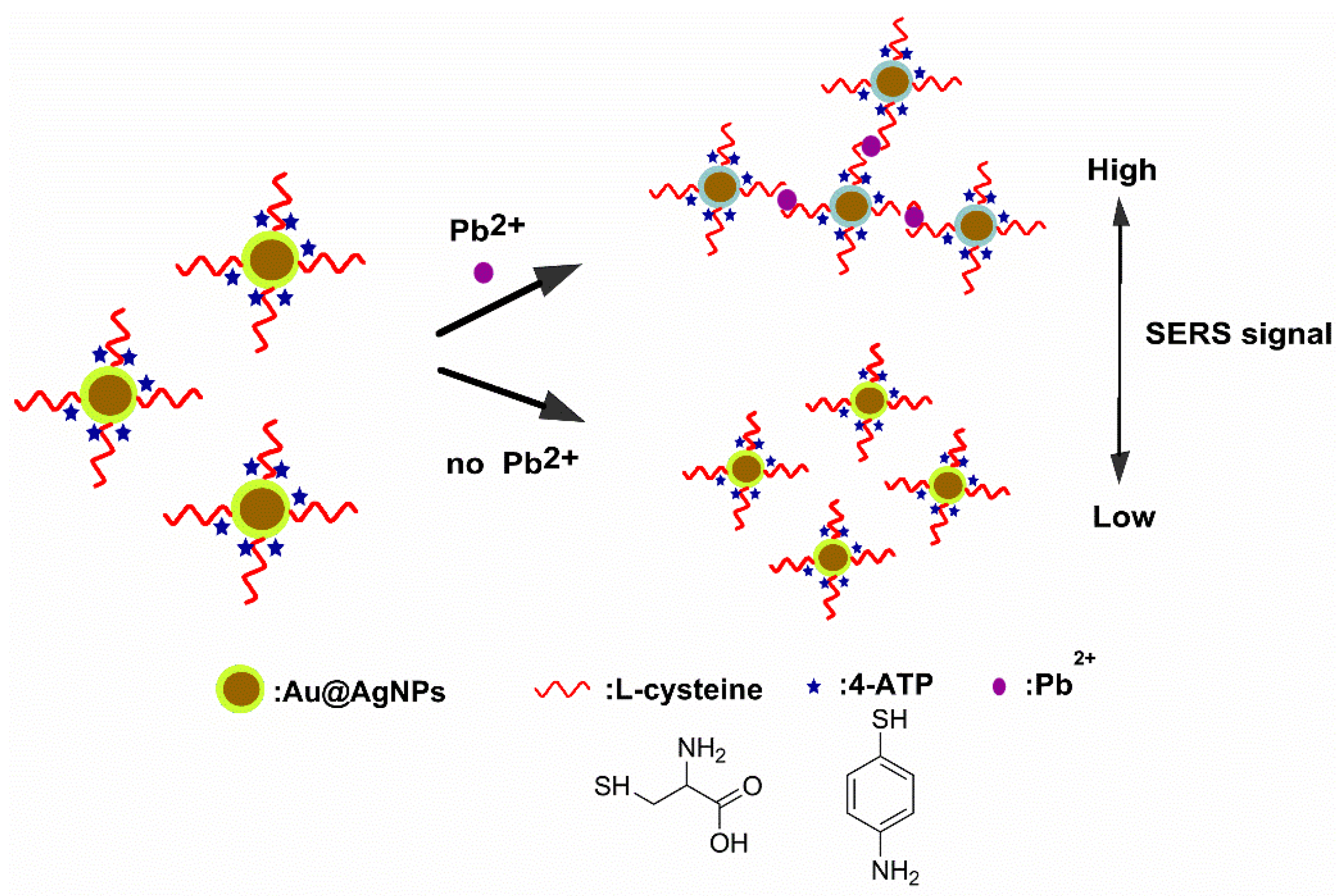
3.1. Detection Rationale
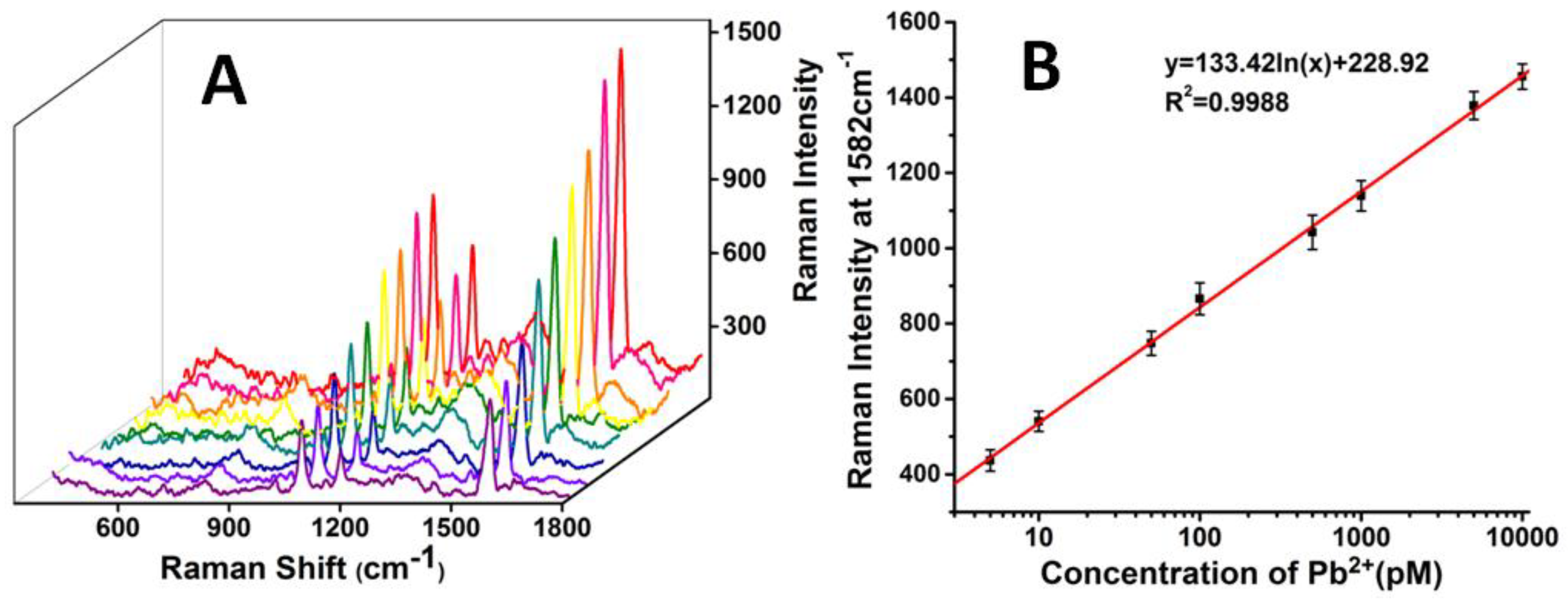
3.2. Establishment of the SERS-Based Aptasensor for Pb2+ Detection
3.3. Selectivity of the SERS Probe for Pb2+
3.4. Recovery of Pb2+ Spiked in Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Diamanti-Kandarakis, E.; Bourguignon, J.-P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Bartrem, C.; Tirima, S.; Von, L.I.; Von, B.M.; Worrell, M.C.; Mohammad, A.S.; Abdullahi, A.; Moller, G. Unknown risk: Co-exposure to lead and other heavy metals among children living in small-scale mining communities in Zamfara State, Nigeria. Int. J. Environ. Health Res. 2014, 24, 304–319. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.; Trevizam, A.R.; Muraoka, T.; Marcante, N.C. Heavy metals in vegetables and potential risk for human health. Sci. Agric. 2012, 69, 54–60. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology. Volume 3: Environmental Toxicology; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; pp. 133–164. [Google Scholar]
- Zhang, W.; Xiong, B.; Chen, L.; Lin, K.; Cui, X.; Bi, H.; Guo, M.; Wang, W. Toxicity assessment of Chlorella vulgaris and Chlorella protothecoides following exposure to Pb(II). Environ. Toxicol. Pharmacol. 2013, 36, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Canário, C.; Ngobeni, P.; Katskov, D.A.; Thomassen, Y. Transverse heated filter atomizer: Atomic absorption spectrometric determination of Pb and Cd in whole blood. J. Anal. At. Spectrom. 2004, 19, 1468–1473. [Google Scholar] [CrossRef]
- Narin, I.; Soylak, M. Enrichment and determinations of nickel(II), cadmium(II), copper(II), cobalt(II) and lead(II) ions in natural waters, table salts, tea and urine samples as pyrrolydine dithiocarbamate chelates by membrane filtration–flame atomic absorption spectrometry combination. Anal. Chim. Acta 2003, 493, 205–212. [Google Scholar]
- Liu, R.; Liu, X.; Tang, Y.; Wu, L.; Hou, X.; Lv, Y. Highly sensitive immunoassay based on immunogold-silver amplification and inductively coupled plasma mass spectrometric detection. Anal. Chem. 2011, 83, 2330–2336. [Google Scholar] [CrossRef] [PubMed]
- Elzinga, E.J.; Rouff, A.A.; Reeder, R.J. The long-term fate of Cu2+, Zn2+, and Pb2+ adsorption complexes at the calcite surface: An X-ray absorption spectroscopy study. Geochim. et Cosmochim. Acta 2006, 70, 2715–2725. [Google Scholar] [CrossRef]
- Taghdisi, S.M.; Danesh, N.M.; Lavaee, P.; Emrani, A.S.; Ramezani, M.; Abnous, K. A novel colorimetric triple-helix molecular switch aptasensor based on peroxidase-like activity of gold nanoparticles for ultrasensitive detection of lead(II). RSC Adv. 2015, 5, 43508–43514. [Google Scholar] [CrossRef]
- Thomas, J.M.; Ting, R.; Perrin, D.M. High affinity DNAzyme-based ligands for transition metal cations—A prototype sensor for Hg2+. Org. Biomol. Chem. 2004, 2, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Guo, Z.; Zeng, G.; Tang, L. Fluorescent and colorimetric sensors for environmental mercury detection. Analyst 2015, 140, 5400–5443. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tang, L.; Ma, W.; Xu, L.; Liu, L.; Kuang, H.; Xu, C. SERS-active Au NR oligomer sensor for ultrasensitive detection of mercury ions. RSC Adv. 2015, 5, 81802–81807. [Google Scholar] [CrossRef]
- Wang, X.; Yang, C.; Zhu, S.; Yan, M.; Ge, S.; Yu, J. 3D origami electrochemical device for sensitive Pb2+ testing based on DNA functionalized iron-porphyrinic metal-organic framework. Biosens. Bioelectron. 2017, 87, 108–115. [Google Scholar]
- Frost, M.S.; Dempsey, M.J.; Whitehead, D.E. Highly Sensitive SERS Detection of Pb2+ Ions in Aqueous Media using Citrate Functionalised Gold Nanoparticles. Sens. Actuators B Chem. 2015, 221, 1003–1008. [Google Scholar] [CrossRef]
- Wu, L.; Xiao, X.; Chen, K.; Yin, W.; Li, Q.; Wang, P.; Lu, Z.; Ma, J.; Han, H. Ultrasensitive SERS detection of Bacillus thuringiensis special gene based on Au@Ag NRs and magnetic beads. Biosens. Bioelectron. 2017, 92, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Zhang, H.; Zhang, L.; Wu, T.; Sun, L.; Jiang, D.; Du, Y. In situ preparation of Ag nanoparticles by laser photoreduction as SERS substrate for determination of Hg2+. J. Raman Spectrosc. 2017, 48, 399–404. [Google Scholar] [CrossRef]
- Indrasekara, A.S.; Meyers, S.; Shubeita, S.; Feldman, L.C.; Gustafsson, T.; Fabris, L. Gold nanostar substrates for SERS-based chemical sensing in the femtomolar regime. Nanoscale 2014, 6, 8891–8899. [Google Scholar] [CrossRef] [PubMed]
- Kabashin, A.V.; Evans, P.; Pastkovsky, S.; Hendren, W.; Wurtz, G.A.; Atkinson, R.; Pollard, R.; Podolskiy, V.A.; Zayats, A.V. Plasmonic nanorod metamaterials for biosensing. Nat. Mater. 2009, 8, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, C.; Jans, H.; Xu, X.; Verellen, N.; Vos, I.; Okumura, Y.; Moshchalkov, V.V.; Lagae, L.; Van, D.P. 300 mm Wafer-level, ultra-dense arrays of Au-capped nanopillars with sub-10 nm gaps as reliable SERS substrates. Nanoscale 2014, 6, 12391–12396. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Han, S.H.; Chung, B.H. Improving Pb2+ detection using DNAzyme-based fluorescence sensors by pairing fluorescence donors with gold nanoparticles. Biosens. Bioelectron. 2011, 26, 2125–2129. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.Z.; Chen, Y.; Li, X.H.; Fang, W.F. Pb2+ induced DNA conformational switch from hairpin to G-quadruplex: Electrochemical detection of Pb2+. Analyst 2011, 136, 2367–2372. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Nam, J.M.; Jeon, K.S.; Lim, D.K.; Kim, H.; Kwon, S.; Lee, H.; Suh, Y.D. Tuning and Maximizing the Single-Molecule Surface-Enhanced Raman Scattering from DNA-Tethered Nanodumbbells. ACS Nano 2012, 6, 9574–9584. [Google Scholar] [CrossRef] [PubMed]
- Ndokoye, P.; Ke, J.; Liu, J.; Zhao, Q.; Li, X. L-Cysteine-Modified Gold Nanostars for SERS-Based Copper Ions Detection in Aqueous Media. Langmuir 2014, 30, 13491–13497. [Google Scholar] [CrossRef] [PubMed]
- Shao, N.; Jin, J.Y.; Cheung, S.M.; Yang, R.H.; Chan, W.H.; Mo, T. A Spiropyran-Based Ensemble for Visual Recognition and Quantification of Cysteine and Homocysteine at Physiological Levels. Angew. Chem. 2006, 45, 4944–4948. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.G.; Zhao, S.; Ma, W.; Wu, X.L.; Li, S.; Kuang, H.; Wang, L.B.; Xu, C.L. Multigaps Embedded Nanoassemblies Enhance in Situ Raman Spectroscopy for Intracellular Telomerase Activity Sensing. Adv. Funct. Mater. 2016, 26, 1602–1608. [Google Scholar] [CrossRef]
- Yan, W.J.; Xu, L.G.; Xu, C.L.; Ma, W.; Kuang, H.; Wang, L.B.; Kotov, N.A. Self-Assembly of Chiral Nanoparticle Pyramids with Strong R/S Optical Activity. J. Am. Chem. Soc. 2012, 134, 15114–15121. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hu, X.; Dong, S. Surfactantless synthesis of multiple shapes of gold nanostructures and their shape-dependent SERS spectroscopy. J. Phys. Chem. B 2006, 110, 16930–16936. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Ok, G.; Choi, S.W.; Jang, H.; Min, D.H. The interfacing structural effect of Ag/graphene oxide nanohybrid films on surface enhanced Raman scattering. Nanoscale 2017, 9, 5872–5878. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, J.J.; Zhao, J.W. The Effect of Dielectric Coating on the Local Electric Field Enhancement of Au-Ag Core-Shell Nanoparticles. Plasmonics 2015, 10, 1–8. [Google Scholar] [CrossRef]
- Chai, F.; Wang, C.; Wang, T.; Li, L.; Su, Z. Colorimetric detection of Pb2+ using glutathione functionalized gold nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 1466–1470. [Google Scholar] [CrossRef] [PubMed]
- Roy, L.E.; Martin, L.R. Theoretical prediction of coordination environments and stability constants of lanthanum lactate complexes in solution. Dalton Trans. 2016, 45, 15517–15522. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.P.; Ding, S.D.; Hu, X.Y.; Li, S.M.; Su, D.P.; Zhang, L.R.; Liu, Y.; Jin, Y.D. Selective extraction of americium(III) over europium(III) ions in nitric acid solution by NTAamide(C8) using a novel water-soluble bisdiglycolamide as a masking agent. Sep. Purif. Technol. 2017, 181, 148–158. [Google Scholar] [CrossRef]
- Bahta, A.; Parker, G.A.; Tuck, D.G. Critical survey of stability constants of complexes of thiocyanate ion. Pure Appl. Chem. 1997, 69, 1489–1548. [Google Scholar] [CrossRef]
- Polavarapu, L.; Perez-Juste, J.; Xu, Q.H.; Liz-Marzán, L.M. Optical sensing of biological, chemical and ionic species through aggregation of plasmonic nanoparticles. J. Mater. Chem. C 2014, 2, 7460–7476. [Google Scholar] [CrossRef]
- Jiang, C.; Guan, Z.; Lim, S.Y.; Polavarapu, L.; Xu, Q.H. Two-photon ratiometric sensing of Hg2+ by using cysteine functionalized Ag nanoparticles. Nanoscale 2011, 3, 3316–3320. [Google Scholar] [CrossRef] [PubMed]


| Sample | Spiked Concentration (nM) | Detected Concentration (nM) | RSD (%) (n = 5) | Recovery (%) (n = 5) |
|---|---|---|---|---|
| Drinking water | 0.1 | 0.0962 | 0.47 | 96.2 |
| 1.0 | 1.0350 | 0.62 | 103.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Zhang, L.; Mei, B.; Tu, J.; Wang, R.; Chen, M.; Cheng, Y. A Rapid Surface-Enhanced Raman Scattering (SERS) Method for Pb2+ Detection Using L-Cysteine-Modified Ag-Coated Au Nanoparticles with Core–Shell Nanostructure. Coatings 2018, 8, 394. https://doi.org/10.3390/coatings8110394
Xu Z, Zhang L, Mei B, Tu J, Wang R, Chen M, Cheng Y. A Rapid Surface-Enhanced Raman Scattering (SERS) Method for Pb2+ Detection Using L-Cysteine-Modified Ag-Coated Au Nanoparticles with Core–Shell Nanostructure. Coatings. 2018; 8(11):394. https://doi.org/10.3390/coatings8110394
Chicago/Turabian StyleXu, Zhou, Linwei Zhang, Bo Mei, Jia Tu, Rong Wang, Maolong Chen, and Yunhui Cheng. 2018. "A Rapid Surface-Enhanced Raman Scattering (SERS) Method for Pb2+ Detection Using L-Cysteine-Modified Ag-Coated Au Nanoparticles with Core–Shell Nanostructure" Coatings 8, no. 11: 394. https://doi.org/10.3390/coatings8110394
APA StyleXu, Z., Zhang, L., Mei, B., Tu, J., Wang, R., Chen, M., & Cheng, Y. (2018). A Rapid Surface-Enhanced Raman Scattering (SERS) Method for Pb2+ Detection Using L-Cysteine-Modified Ag-Coated Au Nanoparticles with Core–Shell Nanostructure. Coatings, 8(11), 394. https://doi.org/10.3390/coatings8110394





