Lignin-Based Thin Films in Emerging Organic Transistor Devices: Challenges, Strategies, and Applications
Abstract
1. Introduction
2. Extraction and Purification of Lignin from Lignocellulosic Biomass
2.1. Extraction Processes
2.1.1. Chemical Methods
Kraft Process
Sulfite Process
Alkaline Extraction (Soda Pulping)
Organosolv Process
Acid Hydrolysis
2.1.2. Physical and Physicochemical Methods. Steam Explosion
2.1.3. Emerging Extraction Methods
Ionic Liquids (ILs)
Deep Eutectic Solvents (DES)
2.2. Lignin Purification Techniques
2.2.1. Solvent Fractionation
2.2.2. Precipitation Methods
2.2.3. Membrane Filtration
2.2.4. Chromatographic Techniques
3. Overcoming Integration Challenges
3.1. Enhancing Solubility and Processability
3.2. Addressing Interfacial Defects and Adhesion
4. Applications of Lignin in Organic Transistor-Based Devices
4.1. Lignin in Transistor Components
4.2. Lignin in Enabling Materials and Systems
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFM | Atomic Force Microscopy |
| ANN | Artificial Neural Network |
| APS | Ammonium PerSulfate |
| APTES | (3-AminoPropyl)TriEtoxySilane |
| ATPS | Artificial Tactile Perception |
| CNC | Cellulose NanoCrystals |
| EDL | Electric Double Layer |
| EGT | Electrolyte-Gated Transistor |
| EPSC | Excitatory PostSynaptic Current |
| FTIR | Fourier-Transformed Infrared spectroscopy |
| GPC | Gel Permeation Chromatography |
| IGZO | Indium–Gallium–Zinc–Oxide |
| Ls | Lignosulfonate |
| MBA | N,N’-Methylene BisAcrylamide |
| MIM | Metal-Insulator-metal |
| NMR | Nuclear Magnetic Resonance Spectroscopy |
| OFET | Organic Field-Effect Transistor |
| PAM | PolyacrylAMide |
| PANI | Polyaniline |
| PEDOT:PSS | Poly(2,3-dihydrothieno-1,4-dioxin)-poly(styrenesulfonate) |
| PMMA | Poly(methyl methacrylate) |
| PNG | Piezoelectric NanoGenerators |
| PPF | Paired-Pulse Facilitation |
| PVA | PolyVinyl Alcohol |
| PVP | PolyVinyl Phenol |
| SAM | Self-Assembled Monolayer |
| SEM | Scanning Electron Microscopy |
| SGFET | Suspended Gate Field-Effect Transistors |
| XPS | X-ray Photoelectron Spectroscopy |
References
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Constant, S.; Wienk, H.L.J.; Frissen, A.E.; Peinder, P.D.; Boelens, R.; van Es, D.S.; Grisel, R.J.H.; Weckhuysen, B.M.; Huijgen, W.J.J.; Gosselink, R.J.A.; et al. New insights into the structure and composition of technical lignins: A comparative characterisation study. Green Chem. 2016, 18, 2651–2665. [Google Scholar] [CrossRef]
- Garlapati, V.K.; Chandel, A.K.; Kumar, S.P.J.; Sharma, S.; Sevda, S.; Ingle, A.P.; Pant, D. Circular economy aspects of lignin: Towards a lignocellulose biorefinery. Renew. Sustain. Energy Rev. 2020, 130, 109977. [Google Scholar] [CrossRef]
- Bruijnincx, P.C.A.; Weckhuysen, B.M. Lignin up for break-down. Nat. Chem. 2014, 6, 1035–1036. [Google Scholar] [CrossRef] [PubMed]
- Tronci, L.; Marrocchi, A. Green gold: Prospects of lignin in organic electronics and bioelectronics. RSC Sustain. 2024, 2, 3769–3781. [Google Scholar] [CrossRef]
- Marrocchi, A. A review of lignin as a precursor for macromonomers: Challenges and opportunities in utilizing agri-food waste. Int. J. Biol. Macromol. 2025, 300, 140332. [Google Scholar] [CrossRef]
- Shorey, R.; Salaghi, A.; Fatehi, P.; Mekonnen, T.H. Valorization of lignin for advanced material applications: A review. RSC Sustain. 2024, 2, 804–831. [Google Scholar] [CrossRef]
- Gaspar, R.; Fardim, P. Lignin-based materials for emerging advanced applications. Curr. Opin. Green Sustain. Chem. 2023, 41, 100834. [Google Scholar] [CrossRef]
- Yu, O.; Kim, K.H. Lignin to Materials: A Focused Review on Recent Novel Lignin Applications. Appl. Sci. 2020, 10, 4626. [Google Scholar] [CrossRef]
- Bertella, S.; Luterbacher, J.S. Lignin Functionalization for the Production of Novel Materials. Trends Chem. 2020, 2, 440–453. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, J.; Lu, X.; Ma, X.; Cao, S.; Pan, X.; Ni, Y. Wearable lignin-based hydrogel electronics: A mini-review. Int. J. Biol. Macromol. 2021, 181, 45–50. [Google Scholar] [CrossRef]
- Li, W.; Zhang, W.; Xu, Y.; Wang, G.; Xu, T.; Nie, S.; Si, C. Lignin-derived materials for triboelectric nanogenerators with emphasis on lignin multifunctionality. Nano Energy 2024, 128, 109912. [Google Scholar] [CrossRef]
- Marrocchi, A. Sustainable Strategies in Organic Electronics: A volume in Woodhead Publishing Series in Electronic and Optical Materials; Woodhead Publishing: Cambridge, UK, 2022; pp. 1–568. [Google Scholar]
- Khan, A.; Nair, V.; Colmenares, J.C.; Gläser, R. Lignin-Based Composite Materials for Photocatalysis and Photovoltaics. Top. Curr. Chem. 2018, 376, 20. [Google Scholar] [CrossRef]
- D’Orsi, R.; Irimia, C.V.; Lucejko, J.J.; Kahraman, B.; Kanbur, Y.; Yumusak, C.; Bednorz, M.; Babudri, F.; Irimia-Vladu, M.; Operamolla, A. Kraft Lignin: From Pulping Waste to Bio-Based Dielectric Polymer for Organic Field-Effect Transistors. Adv. Sustain. Syst. 2022, 6, 2200285. [Google Scholar] [CrossRef]
- Sameni, J.; Krigstin, S.; Sain, M.M. Solubility of lignin and acetylated lignin in organic solvents. Bioresources 2017, 12, 1548–1565. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, H.; Zhao, X.; Xian, D.; Han, X.; Wang, B.; Wang, S.; Zhang, M.; Zhang, C.; Ye, X.; et al. High-Mobility Fungus-Triggered Biodegradable Ultraflexible Organic Transistors. Adv. Sci. 2022, 9, e2105125. [Google Scholar] [CrossRef] [PubMed]
- Dawy, M.; Shabaka, A.A.; Nada, A.M.A. Molecular structure and dielectric properties of some treated lignins. Polym. Degrad. Stab. 1998, 62, 455–462. [Google Scholar] [CrossRef]
- Libretti, C.; Santos Correa, L.; Meier, M.A.R. From waste to resource: Advancements in sustainable lignin modification. Green Chem. 2024, 26, 4358–4386. [Google Scholar] [CrossRef]
- Saadan, R.; Hachimi Alaoui, C.; Ihammi, A.; Chigr, M.; Fatimi, A. A Brief Overview of Lignin Extraction and Isolation Processes: From Lignocellulosic Biomass to Added-Value Biomaterials. Environ. Earth Sci. Proc. 2024, 31, 3. [Google Scholar] [CrossRef]
- Fabbri, F.; Bischof, S.; Mayr, S.; Gritsch, S.; Jimenez Bartolome, M.; Schwaiger, N.; Guebitz, G.M.; Weiss, R. The Biomodified Lignin Platform: A Review. Polymers 2023, 15, 1694. [Google Scholar] [CrossRef]
- Jeffri, N.I.; Mohammad Rawi, N.F.; Mohamad Kassim, M.H.; Abdullah, C.K. Unlocking the potential: Evolving role of technical lignin in diverse applications and overcoming challenges. Int. J. Biol. Macromol. 2024, 274, 133506. [Google Scholar] [CrossRef]
- Tanis, M.H.; Wallberg, O.; Galbe, M.; Al-Rudainy, B. Lignin Extraction by Using Two-Step Fractionation: A Review. Molecules 2024, 29, 98. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.S.; Badri, K.H. Lignin recovery from alkaline hydrolysis and glycerolysis of oil palm fiber. AIP Conf. Proc. 2014, 1614, 433–438. [Google Scholar] [CrossRef]
- Sujatha, M.; Aparna, R. Extraction of Lignin from Agro-Waste Coir Fiber by Mild Alkali Treatment: A Statistical Approach for Process Optimization through Response Surface Methodology. Asian J. Chem. 2023, 36, 81–86. [Google Scholar] [CrossRef]
- D’Orsi, R.; Di Fidio, N.; Antonetti, C.; Raspolli Galletti, A.M.; Operamolla, A. Isolation of Pure Lignin and Highly Digestible Cellulose from Defatted and Steam-Exploded Cynara cardunculus. ACS Sustain. Chem. Eng. 2023, 11, 1875–1887. [Google Scholar] [CrossRef]
- Sheridan, E.; Filonenko, S.; Volikov, A.; Sirviö, J.A.; Antonietti, M. A systematic study on the processes of lignin extraction and nanodispersion to control properties and functionality. Green Chem. 2024, 26, 2967–2984. [Google Scholar] [CrossRef]
- Serrano-Martínez, V.M.; Pérez-Aguilar, H.; Carbonell-Blasco, M.P.; Arán-Ais, F.; Orgilés-Calpena, E. Steam Explosion-Based Method for the Extraction of Cellulose and Lignin from Rice Straw Waste. Appl. Sci. 2024, 14, 2059. [Google Scholar] [CrossRef]
- He, Y.-C.; Liu, F.; Gong, L.; Zhu, Z.-Z.; Ding, Y.; Wang, C.; Xue, Y.-F.; Rui, H.; Tao, Z.-C.; Zhang, D.-P.; et al. Significantly improving enzymatic saccharification of high crystallinity index’s corn stover by combining ionic liquid [Bmim]Cl–HCl–water media with dilute NaOH pretreatment. Bioresour. Technol. 2015, 189, 421–425. [Google Scholar] [CrossRef]
- Asim, A.M.; Uroos, M.; Muhammad, N. Extraction of lignin and quantitative sugar release from biomass using efficient and cost-effective pyridinium protic ionic liquids. RSC Adv. 2020, 10, 44003–44014. [Google Scholar] [CrossRef]
- Sun, Y.-C.; Liu, X.-N.; Wang, T.-T.; Xue, B.-L.; Sun, R.-C. Green Process for Extraction of Lignin by the Microwave-Assisted Ionic Liquid Approach: Toward Biomass Biorefinery and Lignin Characterization. ACS Sustain. Chem. Eng. 2019, 7, 13062–13072. [Google Scholar] [CrossRef]
- Tolesa, L.D.; Gupta, B.S.; Lee, M.-J. Treatment of Coffee Husk with Ammonium-Based Ionic Liquids: Lignin Extraction, Degradation, and Characterization. ACS Omega 2018, 3, 10866–10876. [Google Scholar] [CrossRef] [PubMed]
- Achinivu, E.C. Protic Ionic Liquids for Lignin Extraction-A Lignin Characterization Study. Int. J. Mol. Sci. 2018, 19, 428. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Oduro, I.; Yu, Z.; Cheng, C.; Cronin, D.; Sanyal, U.; Ameli, A.; Liu, W.; Zhao, B.; Zhang, J.; et al. Deep Eutectic Solvent-Extracted Lignin for Flexible Polyurethane Foam Preparation. ACS Sustain. Chem. Eng. 2025, 13, 1304–1315. [Google Scholar] [CrossRef]
- Jančíková, V.; Jablonský, M. Exploiting Deep Eutectic Solvent-like Mixtures for Fractionation Biomass, and the Mechanism Removal of Lignin: A Review. Sustainability 2024, 16, 504. [Google Scholar] [CrossRef]
- Lin, K.T.; Wang, C.; Guo, M.F.; Aprà, E.; Ma, R.; Ragauskas, A.J.; Zhang, X. Lignin with controlled structural properties by N-heterocycle-based deep eutectic solvent extraction. Proc. Natl. Acad. Sci. USA 2023, 120, e2307323120. [Google Scholar] [CrossRef] [PubMed]
- Mattonai, M.; Messina, G.S.; Nardella, F.; Ribechini, E. New Parameters to Model Microwave-Assisted Deep Eutectic Solvent Extraction of Lignin Using Analytical Pyrolysis–GC/MS. ACS Sustain. Chem. Eng. 2022, 10, 15660–15669. [Google Scholar] [CrossRef]
- Hu, M.; Yu, Y.; Li, X.; Wang, X.; Liu, Y. The dawn of aqueous deep eutectic solvents for lignin extraction. Green Chem. 2023, 25, 10235–10262. [Google Scholar] [CrossRef]
- Lobato-Rodríguez, Á.; Gullón, B.; Romaní, A.; Ferreira-Santos, P.; Garrote, G.; Del-Río, P.G. Recent advances in biorefineries based on lignin extraction using deep eutectic solvents: A review. Bioresour. Technol. 2023, 388, 129744. [Google Scholar] [CrossRef]
- D Modi, U.; Ghodadara, V.; Mitchla, S.; Lakdawala, M.; Taqa, A. Advanced Techniques in the Purification of Lignin: Challenges, Methods, and Industrial Applications—A Review. Int. J. All Res. Educ. Sci. Methods 2024, 12, 2455–6211. [Google Scholar] [CrossRef]
- Duval, A.; Vilaplana, F.; Crestini, C.; Lawoko, M. Solvent screening for the fractionation of industrial kraft lignin. Holzforschung 2015, 70, 11–20. [Google Scholar] [CrossRef]
- Nadányi, R.; Zinovyev, G.; Majerčiak, M.; Štosel, M.; Jablonský, M.; Ház, A. Optimizing Hardwood Lignin Precipitation from Kraft Black Liquor: A Study of Temperature and pH Effects. Forests 2024, 15, 1028. [Google Scholar] [CrossRef]
- Gilarranz, M.A.; Rodriguez, F.; Oliet, M.; Revenga, J.A. Acid Precipitation and Purification of Wheat Straw Lignin. Sep. Sci. Technol. 1998, 33, 1359–1377. [Google Scholar] [CrossRef]
- Weidener, D.; Holtz, A.; Klose, H.; Jupke, A.; Leitner, W.; Grande, P.M. Lignin Precipitation and Fractionation from OrganoCat Pulping to Obtain Lignin with Different Sizes and Chemical Composition. Molecules 2020, 25, 3330. [Google Scholar] [CrossRef]
- Humpert, D.; Ebrahimi, M.; Czermak, P. Membrane Technology for the Recovery of Lignin: A Review. Membranes 2016, 6, 42. [Google Scholar] [CrossRef]
- Pham, N.T.-T.; Beaufils, N.; Peydecastaing, J.; Behra, P.; Pontalier, P.-Y. Lignin Purification from Mild Alkaline Sugarcane Extract via Membrane Filtration. Clean Technol. 2024, 6, 750–766. [Google Scholar] [CrossRef]
- Choi, H.; Alherech, M.; Jang, J.H.; Woodworth, S.P.; Ramirez, K.J.; Karp, E.M.; Beckham, G.T. Counter-current chromatography for lignin monomer–monomer and monomer–oligomer separations from reductive catalytic fractionation oil. Green Chem. 2024, 26, 5900–5913. [Google Scholar] [CrossRef]
- Campana, F.; Lanari, D.; Marrocchi, A.; Vaccaro, L. 12-Green solvents for organic electronics processing. In Sustainable Strategies in Organic Electronics; Marrocchi, A., Ed.; Woodhead Publishing: Cambridge, UK, 2022; pp. 425–462. [Google Scholar]
- Ho, D.; Lee, J.; Park, S.; Park, Y.; Cho, K.; Campana, F.; Lanari, D.; Facchetti, A.; Seo, S.; Kim, C.; et al. Green solvents for organic thin-film transistor processing. J. Mater. Chem. C 2020, 8, 5786–5794. [Google Scholar] [CrossRef]
- Campana, F.; Kim, C.; Marrocchi, A.; Vaccaro, L. Green solvent-processed organic electronic devices. J. Mater. Chem. C 2020, 8, 15027–15047. [Google Scholar] [CrossRef]
- Lee, M.; Yun, S.; Ho, D.; Earmme, T.; Marrocchi, A.; Vaccaro, L.; Kim, C. Green solvent-processed complementary-like inverters based on ambipolar organic thin-film transistors. J. Ind. Eng. Chem. 2022, 105, 231–237. [Google Scholar] [CrossRef]
- Lee, Y.; Ho, D.; Valentini, F.; Earmme, T.; Marrocchi, A.; Vaccaro, L.; Kim, C. Improving the charge transport performance of solution-processed organic field-effect transistors using green solvent additives. J. Mater. Chem. C 2021, 9, 16506–16515. [Google Scholar] [CrossRef]
- Yun, S.; Kim, Y.; Lee, S.; Ho, D.; Kim, J.; Kim, H.; Marconi, O.; Marrocchi, A.; Kim, C. Brewers’ spent grain (BSG)-based green dielectric materials for low-voltage operating solution-processed organic field-effect transistors. J. Mater. Chem. C 2022, 10, 15194–15199. [Google Scholar] [CrossRef]
- Bracciale, M.P.; Kim, C.; Marrocchi, A. 1-Organic electronics: An overview of key materials, processes, and devices. In Sustainable Strategies in Organic Electronics; Marrocchi, A., Ed.; Woodhead Publishing: Cambridge, UK, 2022; pp. 3–71. [Google Scholar]
- Chandna, S.; Olivares, M.C.A.; Baranovskii, E.; Engelmann, G.; Böker, A.; Tzschucke, C.C.; Haag, R. Lignin Upconversion by Functionalization and Network Formation. Angew. Chem. Int. Ed. 2024, 63, e202313945. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Huang, M.; Zhou, X.; Luo, R.; Li, L.; Li, X. Research Status of Lignin-Based Polyurethane and Its Application in Flexible Electronics. Polymers 2024, 16, 2340. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Zhu, M.; Zhao, J.; Cai, D.; Cao, H. Valorization of lignin for renewable non-isocyanate polyurethanes: A state-of-the-art review. Mater. Today Sustain. 2023, 22, 100367. [Google Scholar] [CrossRef]
- Larsen, C.; Lundberg, P.; Tang, S.; Ràfols-Ribé, J.; Sandström, A.; Mattias Lindh, E.; Wang, J.; Edman, L. A tool for identifying green solvents for printed electronics. Nat. Commun. 2021, 12, 4510. [Google Scholar] [CrossRef]
- Huo, L.; Lu, Y.; Ding, W.-L.; Wang, Y.; Li, X.; He, H. Recent advances in the preparation, properties, and applications of lignin-based hydrogels and adhesives. Green Chem. 2025, 27, 1895–1908. [Google Scholar] [CrossRef]
- Alonzi, M.; Lanari, D.; Marrocchi, A.; Petrucci, C.; Vaccaro, L. Synthesis of polymeric semiconductors by a surface-initiated approach. RSC Adv. 2013, 3, 23909–23923. [Google Scholar] [CrossRef]
- Baloch, M.; Labidi, J. Lignin biopolymer: The material of choice for advanced lithium-based batteries. RSC Adv. 2021, 11, 23644–23653. [Google Scholar] [CrossRef]
- Ajjan, F.N.; Casado, N.; Rębiś, T.; Elfwing, A.; Solin, N.; Mecerreyes, D.; Inganäs, O. High performance PEDOT/lignin biopolymer composites for electrochemical supercapacitors. J. Mater. Chem. A 2016, 4, 1838–1847. [Google Scholar] [CrossRef]
- Xiao, R.; Yu, G.; Xu, B.B.; Wang, N.; Liu, X. Fiber Surface/Interfacial Engineering on Wearable Electronics. Small 2021, 17, 2102903. [Google Scholar] [CrossRef]
- Xu, X. Interfacial Engineering in Organic Photovoltaics: Enhancing Efficiency through Material Innovations. Highlights Sci. Eng. Technol. 2024, 106, 436–442. [Google Scholar] [CrossRef]
- Eraghi Kazzaz, A.; Fatehi, P. Interaction of synthetic and lignin-based sulfonated polymers with hydrophilic, hydrophobic, and charged self-assembled monolayers. RSC Adv. 2020, 10, 36778–36793. [Google Scholar] [CrossRef]
- Wolf, N.R.; Yuan, X.; Hassani, H.; Milos, F.; Mayer, D.; Breuer, U.; Offenhäusser, A.; Wördenweber, R. Surface Functionalization of Platinum Electrodes with APTES for Bioelectronic Applications. ACS Appl. Bio Mater. 2020, 3, 7113–7121. [Google Scholar] [CrossRef] [PubMed]
- Isozaki, K.; Shimoaka, T.; Oshiro, S.; Yamaguchi, A.; Pincella, F.; Ueno, R.; Hasegawa, T.; Watanabe, T.; Takaya, H.; Nakamura, M. Robust Surface Plasmon Resonance Chips for Repetitive and Accurate Analysis of Lignin–Peptide Interactions. ACS Omega 2018, 3, 7483–7493. [Google Scholar] [CrossRef]
- Milczarek, G.; Inganäs, O. Renewable Cathode Materials from Biopolymer/Conjugated Polymer Interpenetrating Networks. Science 2012, 335, 1468–1471. [Google Scholar] [CrossRef]
- Badri, M.A.S.; Noor, N.F.A.M.; Zain, A.R.M.; MatSalleh, M.; Aziz, T.H.T.A. Exfoliated graphene-alkaline lignin-PEDOT: PSS composite as a transparent conductive electrode. Nanomater. Nanotechnol. 2021, 11, 18479804211015009. [Google Scholar] [CrossRef]
- Tian, X.; Zhao, T.; Li, J.; Li, T.; Yuan, L.; Xue, X.; Wang, Z.; Zhang, J. Coplanar-Gate Synaptic Transistor Array With Organic Electrolyte Using Lithographic Process. IEEE Trans. Electron Devices 2022, 69, 2325–2330. [Google Scholar] [CrossRef]
- Zhu, L.Q.; Wan, C.J.; Guo, L.Q.; Shi, Y.; Wan, Q. Artificial synapse network on inorganic proton conductor for neuromorphic systems. Nat. Commun. 2014, 5, 3158. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.Y.; Cheng, L.; Shi, W.; Lei, Y.; Wen, S.; Wang, F.; Jiang, J.; Wen, P.; Zhang, J. Synaptic Transistor Arrays Based on PVA/Lignin Composite Electrolyte Films. IEEE Trans. Electron Devices 2023, 70, 3245–3250. [Google Scholar] [CrossRef]
- Antony Jose, S.; Cowan, N.; Davidson, M.; Godina, G.; Smith, I.; Xin, J.; Menezes, P.L. A Comprehensive Review on Cellulose Nanofibers, Nanomaterials, and Composites: Manufacturing, Properties, and Applications. Nanomaterials 2025, 15, 356. [Google Scholar] [CrossRef]
- Jiang, B.; Chaoji, C.; Liang, Z.; He, S.; Kuang, Y.; Song, J.; Mi, R.; Chen, G.; Miaolun, J.; Hu, L. Lignin as a Wood-Inspired Binder Enabled Strong, Water Stable, and Biodegradable Paper for Plastic Replacement. Adv. Funct. Mater. 2019, 30, 1906307. [Google Scholar] [CrossRef]
- Singha, N.R.; Deb, M.; Chattopadhyay, P.K. 6-Chitin and chitosan-based blends and composites. In Biodegradable Polymers, Blends and Composites; Mavinkere Rangappa, S., Parameswaranpillai, J., Siengchin, S., Ramesh, M., Eds.; Woodhead Publishing: Cambridge, UK, 2022; pp. 123–203. [Google Scholar]
- Irimia-Vladu, M.; Sariciftci, N.S. Natural polymers for emerging technological applications: Cellulose, lignin, shellac and silk. Polym. Int. 2025, 74, 71–86. [Google Scholar] [CrossRef]
- Yuan, L.; Zhao, T.; Zhang, H.; Liu, H.; Zong, Y.; Ding, X.; Zhang, J. An Artificial Tactile Perception System with Spatio-Temporal Recognition Capability. Adv. Mater. Technol. 2024, 9, 2400338. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Zhu, S.; Xu, L.; Jia, C.; Dai, J.; Song, J.; Yao, Y.; Wang, Y.; Li, Y.; et al. Anisotropic, Transparent Films with Aligned Cellulose Nanofibers. Adv. Mater. 2017, 29, 1606284. [Google Scholar] [CrossRef]
- Fu, Q.; Chen, Y.; Sorieul, M. Wood-Based Flexible Electronics. ACS Nano 2020, 14, 3528–3538. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Wang, C.; Jiang, W.; Yoo, C.G.; Ji, X.; Yang, G.; Chen, J.; Lyu, G. Lignin-silver triggered multifunctional conductive hydrogels for skinlike sensor applications. Int. J. Biol. Macromol. 2022, 221, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Stapf, M.; Komenko, V.; Nong, J.P.; Adam, J.; Selbmann, F.; Kravchenko, A.; Bremer, M.; Fischer, S.; Knobloch, K.; Joseph, Y. Lignin Hydrogels as a Use Case for a New Miniaturized Chemical Sensing Platform Based on Suspended Gate Field Effect Transistors. Adv. Sens. Res. 2024, 3, 2400040. [Google Scholar] [CrossRef]
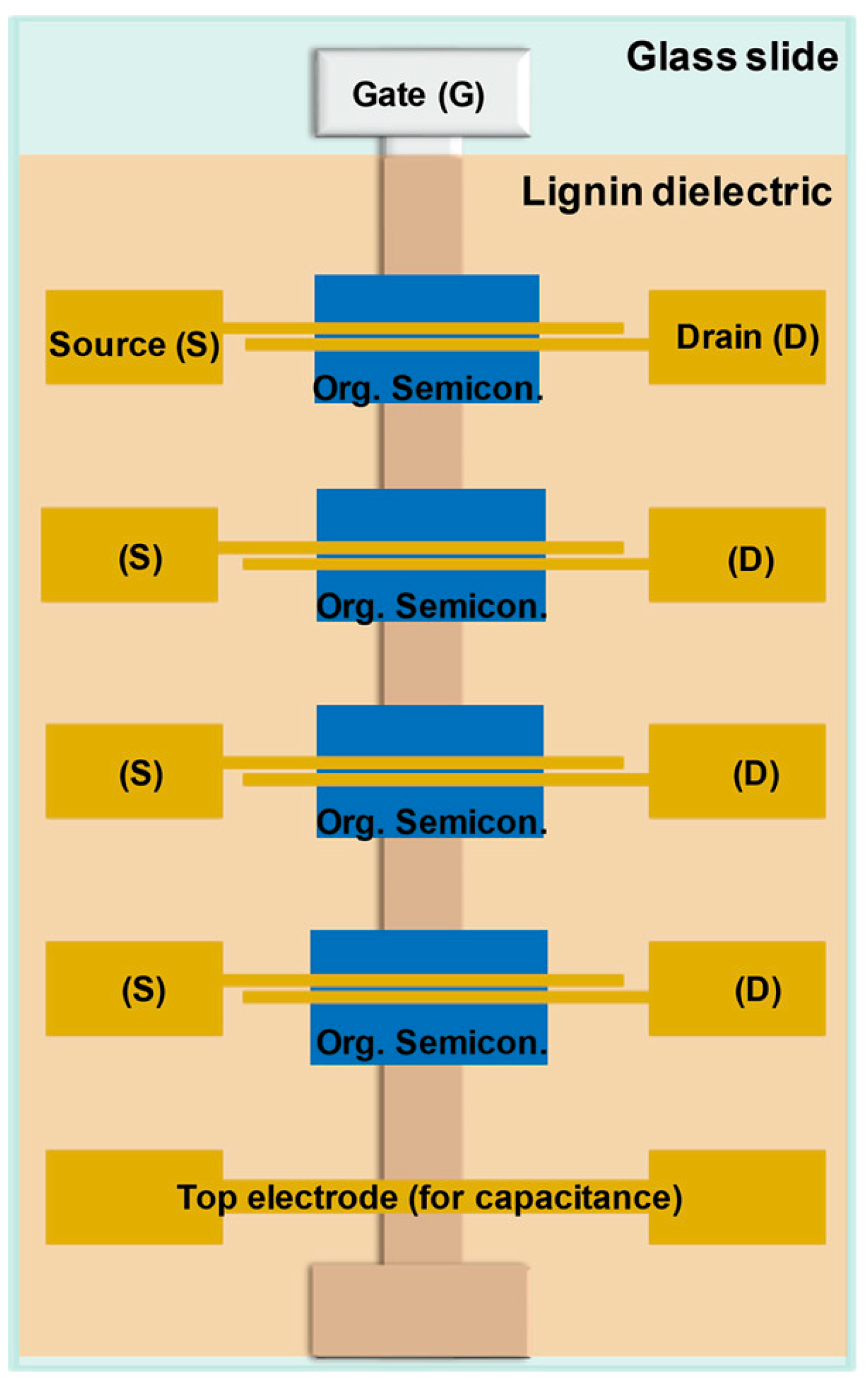
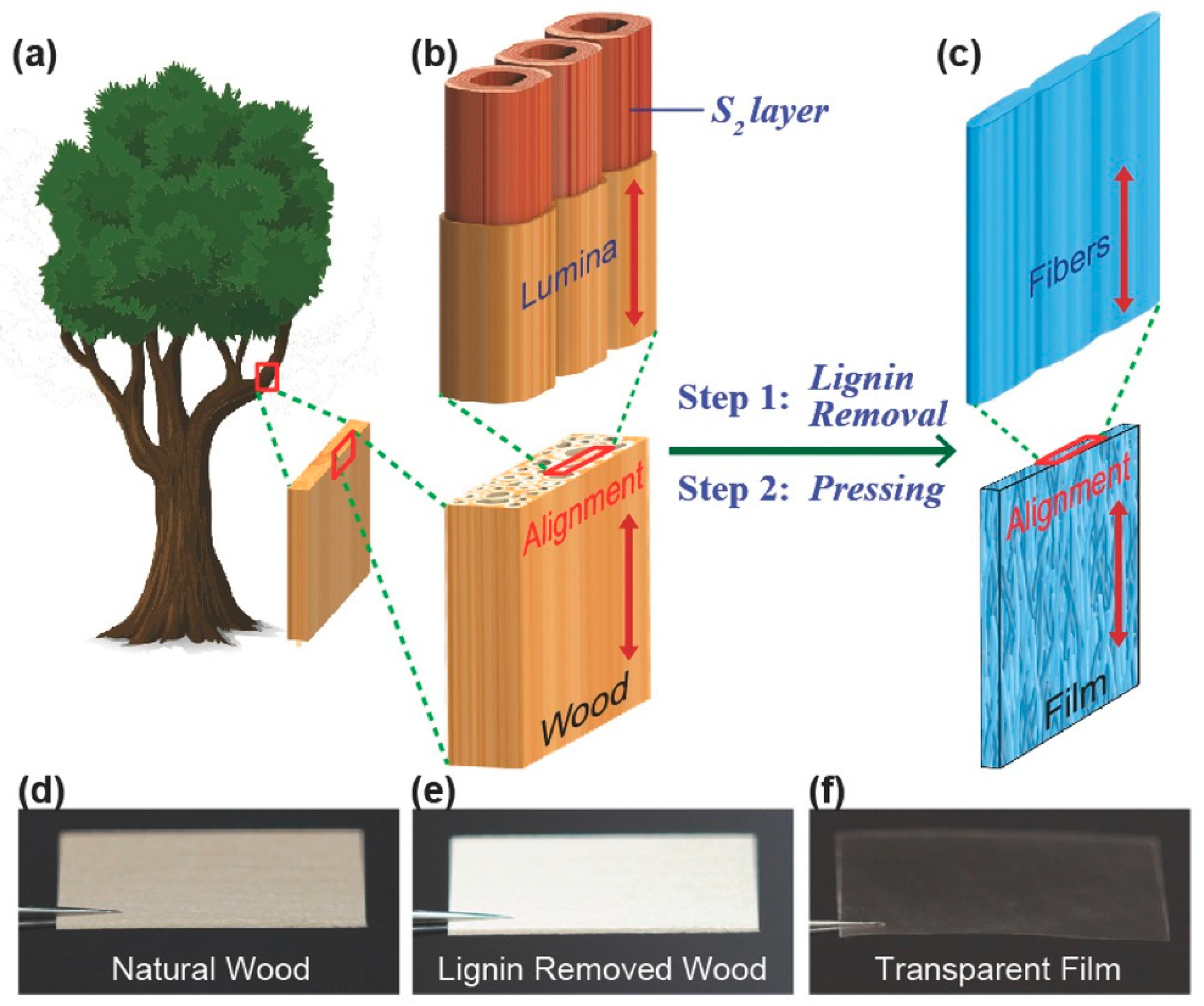
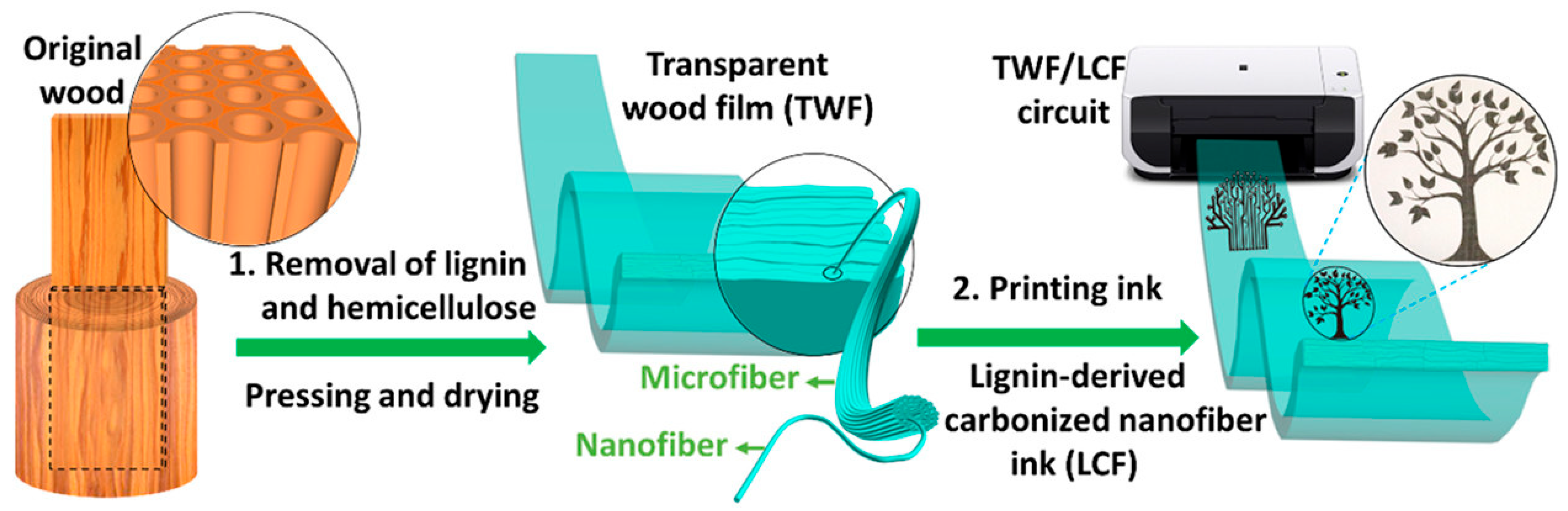
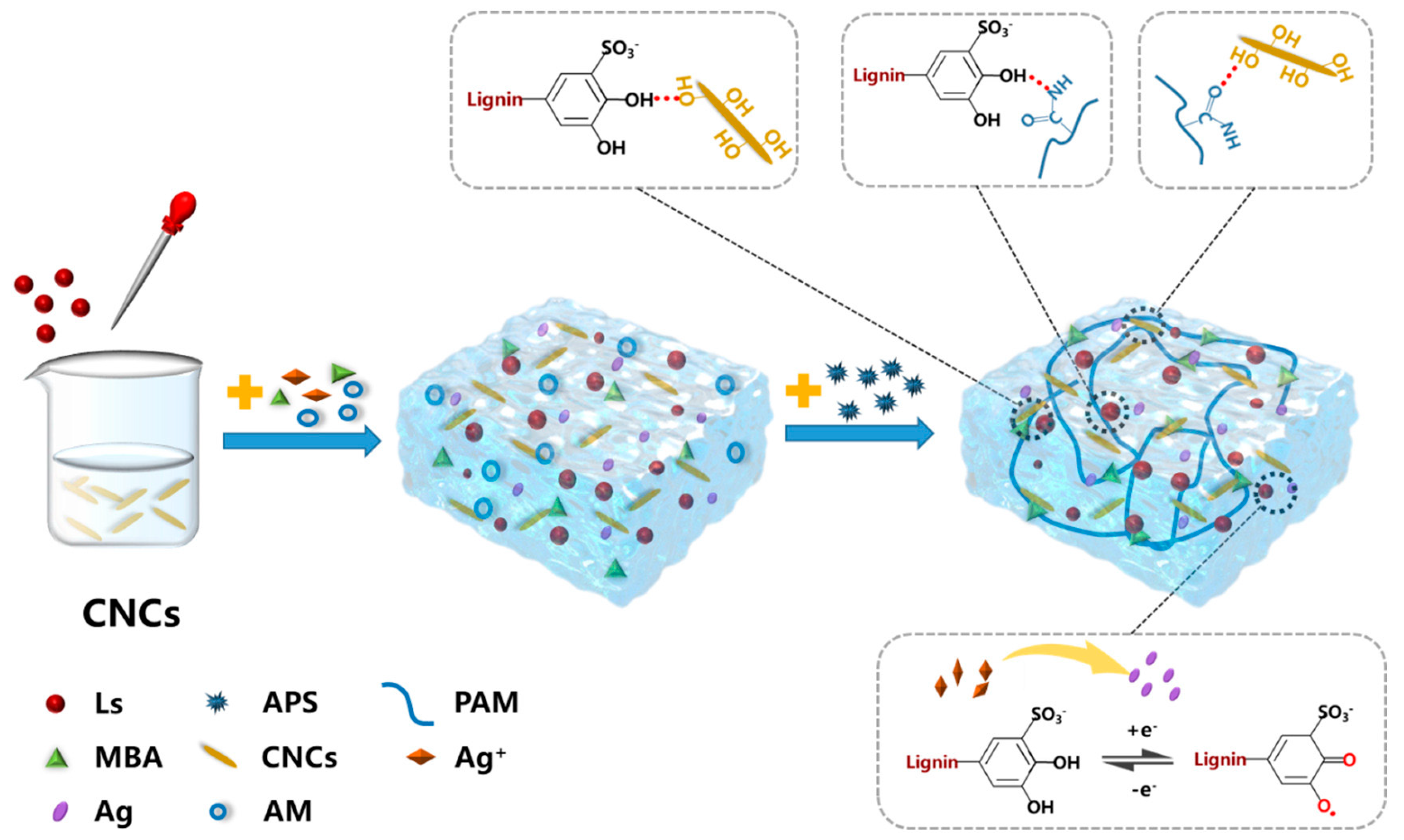

| Semiconductor | Dielectric | Mobility (cm2 V−1 s−1) |
|---|---|---|
| C60 | L1 on Al2O3 | 7 × 10−2 |
| C60 | L1 | 5 × 10−3 |
| C60 | L2 on Al2O3 | 8 × 10−3 cm2 |
| C60 | L2 | 1.5 × 10−2 |
| Pentacene | L1 on Al2O3 | 2 × 10−2 |
| Pentacene | L1 | 4 × 10−3 |
| Pentacene | L2 on Al2O3 | 7 × 10−3 |
| Pentacene | L2 | 3.2 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tronci, L.; Marrocchi, A. Lignin-Based Thin Films in Emerging Organic Transistor Devices: Challenges, Strategies, and Applications. Coatings 2025, 15, 642. https://doi.org/10.3390/coatings15060642
Tronci L, Marrocchi A. Lignin-Based Thin Films in Emerging Organic Transistor Devices: Challenges, Strategies, and Applications. Coatings. 2025; 15(6):642. https://doi.org/10.3390/coatings15060642
Chicago/Turabian StyleTronci, Laura, and Assunta Marrocchi. 2025. "Lignin-Based Thin Films in Emerging Organic Transistor Devices: Challenges, Strategies, and Applications" Coatings 15, no. 6: 642. https://doi.org/10.3390/coatings15060642
APA StyleTronci, L., & Marrocchi, A. (2025). Lignin-Based Thin Films in Emerging Organic Transistor Devices: Challenges, Strategies, and Applications. Coatings, 15(6), 642. https://doi.org/10.3390/coatings15060642








