Hybrid Sol-Gel Coatings: Smart and Green Materials for Corrosion Mitigation
Abstract
:1. Introduction
- Adopting metals with elements that enrich the surface with a corrosion-resistant component during the corrosion process;
- Addition of aqueous inhibitors, which adsorb strongly on the metal surface and prevent the reaction with the oxidizing agent; and
- Deposition of protective coatings.
2. General Background of Sol-Gel Coatings for Corrosion Mitigation
2.1. Corrosion Protective Sol-Gel Coatings in Steel Substrates
2.2. Corrosion Protective Sol-Gel Coatings in Aluminium Substrates
3. Future Hybrid Coating Systems and Approaches
Author Contributions
Conflicts of Interest
References
- Davis, J.R. Corrosion Understanding the Basics; ASM International: Materials Park, OH, USA, 2000. [Google Scholar]
- Bierwagen, G. Corrosion and Its Control by Coatings; American Chemical Society: Washington, DC, USA, 1998; pp. 1–8. [Google Scholar]
- Böhni, H. Corrosion in Reinforced Concrete Structures; Böhni, H., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2005. [Google Scholar]
- Dean, S.W.; Products, A.; Rhea, E.C. Atmospheric Corrosion of Metals; ASTM International: West Conshohocken, PA, USA, 1980. [Google Scholar]
- Uhlig, H.H.; Revie, R.W. Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering; Wiley: New York, NY, USA, 1991. [Google Scholar]
- Smallman, R.E.; Ngan, A.H.W. Chapter 9: Oxidation, Corrosion and Surface Treatment. In Physical Metallurgy and Advanced Materials Engineering, 7th ed.; Smallman, R.E., Ngan, A.H.W., Eds.; Butterworth-Heinemann: Oxford, UK, 2007; pp. 481–511. [Google Scholar]
- Raupach, M.; Elsener, B.; Polder, R.; Mietz, J. Corrosion of Reinforcement in Concrete: Mechanisms, Monitoring, Inhibitors and Rehabilitation Techniques; CRC Press: Cambridge, MA, USA; Boca Raton, FL, USA, 2007. [Google Scholar]
- Kaesche, H. Corrosion of Metals: Physicochemical Principles and Current Problems; Springer: New York, NY, USA, 2003. [Google Scholar]
- Ghali, E.; Sastri, V.S.; Elboujdaini, M. Corrosion Prevention and Protection: Practical Solutions; John Wiley & Sons: New York, NY, USA, 2007. [Google Scholar]
- Bertolini, L.; Elsener, B.; Pedeferri, P. Corrosion of Steel in Concrete: Prevention, Diagnosis, Repair, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Roberge, P. Handbook of Corrosion Engineering; McGraw-Hill Professional: New York, NY, USA, 1999. [Google Scholar]
- Bramfitt, B.L. Carbon and Alloy Steels. In Handbook of Materials Selection; Kutz, M., Ed.; John Wiley & Sons: Inc.: New York, NY, USA, 2007; pp. 25–65. [Google Scholar]
- Eppensteiner, F.W.; Jennkind, M.R. Chromate conversion coatings. Met. Finish. 2007, 105, 413–424. [Google Scholar] [CrossRef]
- Magalhães, A.A.O.; Tribollet, B.; Mattos, O.R.; Margarit, I.C.P.; Barcia, O.E. Chromate Conversion Coatings Formation on Zinc Studied by Electrochemical and Electrohydrodynamical Impedances. J. Electrochem. Soc. 2003, 150, B16–B25. [Google Scholar] [CrossRef]
- Osborne, J.H. Observations on chromate conversion coatings from a sol-gel perspective. Prog. Org. Coat. 2001, 41, 280–286. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Boston, MA, USA, 1990. [Google Scholar]
- Brinker, C.; Hurd, A.; Schunk, P.; Frye, G. Review of sol-gel thin film formation. J. Noncryst. Solids 1992, 148, 424–436. [Google Scholar] [CrossRef]
- Aegerter, M.A.; Mennig, M. Sol-Gel Technologies for Glass Producers and Users; Kluwer Academic Publishers: Boston, MA, USA, 2004. [Google Scholar]
- Balgude, D.; Sabnis, A. Sol-gel derived hybrid coatings as an environment friendly surface treatment for corrosion protection of metals and their alloys. J. Sol-Gel Sci. Technol. 2012, 64, 124–134. [Google Scholar] [CrossRef]
- Kroschwitz, J.I.; Seidel, A. Kirk-Othmer Encyclopedia of Chemical Technology, 5th ed.; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Han, Y.-H.; Taylor, A.; Mantle, M.D.; Knowles, K.M. UV curing of organic–inorganic hybrid coating materials. J. Sol-Gel Sci. Technol. 2007, 43, 111–123. [Google Scholar] [CrossRef]
- Aklalouch, M.; Calleja, A.; Granados, X.; Ricart, S.; Boffa, V.; Ricci, F.; Puig, T.; Obradors, X. Hybrid sol-gel layers containing CeO2 nanoparticles as UV-protection of plastic lenses for concentrated photovoltaics. Sol. Energy Mater. Sol. Cells 2014, 120, 175–182. [Google Scholar] [CrossRef]
- Buskens, P.; Wouters, M.; Rentrop, C.; Vroon, Z. A brief review of environmentally benign antifouling and foul-release coatings for marine applications. J. Coat. Technol. Res. 2013, 10, 29–36. [Google Scholar] [CrossRef]
- Detty, M.R.; Ciriminna, R.; Bright, F.V.; Pagliaro, M. Environmentally benign sol-gel antifouling and foul-releasing coatings. Acc. Chem. Res. 2014, 47, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Chen, D. Anti-reflection (AR) coatings made by sol-gel processes: A review. Sol. Energy Mater. Sol. Cells 2001, 68, 313–336. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, Y.; Zhang, H.; Yan, H.; Lv, H.; Jiang, B. Sol-gel preparation of hydrophobic silica antireflective coatings with low refractive index by base/acid two-step catalysis. ACS Appl. Mater. Interfaces 2014, 6, 11470–11475. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, J. A review of contamination-resistant antireflective sol-gel coatings. J. Sol-Gel Sci. Technol. 2012, 61, 206–212. [Google Scholar] [CrossRef]
- Philipavičius, J.; Kazadojev, I.; Beganskienė, A.; Melninkaitis, A.; Sirutkaitis, V.; Kareiva, A. Hydrophobic antireflective silica coatings via sol-gel process. Mater. Sci. 2008, 14, 283–287. [Google Scholar]
- Zhang, Q.; Wei, Y.; Yang, W.; Hui, H.; Deng, X.; Wang, J.; Xu, Q.; Shen, J. Improvement on contamination resistance to volatile organics and moisture of sol-gel silica antireflective coating for 351 nm laser system by structural modulation with fluorinated compounds. RSC Adv. 2014, 5, 4529–4536. [Google Scholar] [CrossRef]
- Avci, N.; Cimieri, I.; Smet, P.F.; Poelman, D. Stability improvement of moisture sensitive CaS:Eu2+ micro-particles by coating with sol-gel alumina. Opt. Mater. 2011, 33, 1032–1035. [Google Scholar] [CrossRef]
- Islam, T.; Kumar, L.; Khan, S.A. A novel sol-gel thin film porous alumina based capacitive sensor for measuring trace moisture in the range of 2.5–25 ppm. Sens. Actuators B Chem. 2012, 173, 377–384. [Google Scholar] [CrossRef]
- Mistry, K.K.; Saha, D.; Sengupta, K. Sol-gel processed Al2O3 thick film template as sensitive capacitive trace moisture sensor. Sens. Actuators B Chem. 2005, 106, 258–262. [Google Scholar] [CrossRef]
- Saha, D.; Mistry, K.K.; Giri, R.; Guha, A.; Sensgupta, K. Dependence of moisture absorption property on sol-gel process of transparent nano-structured γ-Al2O3 ceramics. Sens. Actuators B Chem. 2005, 109, 363–366. [Google Scholar] [CrossRef]
- Abdolah Zadeh, M.; van der Zwaag, S.; Garcia, S.J. Routes to extrinsic and intrinsic self-healing corrosion protective sol-gel coatings: A review. Self Heal. Mater. 2013, 1, 1–18. [Google Scholar] [CrossRef]
- Habazaki, H.; Kimura, T.; Aoki, Y.; Tsuji, E.; Yano, T. Highly enhanced corrosion resistance of stainless steel by sol-gel layer-by-layer aluminosilicate thin coatings. J. Electrochem. Soc. 2013, 161, C57–C61. [Google Scholar] [CrossRef]
- Rosero-Navarro, N.C.; Pellice, S.A.; Durán, A.; Ceré, S.; Aparicio, M. Corrosion protection of aluminium alloy AA2024 with cerium doped methacrylate-silica coatings. J. Sol Gel Sci. Technol. 2009, 52, 31–40. [Google Scholar] [CrossRef]
- Tiwari, A.; Hihara, L.H. Chapter 10—Sol-Gel Route for the Development of Smart Green Conversion Coatings for Corrosion Protection of Metal Alloys. In Intelligent Coatings for Corrosion Control; Tiwari, A., Rawlins, J., Hihara, L.H., Eds.; Butterworth-Heinemann: Boston, MA, USA, 2015; pp. 363–407. [Google Scholar]
- Zheng, S.; Li, J. Inorganic-organic sol gel hybrid coatings for corrosion protection of metals. J. Sol-Gel Sci. Technol. 2010, 54, 174–187. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Tedim, J.; Ferreira, M.G.S. “Smart” coatings for active corrosion protection based on multi-functional micro and nanocontainers. Electrochimica. Acta 2012, 82, 314–323. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Shchukin, D.G.; Yasakau, K.A.; Möhwald, H.; Ferreira, M.G.S. Anticorrosion coatings with self-healing effect based on nanocontainers impregnated with corrosion inhibitor. Chem. Mater. 2007, 19, 402–411. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Kallip, S.; Zheludkevich, M.L.; Ferreira, M.G.S. Active corrosion protection of AA2024 by sol-gel coatings with cerium molybdate nanowires. Electrochimica. Acta 2013, 112, 236–246. [Google Scholar] [CrossRef]
- Carneiro, J.; Tedim, J.; Fernandes, S.C.M.; Freire, C.S.R.; Gandini, A.; Ferreira, M.G.S.; Zheludkevich, M.L. Chitosan as a smart coating for controlled release of corrosion inhibitor 2-mercaptobenzothiazole. ECS Electrochem. Lett. 2013, 2, C19–C22. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.; Pereira, E.V.; Salta, M.M. Ureasilicate hybrid coatings for corrosion protection of galvanized steel in cementitious media. J. Electrochem. Soc. 2013, 160, C467–C479. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.; Pereira, E.V.; Salta, M.M. Alcohol-aminosilicate hybrid coatings for corrosion protection of galvanized steel in mortar. J. Electrochem. Soc. 2014, 161, C349–C362. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Hot-dip galvanized steel dip-coated with ureasilicate hybrid in simulated concrete pore solution: Assessment of coating morphology and corrosion protection efficiency. Prog. Org. Coat. 2015, 88, 245–255. [Google Scholar] [CrossRef]
- Pepe, A.; Galliano, P.; Aparicio, M.; Durán, A.; Ceré, S. Sol-gel coatings on carbon steel: Electrochemical evaluation. Surf. Coat. Technol. 2006, 200, 3486–3491. [Google Scholar] [CrossRef]
- Facult, C.A.; Ii, M.; Zirconia, F.; Ultrasonics, H.S. Protection of 316L stainless steel by zirconia sol-gel coatings in 15% H2SO4 solutions. J. Mater. Sci. Lett. 1995, 14, 178–181. [Google Scholar]
- Conde, A.; Damborenea, J.; Durán, A.; Menning, M. Protective properties of a sol-gel coating on zinc coated steel. J. Sol Gel Sci. Technol. 2006, 37, 79–85. [Google Scholar] [CrossRef]
- Galio, A.F.; Lamaka, S.V.; Zheludkevich, M.L.; Dick, L.F.P.; Müller, I.L.; Ferreira, M.G.S. Inhibitor-doped sol-gel coatings for corrosion protection of magnesium alloy AZ31. Surf. Coat. Technol. 2010, 204, 1479–1486. [Google Scholar] [CrossRef]
- Zomorodian, A.; Brusciotti, F.; Fernandes, A.; Carmezim, M.J.; Moura e Silva, T.; Fernandes, J.C.S.; Montemor, M.F. Anti-corrosion performance of a new silane coating for corrosion protection of AZ31 magnesium alloy in Hank’s solution. Surf. Coat. Technol. 2012, 206, 4368–4375. [Google Scholar] [CrossRef]
- Honkanen, M.; Hoikkanen, M.; Vippola, M.; Vuorinen, J.; Lepistö, T. Aminofunctional silane layers for improved copper–polymer interface adhesion. J. Mater. Sci. 2011, 46, 6618–6626. [Google Scholar] [CrossRef]
- Karthik, N.; Sethuraman, M.G. Surface protection of copper by allyl thiourea and hybrid sol-gel coatings. Prog. Org. Coat. 2016, 90, 380–389. [Google Scholar] [CrossRef]
- Seifzadeh, D.; Golmoghani-Ebrahimi, E. Formation of novel and crack free nanocomposites based on sol gel process for corrosion protection of copper. Surf. Coat. Technol. 2012, 210, 103–112. [Google Scholar] [CrossRef]
- Sanchez, C.; Belleville, P.; Popall, M.; Nicole, L. Hybrid materials themed issue. Chem. Soc. Rev. 2011, 40, 453–1152. [Google Scholar]
- Wang, D.; Bierwagen, G.P. Sol–gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Sanchez, C.; Ribot, F. Design of hybrid organic-inorganic materials synthesized via sol-gel chemistry. New J. Chem. 1994, 18, 1007–1047. [Google Scholar]
- Messaddeq, S.H.; Pulcinelli, S.H.; Santilli, C.V.; Guastaldi, A.C.; Messaddeq, Y. Microstructure and corrosion resistance of inorganic–organic (ZrO2–PMMA) hybrid coating on stainless steel1. J. Noncryst. Solids 1999, 247, 164–170. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Organic–inorganic hybrid sol-gel coatings for metal corrosion protection: A review of recent progress. J. Coat. Technol. Res. 2015, 12, 1–35. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Lei, X.; Liu, M. Effect of solution concentration on the structures and corrosion inhibition behavior of γ-APS films fabricated on surface of low carbon steel. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2013, 28, 224–230. [Google Scholar] [CrossRef]
- Santana, I.; Pepe, A.; Jimenez-Pique, E.; Pellice, S.; Milošev, I.; Ceré, S. Corrosion protection of carbon steel by silica-based hybrid coatings containing cerium salts: Effect of silica nanoparticle content. Surf. Coat. Technol. 2015, 265, 106–116. [Google Scholar] [CrossRef]
- Santana, I.; Pepe, A.; Jimenez-Pique, E.; Pellice, S.; Ceré, S. Silica-based hybrid coatings for corrosion protection of carbon steel. Part I: Effect of pretreatment with phosphoric acid. Surf. Coat. Technol. 2013, 236, 476–484. [Google Scholar] [CrossRef]
- Qian, M.; Mcintosh Soutar, A.; Tan, X.H.; Zeng, X.T.; Wijesinghe, S.L. Two-part epoxy-siloxane hybrid corrosion protection coatings for carbon steel. Thin Solid Films 2009, 517, 5237–5242. [Google Scholar] [CrossRef]
- Joncoux-Chabrol, K.; Bonino, J.-P.; Gressier, M.; Menu, M.-J.; Pébère, N. Improvement of barrier properties of a hybrid sol-gel coating by incorporation of synthetic talc-like phyllosilicates for corrosion protection of a carbon steel. Surf. Coat. Technol. 2012, 206, 2884–2891. [Google Scholar] [CrossRef]
- Subasri, R.; Malathi, R.; Jyothirmayi, A.; Hebalkar, N.Y. Synthesis and characterization of CuO-hybrid silica nanocomposite coatings on SS 304. Ceram. Int. 2012, 38, 5731–5740. [Google Scholar] [CrossRef]
- Zandi Zand, R.; Verbeken, K.; Adriaens, A. Corrosion resistance performance of cerium doped silica sol-gel coatings on 304L stainless steel. Prog. Org. Coat. 2012, 75, 463–473. [Google Scholar] [CrossRef]
- Gallardo, J.; Durán, A.; de Damborenea, J.J. Electrochemical and in vitro behaviour of sol-gel coated 316L stainless steel. Corros. Sci. 2004, 46, 795–806. [Google Scholar] [CrossRef]
- Hosseinalipour, S.M.; Ershad-langroudi, A.; Hayati, A.N.; Nabizade-Haghighi, A.M. Characterization of sol-gel coated 316L stainless steel for biomedical applications. Prog. Org. Coat. 2010, 67, 371–374. [Google Scholar] [CrossRef]
- Kumar, N.; Jyothirmayi, A.; Soma Raju, K.R.C.; Subasri, R. Effect of functional groups (methyl, phenyl) on organic–inorganic hybrid sol-gel silica coatings on surface modified SS 316. Ceram. Int. 2012, 38, 6565–6572. [Google Scholar] [CrossRef]
- Motalebi, A.; Nasr-Esfahani, M. Electrochemical and In Vitro Behavior of Nanostructure Sol-Gel Coated 316L Stainless Steel Incorporated with Rosemary Extract. J. Mater. Eng. Perform. 2012, 22, 1756–1764. [Google Scholar] [CrossRef]
- Chou, T.; Chandrasekaran, C.; Cao, G.Z. Sol-gel-derived hybrid coatings for corrosion protection. J. Sol-Gel Sci. Technol. 2003, 26, 321–327. [Google Scholar] [CrossRef]
- Gunji, T.; Iizuka, Y.; Arimitsu, K.; Abe, Y. Preparation and properties of alkoxy(methyl)silsesquioxanes as coating agents. J. Polym. Sci. A Polym. Chem. 2004, 42, 3676–3684. [Google Scholar] [CrossRef]
- Castro, Y.; Ferrari, B.; Moreno, R.; Durán, A. Corrosion behaviour of silica hybrid coatings produced from basic catalysed particulate sols by dipping and EPD. Surf. Coat. Technol. 2005, 191, 228–235. [Google Scholar] [CrossRef]
- Juan-Díaz, M.J.; Martínez-Ibáñez, M.; Hernández-Escolano, M.; Cabedo, L.; Izquierdo, R.; Suay, J.; Gurruchaga, M.; Goñi, I. Study of the degradation of hybrid sol-gel coatings in aqueous medium. Prog. Org. Coat. 2014, 77, 1799–1806. [Google Scholar] [CrossRef]
- Jianguo, L.; Gaoping, G.; Chuanwei, Y. Enhancement of the erosion–corrosion resistance of Dacromet with hybrid SiO2 sol-gel. Surf. Coat. Technol. 2006, 200, 4967–4975. [Google Scholar] [CrossRef]
- Wang, H.; Akid, R. Encapsulated cerium nitrate inhibitors to provide high-performance anti-corrosion sol-gel coatings on mild steel. Corros. Sci. 2008, 50, 1142–1148. [Google Scholar] [CrossRef]
- Kannan, A.G.; Choudhury, N.R.; Dutta, N.K. Electrochemical performance of sol-gel derived phospho-silicate-methacrylate hybrid coatings. J. Electroanal. Chem. 2010, 641, 28–34. [Google Scholar] [CrossRef]
- Guin, A.K.; Nayak, S.K.; Rout, T.K.; Bandyopadhyay, N.; Sengupta, D.K. Corrosion behavior of nanohybrid titania–silica composite coating on phosphated steel sheet. J. Coat. Technol. Res. 2011, 9, 97–106. [Google Scholar] [CrossRef]
- Kirtay, S. Preparation of hybrid silica sol-gel coatings on mild steel surfaces and evaluation of their corrosion resistance. Prog. Org. Coat. 2014, 77, 1861–1866. [Google Scholar] [CrossRef]
- Criado, M.; Sobrados, I.; Sanz, J.; Bastidas, J.M. Steel protection using sol-gel coatings in simulated concrete pore solution contaminated with chloride. Surf. Coat. Technol. 2014, 258, 485–494. [Google Scholar] [CrossRef]
- Criado, M.; Sobrados, I.; Bastidas, J.M.; Sanz, J. Steel corrosion in simulated carbonated concrete pore solution its protection using sol-gel coatings. Prog. Org. Coat. 2015, 88, 228–236. [Google Scholar] [CrossRef]
- Phan, T.T.; Bentiss, F.; Jama, C. Structural and anticorrosion performance characterization of phosphosilicate sol-gel coatings prepared from 3-(trimethoxysilyl) propyl methacrylate and bis[2-(methacryloyloxy)ethyl] phosphate. Prog. Org. Coat. 2015, 89, 123–131. [Google Scholar] [CrossRef]
- Annual Report 2014—Activity Report; European Aluminium Association: Brussels, Belgium, 2014.
- Conde, A.; Durán, A.; de Damborenea, J. Polymeric sol-gel coatings as protective layers of aluminium alloys. Prog. Org. Coat. 2003, 46, 288–296. [Google Scholar]
- Zheludkevich, M.L.; Salvado, I.M.; Ferreira, M.G.S. Sol–gel coatings for corrosion protection of metals. J. Mater. Chem. 2005, 15, 5099–5111. [Google Scholar] [CrossRef]
- Parkhill, R.; Knobbe, E.; Donley, M. Application and evaluation of environmentally compliant spray-coated ormosil films as corrosion resistant treatments for aluminum 2024-T3. Prog. Org. Coat. 2001, 41, 261–265. [Google Scholar] [CrossRef]
- Varma, P.C.R.; Colreavy, J.; Cassidy, J.; Oubaha, M.; McDonagh, C.; Duffy, B. Corrosion protection of AA 2024-T3 aluminium alloys using 3,4-diaminobenzoic acid chelated zirconium–silane hybrid sol-gels. Thin Solid Films 2010, 518, 5753–5761. [Google Scholar] [CrossRef]
- Akid, R.; Gobara, M.; Wang, H. Corrosion protection performance of novel hybrid polyaniline/sol-gel coatings on an aluminium 2024 alloy in neutral, alkaline and acidic solutions. Electrochim. Acta 2011, 56, 2483–2492. [Google Scholar] [CrossRef]
- Andreatta, F.; Paussa, L.; Lanzutti, A.; Rosero Navarro, N.C.; Aparicio, M.; Castro, Y.; Duran, A.; Ondratschek, D.; Fedrizzi, L. Development and industrial scale-up of ZrO2 coatings and hybrid organic–inorganic coatings used as pre-treatments before painting aluminium alloys. Prog. Org. Coat. 2011, 72, 3–14. [Google Scholar] [CrossRef]
- Kozhukharov, S.; Kozhukharov, V.; Schem, M.; Aslan, M.; Wittmar, M.; Wittmar, A.; Veith, M. Protective ability of hybrid nano-composite coatings with cerium sulphate as inhibitor against corrosion of AA2024 aluminium alloy. Prog. Org. Coat. 2012, 73, 95–103. [Google Scholar] [CrossRef]
- Li, Y.-S.; Lu, W.; Wang, Y.; Tran, T. Studies of (3-mercaptopropyl)trimethoxylsilane and bis(trimethoxysilyl)ethane sol-gel coating on copper and aluminum. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 73, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Lampke, T.; Darwich, S.; Nickel, D.; Wielage, B. Development and characterization of sol-gel composite coatings on aluminum alloys for corrosion protection. Materialwiss. Werkst. 2008, 39, 914–919. [Google Scholar] [CrossRef]
- Fedel, M.; Callone, E.; Diré, S.; Deflorian, F.; Olivier, M.-G.; Poelman, M. Effect of Na-Montmorillonite sonication on the protective properties of hybrid silica coatings. Electrochim. Acta 2014, 124, 90–99. [Google Scholar] [CrossRef]
- Capelossi, V.R.; Poelman, M.; Recloux, I.; Hernandez, R.P.B.; de Melo, H.G.; Olivier, M.G. Corrosion protection of clad 2024 aluminum alloy anodized in tartaric-sulfuric acid bath and protected with hybrid sol-gel coating. Electrochim. Acta 2014, 124, 69–79. [Google Scholar] [CrossRef]
- Rodič, P.; Iskra, J.; Milošev, I. Study of a sol-gel process in the preparation of hybrid coatings for corrosion protection using FTIR and 1H NMR methods. J. Noncryst. Solids 2014, 396, 25–35. [Google Scholar] [CrossRef]
- Voevodin, N.N.; Kurdziel, J.W.; Mantz, R. Corrosion protection for aerospace aluminum alloys by Modified Self-assembled NAnophase Particle (MSNAP) sol-gel. Surf. Coat. Technol. 2006, 201, 1080–1084. [Google Scholar] [CrossRef]
- Metroke, T.L.; Gandhi, J.S.; Apblett, A. Corrosion resistance properties of Ormosil coatings on 2024-T3 aluminum alloy. Prog. Org. Coat. 2004, 50, 231–246. [Google Scholar] [CrossRef]
- Metroke, T.; Kachurina, O.; Knobbe, E.T. Spectroscopic and corrosion resistance characterization of GLYMO–TEOS Ormosil coatings for aluminum alloy corrosion inhibition. Prog. Org. Coat. 2002, 44, 295–305. [Google Scholar] [CrossRef]
- Pirhady Tavandashti, N.; Sanjabi, S.; Shahrabi, T. Corrosion protection evaluation of silica/epoxy hybrid nanocomposite coatings to AA2024. Prog. Org. Coat. 2009, 65, 182–186. [Google Scholar] [CrossRef]
- Feng, Z.; Liu, Y.; Thompson, G.E.; Skeldon, P. Sol–gel coatings for corrosion protection of 1050 aluminium alloy. Electrochim. Acta 2010, 55, 3518–3527. [Google Scholar] [CrossRef]
- Collazo, A.; Covelo, A.; Nóvoa, X.R.; Pérez, C. Corrosion protection performance of sol-gel coatings doped with red mud applied on AA2024-T3. Prog. Org. Coat. 2012, 74, 334–342. [Google Scholar] [CrossRef]
- Álvarez, P.; Collazo, A.; Covelo, A.; Nóvoa, X.R.; Pérez, C. The electrochemical behaviour of sol-gel hybrid coatings applied on AA2024-T3 alloy: Effect of the metallic surface treatment. Prog. Org. Coat. 2010, 69, 175–183. [Google Scholar] [CrossRef]
- Álvarez, D.; Collazo, A.; Hernández, M.; Nóvoa, X.R.; Pérez, C. Characterization of hybrid sol-gel coatings doped with hydrotalcite-like compounds to improve corrosion resistance of AA2024-T3 alloys. Prog. Org. Coat. 2010, 67, 152–160. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Zheludkevich, M.L.; Yasakau, K.A.; Montemor, M.F.; Ferreira, M.G.S. High effective organic corrosion inhibitors for 2024 aluminium alloy. Electrochim. Acta 2007, 52, 7231–7247. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Zheludkevich, M.L.; Lamaka, S.V.; Ferreira, M.G.S. Mechanism of Corrosion Inhibition of AA2024 by Rare-Earth Compounds. J. Phys. Chem. B 2006, 110, 5515–5528. [Google Scholar] [CrossRef] [PubMed]
- Yasakau, K.A.; Carneiro, J.; Zheludkevich, M.L.; Ferreira, M.G.S. Influence of sol-gel process parameters on the protection properties of sol-gel coatings applied on AA2024. Surf. Coat. Technol. 2014, 246, 6–16. [Google Scholar] [CrossRef]
- Gobara, M.; Kamel, H.; Akid, R.; Baraka, A. Corrosion behaviour of AA2024 coated with an acid-soluble collagen/hybrid silica sol-gel matrix. Prog. Org. Coat. 2015, 89, 57–66. [Google Scholar] [CrossRef]
- Bera, S.; Rout, T.K.; Udayabhanu, G.; Narayan, R. Comparative Study of Corrosion Protection of Sol–Gel Coatings with Different Organic Functionality on Al-2024 substrate. Prog. Org. Coat. 2015, 88, 293–303. [Google Scholar] [CrossRef]
- Rodič, P.; Mertelj, A.; Borovšak, M.; Benčan, A.; Mihailović, D.; Malič, B.; Milošev, I. Composition, structure and morphology of hybrid acrylate-based sol-gel coatings containing Si and Zr composed for protective applications. Surf. Coat. Technol. 2016, 286, 388–396. [Google Scholar] [CrossRef]
- Sheffer, M.; Groysman, A.; Mandler, D. Electrodeposition of sol-gel films on Al for corrosion protection. Corros. Sci. 2003, 45, 2893–2904. [Google Scholar] [CrossRef]
- Khramov, A.N.; Johnson, J.A. Phosphonate-functionalized ORMOSIL coatings for magnesium alloys. Prog. Org. Coat. 2009, 65, 381–385. [Google Scholar] [CrossRef]
- Khramov, A.N.; Balbyshev, V.N.; Kasten, L.S.; Mantz, R.A. Sol–gel coatings with phosphonate functionalities for surface modification of magnesium alloys. Thin Solid Films 2006, 514, 174–181. [Google Scholar] [CrossRef]
- Davis, S.R.; Brough, A.R.; Atkinson, A. Formation of silica/epoxy hybrid network polymers. J. Noncryst. Solids 2003, 315, 197–205. [Google Scholar] [CrossRef]
- Du, Y.J.; Damron, M.; Tang, G.; Zheng, H.; Chu, C.-J.; Osborne, J.H. Inorganic/Organic Hybrid Coatings for Aircraft Aluminum Alloy Substrates. Prog. Org. Coat. 2001, 41, 226–232. [Google Scholar]
- Fontinha, I.R.; Salta, M.M.; Zheludkevich, M.L.; Ferreira, M.G.S. EIS Study of Amine Cured Epoxy-silica-zirconia Sol-gel Coatings for Corrosion Protection of the Aluminium Alloy EN AW 6063. Port. Electrochim. Acta 2013, 31, 307–319. [Google Scholar] [CrossRef]
- Vreugdenhil, A.J.; Balbyshev, V.N.; Donley, M.S. Nanostructured silicon sol-gel surface treatments for Al 2024-T3 protection. J. Coat. Technol. 2001, 73, 35–43. [Google Scholar] [CrossRef]
- Donley, M.S.; Mantz, R.A.; Khramov, A.N.; Balbyshev, V.N.; Kasten, L.S.; Gaspar, D.J. The self-assembled nanophase particle (SNAP) process: A nanoscience approach to coatings. Prog. Org. Coat. 2003, 47, 401–415. [Google Scholar] [CrossRef]
- Pathak, S.S.; Khanna, A.S. Investigation of anti-corrosion behavior of waterborne organosilane-polyester coatings for AA6011 aluminum alloy. Prog. Org. Coat. 2009, 65, 288–294. [Google Scholar] [CrossRef]
- Roussi, E.; Tsetsekou, A.; Tsiourvas, D.; Karantonis, A. Novel hybrid organo-silicate corrosion resistant coatings based on hyperbranched polymers. Surf. Coat. Technol. 2011, 205, 3235–3244. [Google Scholar] [CrossRef]
- Rosero-Navarro, N.C.; Pellice, S.A.; Durán, A.; Aparicio, M. Effects of Ce-containing sol-gel coatings reinforced with SiO2 nanoparticles on the protection of AA2024. Corros. Sci. 2008, 50, 1283–1291. [Google Scholar] [CrossRef]
- Voevodin, N.N.; Grebasch, N.T.; Soto, W.S.; Kasten, L.S.; Grant, J.T.; Arnold, F.E.; Donley, M.S. An organically modified zirconate film as a corrosion-resistant treatment for aluminum 2024-T3. Prog. Org. Coat. 2001, 41, 287–293. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Serra, R.; Montemor, M.F.; Salvado, I.M.M.; Ferreira, M.G.S. Corrosion protective properties of nanostructured sol-gel hybrid coatings to AA2024-T3. Surf. Coat. Technol. 2006, 200, 3084–3094. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Zheludkevich, M.L.; Yasakau, K.A.; Montemor, M.F.; Cecílio, P.; Ferreira, M.G.S. TiOx self-assembled networks prepared by templating approach as nanostructured reservoirs for self-healing anticorrosion pre-treatments. Electrochem. Commun. 2006, 8, 421–428. [Google Scholar] [CrossRef]
- Fontinha, I.R. Revestimentos Nanoestruturados para Protecção de Liga de Alumínio; Tese de Doutoramento: Aveiro, Portugal, 2012. [Google Scholar]
- Rodič, P.; Milošev, I. Electrochemical and Salt Spray Testing of Hybrid Coatings Based on Si and Zr Deposited on Aluminum and Its Alloys. J. Electrochem. Soc. 2015, 162, C592–C600. [Google Scholar] [CrossRef]
- Schem, M.; Schmidt, T.; Gerwann, J.; Wittmar, M.; Veith, M.; Thompson, G.E.; Molchan, I.S.; Hashimoto, T.; Skeldon, P.; Phani, A.R.; et al. CeO2-filled sol-gel coatings for corrosion protection of AA2024-T3 aluminium alloy. Corros. Sci. 2009, 51, 2304–2315. [Google Scholar] [CrossRef]
- Dias, S.A.S.; Lamaka, S.V.; Nogueira, C.A.; Diamantino, T.C.; Ferreira, M.G.S. Sol–gel coatings modified with zeolite fillers for active corrosion protection of AA2024. Corros. Sci. 2012, 62, 153–162. [Google Scholar] [CrossRef]
- Cambon, J.-B.; Esteban, J.; Ansart, F.; Bonino, J.-P.; Turq, V.; Santagneli, S.H.; Santilli, C.V.; Pulcinelli, S.H. Effect of cerium on structure modifications of a hybrid sol-gel coating, its mechanical properties and anti-corrosion behavior. Mater. Res. Bull. 2012, 47, 3170–3176. [Google Scholar] [CrossRef]
- Raps, D.; Hack, T.; Wehr, J.; Zheludkevich, M.L.; Bastos, A.C.; Ferreira, M.G.S.; Nuyken, O. Electrochemical study of inhibitor-containing organic–inorganic hybrid coatings on AA2024. Corros. Sci. 2009, 51, 1012–1021. [Google Scholar] [CrossRef]
- Khramov, A.N.; Voevodin, N.N.; Balbyshev, V.N.; Mantz, R.A. Sol–gel-derived corrosion-protective coatings with controllable release of incorporated organic corrosion inhibitors. Thin Solid Films 2005, 483, 191–196. [Google Scholar] [CrossRef]
- Andreeva, D.V.; Shchukin, D.G. Smart self-repairing Nanocontainers with a shell possessing controlled release properties can. Mater. Today 2008, 11, 24–30. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Poznyak, S.K.; Rodrigues, L.M.; Raps, D.; Hack, T.; Dick, L.F.; Nunes, T.; Ferreira, M.G.S. Active protection coatings with layered double hydroxide nanocontainers of corrosion inhibitor. Corros. Sci. 2010, 52, 602–611. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Zheludkevich, M.; Möhwald, H. Feedback active coatings based on incorporated nanocontainers. J. Mater. Chem. 2006, 16, 4561–4566. [Google Scholar] [CrossRef]
- Tedim, J.; Poznyak, S.K.; Kuznetsova, A.; Raps, D.; Hack, T.; Zheludkevich, M.L.; Ferreira, M.G.S. Enhancement of Active Corrosion Protection via Combination of Inhibitor-Loaded Nanocontainers. ACS Appl. Mater. Interfaces 2010, 2, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Shchukin, D.G.; Lamaka, S.V.; Yasakau, K.A.; Zheludkevich, M.L.; Ferreira, M.G.S.; Möhwald, H. Active Anticorrosion Coatings with Halloysite Nanocontainers. J. Phys. Chem. C 2008, 112, 958–964. [Google Scholar] [CrossRef]
- Mekeridis, E.D.; Kartsonakis, I.A.; Kordas, G.C. Multilayer organic–inorganic coating incorporating TiO2 nanocontainers loaded with inhibitors for corrosion protection of AA2024-T3. Prog. Org. Coat. 2012, 73, 142–148. [Google Scholar] [CrossRef]
- Maia, F.; Tedim, J.; Lisenkov, A.D.; Salak, A.N.; Zheludkevich, M.L.; Ferreira, M.G.S. Silica nanocontainers for active corrosion protection. Nanoscale 2012, 4, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Kartsonakis, I.A.; Koumoulos, E.P.; Balaskas, A.C.; Pappas, G.S.; Charitidis, C.A.; Kordas, G.C. Hybrid organic–inorganic multilayer coatings including nanocontainers for corrosion protection of metal alloys. Corros. Sci. 2012, 57, 56–66. [Google Scholar] [CrossRef]
- Kartsonakis, I.A.; Balaskas, A.C.; Koumoulos, E.P.; Charitidis, C.A.; Kordas, G.C. Incorporation of ceramic nanocontainers into epoxy coatings for the corrosion protection of hot dip galvanized steel. Corros. Sci. 2012, 57, 30–41. [Google Scholar] [CrossRef]
- Borisova, D.; Möhwald, H.; Shchukin, D.G. Influence of embedded nanocontainers on the efficiency of active anticorrosive coatings for aluminum alloys part II: Influence of nanocontainer position. ACS Appl. Mater. Interfaces 2013, 5, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Balaskas, A.C.; Kartsonakis, I.A.; Tziveleka, L.-A.; Kordas, G.C. Improvement of anti-corrosive properties of epoxy-coated AA 2024-T3 with TiO2 nanocontainers loaded with 8-hydroxyquinoline. Prog. Org. Coat. 2012, 74, 418–426. [Google Scholar] [CrossRef]
- Arunchandran, C.; Ramya, S.; George, R.P.; Mudali, U.K. Self-Healing Corrosion Resistive Coatings Based on Inhibitor Loaded TiO2 Nanocontainers. J. Electrochem. Soc. 2012, 159, C552–C559. [Google Scholar] [CrossRef]
- Khramov, A.N.; Voevodin, N.N.; Balbyshev, V.N.; Donley, M.S. Hybrid organo-ceramic corrosion protection coatings with encapsulated organic corrosion inhibitors. Thin Solid Films 2004, 447, 549–557. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Zheludkevich, M.; Yasakau, K.; Lamaka, S.; Ferreira, M.G.S.; Möhwald, H. Layer-by-Layer Assembled Nanocontainers for Self-Healing Corrosion Protection. Adv. Mater. 2006, 18, 1672–1678. [Google Scholar] [CrossRef]
- Skorb, E.V.; Fix, D.; Andreeva, D.V.; Möhwald, H.; Shchukin, D.G. Surface-Modified Mesoporous SiO2 Containers for Corrosion Protection. Adv. Funct. Mater. 2009, 19, 2373–2379. [Google Scholar] [CrossRef]
- Álvarez, D.; Collazo, A.; Nóvoa, X.R.; Pérez, C. Assessment of ZnO nanoparticles as anticorrosive pigment in hybrid sol-gel films. Prog. Org. Coat. 2015. [Google Scholar] [CrossRef]
- Rosero-Navarro, N.C.; Paussa, L.; Andreatta, F.; Castro, Y.; Durán, A.; Aparicio, M.; Fedrizzi, L. Optimization of hybrid sol-gel coatings by combination of layers with complementary properties for corrosion protection of AA2024. Prog. Org. Coat. 2010, 69, 167–174. [Google Scholar] [CrossRef]
- Maia, F.; Yasakau, K.A.; Carneiro, J.; Kallip, S.; Tedim, J.; Henriques, T.; Cabral, A.; Venâncio, J.; Zheludkevich, M.L.; Ferreira, M.G.S. Corrosion protection of AA2024 by sol-gel coatings modified with MBT-loaded polyurea microcapsules. Chem. Eng. J. 2016, 283, 1108–1117. [Google Scholar] [CrossRef]
- Poznyak, S.K.; Zheludkevich, M.L.; Raps, D.; Gammel, F.; Yasakau, K.A.; Ferreira, M.G.S. Preparation and corrosion protective properties of nanostructured titania-containing hybrid sol-gel coatings on AA2024. Prog. Org. Coat. 2008, 62, 226–235. [Google Scholar] [CrossRef]
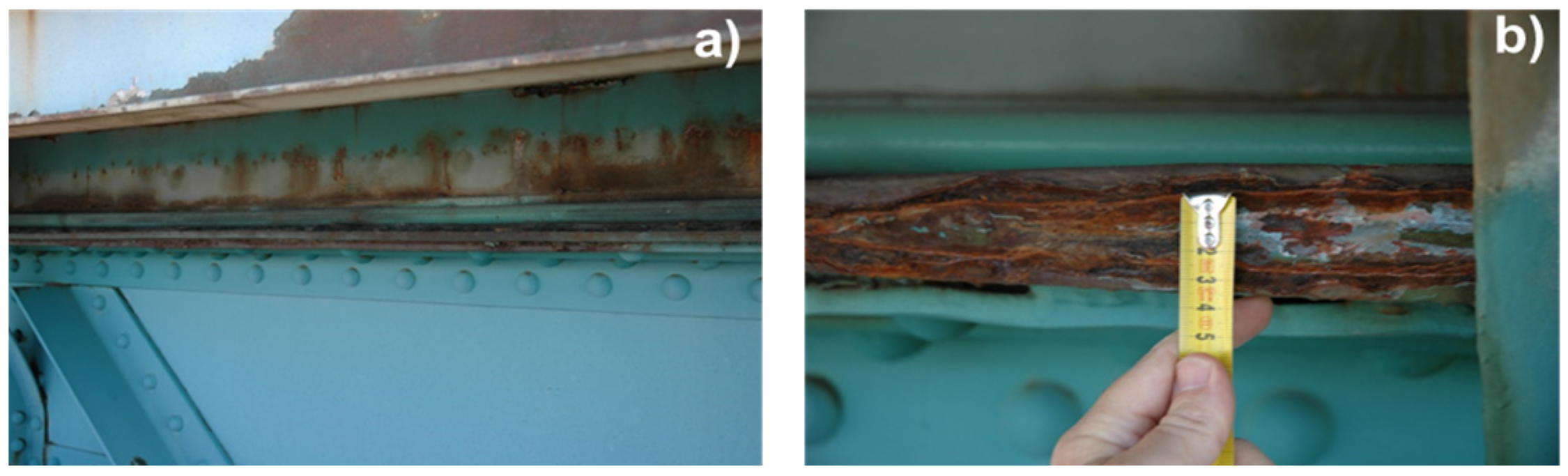



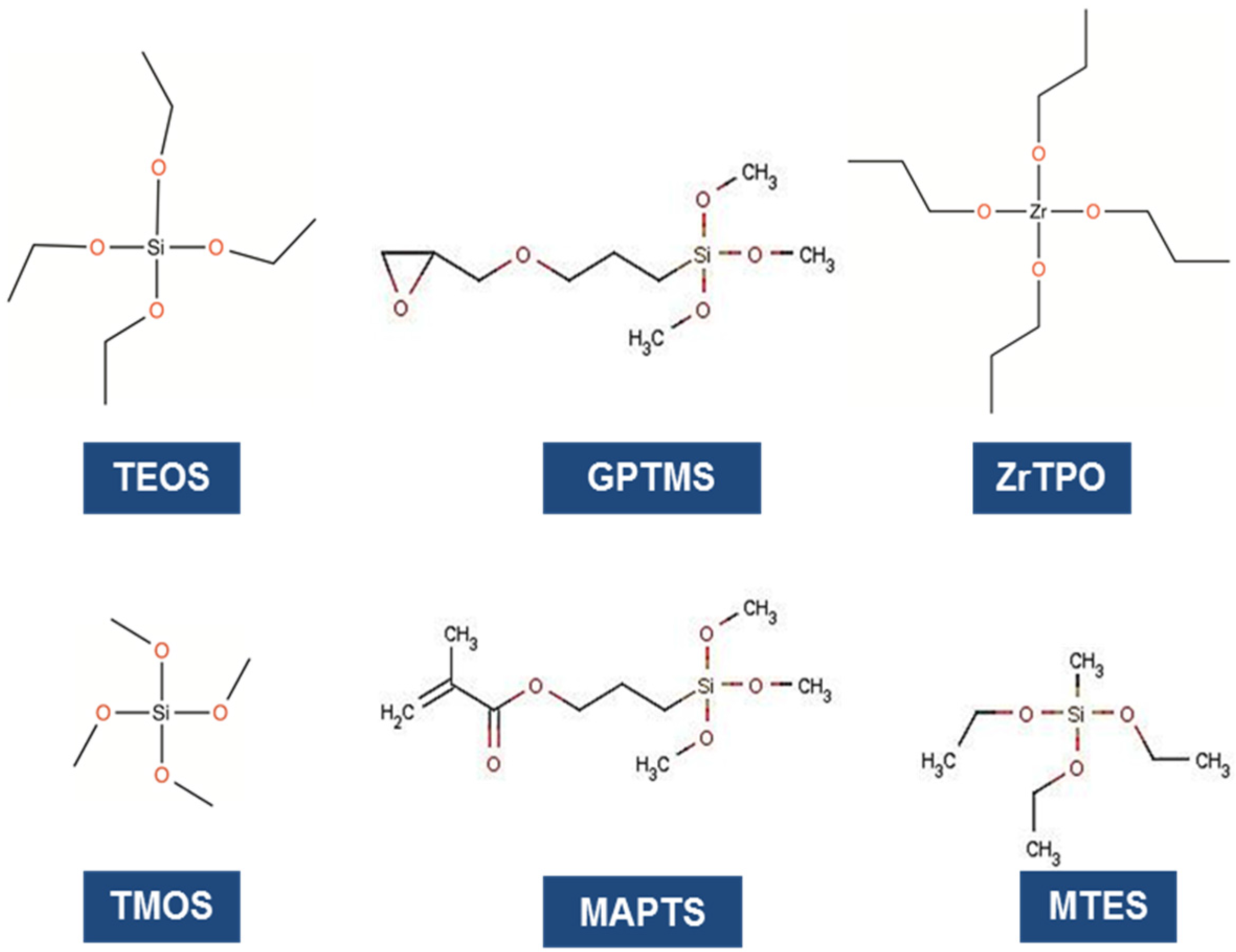
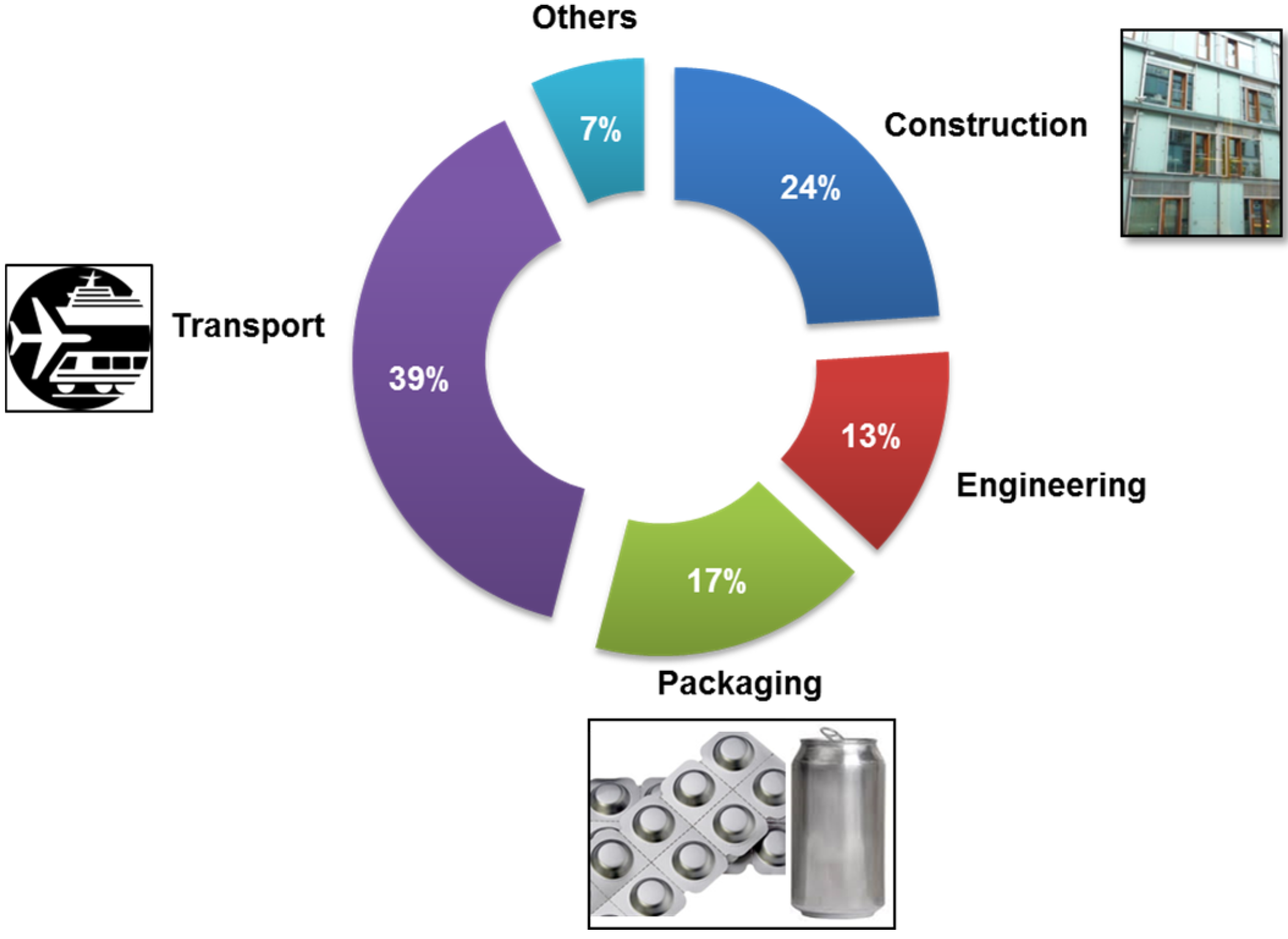
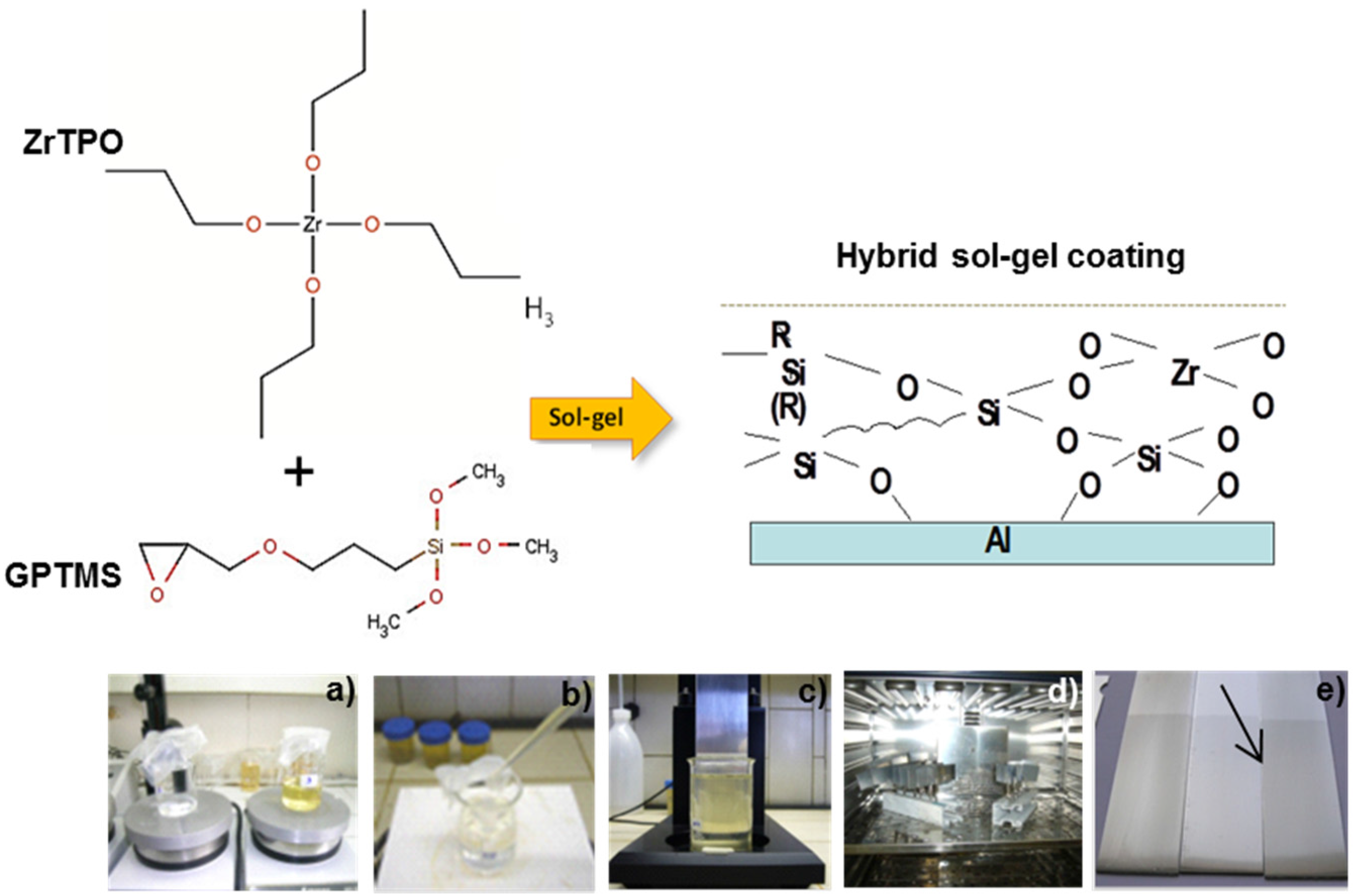
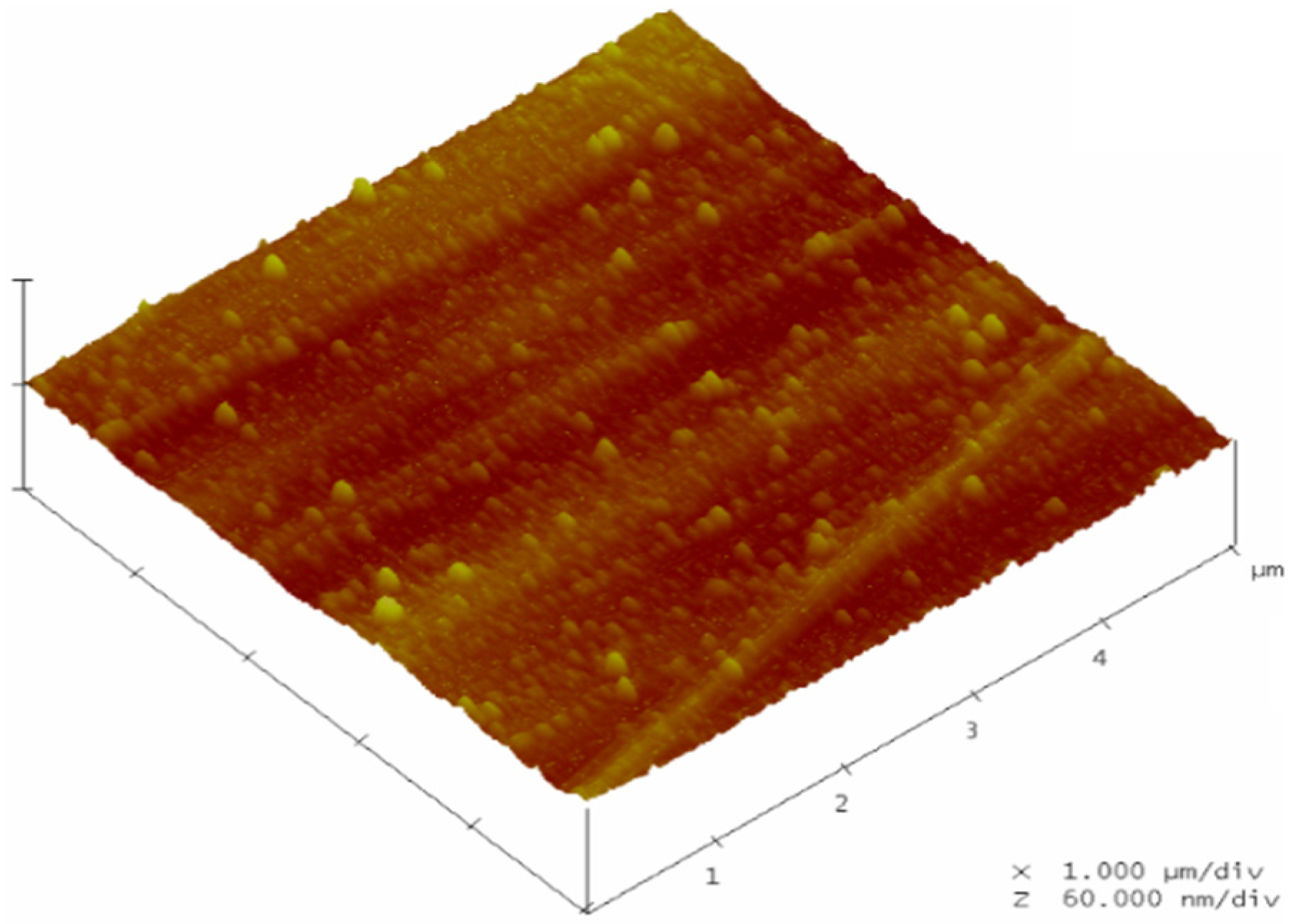
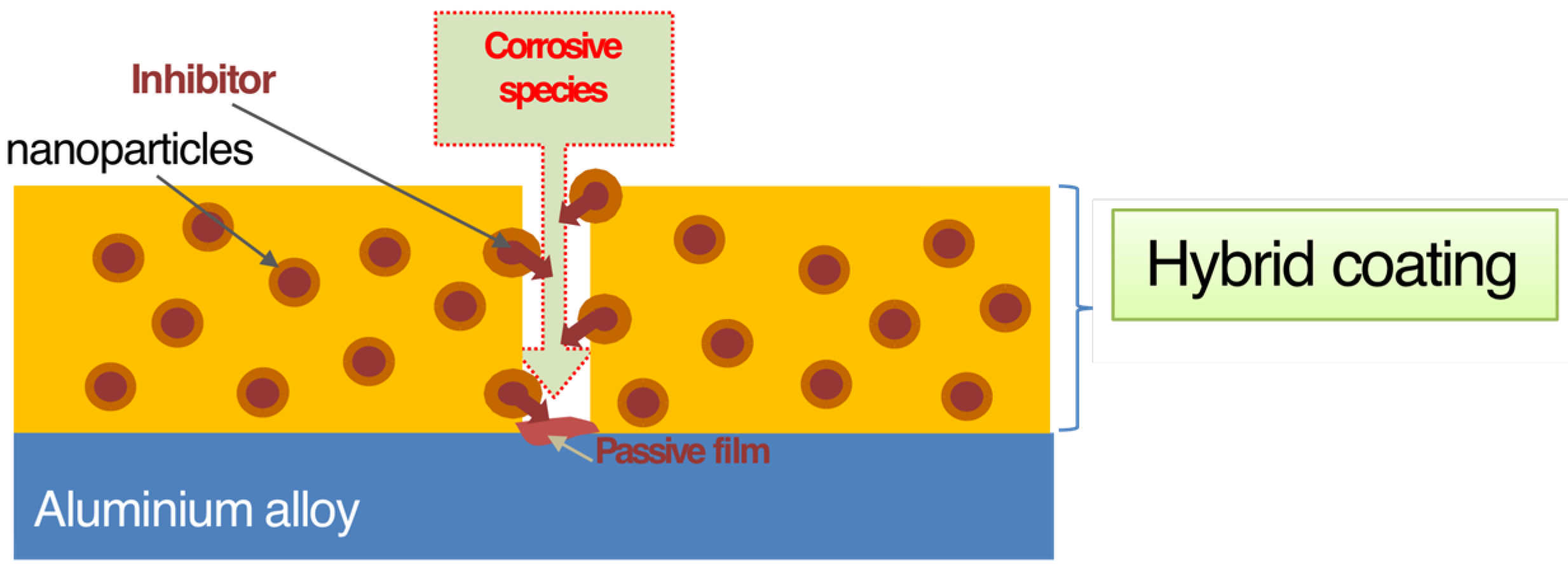
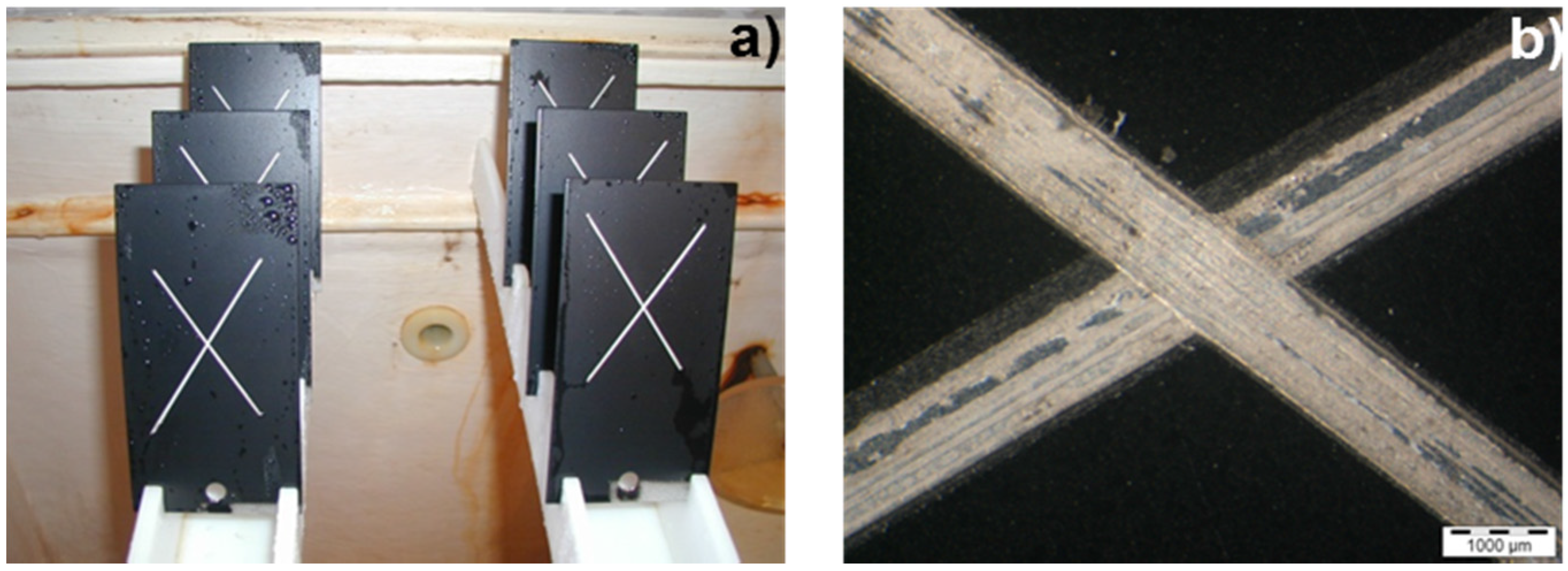
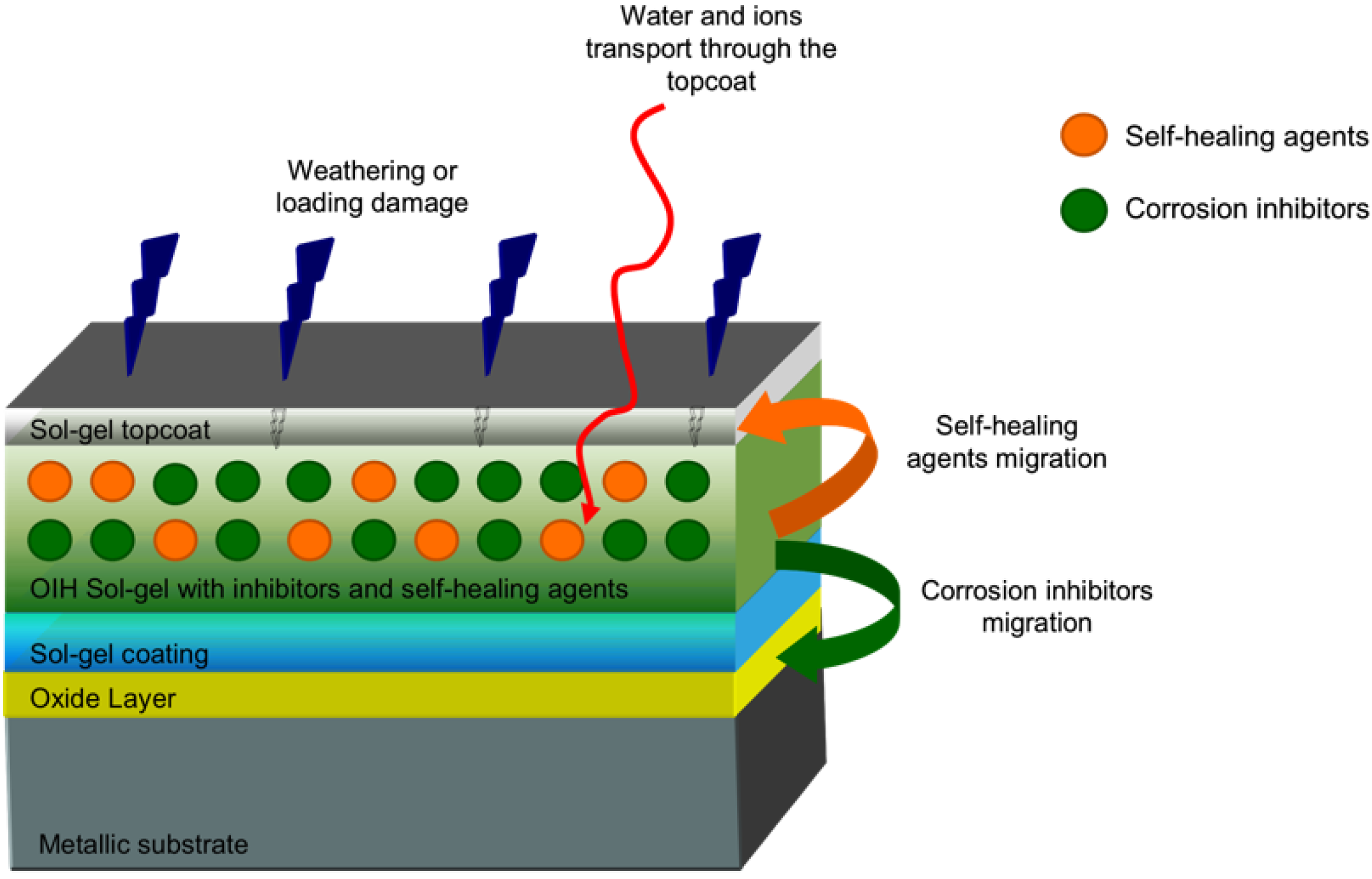
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueira, R.B.; Fontinha, I.R.; Silva, C.J.R.; Pereira, E.V. Hybrid Sol-Gel Coatings: Smart and Green Materials for Corrosion Mitigation. Coatings 2016, 6, 12. https://doi.org/10.3390/coatings6010012
Figueira RB, Fontinha IR, Silva CJR, Pereira EV. Hybrid Sol-Gel Coatings: Smart and Green Materials for Corrosion Mitigation. Coatings. 2016; 6(1):12. https://doi.org/10.3390/coatings6010012
Chicago/Turabian StyleFigueira, Rita B., Isabel R. Fontinha, Carlos J. R. Silva, and Elsa V. Pereira. 2016. "Hybrid Sol-Gel Coatings: Smart and Green Materials for Corrosion Mitigation" Coatings 6, no. 1: 12. https://doi.org/10.3390/coatings6010012
APA StyleFigueira, R. B., Fontinha, I. R., Silva, C. J. R., & Pereira, E. V. (2016). Hybrid Sol-Gel Coatings: Smart and Green Materials for Corrosion Mitigation. Coatings, 6(1), 12. https://doi.org/10.3390/coatings6010012






