Effect of Gas Velocity on Thickness Uniformity of TiNxOy Thin Film in Atomic Layer Deposition Process
Abstract
1. Introduction
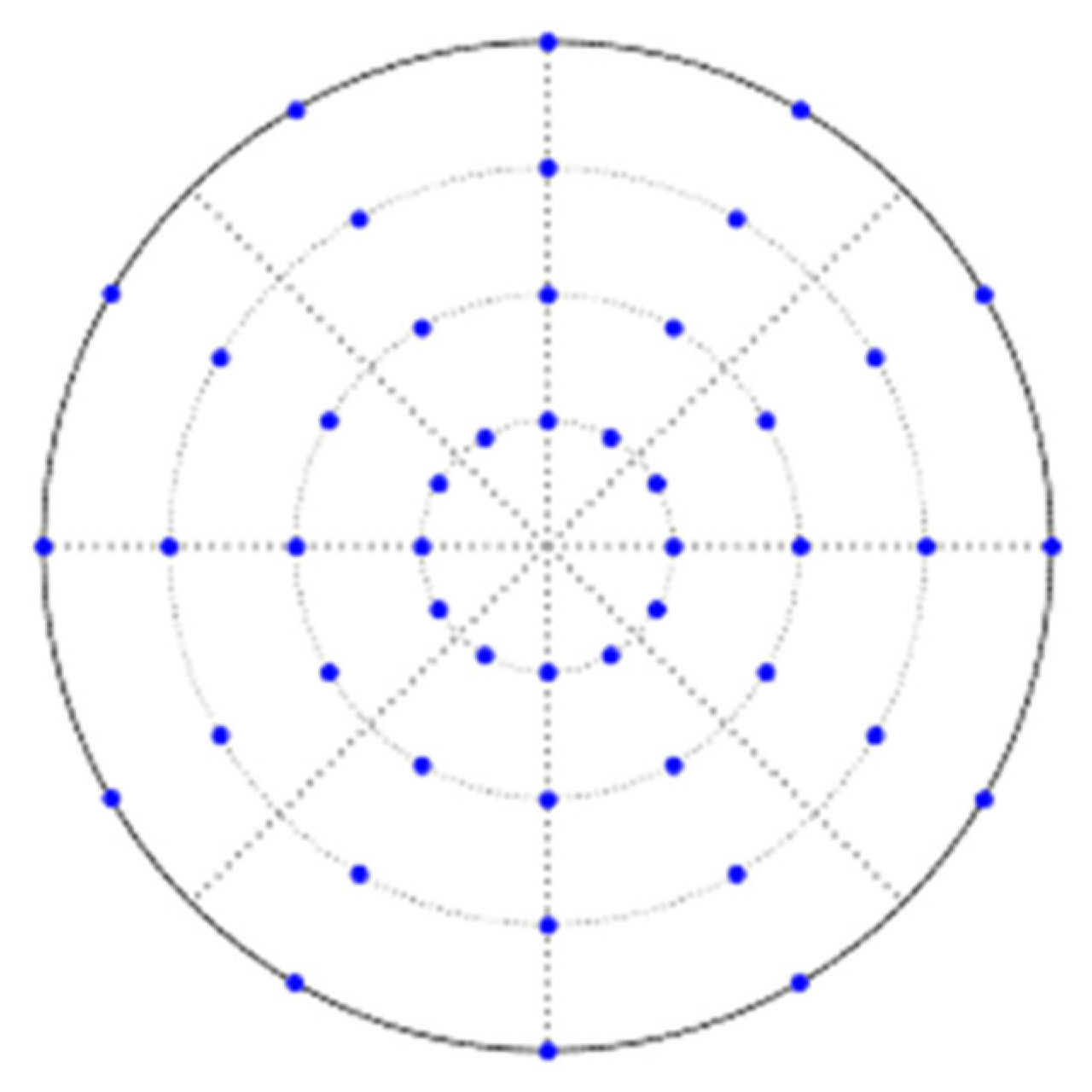
2. Simulation Methods
3. Experimental Method
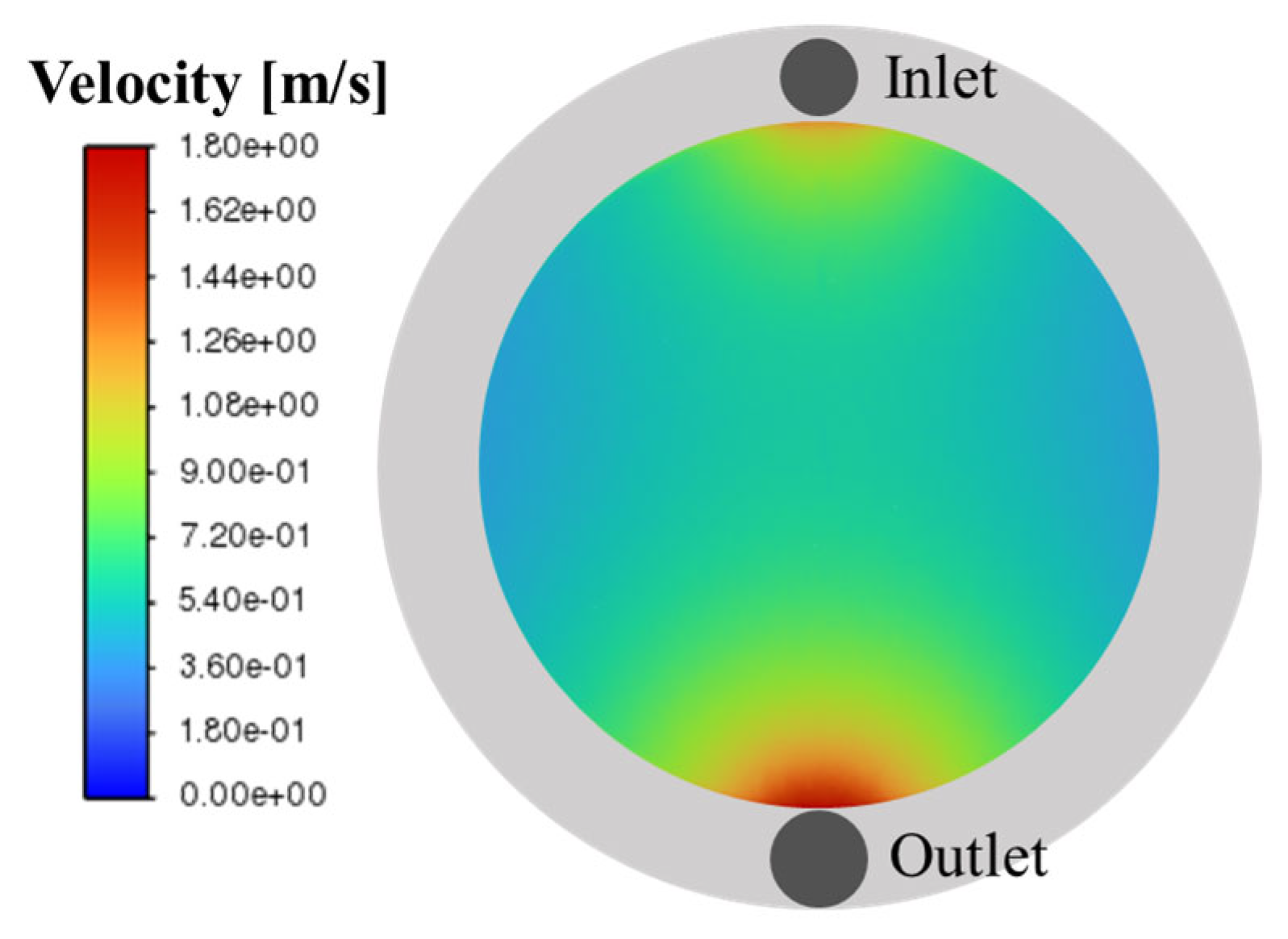
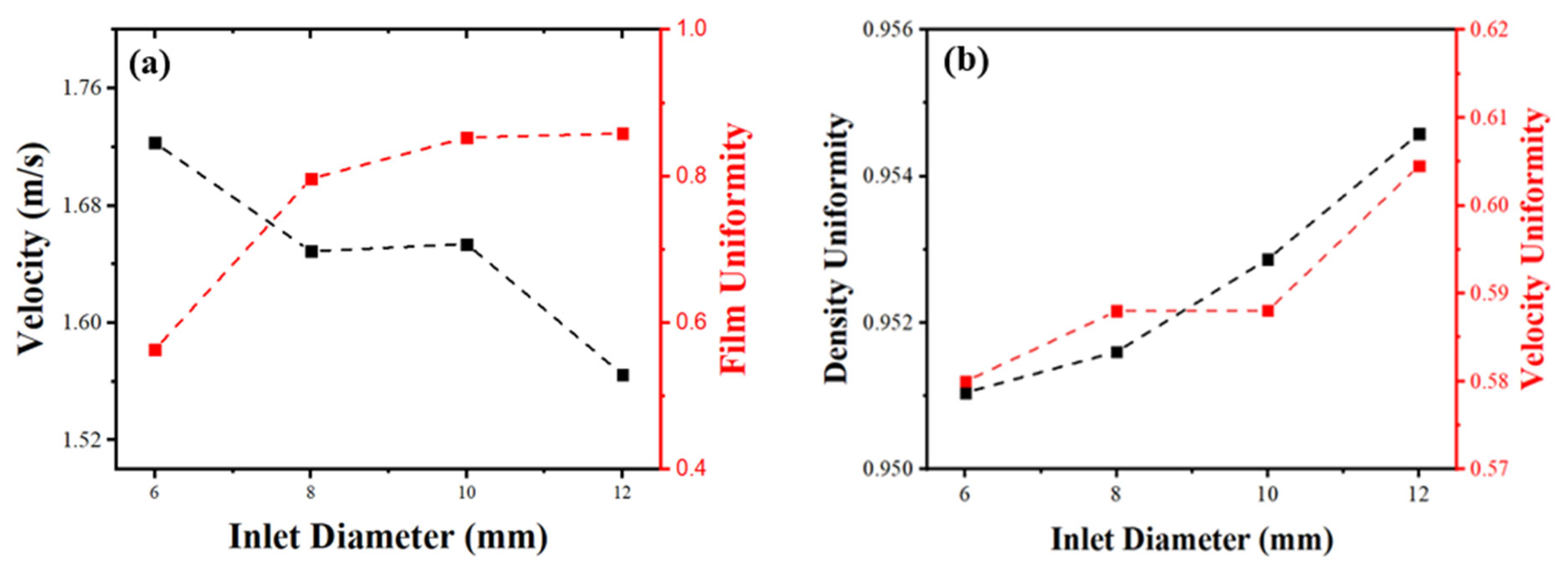
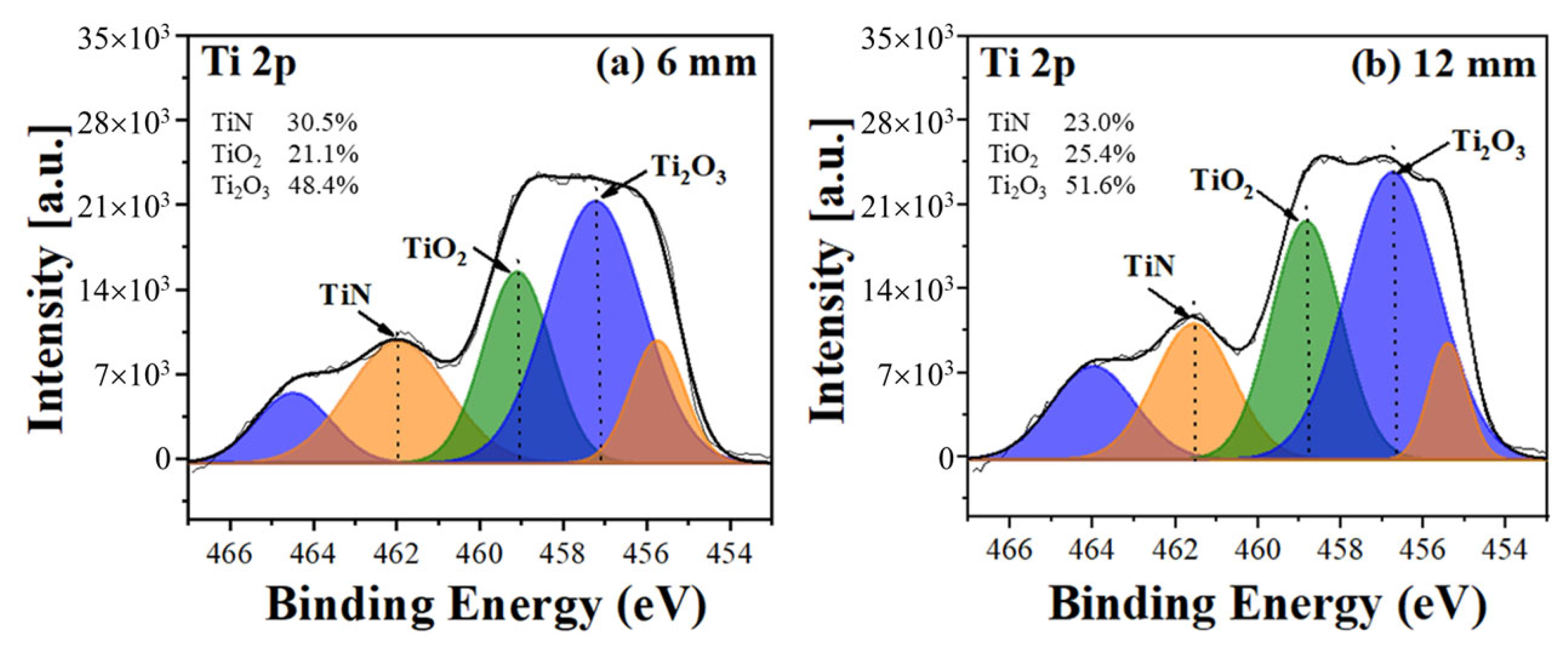
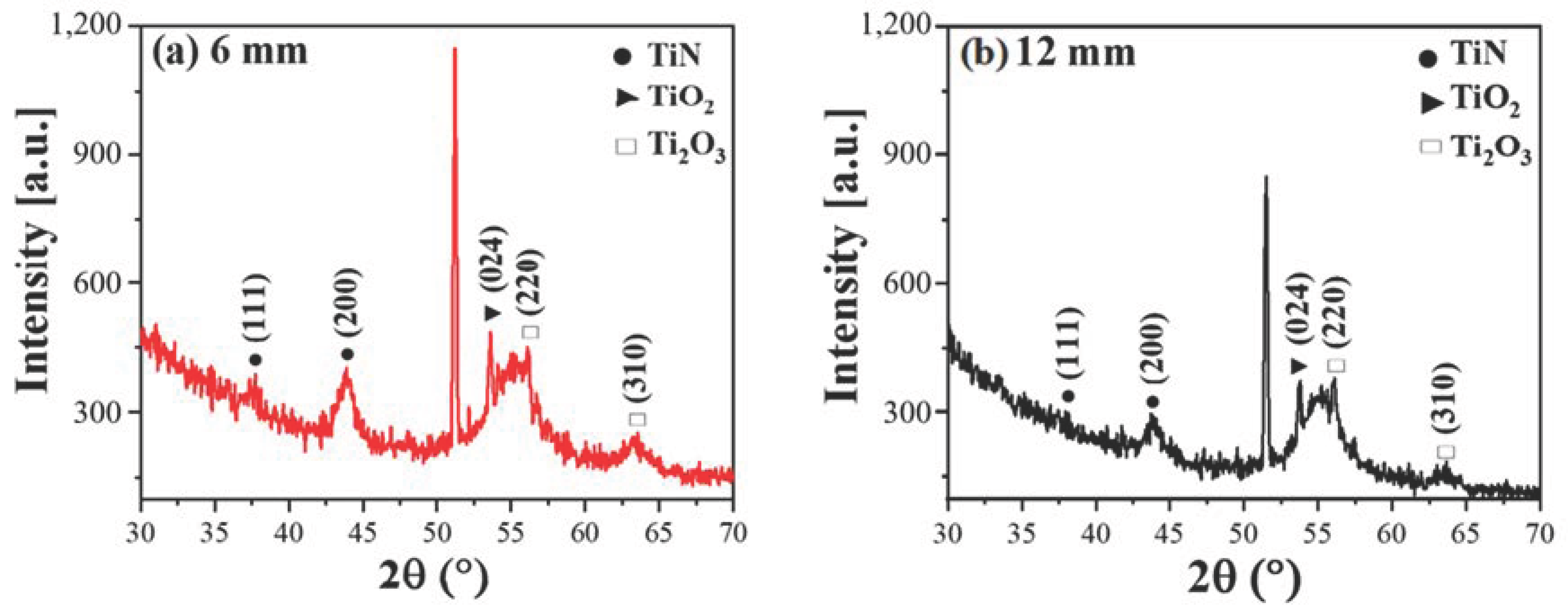
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, P.; Luc, Q.H.; Hernandez, A.S.; Ko, H.L.; Wu, J.Y.; Tran, N.A.; Collaert, N.; Chang, E.Y. The Impact of Width Downscaling on the High-Frequency Characteristics of InGaAs Nanowire FETs. IEEE J. Electron. Devices Soc. 2022, 10, 854–859. [Google Scholar] [CrossRef]
- Colalongo, L.; Comensoli, S.; Richelli, A. A Unified Core Model of Double-gate and Surrounding-gate MOSFETs for Circuit Simulation. Solid-State Electron. 2024, 23, 108849. [Google Scholar] [CrossRef]
- Sharma, V.K. A Survey of Leakage Reduction Techniques in CMOS Digital Circuits for Nanoscale Regime. Aust. J. Electr. Electron. Eng. 2021, 18, 217–236. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C.; Wei, Y.; Feng, S.; Sun, D.; Cheng, H. Three-Dimensional Transistors and Integration based on Low-Dimensional Materials for the Post-Moore’s Law Era. Mater. Today 2023, 63, 170–187. [Google Scholar] [CrossRef]
- Ratnesh, R.K.; Goel, A.; Kaushik, G.; Garg, H.; Chandan; Singh, M.; Prasad, B. Advancement and Challenges in MOSFET Scaling. Mater. Sci. Semicond. Process. 2021, 134, 106002. [Google Scholar] [CrossRef]
- Li, J.; Chai, G.; Wang, X. Atomic Layer Deposition of Thin Films: From a Chemistry Perspective. Int. J. Extrem. Manuf. 2023, 5, 032003. [Google Scholar] [CrossRef]
- Chen, R.; Li, Y.C.; Cai, J.M.; Cao, K.; Lee, H.B.R. Atomic Level Deposition to Extend Moore’s Law and Beyond. Int. J. Extrem. Manuf. 2020, 2, 022002. [Google Scholar] [CrossRef]
- Ranjith, R.; Laha, A.; Bugiel, E.; Osten, H.J.; Xu, K.; Milanov, A.P.; Devi, A. Downscaling of Defect-Passivated Gd2O3 Thin Films on p-Si (001) Wafers Grown by H2O-Assisted Atomic Layer Deposition. Semicond. Sci. Technol. 2010, 25, 105001. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Deng, H. Gas Distribution Effect on Thermal ALD AlN Film Thickness Non-Uniformity. In Proceedings of the 2023 China Semiconductor Technology International Conference (CSTIC), Shanghai, China, 26–27 June 2023. [Google Scholar] [CrossRef]
- Lee, B.J.; Kim, Y.S.; Seo, D.W.; Choi, J.W. The Effect of Deposition Temperature of TiN Thin Film Deposition Using Thermal Atomic Layer Deposition. Coatings 2023, 13, 104. [Google Scholar] [CrossRef]
- Rontu, V. Atomic Layer Deposition in Fabrication of Micro-and Nanodevices. Ph.D. Thesis, Department of Chemical and Materials Science, Aalto University, Espoo, Finland, 2020. Available online: https://aaltodoc.aalto.fi/items/156ff697-7d11-4b7d-9a42-a09160b27308 (accessed on 27 April 2025).
- van Ommen, J.R.; Goulas, A.; Puurunen, R.L. Atomic Layer Deposition. Kirk-Othmer Encycl. Chem. Technol. 2021, 1, 1–42. [Google Scholar] [CrossRef]
- Mun, S.H.; Lee, J.S.; Shin, S.W.; Jeon, S.R.; Choi, S.Y.; Kwak, H.M.; Kim, K.P.; Kim, J.W.; Kang, C.M.; Lee, D.S. Improving the Electrical and Optical Characteristics of AlGaInP Red Micro-LEDs by Double Dielectric Passivation. ECS J. Solid State Sci. Technol. 2024, 13, 026002. [Google Scholar] [CrossRef]
- Elers, K.E.; Blomberg, T.; Peussa, M.; Aitchison, B.; Haukka, S.; Marcus, S. Film Uniformity in Atomic Layer Deposition. Chem. Vap. Depos. 2006, 12, 13–24. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ahn, J.H.; Kang, S.W.; Kim, J.H. Step Coverage Modeling of Thin Films in Atomic Layer Deposition. J. Appl. Phys. 2007, 101, 073502. [Google Scholar] [CrossRef]
- Kim, B.; Lee, N.; Lee, J.; Park, T.; Park, H.; Kim, Y.; Jin, C.; Lee, D.; Kim, H.; Jeon, H. Remote Plasma Enhanced Atomic Layer Deposition of Titanium Nitride Film using Metal Organic Precursor (C12H23N3Ti) and N2 Plasma. Appl. Surf. Sci. 2021, 541, 148482. [Google Scholar] [CrossRef]
- Sidhwa, A.; Spinner, C.; Gandy, T.; Goulding, M.; Brown, W.; Naseem, H.; Ulrich, R.; Ang, S.; Charlton, S.; Prasad, V.; et al. Study of the Step Coverage and Contact Resistance by Using Two-Step TiN Barrier and Evolve Simulation. IEEE Trans. Semicond. Manuf. 2005, 18, 163–173. [Google Scholar] [CrossRef]
- Ohshita, Y.; Ogura, A.; Hoshino, A.; Suzuki, T.; Hiiro, S.; Machida, H. Effects of Deposition Conditions on Step-Coverage Quality in Low-Pressure Chemical Vapor Deposition of HfO2. J. Cryst. Growth 2002, 235, 365–370. [Google Scholar] [CrossRef]
- Siimon, H.; Aarik, J. Thickness Profiles of Thin Films Caused by Secondary Reactions in Flow-Type Atomic Layer Deposition Reactors. J. Phys. D Appl. Phys. 1997, 30, 1725. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Y.; Christofides, P.D. Multiscale computational fluid dynamics modeling of thermal atomic layer deposition with application to chamber design. Chem. Eng. Res. Des. 2019, 147, 529–544. [Google Scholar] [CrossRef]
- Sibanda, D.; Oyinbo, S.T.; Jen, T.C. A Review of Atomic Layer Deposition Modelling and Simulation Methodologies: Density Functional Theory and Molecular Dynamics. Nanotechnol. Rev. 2022, 11, 1332–1363. [Google Scholar] [CrossRef]
- Kunene, T.J.; Tartibu, L.K.; Karimzadeh, S.; Oviroh, P.O.; Ukoba, K.; Jen, T.C. Molecular Dynamics of Atomic Layer Deposition: Sticking Coefficient Investigation. Appl. Sci. 2022, 12, 2188. [Google Scholar] [CrossRef]
- Hidayat, R.; Chowdhury, T.; Kim, Y.; Kim, S.; Mayangsari, T.R.; Kim, S.H.; Lee, W.J. Density Functional Theory Study on the Reducing Agents for Atomic Layer Deposition of Tungsten Using Tungsten Chloride Precursor. Appl. Surf. Sci. 2021, 538, 148156. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, M.; Gu, B.; Khan, M.R.; Ko, B.G.; Yasmeen, S.; Kim, C.S.; Kwon, S.H.; Kim, J.; Kwon, J.; et al. Effects of Al Precursors on Deposition Selectivity of Atomic Layer Deposition of Al2O3 Using Ethanethiol Inhibitor. Chem. Mater. 2020, 32, 8921–8929. [Google Scholar] [CrossRef]
- Pan, D. Density Functional Theory (DFT)-Enhanced Computational Fluid Dynamics Modeling of Substrate Movement and Chemical Deposition Process in Spatial Atomic Layer Deposition. Chem. Eng. Sci. 2021, 234, 116447. [Google Scholar] [CrossRef]
- Mousa, M.B.M.; Oldham, C.J.; Jur, J.S.; Parsons, G.N. Effect of Temperature and Gas Velocity on Growth Per Cycle during Al2O3 and ZnO Atomic Layer Deposition at Atmospheric Pressure. J. Vac. Sci. Technol. A 2012, 30, 01A155. [Google Scholar] [CrossRef]
- Elam, J.W.; Schuisky, M.; Ferguson, J.D.; George, S.M. Surface Chemistry and Film Growth during TiN Atomic Layer Deposition Using TDMAT and NH3. Thin Solid Films 2003, 436, 145–156. [Google Scholar] [CrossRef]
- Weiller, B.H.; Calif, S.M. Method of Controlling Reactions between Tetrakis Dialkylamine Titanium and Ammonia for Producing Titanium Nitride Films. U.S. Patent 5763007, 9 June 1998. [Google Scholar]
- Kang, J.E.; Hong, S.J. Effect of NH3 Flow Rate to Titanium Nitride as Etch Hard Mask in Thermal Atomic Layer Deposition. J. Vac. Sci. Technol. A 2024, 42, 062402. [Google Scholar] [CrossRef]
- Gelest, Inc. Titanium Tetrakis(Dimethylamide), 99+% Product Information. Gelest Website. Available online: https://www.gelest.com/wp-content/uploads/OMTI080_TITANIUM-TETRAKISDIMETHYLAMIDE-99_GHS-US_English-US.pdf (accessed on 18 March 2016).
- Lee, W.J.; Yun, E.Y.; Lee, H.B.R.; Hong, S.W.; Kwon, S.H. Ultrathin Effective TiN Protective Films Prepared by Plasma-Enhanced Atomic Layer Deposition for High Performance Metallic Bipolar Plates of Polymer Electrolyte Membrane Fuel Cells. Appl. Surf. Sci. 2020, 519, 146215. [Google Scholar] [CrossRef]
- Abd El-Fattah, H.A.; El- Mahallawi, I.S.; Mostafa, H.S.; Khalifa, W.A. Optical Properties and Microstructure of TiNxOy and TiN Thin Films before and after Annealing at Different Conditions. Coatings 2019, 9, 22. [Google Scholar] [CrossRef]
- Killampalli, A.S.; Ma, P.F.; Engstrom, J.R. The Reaction of Tetrakis(dimethylamido)titanium with Self-Assembled Alkyltrichlorosilane Monolayers Possessing -OH, -NH2, and -CH3 Terminal Groups. J. Am. Chem. Soc. 2005, 127, 6300–6310. [Google Scholar] [CrossRef]
- Dean, J.A. Properties of Atoms, Radicals, and Bonds. In Lange’s Handbook of Chemistry, 16th ed.; McGraw-Hill: New York, NY, USA, 2005; Chapter 4; pp. 4.50–4.52. Available online:http://fptl.ru/biblioteka/spravo4niki/dean.pdf (accessed on 27 April 2025).
- Kia, A.M.; Speulmanns, J.; Bönhardt, S.; Emara, J.; Kühnel, K.; Haufe, N.; Weinreich, W. Spectroscopic Analysis of Ultra-Thin TiN as a Diffusion Barrier for Lithium-Ion Batteries by ToF-SIMS, XPS, and EELS. Appl. Surf. Sci. 2021, 564, 150457. [Google Scholar] [CrossRef]
- Musschoot, J.; Xie, Q.; Deduytsche, D.; Van den Berghe, S.; Van Meirhaeghe, R.L.; Detavernier, C. Atomic layer deposition of titanium nitride from TDMAT precursor. Microelectron. Eng. 2009, 86, 72–77. [Google Scholar] [CrossRef]
- Liang, C.L.; Cheng, G.A.; Zheng, R.T.; Liu, H.P.; Li, J.C.; Zhang, H.F.; Ma, G.J.; Jiang, Y.L. Composition and Texture of TiN Thin Films Fabricated by ECR Enhanced Sputtering Deposition. Surf. Coat. Technol. 2007, 201, 5537–5540. [Google Scholar] [CrossRef]
- Liang, H.; Xu, J.; Zhou, D.; Sun, X.; Chu, S.; Bai, Y. Thickness Dependent Microstructural and Electrical Properties of TiN Thin Films Prepared by DC Reactive Magnetron Sputtering. Ceram. Int. 2016, 42, 2642–2647. [Google Scholar] [CrossRef]
- Je, J.H.; Noh, D.Y.; Kim, H.K.; Liang, K.S. Preferred Orientation of TiN Films Studied by a Real Time Synchrotron X-Ray Scattering. J. Appl. Phys. 1997, 81, 6126–6133. [Google Scholar] [CrossRef]
- Greene, J.E.; Sundgren, J.E.; Hultman, L.; Petrov, I.; Bergstrom, D.B. Development of Preferred Orientation in Polycrystalline TiN Layers Grown by Ultrahigh Vacuum Reactive Magnetron Sputtering. Appl. Phys. Lett. 1995, 67, 2928–2930. [Google Scholar] [CrossRef]
- Staroń, A. Sorption and Photocatalysis of Dyes on an Oil-Based Composite Enriched with Nanometric ZnO and TiO2. Sustainability 2023, 15, 11874. [Google Scholar] [CrossRef]
- Hu, T.; Feng, P.; Guo, L.; Chu, H.; Liu, F. Construction of Built-In Electric Field in TiO2@Ti2O3 Core-Shell Heterojunctions toward Optimized Photocatalytic Performance. Nanomaterials 2023, 13, 2125. [Google Scholar] [CrossRef]
- Trenczek-Zajac, A.; Radecka, M.; Zakrzewska, K.; Brudnik, A.; Kusior, E.; Bourgeois, S.; Marco de Lucas, M.C.; Imhoff, L. Structural and electrical properties of magnetron sputtered Ti (ON) thin films: The case of TiN doped in situ with oxygen. J. Power Sources 2009, 194, 93–103. [Google Scholar] [CrossRef]

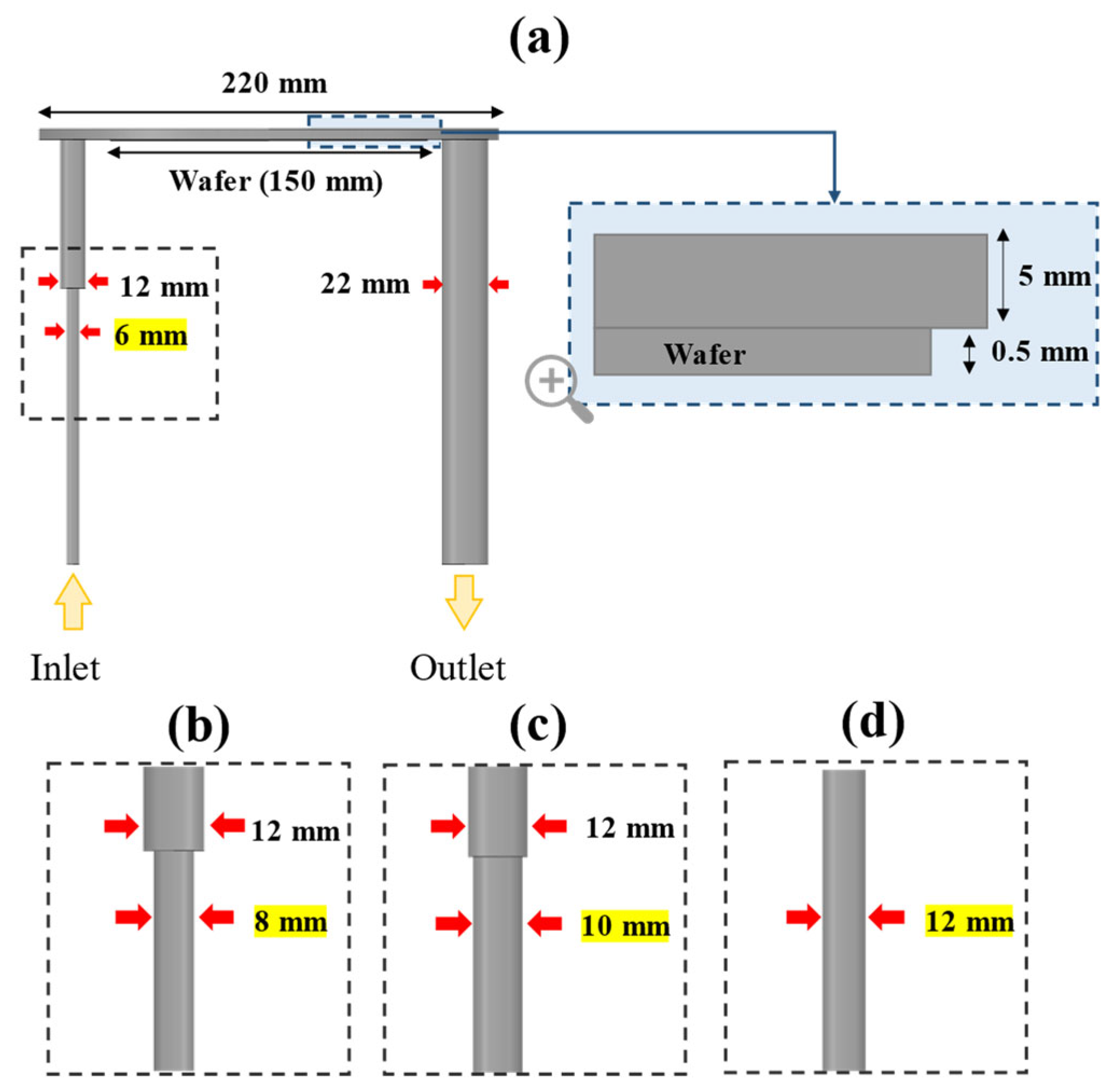
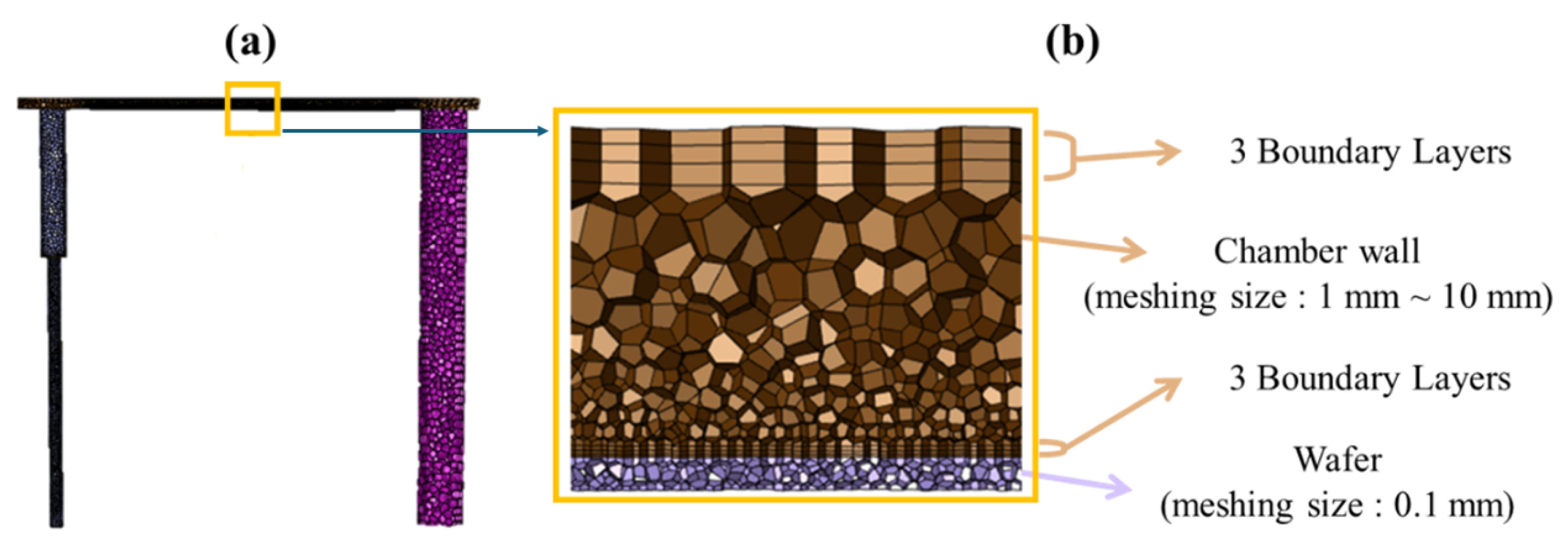
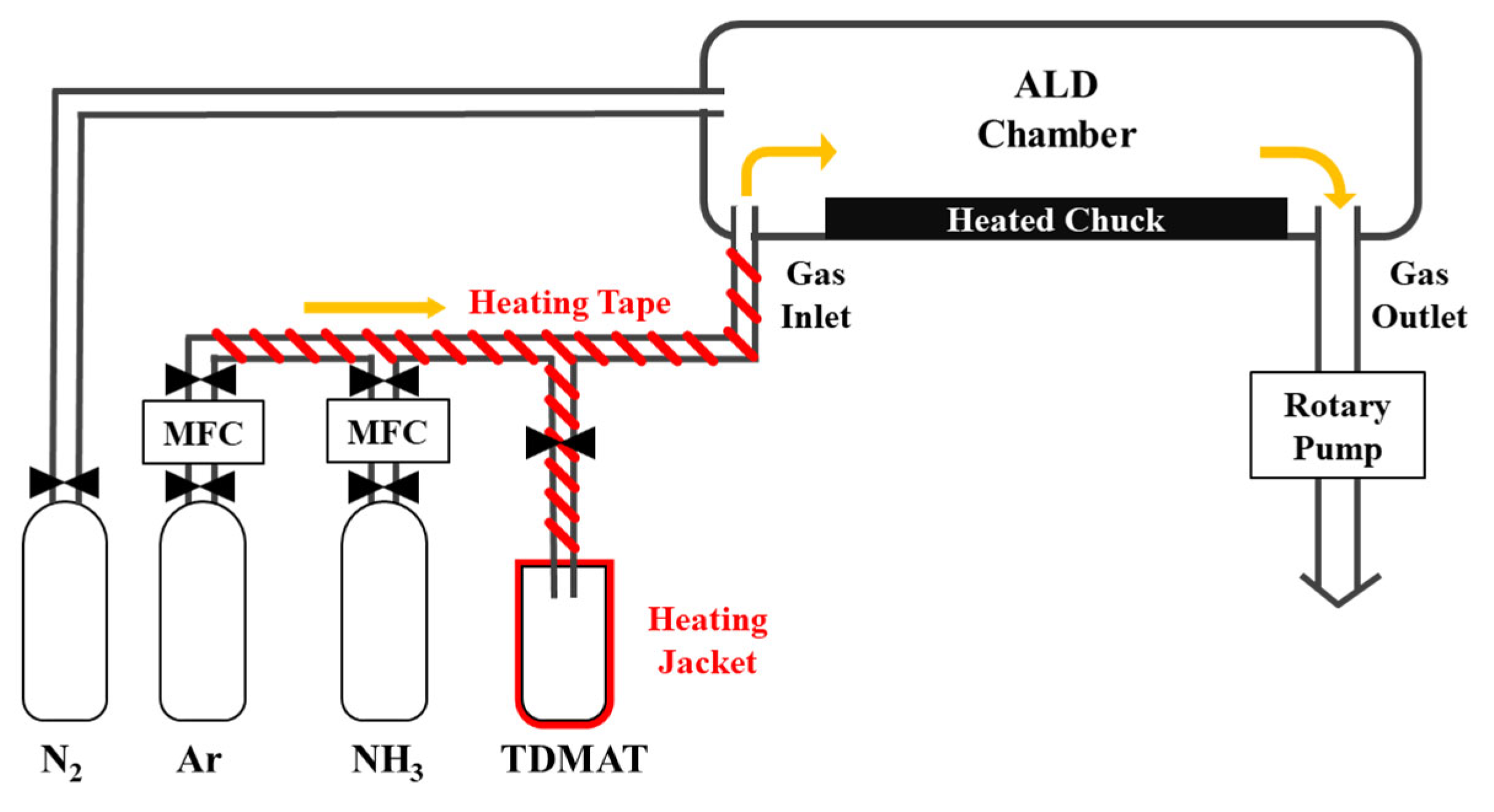

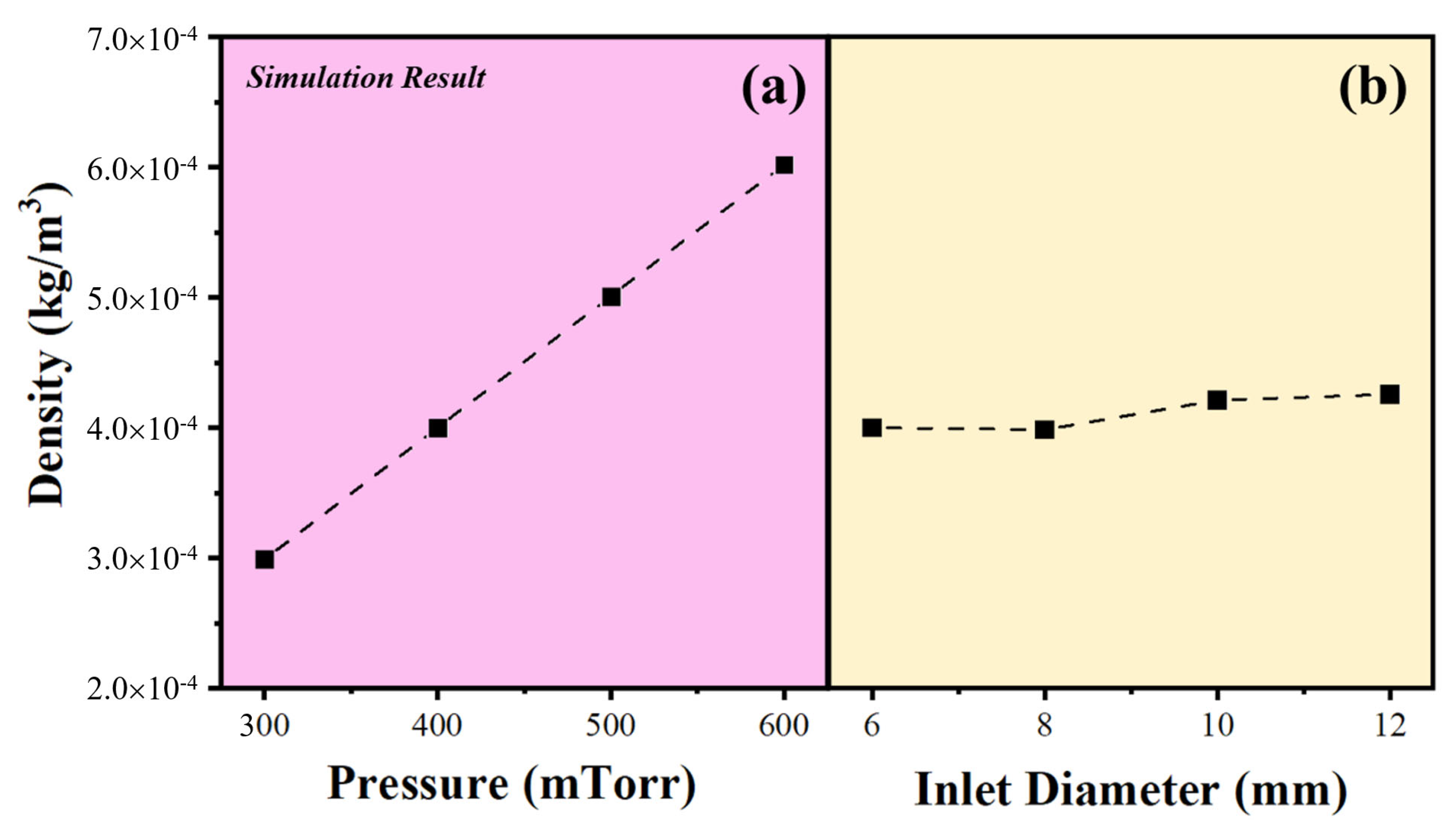
| Type | Reaction |
|---|---|
| Reaction 1 | TDMAT + 2NH* → N2Ti(C2H6)2* + 2HNC2H6 |
| Reaction 2 | N2Ti(N(C2H6))2* + 4/3NH3 → TiN + 2NH* + 2HNC2H6 + 1/6N2 |
| Boundary Conditions | ||
|---|---|---|
| Pressure (mTorr) | 400 | |
| Chuck temperature (K) | 523 | |
| Mass flow rate [kg/s] | TDMAT | 10−6 |
| NH3 | 10−7 | |
| Ar | 10−6 | |
| Gas Line Diameter [mm] | Uniformity (γa) | Non-Uniformity (γb) | Non-Uniformity (γc) [%] |
|---|---|---|---|
| 6 | 0.93 | 2.22 | 16.81 |
| 12 | 0.98 | 0.88 | 8.86 |
| Gas Line Diameter [mm] | Average Film Thickness [nm] |
|---|---|
| 6 | 20.6 (4) |
| 12 | 17.8 (3) |
| Gas Line Diameter [mm] | Concentration [%] | |
|---|---|---|
| O 1s | C 1s | |
| 6 | 43.37 | 7.74 |
| 12 | 46.42 | 7.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.W.; Kim, N.R.; Hong, S.J. Effect of Gas Velocity on Thickness Uniformity of TiNxOy Thin Film in Atomic Layer Deposition Process. Coatings 2025, 15, 707. https://doi.org/10.3390/coatings15060707
Jang JW, Kim NR, Hong SJ. Effect of Gas Velocity on Thickness Uniformity of TiNxOy Thin Film in Atomic Layer Deposition Process. Coatings. 2025; 15(6):707. https://doi.org/10.3390/coatings15060707
Chicago/Turabian StyleJang, Ji Won, Nu Ri Kim, and Sang Jeen Hong. 2025. "Effect of Gas Velocity on Thickness Uniformity of TiNxOy Thin Film in Atomic Layer Deposition Process" Coatings 15, no. 6: 707. https://doi.org/10.3390/coatings15060707
APA StyleJang, J. W., Kim, N. R., & Hong, S. J. (2025). Effect of Gas Velocity on Thickness Uniformity of TiNxOy Thin Film in Atomic Layer Deposition Process. Coatings, 15(6), 707. https://doi.org/10.3390/coatings15060707








