In2S3/C3N4 Nanocomposite and Its Photoelectric Properties in the Broadband Light Spectrum Range
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Graphitic Carbon Nitride
2.3. Preparation of the In2S3/C3N4 Nanocomposite
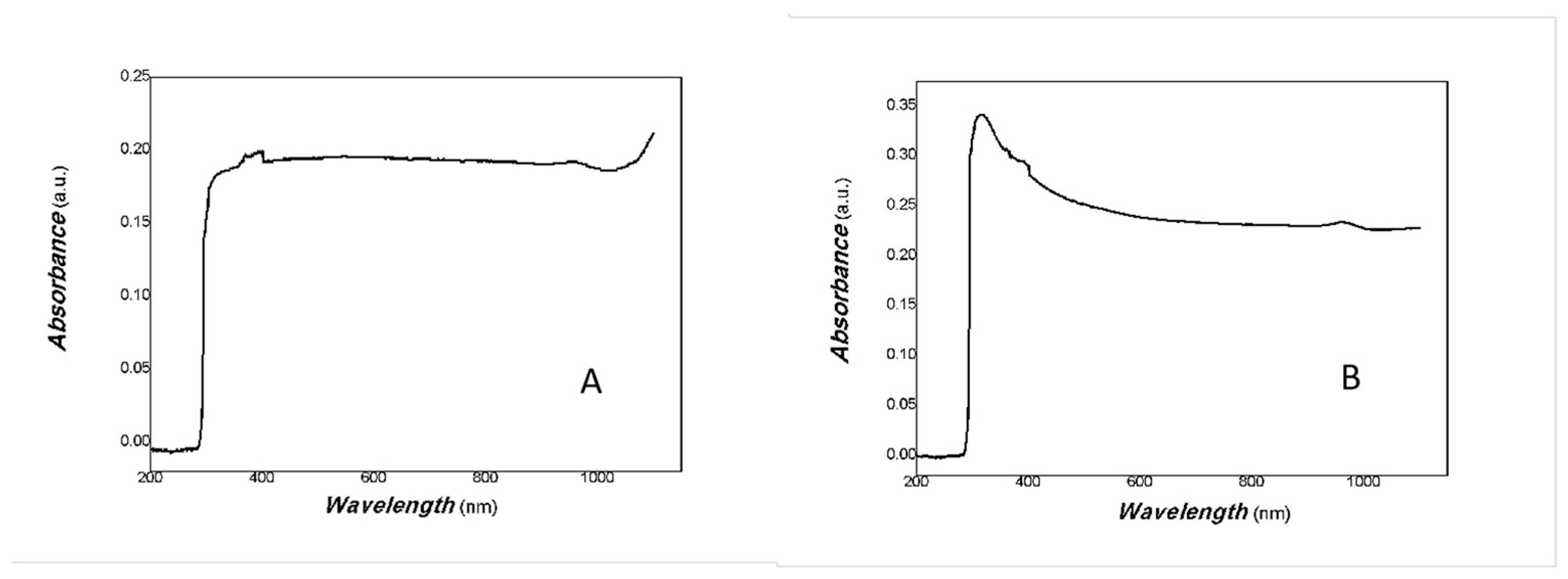
2.4. Characterization of SEM, EDS, EDS Mapping, TEM, UV-Vis-NIR and XRD
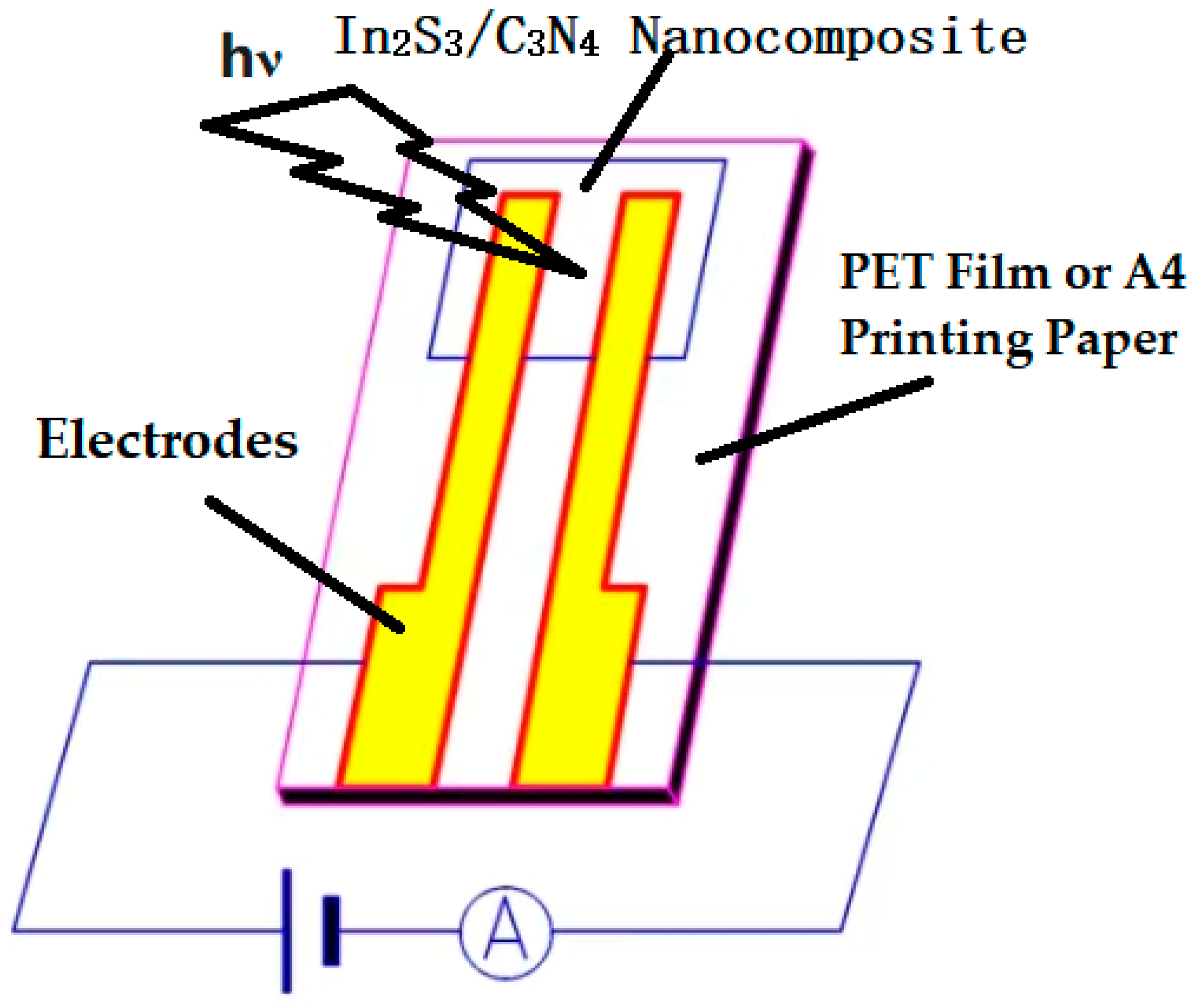
2.5. Optoelectronic Signal Determination of the In2S3/C3N4 Nanocomposite Aggregation States to the Light Sources with Different Wavelengths
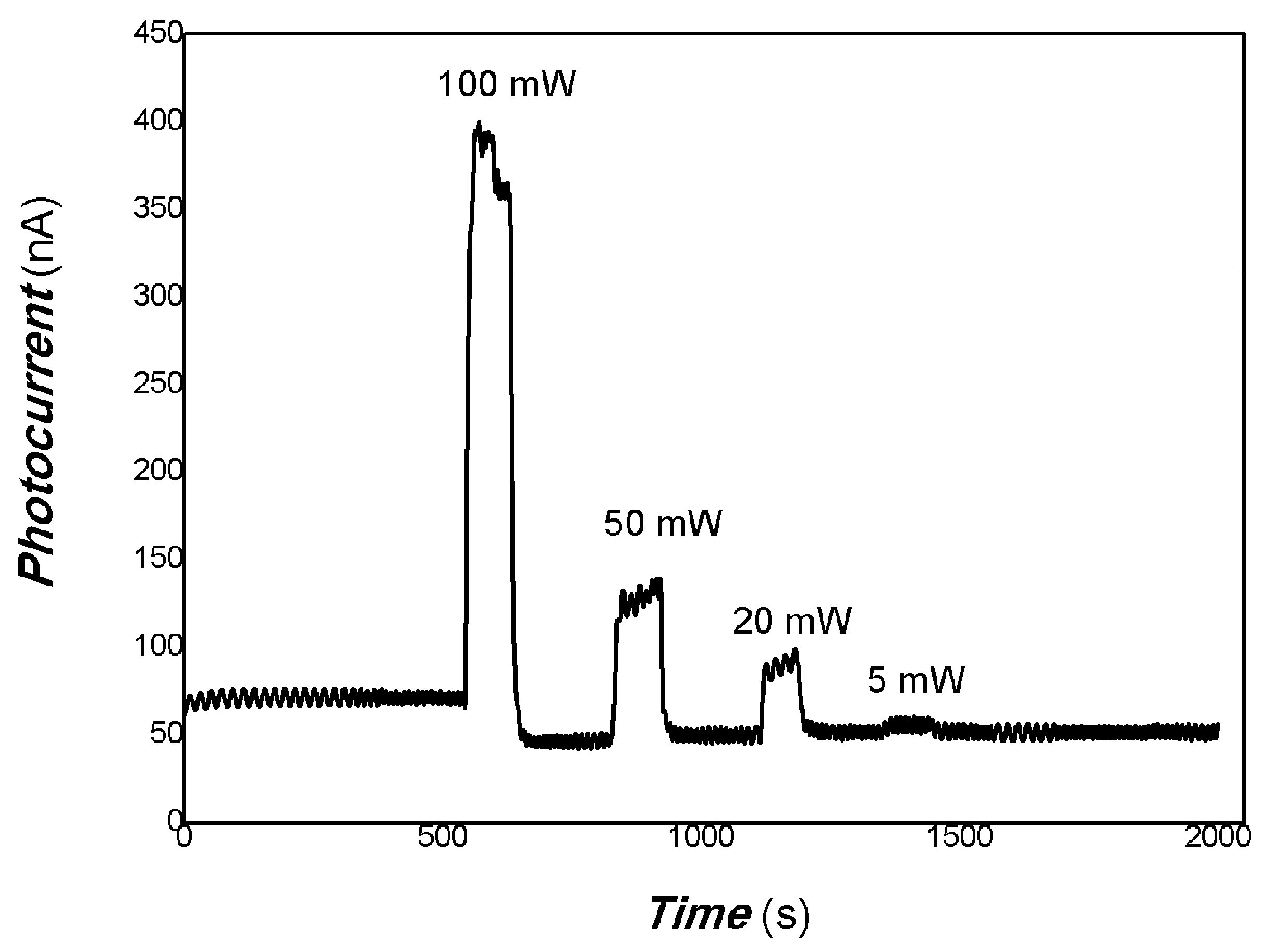
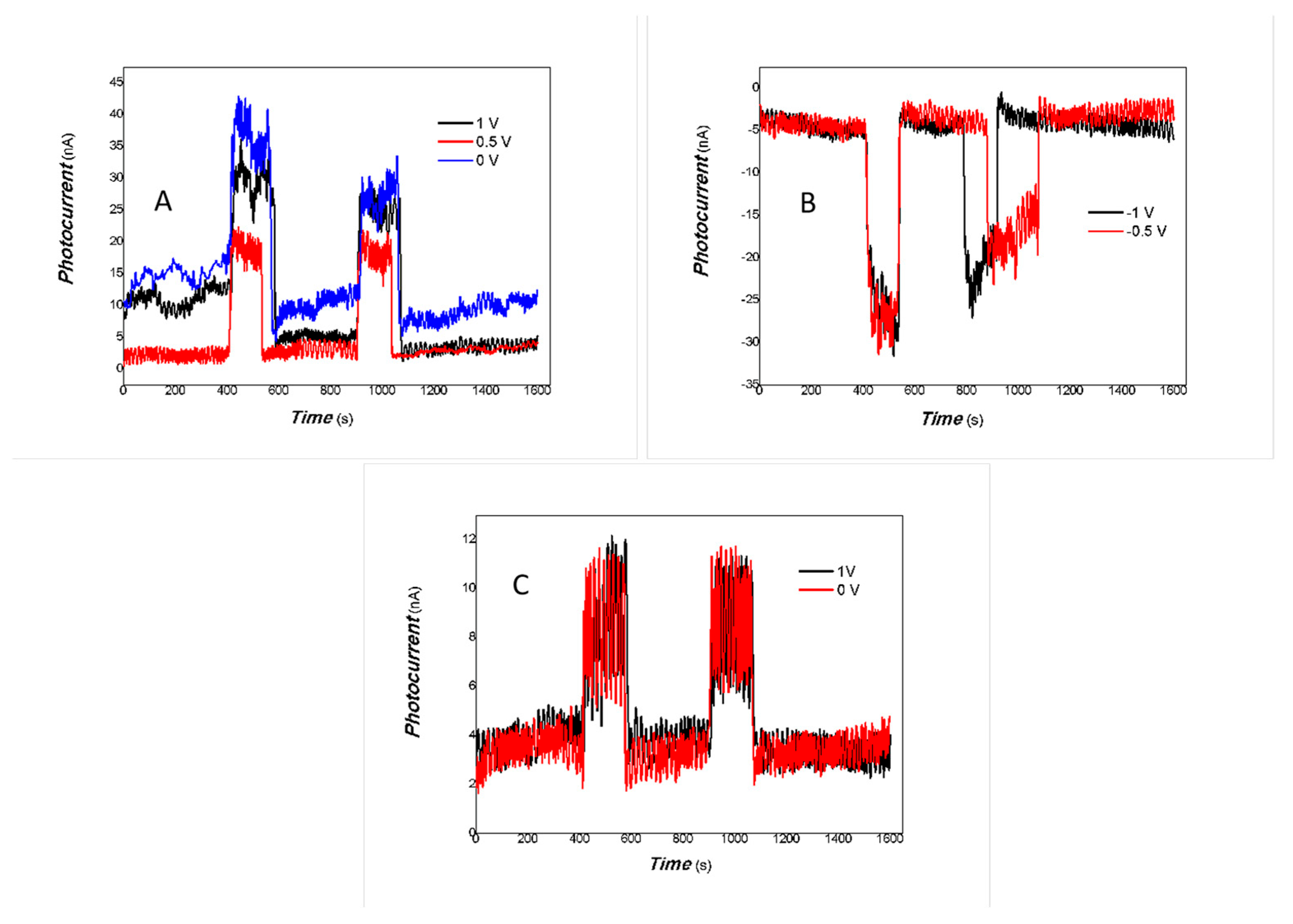
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, C.; Chen, Q.; Deng, X.; Jiang, L.; Tan, H.; Zhou, Y.; Chen, J.; Wang, R. Enhanced Photocatalytic Hydrogen Evolution of In2S3 by Decorating In2O3 with Rich Oxygen Vacancies. Inorg. Chem. 2024, 63, 11125–11134. [Google Scholar] [CrossRef]
- Verma, R.; Rani, R.; Choubey, P.; Sharma, A.; Basu, M. Au Nanoparticles on In2S3/In2O3 Nanopyramids Increase Photoanodic Activity in Photoelectrochemical Water Splitting. ACS Appl. Nano Mater. 2024, 7, 26434–26444. [Google Scholar] [CrossRef]
- Chang, Y.; Syu, S.; Hsu, P. Construction of In2S3–In(OH)3–ZnS nanofibers for boosting photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2024, 86, 24–35. [Google Scholar] [CrossRef]
- Lu, P.; Du, B.; Liu, K.; Luo, Z.; Sikandaier, A.; Diao, L.; Sun, J.; Jiang, L.; Zhu, Y. Heterostructured In2O3/In2S3 hollow fibers enable efficient visible-light driven photocatalytic hydrogen production and 5-hydroxymethylfurfural oxidation. Chin. J. Struct. Chem. 2024, 43, 100361. [Google Scholar] [CrossRef]
- Ai, Y.; Hu, J.; Xiong, X.; Carabineiro, S.A.C.; Li, Y.; Sirotkin, N.; Agafonov, A.; Lv, K. Synergistic interfacial engineering of a S-scheme ZnO/In2S3 photocatalyst with S−O covalent bonds: A dual-functional advancement for tetracycline hydrochloride degradation and H2 evolution. Appl. Catal. B Environ. Energy 2024, 353, 124098. [Google Scholar] [CrossRef]
- Lin, Y.; Ko, F.; Chang, Y. Visible Light-Induced Photocatalytic Hydrogen Generation from Seawater Using Ternary Indium Sulfide/Indium Oxide/Gold Nanocomposites Obtained via Microwave-Assisted Synthesis. ACS Appl. Nano Mater. 2024, 7, 16831–16841. [Google Scholar] [CrossRef]
- Ye, J.; Fan, Z.; Wang, Z.; Wang, Y.; Li, J.; Xie, Y.; Ling, Y.; Chen, Y. In2S3-modified ZnIn2S4 enhanced photogenerated carrier separation efficiency and photocatalytic hydrogen evolution under visible light. Fuel 2024, 373, 132401. [Google Scholar] [CrossRef]
- Zhang, R.; Jia, X.; Sun, M.; Liu, X.; Wang, C.; Yu, X.; Xing, Y. Synergistic manipulation of sulfur vacancies and palladium doping of In2S3 for enhanced photocatalytic H2 production. J. Colloid Interface Sci. 2025, 677, 425–434. [Google Scholar] [CrossRef]
- Cruz, M.R.A.; Kim, J.H.; Torres-Martínez, L.M. Photoelectrocatalytic efficiency of In2S3–In2O3 films as photoelectrodes/photocatalyst in hydrogen production reaction. J. Phys. Chem. Solids 2024, 193, 112218. [Google Scholar] [CrossRef]
- Xia, Y.; Huang, G.; Fan, Y.; Zhao, X.; Wang, L.; Huang, J.; She, H.; Wang, Q. Preparation of 3D/2D ZnIn2S4/Porphyrin(Cu)-COF Type II Heterojunction: An In-Depth Insight into Interfacial Charge Transfer for Efficient Light-to-Hydrogen Conversion. J. Phys. Chem. C 2024, 128, 15000–15011. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, L.; Zhang, J.; Gui, Y.; Liu, L. Hierarchical In2S3 microflowers decorated with WO3 quantum dots: Sculpting S-scheme heterostructure for enhanced photocatalytic H2 evolution and nitrobenzene hydrogenation. J. Mater. Sci. Technol. 2024, 174, 218–225. [Google Scholar] [CrossRef]
- Han, M.; Liu, L.; Guo, B.; Zhang, Z.; Li, X.; Chang, Y.; Luo, S.; Lu, W.; Li, S.; Ye, J. Bi & Mo co-doped In2S3 nano-foam blocks for boosted photocatalytic hydrogen generation. Int. J. Hydrogen Energy 2024, 78, 140–147. [Google Scholar]
- Li, S.; Peng, S.; Li, Y. Constructing an Open-Structured J-Type ZnIn2S4/In(OH)3 Heterojunction for Photocatalytical Hydrogen Generation. J. Phys. Chem. Lett. 2024, 15, 5215–5222. [Google Scholar] [CrossRef]
- Cao, J.; Xu, X.; Que, L.; Li, H.; Wang, J.; Zheng, Y.; Pan, J.; Li, C. The Cd0.8Zn0.2S/In2S3 porous nanotubes heterojunction towards enhanced visible light photocatalytic H2 evolution and photodegradation via MOFs self-template and bimetallic synergism. Renew. Energy 2024, 224, 120212. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.; Li, W.; Shao, Z.; Yu, Y.; Yan, H.; Jiao, S.; Lin, D.; Zhang, W.; Lv, C.; et al. P-doped ultrathin g-C3N4/In2S3 S-scheme heterojunction enhances photocatalytic hydrogen production and degradation of ofloxacin. Phys. B 2024, 685, 416053. [Google Scholar] [CrossRef]
- Yu, Y.; Kipkorir, A.; Choi, M.Y.; Kamat, P.V. Photocatalytic Membrane for Hydrogen Evolution: Directed Electron and Hole Transfer across Pt−AgInS2−Nafion. ACS Mater. Lett. 2024, 6, 1856–1862. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Liu, X.; Wang, K.; Li, G. Small ZnIn2S4 Nanosheets with Abundant Edge-Active Sites Modified with MoS2 Nanosheets for Hydrogen Evolution. ACS Appl. Nano Mater. 2024, 7, 24938–24946. [Google Scholar] [CrossRef]
- Lin, Y.; Chang, Y.; Ko, F. One-pot microwave-assisted synthesis of In2S3/In2O3 nanosheets as highly active visible light photocatalysts for seawater splitting. Int. J. Hydrogen Energy 2024, 52, 953–963. [Google Scholar] [CrossRef]
- Choubey, P.; Verma, R.; Basu, M. Passivation of Surface States in CdIn2S4 via Type II Heterostructure for Boosting Photoelectrochemical Water Splitting Reaction. ACS Appl. Energy Mater. 2024, 7, 9382–9393. [Google Scholar] [CrossRef]
- Xiong, Y.; He, H.; Cui, Y.; Wu, Z.; Ding, S.; Zhang, J.; Peng, B.; Yang, L. Tuning Surface Electronics State of P-Doped In2.77S4/In(OH)3 toward Efficient Photoelectrochemical Water Oxidation. Langmuir 2024, 40, 8533–8541. [Google Scholar] [CrossRef]
- Lv, B.; Wang, Q.; He, Y.; Zhang, L.; Wang, W. In2S3 nanoparticles modify the porous Fe2V4O13 nanostructure photoanode to enhance charge separation efficiency and photoelectrochemical water splitting performance. Surf. Interfaces 2024, 49, 104469. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Li, W.; Ma, H.; Fang, P.; Xiong, R.; Pan, C.; Wei, J. In situ interfacial engineering of MnIn2S4@In2S3 hollow nanotubes for enhanced photocatalytic production of H2O2 and antibiotic degradation. J. Colloid Interface Sci. 2025, 682, 41–49. [Google Scholar] [CrossRef]
- Huang, S.; Gao, J.; Zhou, L.; Lei, J.; Wang, L.; Liu, Y.; Zhang, J. Polydopamine-Loaded ZnIn2S4 Photocatalyst for H2O2 Production in Water. ACS Appl. Nano Mater. 2024, 7, 4481–4490. [Google Scholar] [CrossRef]
- Peng, H.; Li, H.; Ye, B.; Zheng, X. Snowflake-like In2S3/Cu2S heterojunction for simultaneous photocatalytic persulfate oxidation of tetracycline hydrochloride and CO2 photoreduction. Sep. Purif. Technol. 2025, 357, 130242. [Google Scholar] [CrossRef]
- You, F.; Zhou, T.; Li, J.; Huang, S.; Chang, C.; Fan, X.; Zhang, H.; Ma, X.; Gao, D.; Qi, J.; et al. Rich oxygen vacancies in confined heterostructured TiO2@In2S3 hybrid for boosting solar-driven CO2 reduction. J. Colloid Interface Sci. 2024, 660, 77–86. [Google Scholar] [CrossRef]
- Altaf, C.T.; Colak, T.O.; Karagoz, E.; Wang, J.; Liu, Y.; Chen, Y.; Liu, M.; Unal, U.; Sankir, N.D.; Sankir, M. Co-sensitization of Copper Indium Gallium Disulfide and Indium Sulfide on Zinc Oxide Nanostructures: Effect of Morphology in Electrochemical Carbon Dioxide Reduction. ACS Omega 2024, 9, 19209–19218. [Google Scholar] [CrossRef]
- Gu, Z.; Chen, K.; Ding, X.; Zhang, X.; Cheng, Y.; Xiao, X.; Yu, Y. An In2O3/In2S3 photoanode-driven whole-cell biocathode sensor for sensitive detection of nitrate. Sens. Actuators B Chem. 2025, 423, 136761. [Google Scholar] [CrossRef]
- Wang, B.; Sun, Y.; Dong, Y.; Hou, Y.; Lu, Z.; Wei, Z.; Zhang, W.; Suematsu, K.; Hu, J. Chemiresistive flexible gas sensor for NO2 sensing at room-temperature using in situ constructed Au@In2S3/In2O3 hybrid microflowers. Sens. Actuators B Chem. 2025, 422, 136666. [Google Scholar] [CrossRef]
- Ou, R.; Xu, K.; Ha, N.; Ren, G.; Trinh, V.; Hu, Y.; Ma, Q.; Zhang, B.Y.; Wen, X.; Cheng, Y.; et al. Visible-Light-Driven Two-Dimensional Indium Oxysulfide for Sensitive NO2 Detection. ACS Appl. Nano Mater. 2024, 7, 14223–14231. [Google Scholar] [CrossRef]
- Ren, X.; Wang, M.; Chen, J.; Zhao, J.; Wang, H.; Wu, D.; Xu, R.; Zhang, Y.; Ju, H.; Wei, Q. Sulfur defect–engineered Bi2S3−x/In2S3−y mediated signal enhancement of photoelectrochemical sensor for lead ions detection. Talanta 2024, 273, 125871. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Sun, S.; Wang, N.; Huang, B.; Li, X. Hierarchically In2S3@In2O3 nanorods heterojunctions for enhanced NO2 sensing at lower operating temperature. Sens. Actuators B Chem. 2024, 419, 136360. [Google Scholar] [CrossRef]
- Malathi, B.; Parveen, R.A.; Bharathi, P.; Nakamura, A.; Archana, J.; Navaneethan, M.; Harish, S. Interface engineering of 2D/2D MoS2/In2S3 heterostructure for highly sensitive NO2 detection at room temperature gas sensor. J. Environ. Chem. Eng. 2024, 12, 113023. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Q.; Song, P. In situ synthesis of Zn-doped In2S3/In2O3 composites for the monitoring of trace ethanol at low temperature. Vacuum 2024, 222, 112956. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, H.; Yu, H.; Chen, Y.; Ma, X.; Shang, E.; Chen, H.; Kang, X.; Zhang, J.; Tan, R.; et al. ppb-level NO2 gas sensor based on WO3/In2S3 heterostructure by hydrothermal method: A convenient and wearable real-time detection system. Ceram. Int. 2024, 50, 32678–32686. [Google Scholar] [CrossRef]
- Wu, Y.; Tong, Y.; Zhai, C.; Liu, Z.; Du, L.; Li, J.; Li, P.; Jiang, Y. Photocathode Biosensors Based on Au Nanoparticles and ZnIn2S4/In2S3 Composites for the Detection of Exosomal MUC1 in Breast Cancer Diagnosis. ACS Appl. Nano Mater. 2024, 7, 16758–16769. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, N.; Wang, X.; Gao, M.; Chen, D.; Li, Y.; Wu, Q.; Tu, J. TiO2 Nanosheet/In2S3 Nanosheet/Polydopamine Layered Composites for Constructing Enzymatic and Nonenzymatic Dual Sensors under Visible Light. ACS Appl. Nano Mater. 2024, 7, 10234–10242. [Google Scholar] [CrossRef]
- Zhu, F.; Zhang, S.; Zhang, Q.; Ma, K.; Wu, J.; Cai, Y. Defect-rich In2S3/CoS2 heterostructure for rapid storage of sodium ions. Electrochim. Acta 2025, 510, 145383. [Google Scholar] [CrossRef]
- Ahmad, M.; Inayat, A.; Hussain, M.N.; Khan, A.; Adnan; Alam, M.; Sultan, M.; Abbas, S.M. Graphene supported In2S3 nanostructure as electrode material for lithium sulfur batteries and supercapacitors. Mater. Today Sustain. 2024, 25, 100631. [Google Scholar] [CrossRef]
- Mishra, S.R.; Gadore, V.; Yadav, G.; Ahmaruzzaman, M. Next-generation energy storage: In2S3-based materials as high-performance electrodes for alkali-ion batteries. Next Energy 2024, 2, 100071. [Google Scholar] [CrossRef]
- Mishra, S.R.; Gadore, V.; Ahmaruzzaman, M. Next-generation nanotechnology: Exploring the potential of In2S3-based perovskite solar cells. Next Nanotechnol. 2024, 6, 100064. [Google Scholar] [CrossRef]
- Alagarasan, D.; Hegde, S.S.; Naik, R.; Murahari, P.; Shetty, H.D.; Alkallas, F.H.; Trabelsi, A.B.G.; Khan, F.S.; AlFaify, S.; Shkir, M. Fabrication of Bi-doped In2S3 thin films for highly sensitive UV photodetector applications. J. Photochem. Photobiol. A Chem. 2024, 454, 115697. [Google Scholar] [CrossRef]
- Jia, Z.; Li, R.; Bai, S.; Liu, Y.; Zhang, S.; Lin, Q. High-Speed and Low-Noise Photodetectors Based on Solution Processed AgInS2. ACS Photonics 2024, 11, 4349–4356. [Google Scholar] [CrossRef]
- Kipkorir, A.; Ealey, G.; Yu, Y.; Kamat, P.V. AgInS2-Embedded Photocatalytic Membrane: Insights into the Excited State and Electron Transfer Dynamics. Langmuir 2024, 40, 1373–1380. [Google Scholar] [CrossRef]
- Mishra, S.R.; Gadore, V.; Ahmaruzzaman, M. An overview of In2S3 and In2S3-based photocatalyst: Characteristics, synthesis, modifications, design strategies, and catalytic environmental application. J. Environ. Chem. Eng. 2024, 12, 113449. [Google Scholar] [CrossRef]
- Yu, Y.; Kipkorir, A.; Choi, M.Y.; Kamat, P.V. Directional Electron Transfer across In2S3/ZnS-Embedded Photocatalytic Membranes. ACS Appl. Energy Mater. 2024, 7, 681–688. [Google Scholar] [CrossRef]
- Han, S.; Zhu, Z.; Chen, F.; Sun, W.; Tang, Y. Construction of a novel multidimensional 1D/2D heterojunction FeVO4/In2S3 with significantly reinforced charge transport and excellent photocatalytic activity. J. Phys. Chem. Solids 2024, 195, 112303. [Google Scholar] [CrossRef]
- Chen, P.; Ou, X.; Xia, C.; Zhao, K.; Wei, M.; Wang, Y.; Zhang, M. Construction of dual Z-scheme g-C3N4/BiVO4 (040)/In2S3 photocatalyst for efficient photocatalytic degradation and sterilization under solar light irradiation. Appl. Surf. Sci. 2024, 643, 158665. [Google Scholar] [CrossRef]
- Dhamri, M.H.A.; Maridevaru, M.C.; Sillanpaa, M.; Kim, Y.; Selvaraj, R. Fabrication of chrysanthemums like TiO2@In2S3 S-scheme heterojunction nanostructures for the degradation of methylene blue dye present in aqueous solution. Inorg. Chem. Commun. 2024, 168, 11292. [Google Scholar]
- Jain, S.; Kumari, M.; Kumar, N.; Mittal, A.; Kumar, V.; Tahir, M.; Singh, P.; Rani, G.; Kataria, J.; Jogender, C. Fabrication of novel S-type In2S3/Ag2S heterostructures with superior photocatalytic and electrochemical characteristics for remediation of organic contaminants in water. Inorg. Chem. Commun. 2025, 172, 113621. [Google Scholar] [CrossRef]
- Tai, C.; Zhang, Z.; Ji, X.; Ma, J.; Han, X.; Wang, R.; Lu, Q.; Wei, M.; Si, C.; Chen, S.; et al. Ternary heterojunction of 1D CeO2/TiO2 nanofibers decorated with In2S3 nanosheets boosting the photodegradation of organic pollutants. Surf. Interfaces 2024, 46, 104177. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, W.; Zhu, M.; Hu, Z.; Wu, D.; Ji, X.; Zhang, D.; Pu, X. In2S3/In2O3 Nanocomposite In Situ Forming an S-Scheme Heterojunction for the Photocatalytic Degradation of Tetracycline under Visible Light. ACS Appl. Nano Mater. 2024, 7, 20339–20348. [Google Scholar] [CrossRef]
- Wang, C.; Liu, N.; Liu, X.; Tian, Y.; Jiang, Q.; Chen, X.; Hou, B. Sulfur vacancy-enhanced In2S3−x hollow microtubes for photocatalytic Cr (VI) and tetracycline removal. J. Environ. Manag. 2024, 353, 120173. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; He, C.; Yu, H.; Shui, A. Fabrication of the ternary dual S-scheme ZnO/ZnS/In2S3 heterojunction for enhancing pollutant photodegradation. Appl. Surf. Sci. 2024, 652, 159284. [Google Scholar] [CrossRef]
- Wang, C.; Liu, N.; Liu, X.; Jiang, Q.; Tian, Y.; Xie, H.; Chen, X.; Hou, B. Fe doping in In2S3 hollow nanotubes for efficient photo-Fenton degradation of emerging organic pollutants. Sep. Purif. Technol. 2024, 345, 127405. [Google Scholar] [CrossRef]
- Su, T.; Chen, Z.; Luo, X.; Xie, X.; Qin, Z.; Ji, H. Preparation of Fe-doped In2S3/In2O3 Composite for Photocatalytic Degradation of Tetracycline. ACS Chem. Health Saf. 2024, 31, 490–502. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, H.; Yao, B.; Wang, Y. Hollow core-shell B-g-C3N4−x@Bi2S3/In2S3 dual S-scheme heterojunction photothermal nanoreactor: Boosting photothermal catalytic activity in confined space. Chem. Eng. J. 2024, 484, 149399. [Google Scholar] [CrossRef]
- Wu, J.; Wang, T.; Zhang, L.; Du, P. In2S3/Cd0.9Zn0.1S Heterojunction for Efficient Photocatalytic Formic Acid Decomposition to Syngas (H2 + CO) under Visible Light. ACS Sustain. Chem. Eng. 2024, 12, 1625–1631. [Google Scholar] [CrossRef]
- Sharmila, B.; Divyashree, P.; Sharma, S.; Dwivedi, P.; Das, S. Scalable In2S3 based optical memristor devices as artificial synapse for logic realization and neuromorphic computing. Mater. Sci. Semicond. Process. 2025, 185, 108985. [Google Scholar]
- Salam, J.A.; Anand, A.M.; Raj, A.; Nath R, A.; Jayakrishnan, R. Self-powered response in β-In2S3 thin films. J. Sci. Adv. Mater. Devices 2024, 9, 100671. [Google Scholar] [CrossRef]
- Kharbanda, N.; Sachdeva, M.; Ghorai, N.; Kaur, A.; Kumar, V.; Ghosh, H.N. Plasmon-Induced Ultrafast Hot Hole Transfer in Nonstoichiometric CuxInyS/CdS Heteronanocrystals. J. Phys. Chem. Lett. 2024, 15, 5056–5062. [Google Scholar] [CrossRef]
- Guillemeney, L.; Dutta, S.; Valleix, R.; Patriarche, G.; Mahler, B.; Abécassis, B. Ligand Tail Controls the Conformation of Indium Sulfide Ultrathin Nanoribbons. J. Am. Chem. Soc. 2024, 146, 22318–22326. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Havenridge, S.; Zaluzec, N.J.; Kang, D.; Jayaweera, N.P.; Elam, J.W.; Mulfort, K.L.; Liu, C.; Martinson, A.B.F. Vapor Infiltration Synthesis of Indium Sulfide Magic Size Cluster. ACS Nano 2024, 18, 31372–31380. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.; Mohammadi, M.R.; Li, Y. Enhancing photovoltaic performance of dye-sensitized solar cells through TiO2/g-C3N4 nanocomposite photoanodes for improved charge carrier management. Electrochim. Acta 2025, 509, 145331. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Wang, W.; Wang, H.; Chen, Q.; Chen, J.; Chen, D. Constructing functionalized carbon quantum dots on amino-rich graphitic carbon nitride to enhance CO2 photocatalytic reduction: Critical role of functional group modulation. Sep. Purif. Technol. 2025, 355, 129780. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Y.; You, Y.; Jiang, Z. Efficient UV−Vis−NIR Responsive CO2 Reduction Photocatalyst with Black Pinecone-Shaped Carbon Nitride Loaded with Lanthanum Oxide. ACS Appl. Mater. Interfaces 2024, 16, 63509–63519. [Google Scholar] [CrossRef]
- Zhou, F.; Li, W.; Wu, J.; Yang, W.; Sun, Y.; Zhou, H.; Jia, T.; Ling, Y.; He, P.; Pan, W.; et al. Enhanced solar-driven CO2 conversion: The role of Yb-doped CuInS2 quantum dots on g-C3N4 nanosheets. Appl. Catal. B Environ. Energy 2025, 362, 124716. [Google Scholar] [CrossRef]
- Yi, S.; Wang, Z.; Wu, Z.; Yang, X.; Wang, H.; Yang, J.; She, X.; Xu, H. Monodispersed Cu sites assembled on ultrathin 2D C3N4 for efficient electrocatalytic CO2 methanation. Appl. Surf. Sci. 2025, 681, 161492. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Y.; Wang, D.; He, W.; Fang, X.; Zhao, C.; Pan, J.; Liu, D.; Liu, S.; Chen, T.; et al. Nanoengineering construction of g-C3N4/Bi2WO6 S-scheme heterojunctions for cooperative enhanced photocatalytic CO2 reduction and pollutant degradation. Sep. Purif. Technol. 2025, 354, 128893. [Google Scholar] [CrossRef]
- He, Y.; Han, L.; Hou, Y.; Qiao, X.; Zhu, M. P3HT/g-C3N4 composite fiber membranes for high-performance photocatalytic hydrogen evolution. Appl. Surf. Sci. 2025, 682, 161673. [Google Scholar] [CrossRef]
- Yang, D.; Ye, Q.; Qu, C.; Meng, F.; Wang, L.; Li, Y. Visible-light-driven F/C co-doping g-C3N4 nanosheets for efficient hydrogen evolution: Charge redistribution on C4 delocalized large π bond. Appl. Catal. B Environ. Energy 2025, 361, 124637. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, H.; Zhou, Y.; Wu, Y. Construction of g-C3N4-based donor-acceptor conjugated copolymer for photocatalytic selective oxidation of biomass-derived 5-hydroxymethylfurfural under visible light. J. Mol. Struct. 2025, 1322, 140404. [Google Scholar] [CrossRef]
- Jayaprakash, K.; Sivasamy, A. Polymeric graphitic carbon nitride layers decorated with erbium oxide and enhanced photocatalytic performance under visible light irradiation. Environ. Sci. Pollut. Res. 2023, 30, 52561–52575. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Mutahir, S.; Shaheen, I.; Qunhui, Y.; Bououdina, M.; Humayun, M. Recent advances over the doped g-C3N4 in photocatalysis: A review. Coord. Chem. Rev. 2025, 522, 216227. [Google Scholar] [CrossRef]
- Nijad, I.; Pavel, G.; Roopesh, M.; Mathieu, G.; Miroslav, Š. Fine-tuning photoluminescence and photocatalysis: Exploring the effects of carbon quantum dots synthesis and purification on g-C3N4. Colloids Surf. A Physicochem. Eng. Asp. 2025, 706, 135789. [Google Scholar]
- Pei, J.; Li, H.; Zhuang, S.; Zhang, D.; Yu, D. Recent Advances in g-C3N4 Photocatalysts: A Review of Reaction Parameters, Structure Design and Exfoliation Methods. Catalysts 2023, 13, 1402. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Huang, F.; Song, S.; Ai, G.; Xin, X.; Zhao, B.; Zheng, Y.; Zhang, Z. Recent Advances in g-C3N4-Based Materials and Their Application in Energy and Environmental Sustainability. Molecules 2023, 28, 432. [Google Scholar] [CrossRef]
- Sharma, M.D.; Basu, M. Nanosheets of In2S3/S-C3N4-Dots for Solar Water-Splitting in Saline Water. Langmuir 2022, 38, 12981–12990. [Google Scholar] [CrossRef]
- Ma, X.; Li, C.; Zhang, X.; Gao, M.; Wang, Y.; Li, G. Interface Optimisation of the Fe2O3/C3N4 Heterojunction with Metal Nanoparticles and Their Negative and Positive Photoelectric Responses in a Broadband Light Spectrum Range. Coatings 2024, 14, 1595. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, K.; Ai, Q.; Sun, X.; Chen, X.; Yang, J.; Zhu, Y.; Cheng, Z.; Li, B.; Liu, L.; et al. High-performance fully transparent Ga2O3 solar-blind UV photodetector with the embedded indium–tin–oxide electrodes. Mater. Today Phys. 2023, 33, 101034. [Google Scholar] [CrossRef]
- Ferhati, H.; Djeffal, F.; Bendjerad, A.; Saidi, A.; Benhaya, A. Post-annealing effects on RF sputtered all-amorphous ZnO/SiC heterostructure for solar-blind highly-detective and ultralow dark-noise UV photodetector. J. Non-Cryst. Solids 2021, 574, 121168. [Google Scholar] [CrossRef]
- Ferhati, H.; Djeffal, F. High-Responsivity MSM Solar-Blind UV Photodetector Based on Annealed ITO/Ag/ITO Structure Using RF Sputtering. IEEE Sens. J. 2019, 19, 7942–7949. [Google Scholar] [CrossRef]
- Chu, L.; Xu, C.; Liu, D.; Nie, C.; Zhou, X.; Deng, L. A high-performance self-powered broadband photodetector based on vertical MAPbBr3/ZnO heterojunction. Mater. Sci. Semicond. Process. 2024, 169, 107943. [Google Scholar] [CrossRef]
- Lyu, Z.; Zhao, Y.; Duan, R.; Liu, M.; Yao, X.; Liu, S.; Li, S. Enhancing the performance of self-powered heterojunctional ZnO/Cs3Bi2I9 photodetectors through pyroelectric-photovoltaic coupling effect. Nano Energy 2024, 131, 110205. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, C.; Wei, Y.; Ouyang, Y.; Han, J.; Li, C.; Dong, M.; Wang, J. High performance self-powered PbSe/WSe2 p-n heterojunction photodetector for image sensing. J. Mater. Sci. Technol. 2025, 225, 125–132. [Google Scholar] [CrossRef]
- Jia, M.; Tang, L.; Teng, K.S.; Lü, Y. High-performance self-powered ultraviolet photodetector based on PVK/amorphous-WO3 organic-inorganic heterojunction. Appl. Surf. Sci. 2024, 643, 158641. [Google Scholar] [CrossRef]
- Xiao, Y.; He, S.; Fan, X.; Du, Y.; Li, Y.; Zhao, L.; Gao, L. Realizing enhanced photoresponse for self-powered broadband photodetector with asymmetric contacts based MoTe2/WSe2 van der Waals heterostructure. J. Alloys Compd. 2024, 1006, 176358. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X. Self-powered photodetector with GeSe/WS2/MoS2 van der Waals heterojunction. Sens. Actuators A Phys. 2025, 381, 116080. [Google Scholar] [CrossRef]
- Xu, W.; Li, B.; Wu, Y.; Dong, Z.; Zhang, K.; Wang, Q.; Feng, S.; Lu, W. Ultrahigh Bipolar Photoresponse in a Self-Powered Ultraviolet Photodetector Based on GaN and In/Sn-Doped Ga2O3 Nanowires pn junction. ACS Appl. Mater. Interfaces 2024, 16, 35303–35314. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.; Zhao, X.; Zong, L.; Wang, S.; Li, B.; Li, Y.; Ban, G.; Wang, Z.; Ma, Z.; et al. Visible-Light Self-Powered Photodetector with High Sensitivity Based on the Type-II Heterostructure of CdPSe3/MoS2. ACS Appl. Mater. Interfaces 2024, 16, 32334–32343. [Google Scholar] [CrossRef]
- Ban, G.; Song, J.; Wang, Z.; Zhao, X.; Li, Y.; Yang, J.; Ye, C.; Teng, F.; Hu, P.; Fan, H. Visible-near-infrared self-powered photodetector based on FePSe3. Surf. Interfaces 2024, 55, 105319. [Google Scholar] [CrossRef]
- Pérez-Torres, A.F.; Hernández-Barreto, D.F.; Bernal, V.; Giraldo, L.; Moreno-Piraján, J.C.; da Silva, E.A.; Alves, M.D.C.M.; Morais, J.; Hernandez, Y.; Cortés, M.T.; et al. Sulfur-Doped g-C3N4 Heterojunctions for Efficient Visible Light Degradation of Methylene Blue. ACS Omega 2023, 8, 47821–47834. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, X.; Gao, M.; Hu, R.; Wang, Y.; Li, G. The Interface Interaction of C3N4/Bi2S3 Promoted the Separation of Excitons and the Extraction of Free Photogenerated Carriers in the Broadband Light Spectrum Range. Inorganics 2025, 13, 122. [Google Scholar] [CrossRef]














| Excitation Light Wavelength (nm) | In2S3/C3N4 Nanocomposite | Response Time (s) | Recovery Time (s) | Ratio of On/Off |
|---|---|---|---|---|
| 50 mW 405 nm | using sodium thiosulfate precursor as a sulphur source | 19.7 | 12.0 | 2.14 |
| 50 mW 405 nm | using thiourea precursor as a sulphur source | 12.0 | 20.6 | 1.23 |
| 50 mW 532 nm | using sodium thiosulfate precursor as a sulphur source | 32.6 | 16.3 | 2.14 |
| 50 mW 532 nm | using thiourea precursor as a sulphur source | 56.6 | 4.3 | 1.23 |
| 50 mW 650 nm | using sodium thiosulfate precursor as a sulphur source | 24.0 | 12.0 | 2.43 |
| 50 mW 650 nm | using thiourea precursor as a sulphur source | 36.8 | 16.3 | 1.24 |
| 50 mW 780 nm | using sodium thiosulfate precursor as a sulphur source | 7.7 | 12.0 | 1.74 |
| 50 mW 780 nm | using thiourea precursor as a sulphur source | 12.0 | 29.1 | 1.27 |
| 50 mW 808 nm | using sodium thiosulfate precursor as a sulphur source | 12.0 | 16.3 | 1.85 |
| 50 mW 808 nm | using thiourea precursor as a sulphur source | 8.6 | 12.0 | 1.40 |
| 50 mW 980 nm | using sodium thiosulfate precursor as a sulphur source | 4.3 | 12.0 | 1.26 |
| 50 mW 980 nm | using thiourea precursor as a sulphur source | 8.6 | 8.6 | 1.32 |
| 40 mW 1064 nm | using sodium thiosulfate precursor as a sulphur source | 8.6 | 7.7 | 1.24 |
| 40 mW 1064 nm | using thiourea precursor as a sulphur source | 4.3 | 28.3 | 1.16 |
| 25 W, visible light | using sodium thiosulfate precursor as a sulphur source | 20.6 | 20.5 | 3.00 |
| 25 W, visible light | using thiourea precursor as a sulphur source | 12.0 | 16.3 | 1.77 |
| Excitation Light Wavelength (nm) | Response Time (s) | Recovery Time (s) | Ratio of On/Off |
|---|---|---|---|
| 100 mW, 650 nm | 12.0 | 16.3 | 5.67 |
| 50 mW, 650 nm | 5.7 | 4.3 | 2.86 |
| 5 mW, 650 nm | 15.6 | 15.1 | 1.15 |
| 100 mW, 808 nm | 16.3 | 8.1 | 2.11 |
| 50 mW, 808 nm | 20.6 | 8.1 | 1.96 |
| 10 mW, 808 nm | 8.1 | 8.1 | 1.24 |
| 100 mW, 980 nm | 16.3 | 7.7 | 2.03 |
| 50 mW, 980 nm | 8.6 | 8.6 | 1.33 |
| 5 mW, 980 nm | 8.6 | 4.3 | 1.05 |
| Excitation Light Wavelength (nm) | Response Time (s) | Recovery Time (s) | Ratio of On/Off |
|---|---|---|---|
| 50 mW, 405 nm | 6.6 | 16.6 | 1.79 |
| 50 mW, 532 nm | 16.5 | 6.5 | 1.80 |
| 50 mW, 650 nm | 3.4 | 3.1 | 3.40 |
| 50 mW, 780 nm | 16.2 | 3.1 | 3.41 |
| 200 mW, 808 nm | 22.8 | 6.6 | 2.66 |
| 100 mW, 980 nm | 9.7 | 10.0 | 3.17 |
| Nanocomposite Sample | C Element (Mass Percentage (%)) | N Element (Mass Percentage (%)) | In Element (Mass Percentage (%)) | S Element (Mass Percentage (%)) | Total (%) |
|---|---|---|---|---|---|
| In2S3/C3N4 coated on aluminum foil | 2.47 | 0.00 | 3.20 | 1.33 | 100.00 (Otherwise, Al element mass percentage: 91.26%; O element mass percentage: 1.75%) |
| C element (atomic percentage (%)) | N element (atomic percentage (%)) | In element (atomic percentage (%)) | S element (atomic percentage (%)) | Total (%) | |
| In2S3/C3N4 coated on aluminum foil | 5.45 | 0.00 | 0.74 | 1.10 | 100.00 (Otherwise, Al element atomic percentage: 89.81%; O element atomic percentage: 2.90%) |
| C element (mass percentage (%)) | N element (mass percentage (%)) | In element (mass percentage (%)) | S element (mass percentage (%)) | Total (%) | |
| In2S3/C3N4 (remove the effects of aluminum foil) | 5.51 | 0.02 | 81.31 | 13.16 | 100.00 |
| C element (mass percentage (%)) | N element (mass percentage (%)) | In element (mass percentage (%)) | S element (mass percentage (%)) | Total (%) | |
| In2S3/C3N4 (remove the effects of aluminum foil) | 29.07 | 0.08 | 44.85 | 26.00 | 100.00 |
 | |||||
 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Zhang, X.; Gao, M.; Hu, R.; Wang, Y.; Li, G. In2S3/C3N4 Nanocomposite and Its Photoelectric Properties in the Broadband Light Spectrum Range. Coatings 2025, 15, 718. https://doi.org/10.3390/coatings15060718
Ma X, Zhang X, Gao M, Hu R, Wang Y, Li G. In2S3/C3N4 Nanocomposite and Its Photoelectric Properties in the Broadband Light Spectrum Range. Coatings. 2025; 15(6):718. https://doi.org/10.3390/coatings15060718
Chicago/Turabian StyleMa, Xingfa, Xintao Zhang, Mingjun Gao, Ruifen Hu, You Wang, and Guang Li. 2025. "In2S3/C3N4 Nanocomposite and Its Photoelectric Properties in the Broadband Light Spectrum Range" Coatings 15, no. 6: 718. https://doi.org/10.3390/coatings15060718
APA StyleMa, X., Zhang, X., Gao, M., Hu, R., Wang, Y., & Li, G. (2025). In2S3/C3N4 Nanocomposite and Its Photoelectric Properties in the Broadband Light Spectrum Range. Coatings, 15(6), 718. https://doi.org/10.3390/coatings15060718



_Ma.jpg)


