Pitfalls and Challenges in Specific Absorption Rate Evaluation for Functionalized and Coated Magnetic Nanoparticles Used in Magnetic Fluid Hyperthermia
Abstract
1. Magnetic Hyperthermia as a Hope in Cancer Therapy
1.1. Introduction
1.2. MH Application
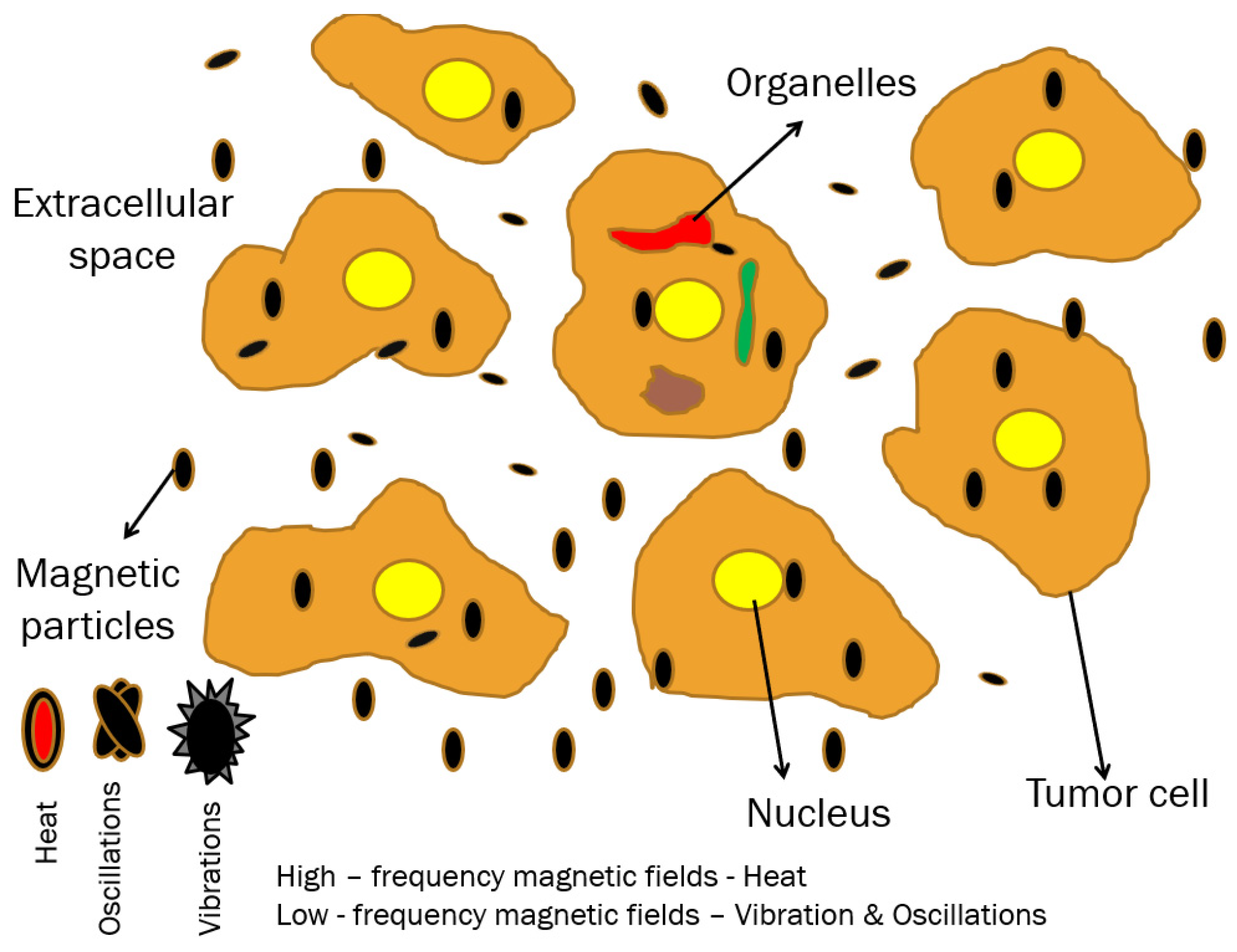
1.3. Cellular MH
1.3.1. Nanoparticle Uptake and Localization
1.3.2. Extracellular/Intracellular Thermal and Mechanical MH Effects
1.4. MNP Synthesis
1.5. Magnetic Nanoparticle and Necrotic Tissue Clearance
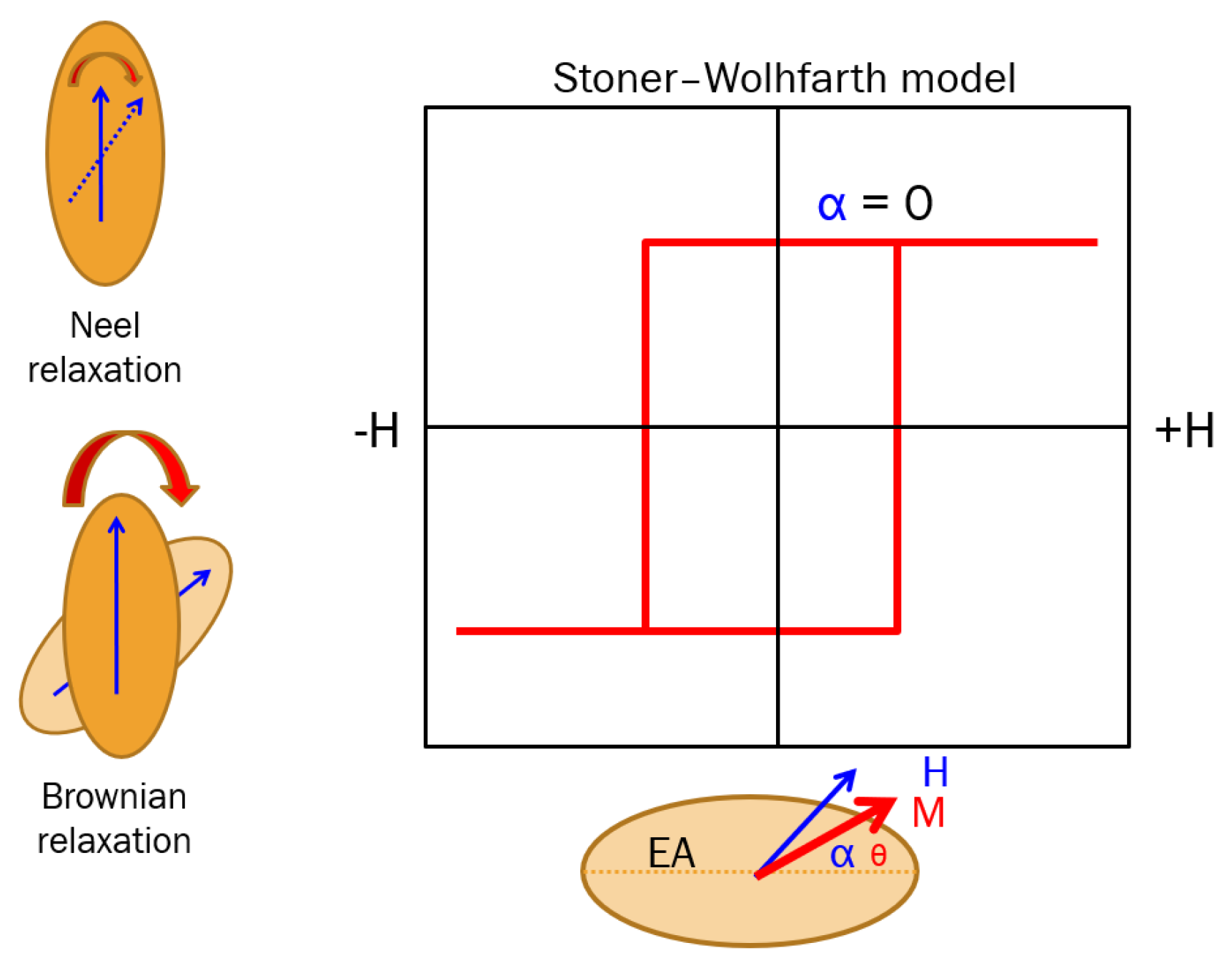
2. Physical Mechanisms Involved in Magnetic Hyperthermia
- Neel relaxation when and magnetic moments fluctuate statistically and coherently around the nanoparticle’s easy axis between the two energy minima. Neel relaxation is defined by relaxation time: , where is a time constant, with . Neel relaxation phenomena take place in both dry and fluid-suspended SPM-NPs.
- Brownian relation when the magnetic moments are strongly bound to the nanoparticle (the case of high values of ) and cannot be driven by the AC field. In this case, the particle rotates as a whole against the fluid viscosity resistance, being characterized by a relaxation time defined as , related to the fluid viscosity () and particle’s hydrodynamic volume.
3. SAR Evaluation Methods in Magnetic Hyperthermia
3.1. Specific Absorption Rate (SAR) Bioheat Equation
3.2. Calorimetric Methods in SAR Evaluation
3.2.1. General Aspects
3.2.2. Initial Slope (IS) Method (Sketched in Figure 3a)
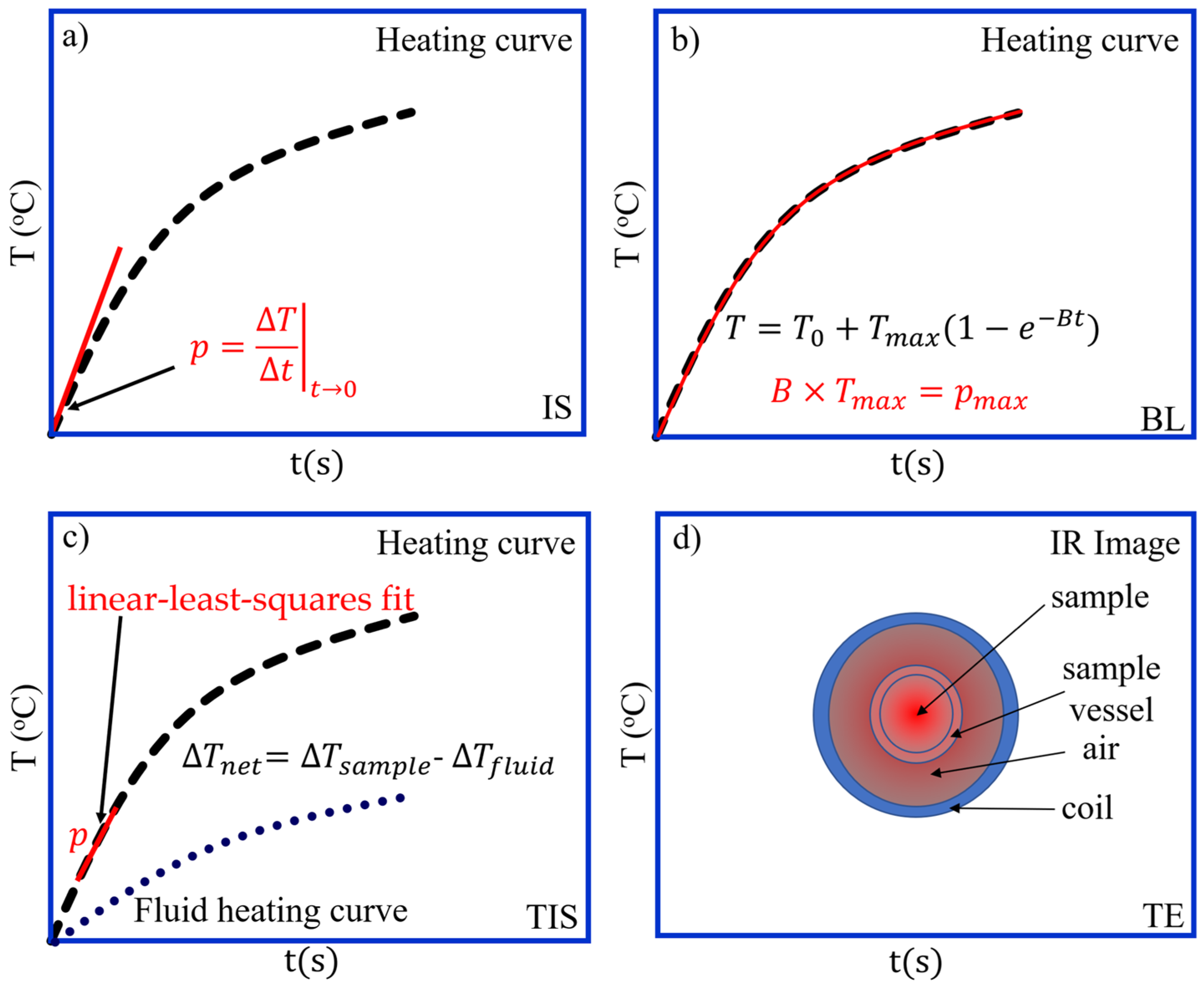
3.2.3. Box–Lucas (BL) Method (Sketched in Figure 3b)
3.2.4. Temperature Increase Slope (TIS) Method (Sketched in Figure 3c)
3.2.5. Thermographic Evaluation (TE) Method (Sketched in Figure 3d)
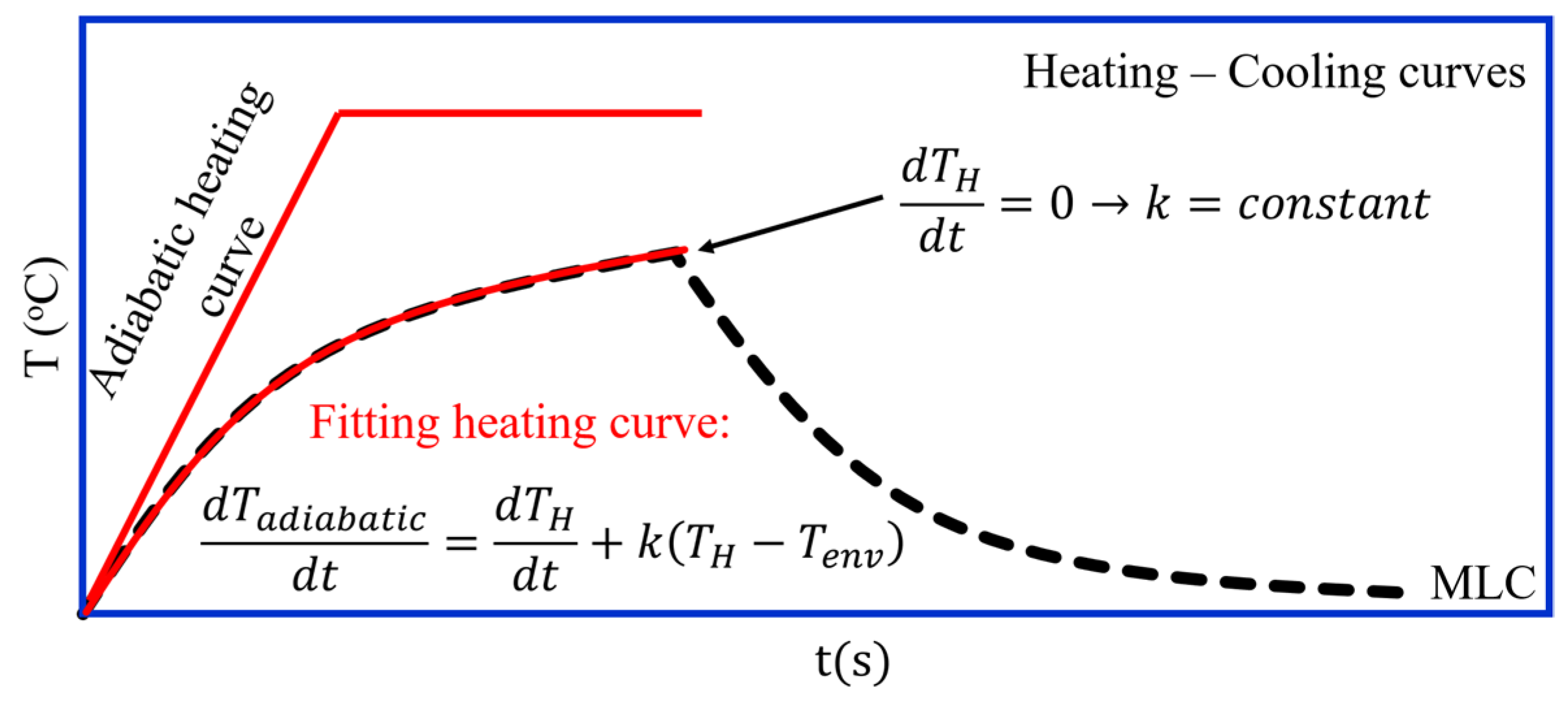
3.2.6. Adiabatic Heating Curve Methods (Sketched in Figure 4a,b)

3.2.7. Modified Law of Cooling (MLC) Method (Sketched in Figure 5)
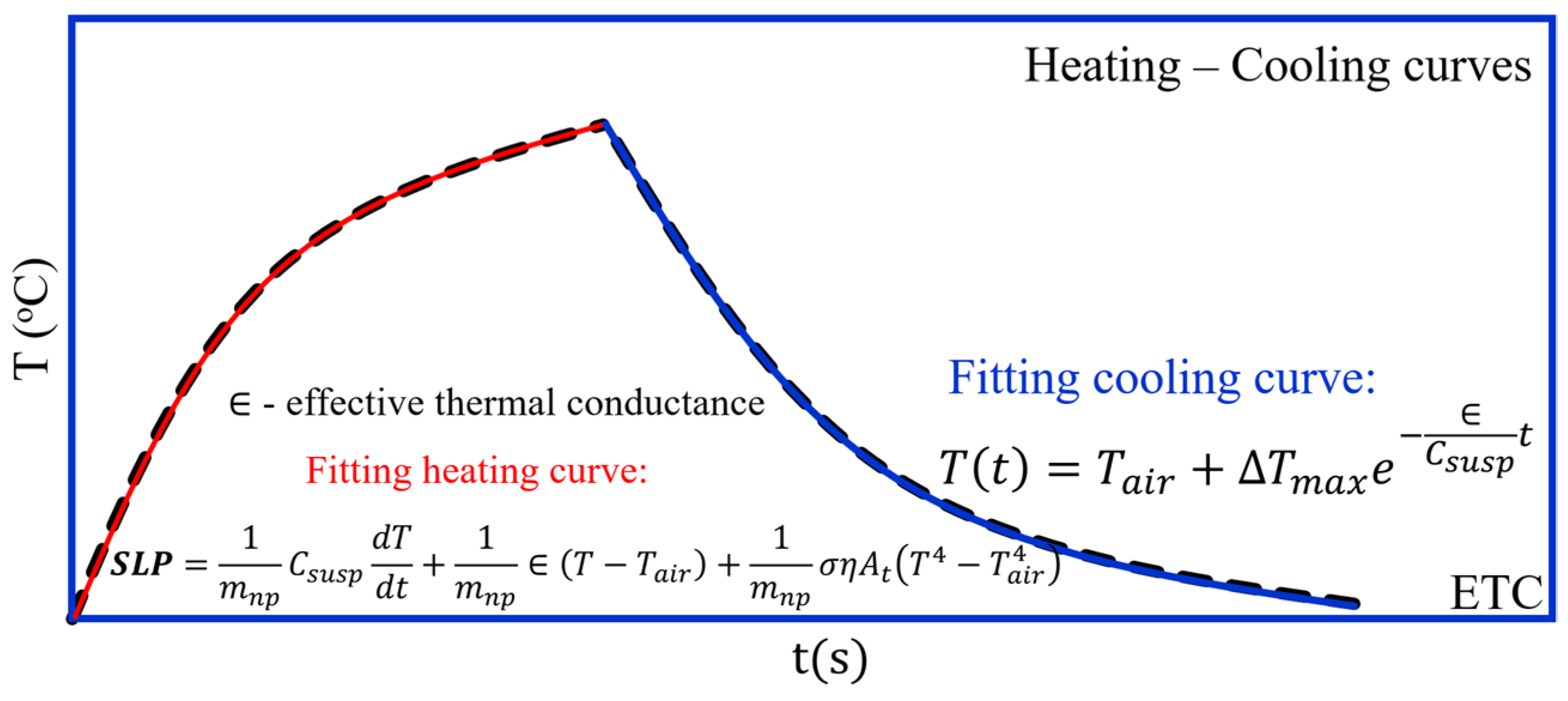
3.2.8. Effective Thermal Conductance (ETC) Method (Sketched in Figure 6)
3.2.9. Evaluation of Nanoheater Temperature (ENT) Method (Sketched in Figure 7)

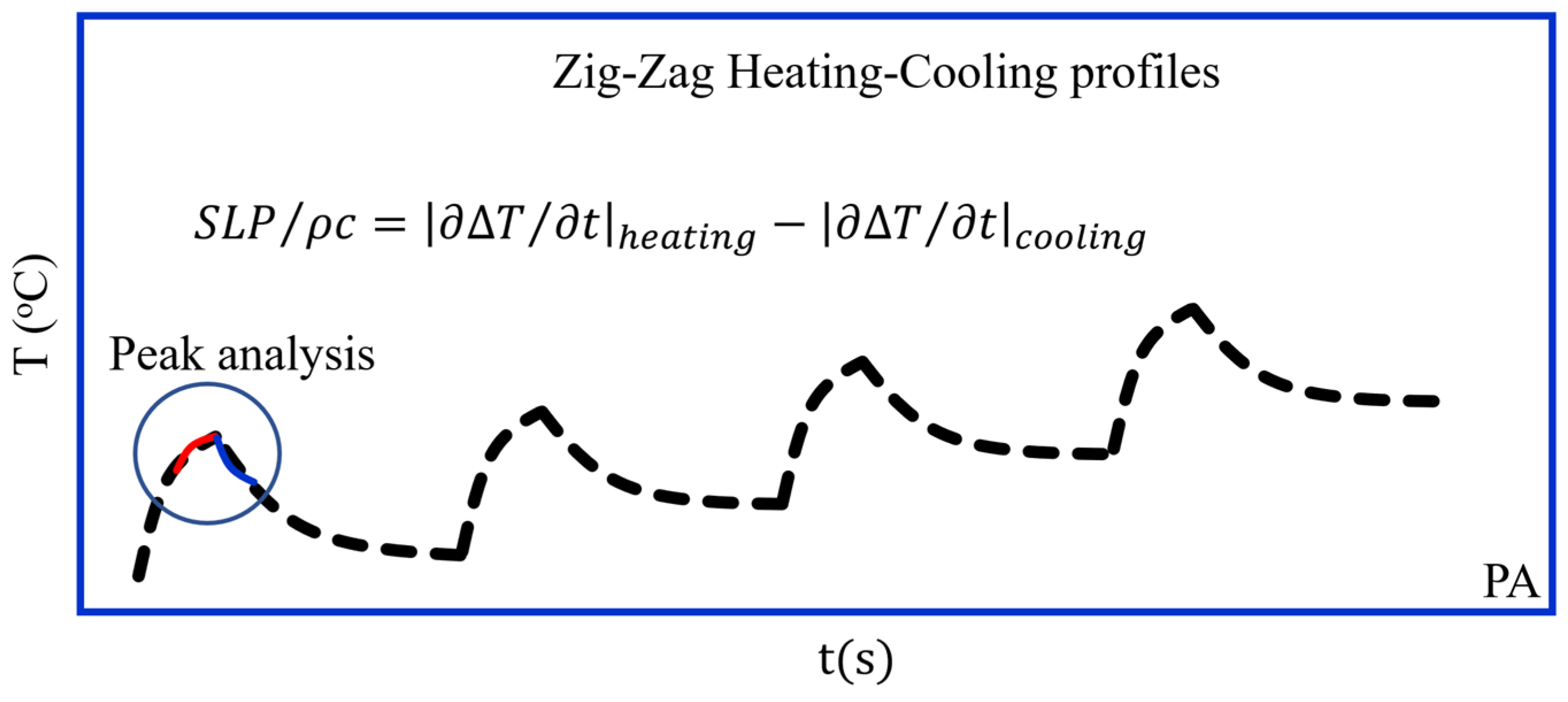
3.2.10. Peak Analysis (PA) Method (Sketched in Figure 8)
3.2.11. Pulse Heating (PH) Method (Sketched in Figure 9)

3.2.12. Discussion
3.3. Magnetic Methods in SAR Evaluation
4. Effects of Coating Layer on SAR
5. SAR at Cellular Level
6. Conclusions and Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moon, J.; Kitty, I.; Renata, K.; Qin, S.; Zhao, F.; Kim, W. DNA Damage and Its Role in Cancer Therapeutics. Int. J. Mol. Sci. 2023, 24, 4741. [Google Scholar] [CrossRef] [PubMed]
- Kay, J.; Thadhani, E.; Samson, L.; Engelward, B. Inflammation-Induced DNA Damage, Mutations and Cancer. DNA Repair 2019, 83, 102673. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA Damage, Repair, and Mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Huang, R.; Zhou, P.-K. DNA Damage Repair: Historical Perspectives, Mechanistic Pathways, and Clinical Translation for Targeted Cancer Therapy. Signal Transduct. Target. Ther. 2021, 6, 254. [Google Scholar] [CrossRef]
- Li, L.; Guan, Y.; Chen, X.; Yang, J.; Cheng, Y. DNA Repair Pathways in Cancer Therapy and Resistance. Front. Pharmacol. 2021, 11, 629266. [Google Scholar] [CrossRef]
- Imashiro, C.; Takeshita, H.; Morikura, T.; Miyata, S.; Takemura, K.; Komotori, J. Development of Accurate Temperature Regulation Culture System with Metallic Culture Vessel Demonstrates Different Thermal Cytotoxicity in Cancer and Normal Cells. Sci. Rep. 2021, 11, 21466. [Google Scholar] [CrossRef]
- De Maio, A.; Alfieri, G.; Mattone, M.; Ghanouni, P.; Napoli, A. High-Intensity Focused Ultrasound Surgery for Tumor Ablation: A Review of Current Applications. Radiol. Imaging Cancer 2023, 6, e230074. [Google Scholar] [CrossRef]
- Ji, Y.; Zhu, J.; Zhu, L.; Zhu, Y.; Zhao, H. High-Intensity Focused Ultrasound Ablation for Unresectable Primary and Metastatic Liver Cancer: Real-World Research in a Chinese Tertiary Center with 275 Cases. Front. Oncol. 2020, 10, 519164. [Google Scholar] [CrossRef]
- Schena, E.; Saccomandi, P.; Fong, Y. Laser Ablation for Cancer: Past, Present and Future. J. Funct. Biomater. 2017, 8, 19. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, L.; Liu, S.; Li, J.; Xia, J.; Qin, X.; Li, Y.; Gao, T.; Tang, X. The State-of-the-Art and Perspectives of Laser Ablation for Tumor Treatment. Cyborg Bionic Syst. 2024, 5, 0062. [Google Scholar] [CrossRef] [PubMed]
- Adibzadeh, F.; Sumser, K.; Curto, S.; Yeo, D.T.B.; Shishegar, A.A.; Paulides, M.M. Systematic review of pre-clinical and clinical devices for magnetic resonance-guided radiofrequency hyperthermia. Int. J. Hyperth. 2020, 37, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Lim, M.; Goos, J.A.C.M.; Qiao, R.; Ng, Y.Y.; Mansfeld, F.M.; Jackson, M.; Davis, T.P.; Kavallaris, M. Biologically Targeted Magnetic Hyperthermia: Potential and Limitations. Front. Pharmacol. 2018, 9, 831. [Google Scholar] [CrossRef]
- Dutz, S.; Hergt, R. Magnetic Particle Hyperthermia—A Promising Tumour Therapy? Nanotechnology 2014, 25, 452001. [Google Scholar] [CrossRef]
- Feddersen, T.V.; Hernandez-Tamames, J.A.; Franckena, M.; van Rhoon, G.C.; Paulides, M.M. Clinical Performance and Future Potential of Magnetic Resonance Thermometry in Hyperthermia. Cancers 2021, 13, 31. [Google Scholar] [CrossRef]
- Gavilán, H.; Kumar Avugadda, S.; Fernández-Cabada, T.; Soni, N.; Cassani, M.; Mai, B.T.; Chantrell, R.; Pellegrino, T. Magnetic Nanoparticles and Clusters for Magnetic Hyperthermia: Optimizing Their Heat Performance and Developing Combinatorial Therapies to Tackle Cancer. Chem. Soc. Rev. 2021, 50, 11614–11667. [Google Scholar] [CrossRef]
- Kok, H.P.; Cressman, E.N.K.; Ceelen, W.; Brace, C.L.; Ivkov, R.; Grüll, H.; ter Haar, G.; Wust, P.; Crezee, J. Heating Technology for Malignant Tumors: A Review. Int. J. Hyperth. 2020, 37, 711–741. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Moreira, J.A.; Monteiro, F.J.; Laranjeira, M.S. Nanomaterials in Cancer: Reviewing the Combination of Hyperthermia and Triggered Chemotherapy. J. Control. Release 2022, 347, 89–103. [Google Scholar] [CrossRef]
- Shirvalilou, S.; Khoei, S.; Esfahani, A.J.; Kamali, M.; Shirvaliloo, M.; Sheervalilou, R.; Mirzaghavami, P. Magnetic Hyperthermia as an Adjuvant Cancer Therapy in Combination with Radiotherapy versus Radiotherapy Alone for Recurrent/Progressive Glioblastoma: A Systematic Review. J. Neurooncol. 2021, 152, 419–428. [Google Scholar] [CrossRef]
- Price, P.M.; Mahmoud, W.E.; Al-Ghamdi, A.A.; Bronstein, L.M. Magnetic Drug Delivery: Where the Field Is Going. Front. Chem. 2018, 6, 619. [Google Scholar] [CrossRef]
- Pusta, A.; Tertis, M.; Crăciunescu, I.; Turcu, R.; Mirel, S.; Cristea, C. Recent Advances in the Development of Drug Delivery Applications of Magnetic Nanomaterials. Pharmaceutics 2023, 15, 1872. [Google Scholar] [CrossRef] [PubMed]
- Toderascu, L.I.; Sima, L.E.; Orobeti, S.; Florian, P.E.; Icriverzi, M.; Maraloiu, V.-A.; Comanescu, C.; Iacob, N.; Kuncser, V.; Antohe, I.; et al. Synthesis and anti-melanoma activity of L-cysteine-coated iron oxide nanoparticles loaded with doxorubicin. Nanomaterials 2023, 13, 621. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic Nanoparticles: Design and Characterization, Toxicity and Biocompatibility, Pharmaceutical and Biomedical Applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef] [PubMed]
- Kush, P.; Kumar, P.; Singh, R.; Kaushik, A. Aspects of High-Performance and Bio-Acceptable Magnetic Nanoparticles for Biomedical Applications. Asian J. Pharm. Sci. 2021, 16, 704–737. [Google Scholar] [CrossRef]
- Bychkova, A.V.; Sorokina, O.N.; Rosenfeld, M.A.; Kovarski, A. Multifunctional biocompatible coatings on magnetic nanoparticles. Russ. Chem. Rev. 2012, 81, 1070–1083. [Google Scholar] [CrossRef]
- Atkinson, W.J.; Brezovich, I.A.; Chakraborty, D.P. Usable frequencies in hyperthermia with thermal seeds. IEEE Trans. Biomed. Eng. 1984, 31, 70. [Google Scholar] [CrossRef]
- Dutz, S.; Hergt, R. Magnetic Nanoparticle Heating and Heat Transfer on a Microscale: Basic Principles, Realities, and Physical Limitations of Hyperthermia for Tumour Therapy. Int. J. Hyperth. 2013, 29, 790–800. [Google Scholar] [CrossRef]
- de la Parte, B.H.; Rodrigo, I.; Gutiérrez-Basoa, J.; Correcher, S.I.; Mar Medina, C.; Echevarría-Uraga, J.J.; Garcia, J.A.; Plazaola, F.; García-Alonso, I. Proposal of New Safety Limits for In Vivo Experiments of Magnetic Hyperthermia Antitumor Therapy. Cancers 2022, 14, 3084. [Google Scholar] [CrossRef]
- Kwok, M.K.Y.; Maley, C.C.J.; Dworkin, A.; Hattersley, S.; Southern, P.; Pankhurst, Q.A. Nonspecific Eddy Current Heating in Magnetic Field Hyperthermia. Appl. Phys. Lett. 2023, 122, 240502. [Google Scholar] [CrossRef]
- Gu, Y.; Piñol, R.; Moreno-Loshuertos, R.; Brites, C.D.S.; Zeler, J.; Martínez, A.; Maurin-Pasturel, G.; Fernández-Silva, P.; Marco-Brualla, J.; Téllez, P.; et al. Local Temperature Increments and Induced Cell Death in Intracellular Magnetic Hyperthermia. ACS Nano 2023, 17, 6822–6832. [Google Scholar] [CrossRef]
- Li, J.; Tran, N.; Webster, T.J. Towards a Better Understanding of the Effects of the Magnetic Nanoparticles Size and Magnetic Field on Cellular Endocytosis. J. Phys. D Appl. Phys. 2020, 53, 175401. [Google Scholar] [CrossRef]
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of Physico-Chemical Properties of Nanoparticles on Their Intracellular Uptake. Int. J. Mol. Sci. 2020, 21, 8019. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yang, Z.; Sun, H.; Li, J.; Lan, X.; Liu, S. Understanding Cellular Uptake, Localization, and Retention for Enhanced Disease Diagnosis and Therapy. Aging Dis. 2025, 16, 168–208. [Google Scholar] [CrossRef] [PubMed]
- Potrč, T.; Kralj, S.; Nemec, S.; Kocbek, P.; Erdani Kreft, M. The Shape Anisotropy of Magnetic Nanoparticles: An Approach to Cell-Type Selective and Enhanced Internalization. Nanoscale 2023, 15, 8611–8618. [Google Scholar] [CrossRef]
- Blanco-Andujar, C.; Ortega, D.; Southern, P.; Nesbitt, S.A.; Thanh, N.T.K.; Pankhurst, Q.A. Real-Time Tracking of Delayed-Onset Cellular Apoptosis Induced by Intracellular Magnetic Hyperthermia. Nanomedicine 2016, 11, 121–136. [Google Scholar] [CrossRef]
- Calatayud, M.P.; Soler, E.; Torres, T.E.; Campos-Gonzalez, E.; Junquera, C.; Ibarra, M.R.; Goya, G.F. Cell damage produced by magnetic fluid hyperthermia on microglial BV2 cells. Sci. Rep. 2017, 7, 8627. [Google Scholar] [CrossRef]
- Beola, L.; Asín, L.; Fratila, R.M.; Herrero, V.; de la Fuente, J.M.; Grazu, V.; Gutiérrez, L. Dual Role of Magnetic Nanoparticles as Intracellular Hotspots and Extracellular Matrix Disruptors Triggered by Magnetic Hyperthermia in 3D Cell Culture Models. ACS Appl. Mater. Interfaces 2018, 10, 44301–44313. [Google Scholar] [CrossRef]
- Hogan, K.J.; Perez, M.R.; Mikos, A.G. Extracellular Matrix Component-Derived Nanoparticles for Drug Delivery and Tissue Engineering. J. Control. Release 2023, 360, 888–912. [Google Scholar] [CrossRef]
- Chen, B.-W.; Chiu, G.-W.; He, Y.-C.; Huang, C.-Y.; Huang, H.-T.; Sung, S.-Y.; Hsieh, C.-L.; Chang, W.-C.; Hsu, M.-S.; Wei, Z.-H.; et al. Extracellular and Intracellular Intermittent Magnetic-Fluid Hyperthermia Treatment of SK-Hep1 Hepatocellular Carcinoma Cells Based on Magnetic Nanoparticles Coated with Polystyrene Sulfonic Acid. PLoS ONE 2021, 16, e0245286. [Google Scholar] [CrossRef]
- Hannon, G.; Bogdanska, A.; Volkov, Y.; Prina-Mello, A. Comparing the Effects of Intracellular and Extracellular Magnetic Hyperthermia on the Viability of BxPC-3 Cells. Nanomaterials 2020, 10, 593. [Google Scholar] [CrossRef]
- Kobayashi, S.; Ohki, A.; Tanoue, M.; Inaoka, Y.; Murase, K. Comparative Study of Extracellular and Intracellular Magnetic Hyperthermia Treatments Using Magnetic Particle Imaging. Open J. Appl. Sci. 2017, 7, 647–660. [Google Scholar] [CrossRef]
- Idiago-López, J.; Ferreira, D.; Asín, L.; Moros, M.; Armenia, I.; Grazú, V.; Fernandes, A.R.; Baptista, P.V.; Fratila, R.M. Membrane-Localized Magnetic Hyperthermia Promotes Intracellular Delivery of Cell-Impermeant Probes. Nanoscale 2024, 16, 15176–15195. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Afonso, Y.; Ruta, S.; Páez-Rodríguez, A.; van Zanten, T.S.; Gleadhall, S.; Fratila, R.M.; Moros, M.; Morales, M.P.; Satoh, A.; Chantrell, R.W.; et al. Reversible Alignment of Nanoparticles and Intracellular Vesicles During Magnetic Hyperthermia Experiments. Adv. Funct. Mater. 2024, 34, 2405334. [Google Scholar] [CrossRef]
- Kim, D.-H.; Rozhkova, E.A.; Ulasov, I.V.; Bader, S.D.; Rajh, T.; Lesniak, M.S.; Novosad, V. Biofunctionalized Magnetic-Vortex Microdiscs for Targeted Cancer-Cell Destruction. Nat. Mater. 2009, 9, 165–171. [Google Scholar] [CrossRef]
- Zhang, E.; Kircher, M.F.; Koch, M.; Eliasson, L.; Goldberg, S.N.; Renström, E. Dynamic Magnetic Fields Remote-Control Apoptosis via Nanoparticle Rotation. ACS Nano 2014, 8, 3192–3201. [Google Scholar] [CrossRef]
- Naud, C.; Thébault, C.; Carrière, M.; Hou, Y.; Morel, R.; Berger, F.; Diény, B.; Joisten, H. Cancer treatment by magneto-mechanical effect of particles, a review. Nanoscale Adv. 2020, 2, 3632–3655. [Google Scholar] [CrossRef]
- Cheng, D.; Li, X.; Zhang, G.; Shi, H. Morphological Effect of Oscillating Magnetic Nanoparticles in Killing Tumor Cells. Nanoscale Res. Lett. 2014, 9, 195. [Google Scholar] [CrossRef]
- Nishita, M.; Park, S.-Y.; Nishio, T.; Kamizaki, K.; Wang, Z.; Tamada, K.; Takumi, T.; Hashimoto, R.; Otani, H.; Pazour, G.J.; et al. Ror2 Signaling Regulates Golgi Structure and Transport Through IFT20 for Tumor Invasiveness. Sci. Rep. 2017, 7, 1. [Google Scholar] [CrossRef]
- Leulmi, S.; Chauchet, X.; Morcrette, M.; Ortiz, G.; Joisten, H.; Sabon, P.; Livache, T.; Hou, Y.; Carrière, M.; Lequien, S.; et al. Triggering the Apoptosis of Targeted Human Renal Cancer Cells by the Vibration of Anisotropic Magnetic Particles Attached to the Cell Membrane. Nanoscale 2015, 7, 15904–15913. [Google Scholar] [CrossRef]
- Dieny, B.; Morel, R.; Joisten, H.; Naud, C.; Nicolas, A.; Visonà, A.; Obeïd, P.; Belin, S.; Berger, F. Magnetism for mechanobiology and related biomedical applications. Phys. Rev. Appl. 2025, 23, 010501. [Google Scholar] [CrossRef]
- Unni, M.; Uhl, A.M.; Savliwala, S.; Savitzky, B.H.; Dhavalikar, R.; Garraud, N.; Arnold, D.P.; Kourkoutis, L.F.; Andrew, J.S.; Rinaldi, C. Thermal Decomposition Synthesis of Iron Oxide Nanoparticles with Diminished Magnetic Dead Layer by Controlled Addition of Oxygen. ACS Nano 2017, 11, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Fokina, V.; Wilke, M.; Dulle, M.; Ehlert, S.; Förster, S. Size Control of Iron Oxide Nanoparticles Synthesized by Thermal Decomposition Methods. J. Phys. Chem. C 2022, 126, 21356–21367. [Google Scholar] [CrossRef]
- Demessie, A.A.; Park, Y.; Singh, P.; Moses, A.S.; Korzun, T.; Sabei, F.Y.; Albarqi, H.A.; Campos, L.; Wyatt, C.R.; Farsad, K.; et al. An Advanced Thermal Decomposition Method to Produce Magnetic Nanoparticles with Ultrahigh Heating Efficiency for Systemic Magnetic Hyperthermia. Small Methods 2022, 6, 2200916. [Google Scholar] [CrossRef] [PubMed]
- Gavilan, H.; Rizzo, G.M.R.; Silvestri, N.; Mai, B.T.; Pellegrino, T. Scale-up approach for the preparation of magnetic ferrite nanocubes and other shapes with benchmark performance for magnetic hyperthermia applications. Nat. Protoc. 2023, 18, 783–809. [Google Scholar] [CrossRef]
- Nițică, S.; Fizeșan, I.; Dudric, R.; Barbu-Tudoran, L.; Pop, A.; Loghin, F.; Vedeanu, N.; Lucaciu, C.M.; Iacovita, C. A Fast, Reliable Oil-In-Water Microemulsion Procedure for Silica Coating of Ferromagnetic Zn Ferrite Nanoparticles Capable of Inducing Cancer Cell Death In Vitro. Biomedicines 2022, 10, 1647. [Google Scholar] [CrossRef]
- Bruschi, M.L.; Toledo, L.A.S. Pharmaceutical Applications of Iron-Oxide Magnetic Nanoparticles. Magnetochemistry 2019, 5, 50. [Google Scholar] [CrossRef]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; Lima, T.M.T.; Delbem, A.C.B.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef]
- Li, B.; Chen, X.; Qiu, W.; Zhao, R.; Duan, J.; Zhang, S.; Pan, Z.; Zhao, S.; Guo, Q.; Qi, Y.; et al. Synchronous Disintegration of Ferroptosis Defense Axis via Engineered Exosome-Conjugated Magnetic Nanoparticles for Glioblastoma Therapy. Adv. Sci. 2022, 9, 2105451. [Google Scholar] [CrossRef]
- Soares, G.A.; Faria, J.V.C.; Pinto, L.A.; Prospero, A.G.; Pereira, G.M.; Stoppa, E.G.; Buranello, L.P.; Bakuzis, A.F.; Baffa, O.; Miranda, J.R.A. Long-Term Clearance and Biodistribution of Magnetic Nanoparticles Assessed by AC Biosusceptometry. Materials 2022, 15, 2121. [Google Scholar] [CrossRef]
- Vandendriessche, S.; Mattos, M.S.; Bialek, E.L.; Schuermans, S.; Proost, P.; Marques, P.E. Complement Activation Drives the Phagocytosis of Necrotic Cell Debris and Resolution of Liver Injury. Front. Immunol. 2024, 15, 1512470. [Google Scholar] [CrossRef]
- Coey, J.M.D. Magnetism and Magnetic Materials; Cambridge University Press: Cambridge, UK, 2010; ISBN 978-0-521-81614-4. [Google Scholar]
- Kuncser, V.; Palade, P.; Kuncser, A.; Greculeasa, S.; Schinteie, G. Engineering Magnetic Properties of Nanostructures via Size Effects and Interphase Interactions. In Size Effects in Nanostructures; Kuncser, V., Miu, L., Eds.; Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 2014; pp. 169–237. [Google Scholar] [CrossRef]
- Bedanta, S.; Kleemann, W. Supermagnetism. J. Phys. D Appl. Phys. 2009, 42, 013001. [Google Scholar] [CrossRef]
- Rajan, A.; Sahu, N.K. Review on Magnetic Nanoparticle-Mediated Hyperthermia for Cancer Therapy. J. Nanopart. Res. 2020, 22, 319. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Heating Magnetic Fluid with Alternating Magnetic Field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Rognin, E.; Brun, P.; Sauvage, E.; Lacombe, J. Computation of Eddy Currents in Highly Conductive Particles Dispersed in a Moderately Conductive Matrix. Int. J. Appl. Electromagn. Mech. 2017, 53, 2241. [Google Scholar] [CrossRef]
- Wheeler, H.A. Formulas for the Skin Effect. Proc. IRE 1942, 30, 412–426. [Google Scholar] [CrossRef]
- Available online: https://www.rfcafe.com/references/electrical/cond-high-freq.htm (accessed on 14 January 2025).
- Iacob, N.; Schinteie, G.; Palade, P.; Kuncser, V. Approach for an Improved Experimental Evaluation of the Specific Absorption Rate in Magnetic Fluid Hyperthermia. J. Nanopart. Res. 2015, 17, 190. [Google Scholar] [CrossRef]
- Coïsson, M.; Barrera, G.; Appino, C.; Celegato, F.; Martino, L.; Safronov, A.P.; Kurlyandskaya, G.V.; Tiberto, P. Specific Loss Power Measurements by Calorimetric and Thermal Methods on γ-Fe2O3 Nanoparticles for Magnetic Hyperthermia. J. Magn. Magn. Mater. 2019, 473, 403–409. [Google Scholar] [CrossRef]
- Takeuchi, M.; Sakai, T.; Andocs, G.; Takao, K.; Nagaoka, R.; Hasegawa, H. Temperature Elevation in Tissue Detected In Vivo Based on Statistical Analysis of Ultrasonic Scattered Echoes. Sci. Rep. 2020, 10, 9030. [Google Scholar] [CrossRef]
- Sun, Y.; Kong, M.; Ke, J.; Yuan, W.; Wen, C.; Gu, Y.; Luo, S.; Feng, W. Nanothermometer for In Vivo Temperature Detection with High Spatial Resolution Based on Core–Shell Rare Earth Nanoparticles. ACS Appl. Nano Mater. 2023, 6, 23173–23183. [Google Scholar] [CrossRef]
- Valdés, D.P.; Torres, T.E.; Maldonado, A.C.M.; Urretavizcaya, G.; Nadal, M.S.; Mansilla, M.V.; Zysler, R.D.; Goya, G.F.; Biasi, E.D.; Lima, E., Jr. Thermographical Method to Assess the Performance of Magnetic Nanoparticles in Hyperthermia Experiments Through Spatiotemporal Temperature Profiles. Phys. Rev. Appl. 2023, 19, 014042. [Google Scholar] [CrossRef]
- Kallumadil, M.; Tada, M.; Nakagawa, T.; Abe, M.; Southern, P.; Pankhurst, Q.A. Suitability of Commercial Colloids for Magnetic Hyperthermia. J. Magn. Magn. Mater. 2009, 321, 1509–1513. [Google Scholar] [CrossRef]
- Iacob, N.; Schinteie, G.; Palade, P.; Ticos, C.M.; Kuncser, V. Stepped Heating Procedure for Experimental SAR Evaluation of Ferrofluids. Eur. Phys. J. E 2015, 38, 57. [Google Scholar] [CrossRef] [PubMed]
- Carrey, J.; Mehdaoui, B.; Respaud, M. Simple Models for Dynamic Hysteresis Loop Calculations of Magnetic Single-Domain Nanoparticles: Application to Magnetic Hyperthermia Optimization. J. Appl. Phys. 2011, 109, 083921. [Google Scholar] [CrossRef]
- Beković, M.; Trlep, M.; Jesenik, M.; Gorican, V.; Hamler, A. An Experimental Study of Magnetic-Field and Temperature Dependence on Magnetic Fluid’s Heating Power. J. Magn. Magn. Mater. 2013, 331, 264–268. [Google Scholar] [CrossRef]
- Gas, P.; Kurgan, E. Cooling Effects Inside Water-Cooled Inductors for Magnetic Fluid Hyperthermia. IEEE Trans. Magn. 2017, 53, 1528–1534. [Google Scholar] [CrossRef]
- Available online: https://www.calculator.net/dew-point-calculator.html (accessed on 15 January 2025).
- Li, J.; Ye, Y.; Lin, Z.; Wang, Z.; Chen, Y.; Li, G.; Ouyang, Z.; Li, J. Application of a New Self-Regulating Temperature Magnetic Material Fe83Zr10B7 in Magnetic Induction Hyperthermia. Int. J. Hyperth. 2023, 40, 2211269. [Google Scholar] [CrossRef]
- Vicentini, M.; Ferrero, R.; Manzin, A. Influence of Coil Geometry, Supply Conditions and Nanoparticle Heating Properties on Magnetic Hyperthermia in Mouse Models. Int. J. Therm. Sci. 2024, 203, 109151. [Google Scholar] [CrossRef]
- Makridis, A.; Curto, S.; van Rhoon, G.C.; Samaras, T.; Angelakeris, M. A Standardisation Protocol for Accurate Evaluation of Specific Loss Power in Magnetic Hyperthermia. J. Phys. D Appl. Phys. 2019, 52, 255001. [Google Scholar] [CrossRef]
- García-Soriano, D.; Milán-Rois, P.; Lafuente-Gómez, N.; Rodríguez-Díaz, C.; Navío, C.; Somoza, Á.; Salas, G. Multicore Iron Oxide Nanoparticles for Magnetic Hyperthermia and Combination Therapy Against Cancer Cells. J. Colloid Interface Sci. 2024, 670, 73–85. [Google Scholar] [CrossRef]
- Tithito, T.; Sillapaprayoon, S.; Chantho, V.; Pimtong, W.; Thongbunchoo, J.; Charoenphandhu, N.; Krishnamra, N.; Yong, N.; Lert-itthiporn, A.; Maneeprakorn, W.; et al. Evaluation of Magnetic Hyperthermia, Drug Delivery and Biocompatibility of Trace Element Co-Doped Hydroxyapatite Combined with Mn–Zn Ferrite for Bone Tissue Applications. RSC Adv. 2024, 14, 29242–29253. [Google Scholar] [CrossRef]
- Zeleňáková, A.; Nagy, Ľ.; Hrubovčák, P.; Barutiak, M.; Lisnichuk, M.; Huntošová, V.; Mrakovič, A.; Gerina, M.; Zákutná, D. Cobalt-Ferrite Nano-Cubes for Magnetic Hyperthermia Applications. J. Alloys Compd. 2024, 989, 174415. [Google Scholar] [CrossRef]
- Soetaert, F.; Kandala, S.K.; Bakuzis, A.; Ivkov, R. Experimental Estimation and Analysis of Variance of the Measured Loss Power of Magnetic Nanoparticles. Sci. Rep. 2017, 7, 6661. [Google Scholar] [CrossRef]
- Schinteie, G.; Palade, P.; Vekas, L.; Iacob, N.; Bartha, C.; Kuncser, V. Volume Fraction Dependent Magnetic Behaviour of Ferrofluids for Rotating Seal Applications. J. Phys. D Appl. Phys. 2013, 46, 395501. [Google Scholar] [CrossRef]
- Chalkidou, A.; Simeonidis, K.; Angelakeris, M.; Samaras, T.; Martinez-Boubeta, C.; Balcells, L.; Papazisis, K.; Dendrinou-Samara, C.; Kalogirou, O. In vitro application of Fe/MgO nanoparticles as magnetically mediated hyperthermia agents for cancer treatment. J. Magn. Magn. Mater. 2011, 323, 775–780. [Google Scholar] [CrossRef]
- Iglesias, C.A.M.; Araújo, J.C.R.; Xavier, J.; Anders, R.L.; de Araújo, J.M.; da Silva, R.B.; Soares, J.M.; Brito, E.L.; Streck, L.; Fonseca, J.L.C.; et al. Magnetic Nanoparticles Hyperthermia in a Non-Adiabatic and Radiating Process. Sci. Rep. 2021, 11, 11867. [Google Scholar] [CrossRef]
- Coïsson, M.; Barrera, G.; Celegato, F.; Martino, L.; Vinai, F.; Martino, P.; Ferraro, G.; Tiberto, P. Specific Absorption Rate Determination of Magnetic Nanoparticles Through Hyperthermia Measurements in Non-Adiabatic Conditions. J. Magn. Magn. Mater. 2016, 415, 2–7. [Google Scholar] [CrossRef]
- Ruta, S.; Fernández-Afonso, Y.; Rannala, S.E.; Morales, M.P.; Veintemillas-Verdaguer, S.; Jones, C.; Gutiérrez, L.; Chantrell, R.W.; Serantes, D. Beyond Newton’s Law of Cooling in Evaluating Magnetic Hyperthermia Performance: A Device-Independent Procedure. Nanoscale Adv. 2024, 6, 4207. [Google Scholar] [CrossRef]
- Natividad, E.; Castro, M.; Mediano, A. Accurate Measurement of the Specific Absorption Rate Using a Suitable Adiabatic Magnetothermal Setup. Appl. Phys. Lett. 2008, 92, 093116. [Google Scholar] [CrossRef]
- Lázaro, M.; Sola-Leyva, A.; Jimenez-Carretero, M.; Carrasco Jiménez, M.P.; Delgado, Á.V.; Iglesias, G.R. The Role of Biocompatible Coatings of Magnetic Nanorods on Their Thermal Response in Hyperthermia: Consequences on Tumor Cell Survival. J. Drug Deliv. Sci. Technol. 2024, 95, 105622. [Google Scholar] [CrossRef]
- Guibert, C.; Fresnais, J.; Peyre, V.; Dupuis, V. Magnetic Fluid Hyperthermia Probed by Both Calorimetric and Dynamic Hysteresis Measurements. J. Magn. Magn. Mater. 2017, 421, 384–392. [Google Scholar] [CrossRef]
- Coïsson, M.; Barrera, G.; Celegato, F.; Martino, L.; Kane, S.N.; Raghuvanshi, S.; Vinai, F.; Tiberto, P. Hysteresis Losses and Specific Absorption Rate Measurements in Magnetic Nanoparticles for Hyperthermia Applications. Biochim. Biophys. Acta 2017, 1861, 1545–1558. [Google Scholar] [CrossRef] [PubMed]
- Garaio, E.; Collantes, J.M.; Garcia, J.A.; Plazaola, F.; Mornet, S.; Couillaud, F.; Sandre, O. A Wide-Frequency Range AC Magnetometer to Measure the Specific Absorption Rate in Nanoparticles for Magnetic Hyperthermia. J. Magn. Magn. Mater. 2014, 368, 432–437. [Google Scholar] [CrossRef]
- Rodrigo, I.; Castellanos-Rubio, I.; Garaio, E.; Arriortua, O.K.; Insausti, M.; Orue, I.; Garcia, J.A.; Plazaola, F. Exploring the Potential of Dynamic Hysteresis Loops via High Field, High Frequency, and Temperature-Adjustable AC Magnetometer for Magnetic Hyperthermia Characterization. Int. J. Hyperth. 2020, 37, 976–991. [Google Scholar] [CrossRef]
- Sámano, A.H.; Rosales, S.; Mazon, E.E.; Casillas, N.; Topete, A.; Paz, J.A.; Quintero, L.H.; Estrada, J.C.; Cano, M.E. A Dynamic Hysteresis Meter for Studying Ferrofluids Designed for Magnetic Hyperthermia. Meas. Sci. Technol. 2020, 31, 055902. [Google Scholar] [CrossRef]
- Zapata, J.C.; Restrepo, J. Dynamic Hysteretic Properties and Specific Loss Power of Magnetic Nanoparticles in a Viscous Medium at Different Thermal Baths. AIP Adv. 2023, 13, 105110. [Google Scholar] [CrossRef]
- Glöck, G.; Hergt, R.; Zeisberger, M.; Dutz, S.; Nagel, S.; Weitschies, W. The Effect of Field Parameters, Nanoparticle Properties, and Immobilization on the Specific Heating Power in Magnetic Particle Hyperthermia. J. Phys. Condens. Matter 2006, 18, S2935–S2949. [Google Scholar] [CrossRef]
- Nakamura, K.; Ueda, K.; Tomitaka, A.; Yamada, T.; Takemura, Y. Self-Heating Temperature and AC Hysteresis of Magnetic Iron Oxide Nanoparticles and Their Dependence on Secondary Particle Size. IEEE Trans. Magn. 2013, 49, 240–243. [Google Scholar] [CrossRef]
- Ognjanović, M.; Stanković, D.M.; Jaćimović, Ž.K.; Kosović-Perutović, M.; Dojčinović, B.; Antić, B. The Effect of Surface-Modifier of Magnetite Nanoparticles on Electrochemical Detection of Dopamine and Heating Efficiency in Magnetic Hyperthermia. J. Alloys Compd. 2021, 884, 161075. [Google Scholar] [CrossRef]
- Ferreira-Filho, V.C.; Morais, B.; Vieira, B.J.C.; Waerenborgh, J.C.; Carmezim, M.J.; Tóth, C.N.; Même, S.; Lacerda, S.; Jaque, D.; Sousa, C.T.; et al. Influence of SPION Surface Coating on Magnetic Properties and Theranostic Profile. Molecules 2024, 29, 1824. [Google Scholar] [CrossRef]
- Vassallo, M.; Martella, D.; Barrera, G.; Celegato, F.; Coïsson, M.; Ferrero, R.; Olivetti, E.S.; Troia, A.; Sözeri, H.; Parmeggiani, C.; et al. Improvement of Hyperthermia Properties of Iron Oxide Nanoparticles by Surface Coating. ACS Omega 2023, 8, 2143–2154. [Google Scholar] [CrossRef]
- Khan, F.; Lahiri, B.B.; Srujana, M.; Vidya, R.; Philip, J. Induction Heating of Magnetic Nanoparticles: Role of Thermo-Physical Properties of the Coating Moieties. Colloids Surf. A Physicochem. Eng. Asp. 2024, 692, 133982. [Google Scholar] [CrossRef]
- Osaci, M.; Cacciola, M. About the Influence of the Colloidal Magnetic Nanoparticles Coating on the Specific Loss Power in Magnetic Hyperthermia. J. Magn. Magn. Mater. 2021, 519, 167451. [Google Scholar] [CrossRef]
- Rennick, J.J.; Johnston, A.P.R.; Parton, R.G. Key Principles and Methods for Studying the Endocytosis of Biological and Nanoparticle Therapeutics. Nat. Nanotechnol. 2021, 16, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Upadhyay, R.V.; Parekh, K.; Reis, D.; Oliveira, C.L.P.; Neto, A.M.F. Optimized Mn0.5Zn0.5Fe2O4 Nanoflowers Based Magnetic Fluids for Potential Biomedical Applications. J. Magn. Magn. Mater. 2024, 590, 171656. [Google Scholar] [CrossRef]
- Bejko, M.; Al Yaman, Y.; Keyes, A.; Bagur, A.; Rosa, P.; Gayot, M.; Weill, F.; Mornet, S.; Sandre, O. Structure-Function Relationship of Iron Oxide Nanoflowers: Optimal Sizes for Magnetic Hyperthermia Depending on Alternating Magnetic Field Conditions. ChemPhysChem 2024, 25, e202400023. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacob, N. Pitfalls and Challenges in Specific Absorption Rate Evaluation for Functionalized and Coated Magnetic Nanoparticles Used in Magnetic Fluid Hyperthermia. Coatings 2025, 15, 345. https://doi.org/10.3390/coatings15030345
Iacob N. Pitfalls and Challenges in Specific Absorption Rate Evaluation for Functionalized and Coated Magnetic Nanoparticles Used in Magnetic Fluid Hyperthermia. Coatings. 2025; 15(3):345. https://doi.org/10.3390/coatings15030345
Chicago/Turabian StyleIacob, Nicusor. 2025. "Pitfalls and Challenges in Specific Absorption Rate Evaluation for Functionalized and Coated Magnetic Nanoparticles Used in Magnetic Fluid Hyperthermia" Coatings 15, no. 3: 345. https://doi.org/10.3390/coatings15030345
APA StyleIacob, N. (2025). Pitfalls and Challenges in Specific Absorption Rate Evaluation for Functionalized and Coated Magnetic Nanoparticles Used in Magnetic Fluid Hyperthermia. Coatings, 15(3), 345. https://doi.org/10.3390/coatings15030345





