Mechanism and Pathway of Atrazine Degradation by Peroxymonosulfate Activated by CoNiFe-Layered Double Hydroxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
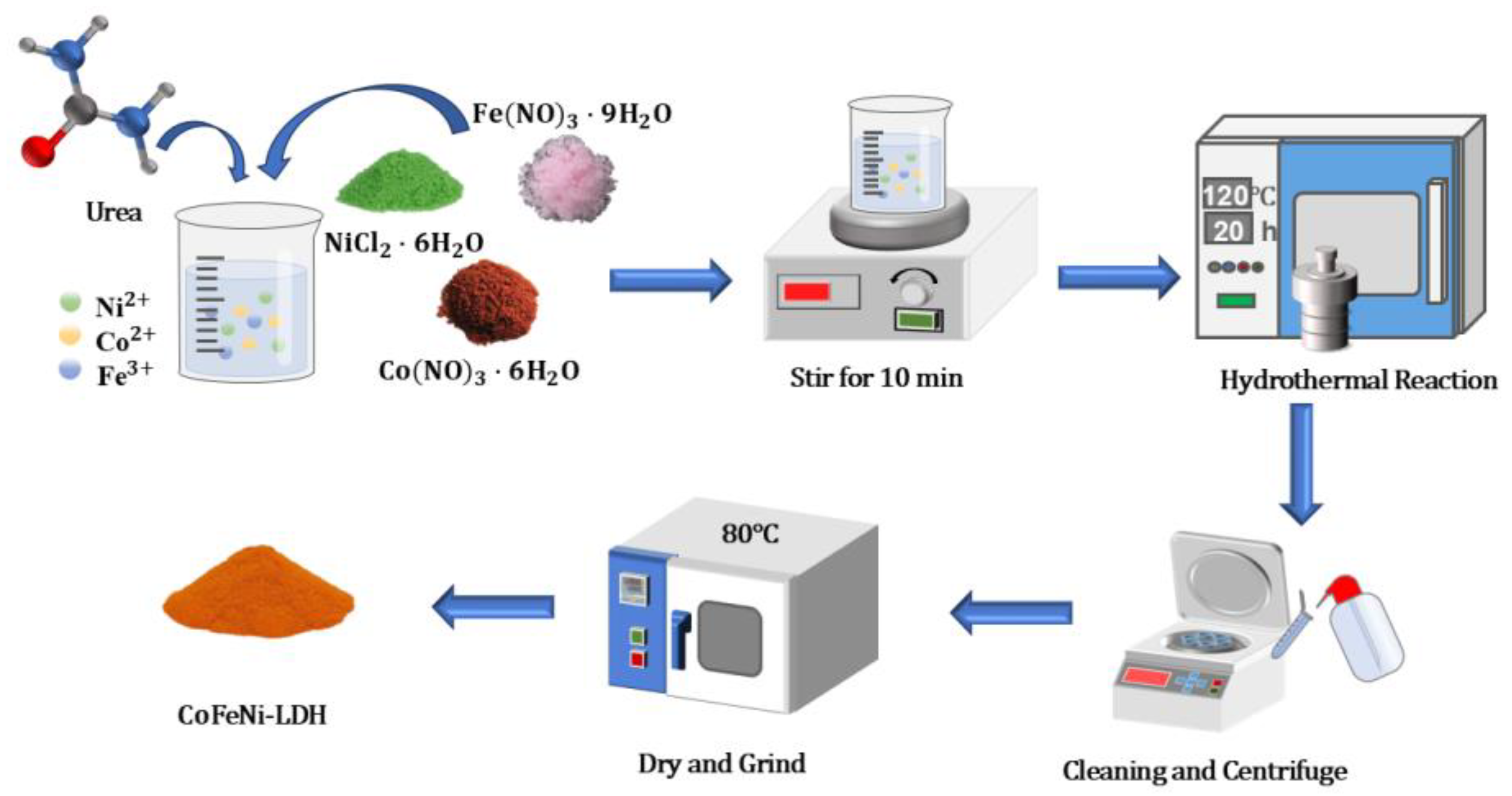
2.2. Preparation
2.3. Characterization
3. Results
3.1. Characterization of CoNiFe-LDH Activator
3.1.1. SEM
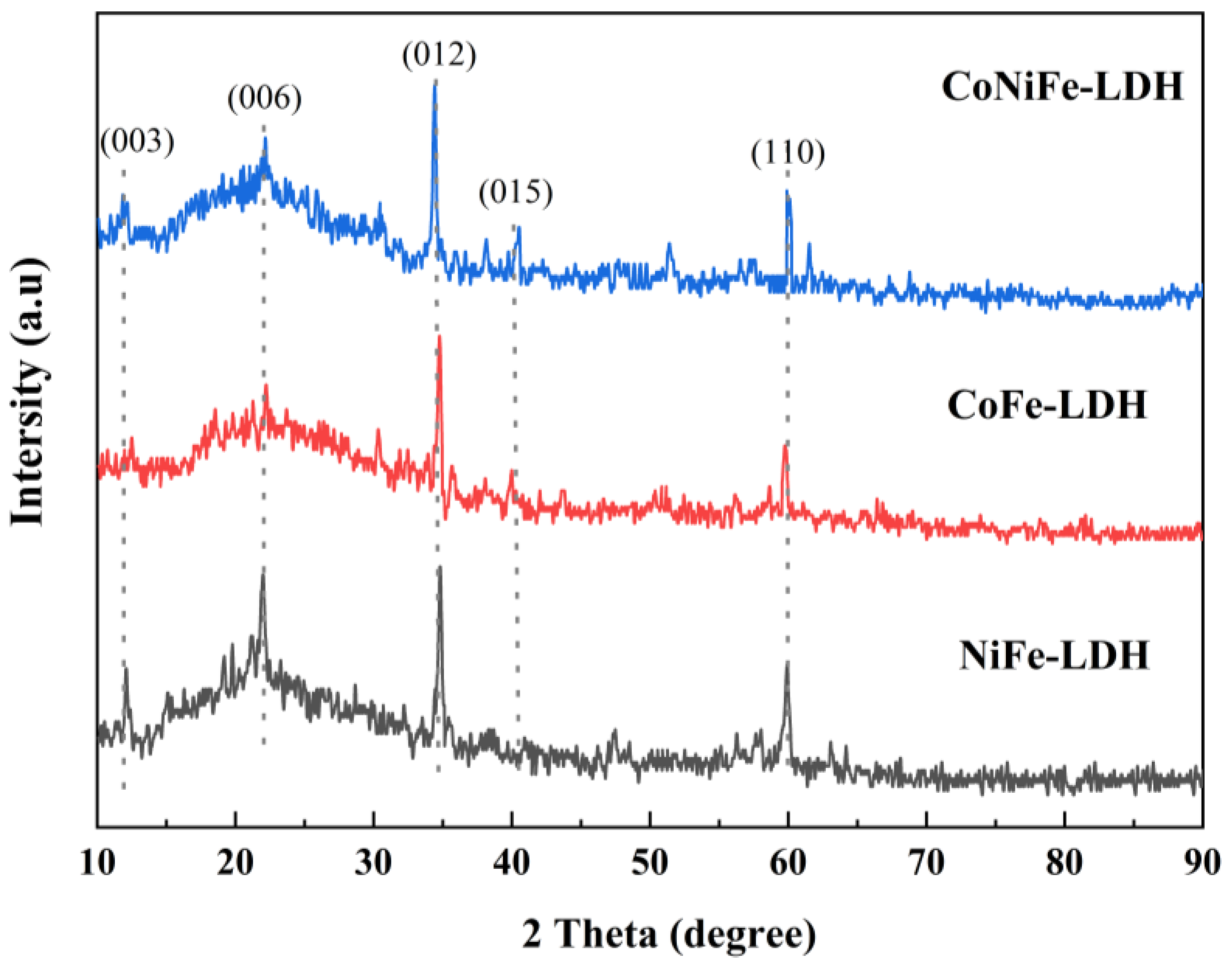
3.1.2. XRD
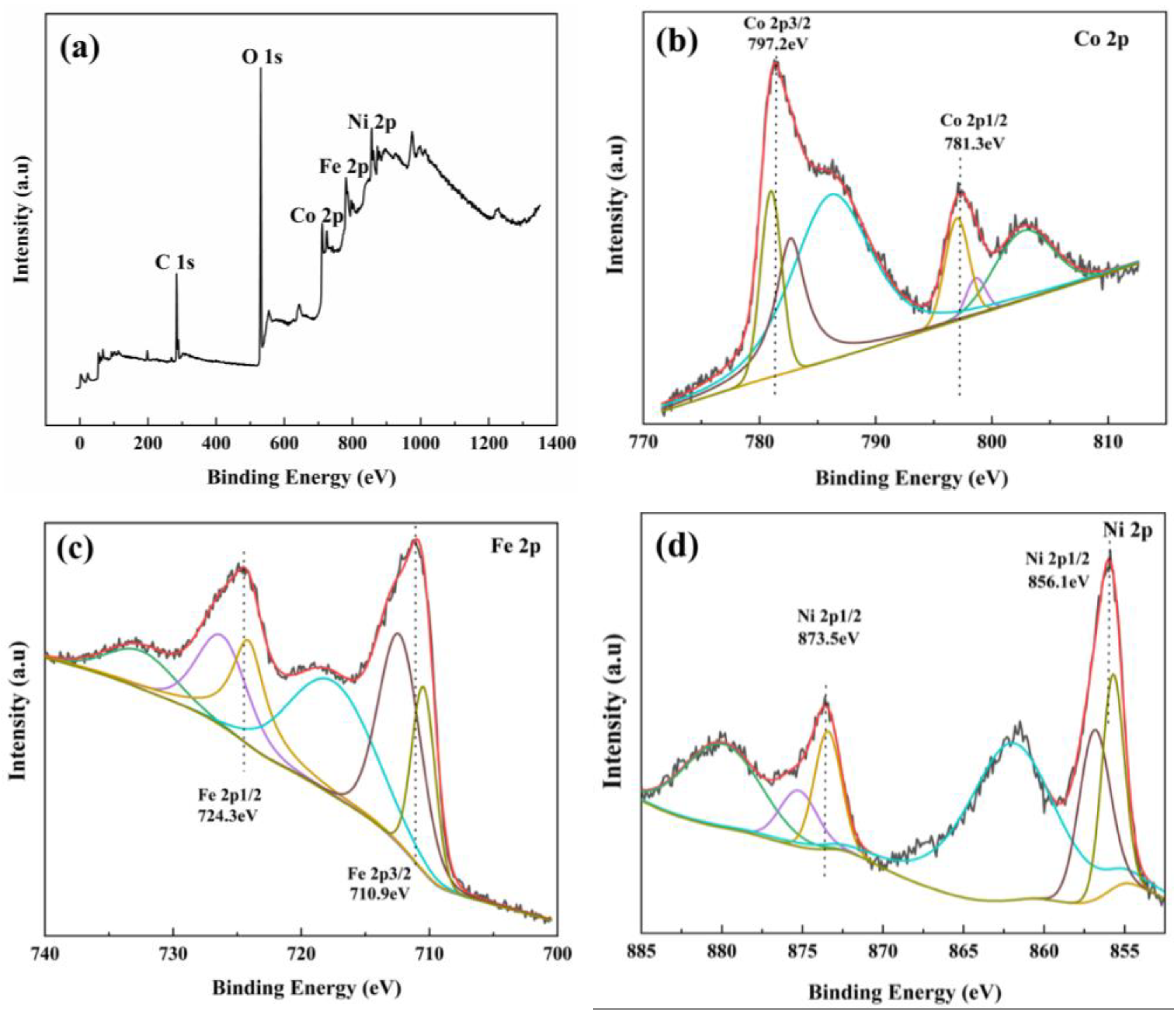
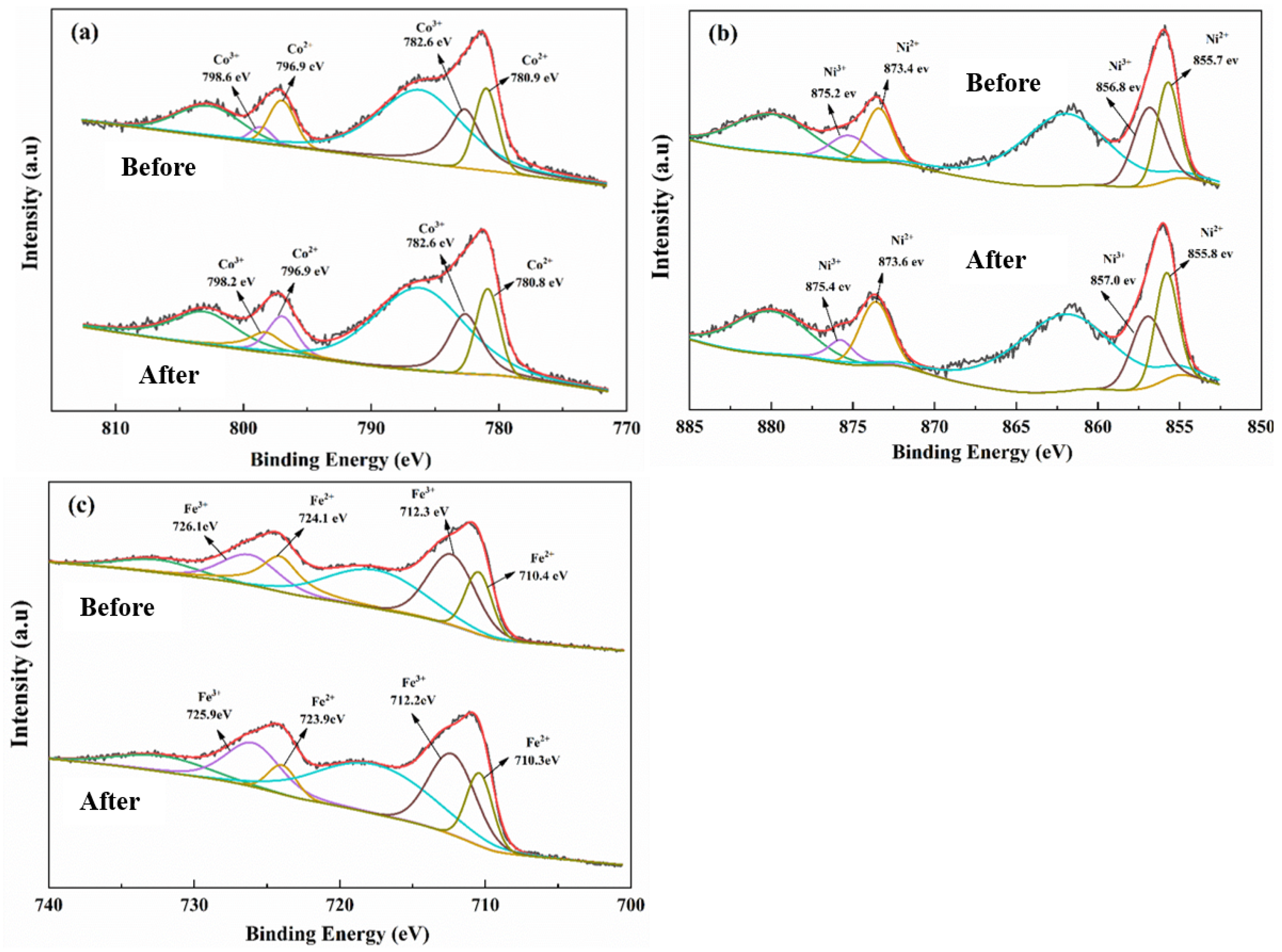
3.1.3. XPS
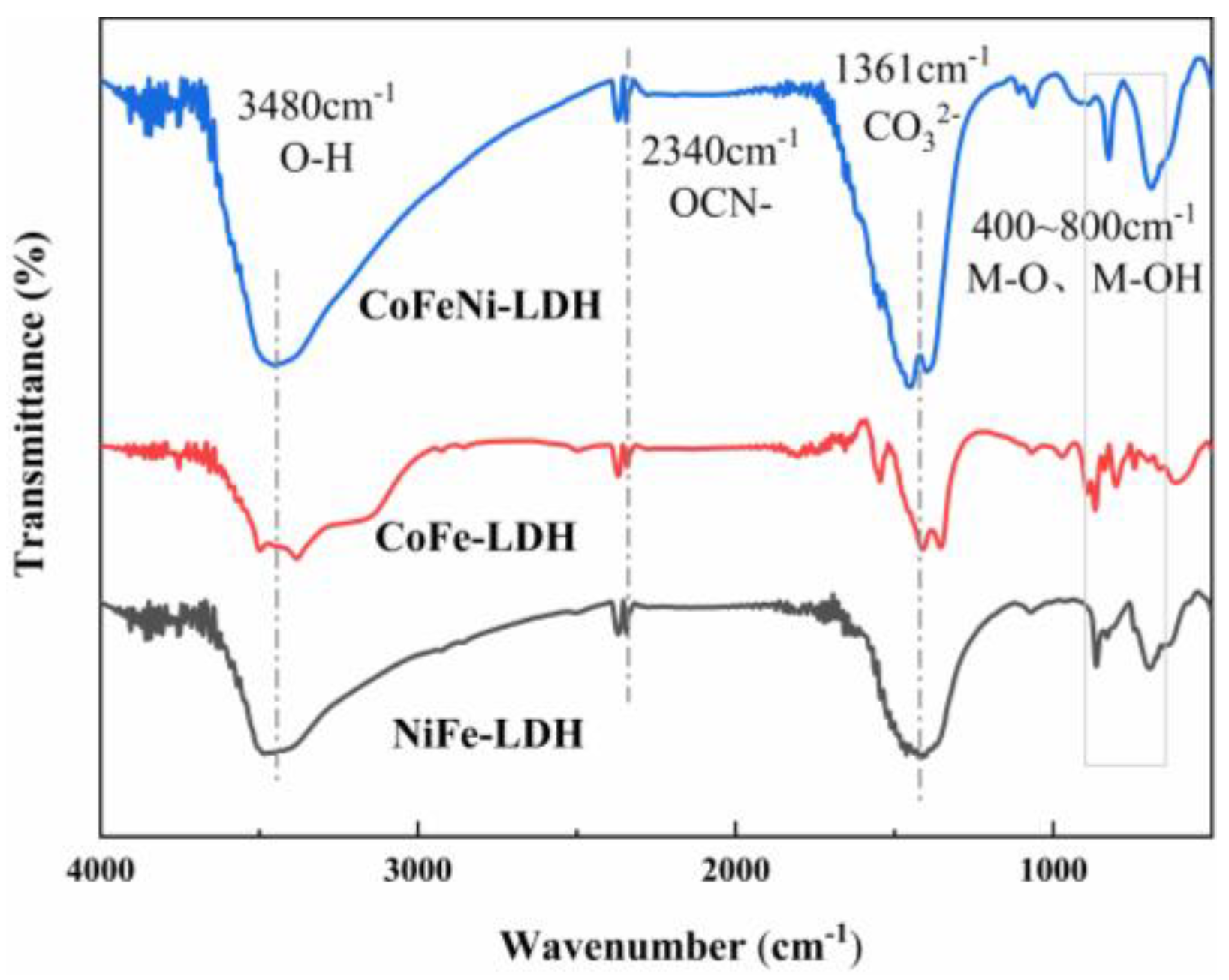
3.1.4. FT-IR
3.1.5. BET
3.2. CoNiFe-LDH Activation Performance
3.2.1. ATZ Degradation in PMS/CoNiFe-LDH System
3.2.2. Effect of Inorganic Anions and Humic Acid
3.3. Structural Stability and Reusability
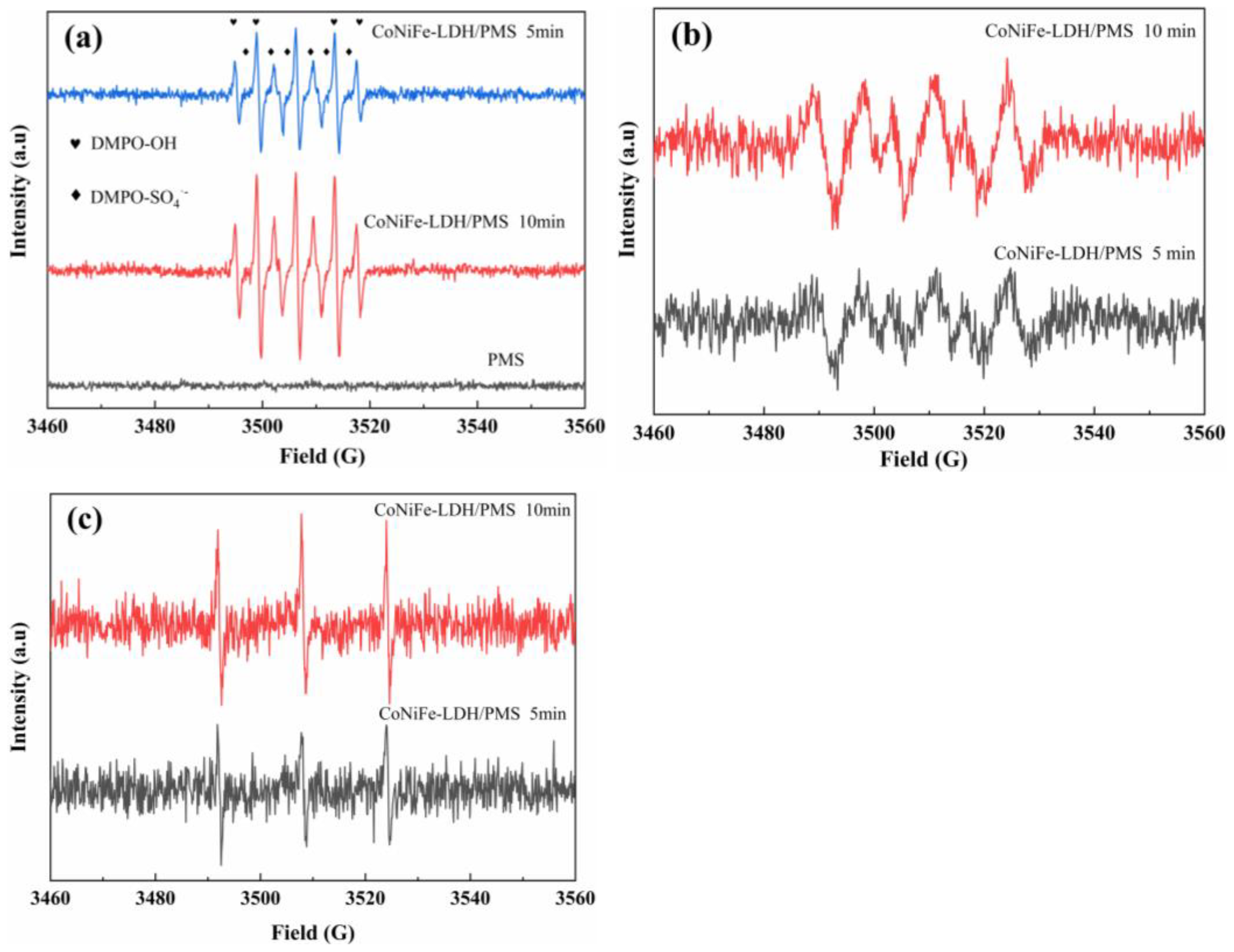
3.4. Free Radical Types
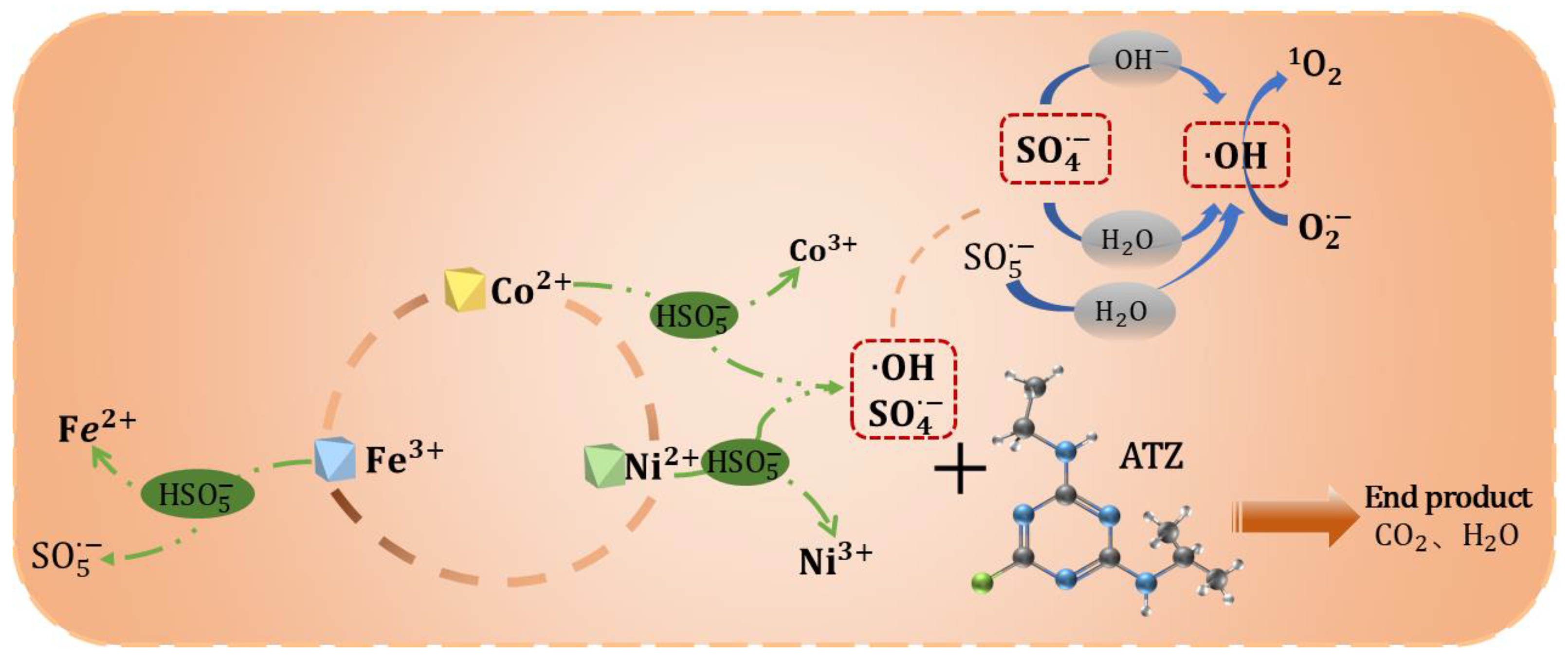
3.5. Mechanisms
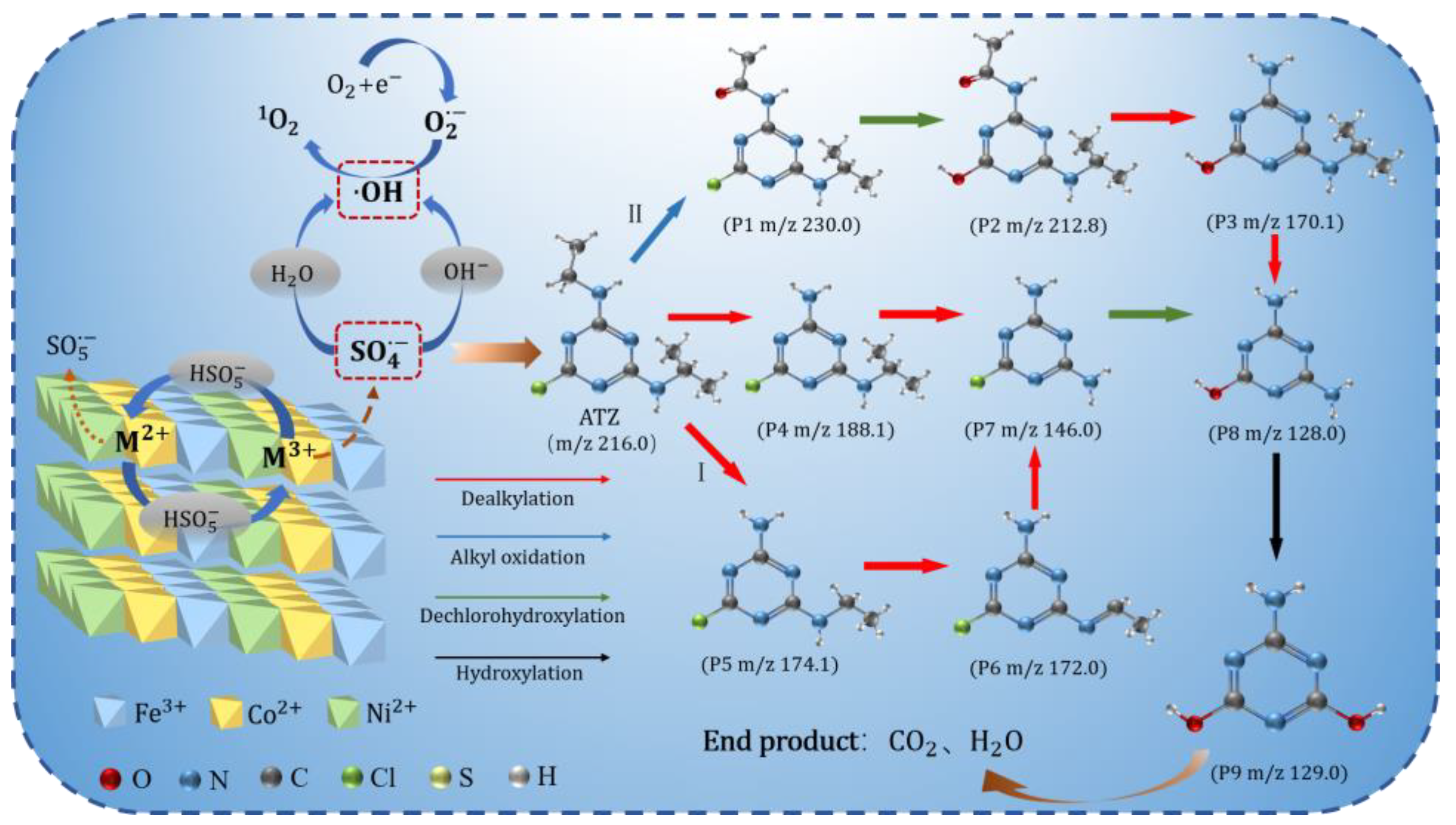
3.6. Pathways
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belver, C.; Han, C.; Rodriguez, J.J.; Dionysiou, D.D. Innovative W-doped titanium dioxide anchored on clay for photocatalytic removal of atrazine. Catal. Today 2017, 280, 21–28. [Google Scholar] [CrossRef]
- Gao, T.; Tian, H.; Wang, Z.; Shi, J.; Yang, R.; Wang, F.; Xiang, L.; Dai, Y.; Megharaj, M. Effects of atrazine on microbial metabolic limitations in black soils: Evidence from enzyme stoichiometry. Chemosphere 2023, 334, 139045. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Li, Y.; Cao, M.; Guo, Y.; Qiu, G.; Tu, S.; Xiong, S.; Fang, D. Amino acid promoted oxidation of atrazine by Fe3O4/persulfate. Heliyon 2024, 10, e23371. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.A.; He, X.; Shah, N.S.; Khan, H.M.; Hapeshi, E.; Fatta-Kassinos, D.; Dionysiou, D.D. Kinetic and mechanism investigation on the photochemical degradation of atrazine with activated H2O2, S2O82− and HSO5−. Chem. Eng. J. 2014, 252, 393–403. [Google Scholar] [CrossRef]
- Arlos, M.J.; Bragg, L.M.; Parker, W.J.; Servos, M.R. Distribution of selected antiandrogens and pharmaceuticals in a highly impacted watershed. Water Res. 2015, 72, 40–50. [Google Scholar] [CrossRef]
- Vizioli, B.D.C.; da Silva, G.S.; de Medeiros, J.F.; Montagner, C.C. Atrazine and its degradation products in drinking water source and supply: Risk assessment for environmental and human health in Campinas, Brazil. Chemosphere 2023, 336, 139289. [Google Scholar] [CrossRef]
- Bachetti, R.A.; Urseler, N.; Morgante, V.; Damilano, G.; Porporatto, C.; Agostini, E.; Morgante, C. Monitoring of atrazine pollution and its spatial-seasonal variation on surface water sources of an agricultural river basin. Bull. Environ. Contam. Toxicol. 2021, 106, 929–935. [Google Scholar] [CrossRef]
- Centanni, M.; Ricci, G.F.; De Girolamo, A.M.; Romano, G.; Gentile, F. A review of modeling pesticides in freshwaters: Current status, progress achieved and desirable improvements. Environ. Pollut. 2023, 316, 120553. [Google Scholar] [CrossRef]
- Silveyra, G.R.; Canosa, I.S.; Rodriguez, E.M.; Medesani, D.A. Effects of atrazine on ovarian growth, in the estuarine crab. Neohelice granulata. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 192, 1–6. [Google Scholar] [CrossRef]
- Wang, C.; Liu, B.; Chen, J.; Hao, X.; Yang, Q. Self-cycling of Fe (II)/Fe (III) and the important role of Fe (IV) in promoting efficient degradation of atrazine (ATZ) in a wider pH range. Chem. Eng. Res. Des. 2024, 202, 60–73. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, J.; Li, J.; Cao, B.; Chen, Y.; Liu, D.; Wang, X.; Zhang, Y. Exogenous Zn2+ enhance the biodegradation of atrazine by regulating the chlorohydrolase gene trzN transcription and membrane permeability of the degrader Arthrobacter sp. DNS10. Chemosphere 2020, 238, 124594. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Deng, S.; Wang, L.; Hu, Y.; Cao, B.; Lv, J.; Qu, J.; Wang, L.; Wang, Y.; Zhang, Y. Nicosulfuron inhibits atrazine biodegradation by Arthrobacter sp. DNS10: Influencing mechanisms insight from bacteria viability, gene transcription and reactive oxygen species production. Environ. Pollut. 2021, 273, 116517. [Google Scholar] [CrossRef]
- Alattas, S.G.; Zabermawi, N.M.; El Bestawy, E. Biodegradation of atrazine using selected marine bacteria: Possibilities for treating pesticide-contaminated wastewater. J. King Saud Univ.-Sci. 2023, 35, 102721. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, B.; Zhao, L.; Sun, L.; Gao, Y.; Li, J.; Yang, F. Biochar-supported reduced graphene oxide composite for adsorption and coadsorption of atrazine and lead ions. Appl. Surf. Sci. 2018, 427, 147–155. [Google Scholar] [CrossRef]
- Do Nascimento, C.T.; Vieira, M.G.A.; Scheufele, F.B.; Palú, F.; da Silv, E.A.; Borba, C.E. Adsorption of atrazine from aqueous systems on chemically activated biochar produced from corn straw. J. Environ. Chem. Eng. 2022, 10, 107039. [Google Scholar] [CrossRef]
- Chandra, P.N.; Usha, K. Removal of atrazine herbicide from water by polyelectrolyte multilayer membranes. Mater. Today Proc. 2021, 41, 622–627. [Google Scholar] [CrossRef]
- Cai, Y.L.; Xu, Y.H.; Xiang, J.Z.; Zhang, Z.; He, Q.; Li, Y.; Lü, J. Iron-doped bismuth oxybromides as visible-light-responsive Fenton catalysts for the degradation of atrazine in aqueous phases. J. Environ. Sci. 2024, 137, 321–332. [Google Scholar] [CrossRef]
- Pan, J. Atrazine degradation in the ultraviolet (UV) irradiation/hydrogen peroxide (H2O2) treatment. Adv. Mater. Res. 2013, 726, 1823–1826. [Google Scholar] [CrossRef]
- Ding, X.; Wang, S.; Shen, W.; Mu, Y.; Wang, L.; Chen, H.; Zhang, L. Fe@ Fe2O3 promoted electrochemical mineralization of atrazine via a triazinon ring opening mechanism. Water Res. 2017, 112, 9–18. [Google Scholar] [CrossRef]
- Dong, G.; Chen, B.; Liu, B.; Hounjet, L.J.; Cao, Y.; Stoyanov, S.R.; Yang, M.; Zhang, B. Advanced oxidation processes in microreactors for water and wastewater treatment: Development, challenges, and opportunities. Water Res. 2022, 211, 118047. [Google Scholar] [CrossRef]
- Buthiyappan, A.; Abdul Aziz, A.R.; Wan Daud, W.M.A. Recent advances and prospects of catalytic advanced oxidation process in treating textile effluents. Rev. Chem. Eng. 2016, 32, 1–47. [Google Scholar] [CrossRef]
- Rasool, R.T.; Ashraf, G.A.; Fadhali, M.M.; Al-Sulaimi, S.; Ghernaout, D.; El Jery, A.; Aldrdery, M.; Elkhaleefa, A.; Hassan, N.; Ajmal, Z.; et al. Peroxymonosulfate-based photodegradation of naproxen by stimulating (Mo, V, and Zr)-carbide nanoparticles. J. Water Process Eng. 2023, 54, 104027. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Quan, X.; Zhang, J.; Chen, S.; Afzal, S. Enhanced anaerobic fermentation with azo dye as electron acceptor: Simultaneous acceleration of organics decomposition and azo decolorization. J. Environ. Sci. 2014, 26, 1970–1976. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Khosla, A.; Sharma, S.K.; Dhiman, P.; Sharma, G.; Gnanasekaran, L.; Naushad, M.; Stadler, F.J. A review on S-scheme and dual S-scheme heterojunctions for photocatalytic hydrogen evolution, water detoxification and CO2 reduction. Fuel 2023, 333, 126267. [Google Scholar] [CrossRef]
- Derbalah, A.; Sakugawa, H. Sulfate radical-based advanced oxidation technology to remove pesticides from water a review of the most recent technologies. Int. J. Environ. Res. 2024, 18, 11. [Google Scholar] [CrossRef]
- Chen, J.; Xu, J.; Liu, T.; Qian, Y.; Zhou, X.; Xiao, S.; Zhang, Y. Selective oxidation of tetracyclines by peroxymonosulfate in livestock wastewater: Kinetics and non-radical mechanism. J. Hazard. Mater. 2020, 386, 121656. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.; Wang, Y.; Le Roux, J.; Yang, Y.; Croué, J.P. Efficient peroxydisulfate activation process not relying on sulfate radical generation for water pollutant degradation. Environ. Sci. Technol. 2014, 48, 5868–5875. [Google Scholar] [CrossRef]
- Zhu, S.; Li, X.; Kang, J.; Duan, X.; Wang, S. Persulfate activation on crystallographic manganese oxides: Mechanism of singlet oxygen evolution for nonradical selective degradation of aqueous contaminants. Environ. Sci. Technol. 2018, 53, 307–315. [Google Scholar] [CrossRef]
- Guan, Y.H.; Ma, J.; Ren, Y.M.; Liu, Y.L.; Xiao, J.Y.; Lin, L.Q.; Zhang, C. Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals. Water Res. 2013, 47, 5431–5438. [Google Scholar] [CrossRef]
- Xie, Z.H.; Zhou, H.Y.; He, C.S.; Pan, Z.C.; Yao, G.; Lai, B. Synthesis, application and catalytic performance of layered double hydroxide based catalysts in advanced oxidation processes for wastewater decontamination: A review. Chem. Eng. J. 2021, 414, 128713. [Google Scholar] [CrossRef]
- Tang, X.; Tang, R.; Xiong, S.; Zheng, J.; Li, L.; Zhou, Z.; Gong, D.; Deng, Y.; Su, L.; Liao, C. Application of natural minerals in photocatalytic degradation of organic pollutants: A review. Sci. Total Environ. 2022, 812, 152434. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Chen, Y.; Qian, L.; Gao, W.; Ouyang, D.; Chen, M. Heterogeneously catalyzed persulfate with a CuMgFe layered double hydroxide for the degradation of ethylbenzene. J. Hazard. Mater. 2017, 338, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Farhan, A.; Khalid, A.; Maqsood, N.; Iftekhar, S.; Sharif, H.M.A.; Qi, F.; Sillanpää, M.A.; Asif, M.B. Progress in layered double hydroxides (LDHs): Synthesis and application in adsorption, catalysis and photoreduction. Sci. Total Environ. 2023, 912, 169160. [Google Scholar] [CrossRef] [PubMed]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, C.; Luo, M.; Wang, Q.; Wang, J.; Liao, Z.; Chen, Z.; Chen, Z. Understanding the synergetic effect from foreign metals in bimetallic oxides for PMS activation: A common strategy to increase the stoichiometric efficiency of oxidants. Chem. Eng. J. 2020, 381, 122587. [Google Scholar] [CrossRef]
- Liu, L.; Deng, Q.; White, P.; Dong, S.; Cole, I.S.; Dong, J.; Chen, X.B. Hydrothermally prepared layered double hydroxide coatings for corrosion protection of Mg alloys—A critical review. Corros. Commun. 2022, 8, 40–48. [Google Scholar] [CrossRef]
- Wang, J.; Ding, Q.; Bai, C.; Wang, F.; Sun, S.; Xu, Y.; Li, H. Synthesis of CNTs/CoNiFe-LDH nanocomposite with high specific surface area for asymmetric supercapacitor. Nanomaterials 2021, 11, 2155. [Google Scholar] [CrossRef]
- Zeng, H.; Zhang, W.; Deng, L.; Luo, J.; Zhou, S.; Liu, X.; Pei, Y.; Shi, Z.; Crittenden, J. Degradation of dyes by peroxymonosulfate activated by ternary CoFeNi-layered double hydroxide: Catalytic performance, mechanism and kinetic modeling. J. Colloid Interface Sci. 2018, 515, 92–100. [Google Scholar] [CrossRef]
- Liao, F.; Yang, G.; Cheng, Q.; Mao, L.; Zhao, X.; Chen, L. Rational design and facile synthesis of Ni-Co-Fe ternary LDH porous sheets for high-performance aqueous asymmetric supercapacitor. Electrochim. Acta 2022, 428, 140939. [Google Scholar] [CrossRef]
- Deng, L.; Shi, Z.; Peng, X.; Zhou, S. Magnetic calcinated cobalt ferrite/magnesium aluminum hydrotalcite composite for enhanced adsorption of methyl orange. J. Alloys Compd. 2016, 688, 101–112. [Google Scholar] [CrossRef]
- Yao, J.; Xu, D.; Ma, X.; Xiao, J.; Zhang, M.; Gao, H. Trimetallic CoNiFe-layered double hydroxides: Electronic coupling effect and oxygen vacancy for boosting water splitting. J. Power Sources 2022, 524, 231068. [Google Scholar] [CrossRef]
- Li, J.; Shu, C.; Ran, Z.; Li, M.; Zheng, R.; Long, J. Heteroatom-induced electronic structure modulation of vertically oriented oxygen vacancy-rich NiFe layered double oxide nanoflakes to boost bifunctional catalytic activity in Li–O2 battery. ACS Appl. Mater. Interfaces 2019, 11, 29868–29878. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zuo, X.; Yang, S.; Cai, T.; Ding, D. Rational design and synthesis of hollow Co3O4@ Fe2O3 core-shell nanostructure for the catalytic degradation of norfloxacin by coupling with peroxymonosulfate. Chem. Eng. J. 2019, 359, 373–384. [Google Scholar] [CrossRef]
- Lu, H.; Sui, M.; Yuan, B.; Wang, J.; Lv, Y. Efficient degradation of nitrobenzene by Cu-Co-Fe-LDH catalyzed peroxymonosulfate to produce hydroxyl radicals. Chem. Eng. J. 2019, 357, 140–149. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Wang, X.G.; Xie, Z.; Zhou, Z. NiFe2O4–CNT composite: An efficient electrocatalyst for oxygen evolution reactions in Li–O2 batteries guided by computations. J. Mater. Chem. A 2016, 4, 9390–9393. [Google Scholar] [CrossRef]
- Li, X.; Xiong, S.; Tang, X.; Tang, R.; Deng, Y.; Gong, D. Facile synthesis of CoFeAl-layered double hydroxides activated by peroxymonosulfate for enhanced ciprofloxacin degradation. J. Environ. Chem. Eng. 2024, 12, 112483. [Google Scholar] [CrossRef]
- Sun, D.; Li, C.; Lu, S.; Yang, Q.; He, C. Magnetic Fe3O4@CoFe-LDH nanocomposite heterogeneously activated peroxymonosulfate for degradation of azo-dye AO7. RSC Adv. 2021, 11, 20258–20267. [Google Scholar] [CrossRef]
- Oliva, M.Á.; Giraldo, D.; Almodóvar, P.; Martín, F.; López, M.L.; Pavlovic, I.; Sánchez, L. Designing a NiFe-LDH/MnO2 heterojunction to improve the photocatalytic activity for NOx removal under visible light. Chem. Eng. J. 2024, 489, 151241. [Google Scholar] [CrossRef]
- Ge, L.; Shao, B.; Liang, Q.; Huang, D.; Liu, Z.; He, Q.; Wu, T.; Luo, S.; Pan, Y.; Zhao, C.; et al. Layered double hydroxide based materials applied in persulfate based advanced oxidation processes: Property, mechanism, application and perspectives. J. Hazard. Mater. 2022, 424, 127612. [Google Scholar] [CrossRef]
- Wu, B.Q.; Cheng, S.T.; Shen, X.F.; Pang, Y.H. Conversion of CoNiFe-LDH to CoNiFe-MOF/LDH as catalyst for efficient heterogeneous electro-Fenton degradation of sulfonamide antibiotics. J. Environ. Chem. Eng. 2024, 12, 112426. [Google Scholar] [CrossRef]
- Inayat, A.; Klumpp, M.; Schwieger, W. The urea method for the direct synthesis of ZnAl layered double hydroxides with nitrate as the interlayer anion. Appl. Clay Sci. 2011, 51, 452–459. [Google Scholar] [CrossRef]
- Deng, J.; Xu, M.; Qiu, C.; Chen, Y.; Ma, X.; Gao, N.; Li, X. Magnetic MnFe2O4 activated peroxymonosulfate processes for degradation of bisphenol A: Performance, mechanism and application feasibility. Appl. Surf. Sci. 2018, 459, 138–147. [Google Scholar] [CrossRef]
- Wang, R.; Yu, Y.; Zhang, R.; Ren, X.; Guo, W. Elucidating the origin mechanism of a morphology-dependent layered double hydroxide catalyst toward organic contaminant oxidation via persulfate activation. Environ. Sci. Pollut. Res. 2022, 29, 79126–79139. [Google Scholar] [CrossRef]
- GB 25467-2010; Emission Standard of Pollutants for Copper, Nickel, Cobalt Industry. China Environmental Science Press: Beijing, China, 2010.
- GB 13456-2012; Discharge Standard of Water Pollutants for Iron and Steel Industry. Ministry of Ecology and Environment: Beijing, China, 2012.
- Huang, Y.; Han, C.; Liu, Y.; Nadagouda, M.N.; Machala, L.; O’Shea, K.E.; Sharma, V.K.; Dionysiou, D.D. Degradation of atrazine by ZnxCu1−xFe2O4 nanomaterial-catalyzed sulfite under UV–vis light irradiation: Green strategy to generate SO4−. Appl. Catal. B Environ. 2018, 221, 380–392. [Google Scholar] [CrossRef]
- Tang, R.; Gong, D.; Deng, Y.; Xiong, S.; Deng, J.; Li, L.; Zhou, Z.; Zheng, J.; Su, L.; Yang, L.; et al. π-π Stacked step-scheme PDI/g-C3N4/TiO2@ Ti3C2 photocatalyst with enhanced visible photocatalytic degradation towards atrazine via peroxymonosulfate activation. Chem. Eng. J. 2022, 427, 131809. [Google Scholar] [CrossRef]
- Jawad, A.; Lang, J.; Liao, Z.; Khan, A.; Ifthikar, J.; Lv, Z.; Long, S.; Chen, Z.; Chen, Z. Activation of persulfate by CuOx@ Co-LDH: A novel heterogeneous system for contaminant degradation with broad pH window and controlled leaching. Chem. Eng. J. 2018, 335, 548–559. [Google Scholar] [CrossRef]
- Zhu, L.; Shi, Z.; Deng, L.; Duan, Y. Efficient degradation of sulfadiazine using magnetically recoverable MnFe2O4/δ-MnO2 hybrid as a heterogeneous catalyst of peroxymonosulfate. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125637. [Google Scholar] [CrossRef]
- Koo, P.L.; Jaafar, N.F.; Yap, P.S.; Oh, W.D. A review on the application of perovskite as peroxymonosulfate activator for organic pollutants removal. J. Environ. Chem. Eng. 2022, 10, 107093. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, G.; Chen, Y.; Han, B.; Xia, K.; Zhou, C. Utilizing cobalt-doped materials as heterogeneous catalysts to activate peroxymonosulfate for organic pollutant degradation: A critical review. Environ. Sci. Water Res. Technol. 2021, 7, 1197–1211. [Google Scholar] [CrossRef]
- Abd El-Monaem, E.M.; Elshishini, H.M.; Bakr, S.S.; El-Aqapa, H.G.; Hosny, M.; Andaluri, G.; El-Subruiti, G.M.; Omer, A.M.; Eltaweil, A.S. A comprehensive review on LDH-based catalysts to activate persulfates for the degradation of organic pollutants. npj Clean Water 2023, 6, 34. [Google Scholar] [CrossRef]
- Zheng, H.; Bao, J.; Huang, Y.; Xiang, L.; Faheem, M.; Ren, B.; Du, J.; Nadagouda, M.N.; Dionysiou, D.D. Efficient degradation of atrazine with porous sulfurized Fe2O3 as catalyst for peroxymonosulfate activation. Appl. Catal. B Environ. 2019, 259, 118056. [Google Scholar] [CrossRef]
- Niu, B.; Cai, J.; Song, W.; Zhao, G. Intermediate accumulation and toxicity reduction during the selective photoelectrochemical process of atrazine in complex water bodies. Water Res. 2021, 205, 117663. [Google Scholar] [CrossRef] [PubMed]
- Ralston-Hooper, K.; Hardy, J.; Hahn, L.; Ochoa-Acuña, H.; Lee, L.S.; Mollenhauer, R.; Sepúlveda, M.S. Acute and chronic toxicity of atrazine and its metabolites deethylatrazine and deisopropylatrazine on aquatic organisms. Ecotoxicology 2009, 18, 899–905. [Google Scholar] [CrossRef] [PubMed]

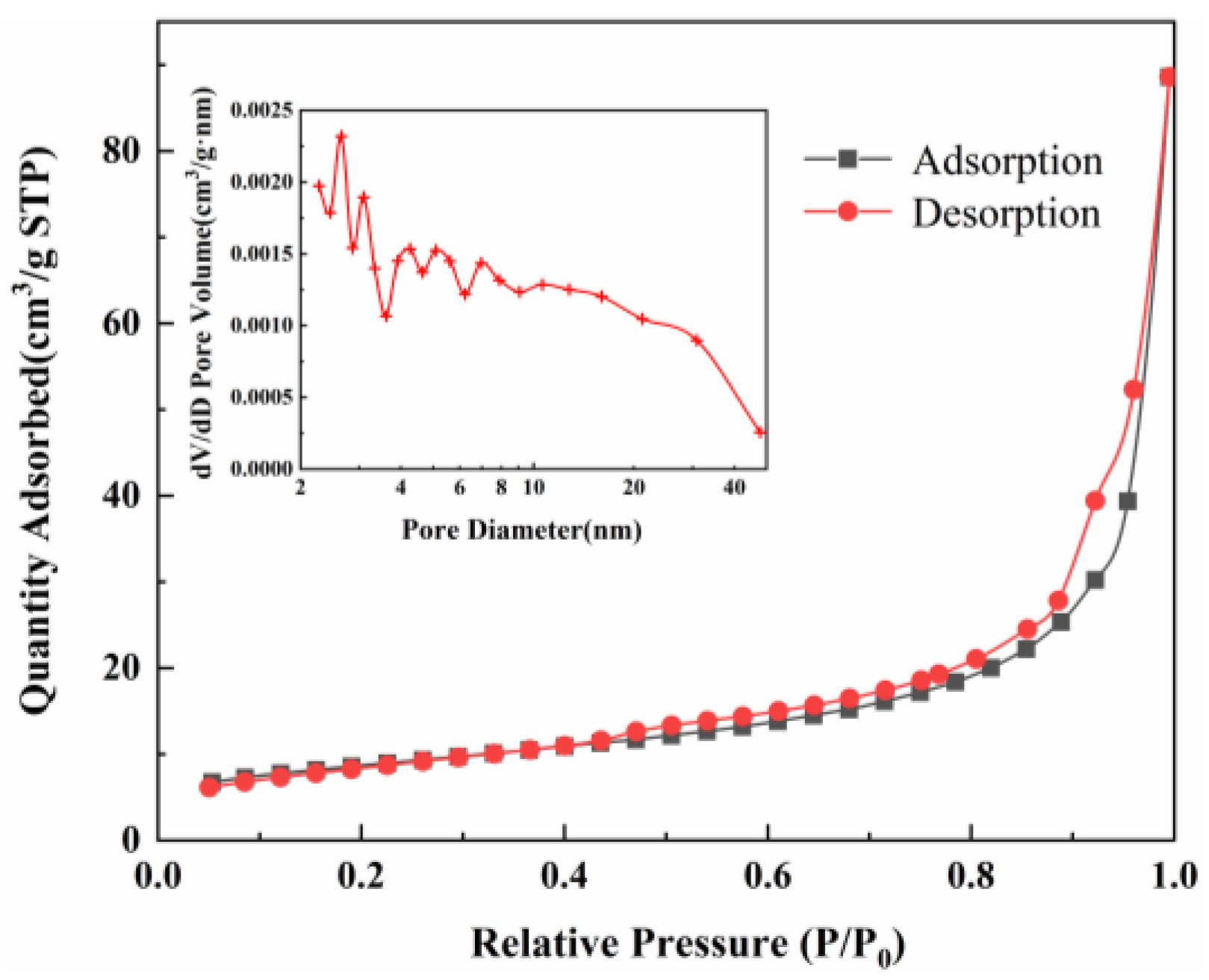
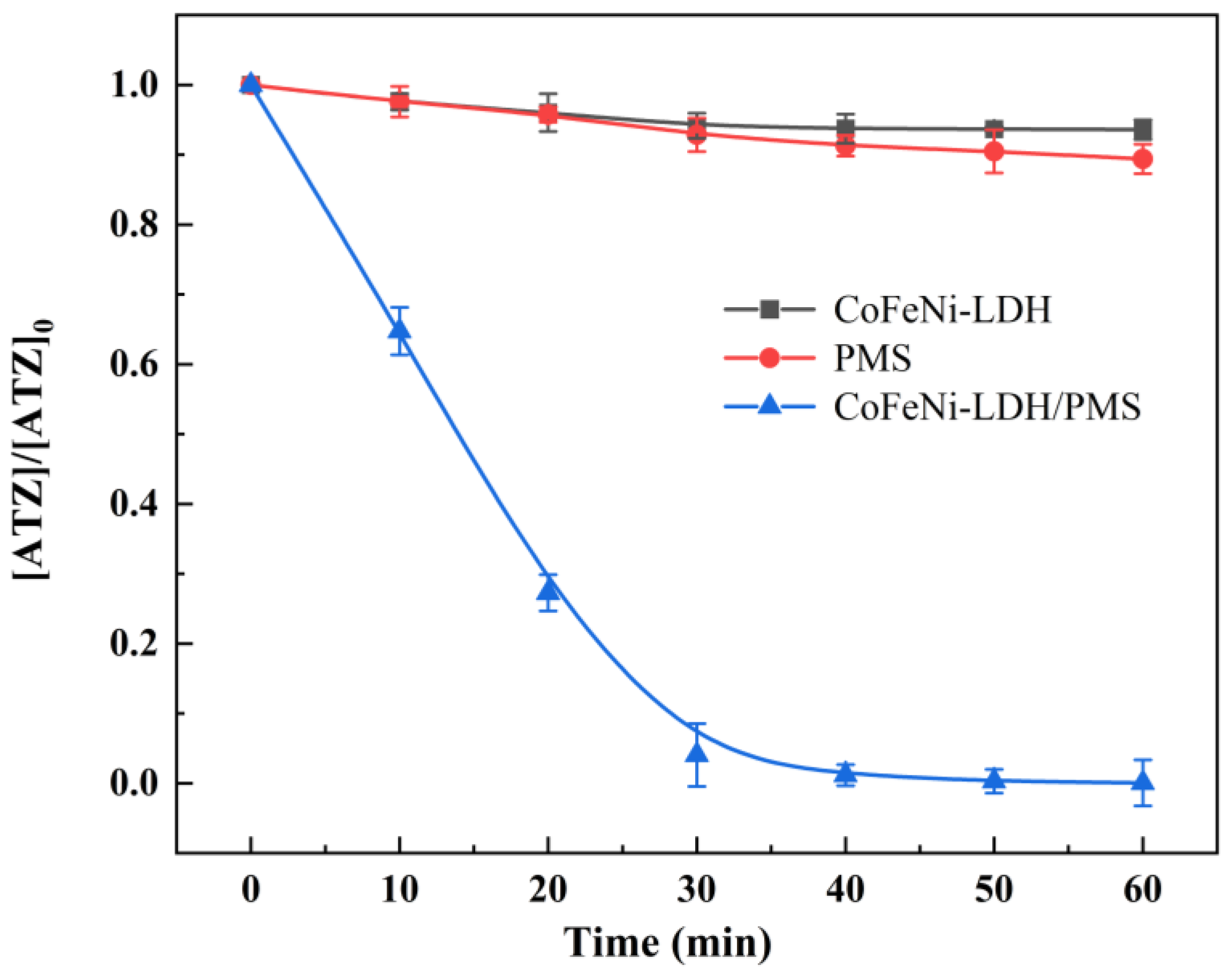
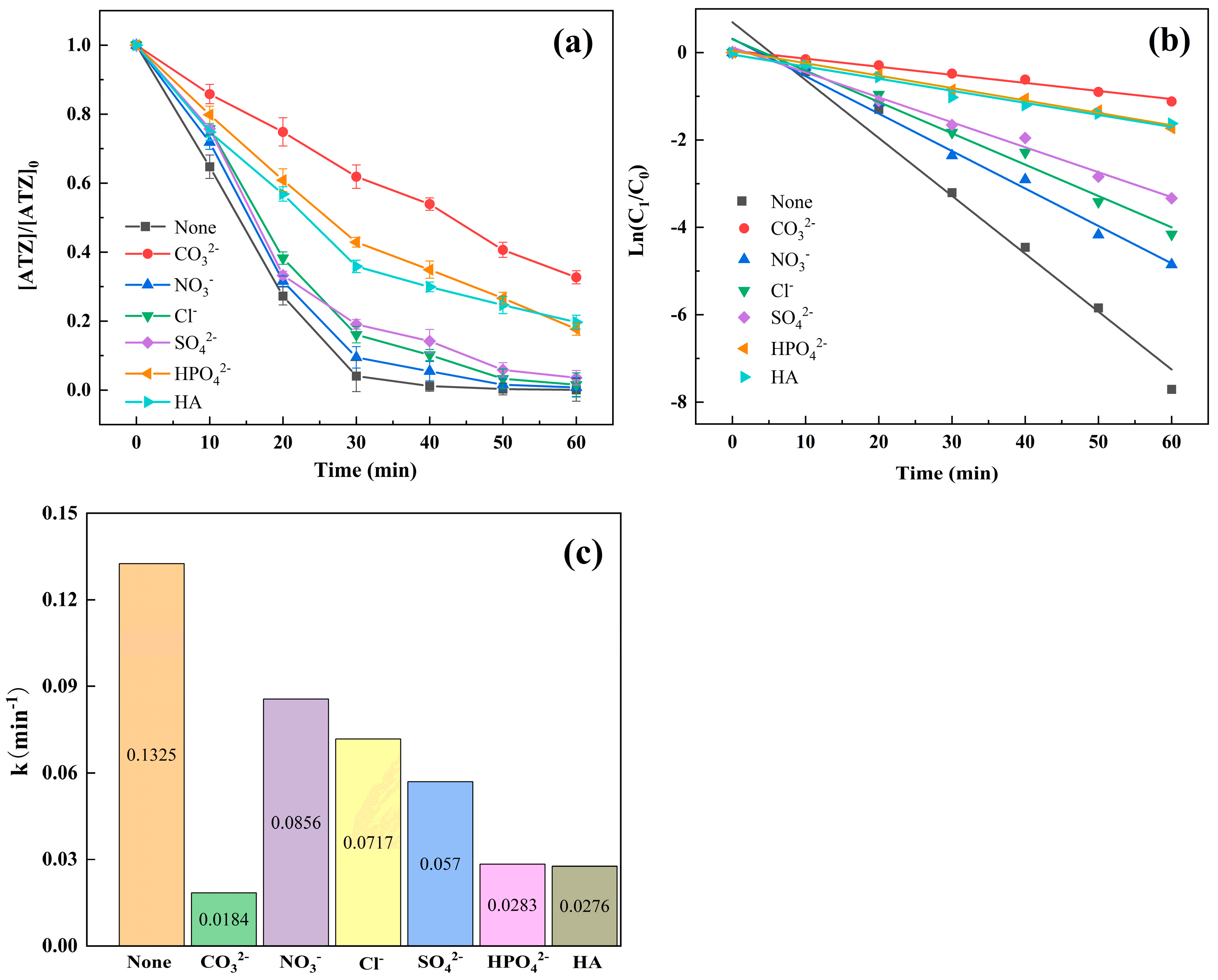
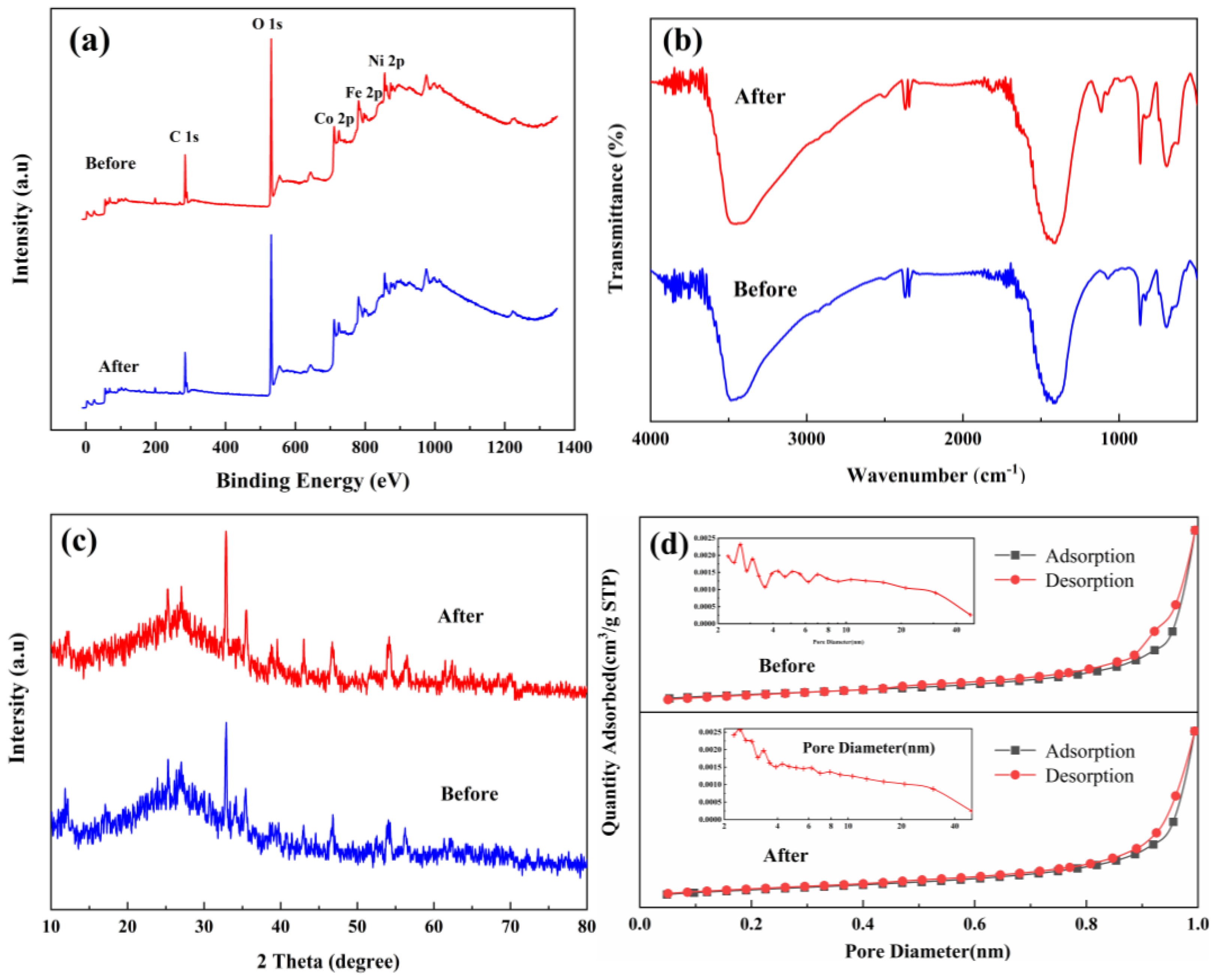


| Material | SBET * (m2·g−1) | Smicro (m2·g−1) | Smeso (m2·g−1) | Vtot (cm2·g−1) | Vmicro (cm2·g−1) | Vmeso (cm2·g−1) |
|---|---|---|---|---|---|---|
| CoNiFe-LDH | 31.1249 | 5.5202 | 25.6047 | 0.1370 | 0.0022 | 0.1348 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Li, X.; Deng, Y.; Zhang, Y.; Huang, Y.; Zheng, H. Mechanism and Pathway of Atrazine Degradation by Peroxymonosulfate Activated by CoNiFe-Layered Double Hydroxide. Coatings 2025, 15, 346. https://doi.org/10.3390/coatings15030346
Zhang Z, Li X, Deng Y, Zhang Y, Huang Y, Zheng H. Mechanism and Pathway of Atrazine Degradation by Peroxymonosulfate Activated by CoNiFe-Layered Double Hydroxide. Coatings. 2025; 15(3):346. https://doi.org/10.3390/coatings15030346
Chicago/Turabian StyleZhang, Zhanmei, Xinyue Li, Yang Deng, Yi Zhang, Yunxuan Huang, and Huaili Zheng. 2025. "Mechanism and Pathway of Atrazine Degradation by Peroxymonosulfate Activated by CoNiFe-Layered Double Hydroxide" Coatings 15, no. 3: 346. https://doi.org/10.3390/coatings15030346
APA StyleZhang, Z., Li, X., Deng, Y., Zhang, Y., Huang, Y., & Zheng, H. (2025). Mechanism and Pathway of Atrazine Degradation by Peroxymonosulfate Activated by CoNiFe-Layered Double Hydroxide. Coatings, 15(3), 346. https://doi.org/10.3390/coatings15030346







