Biomass-Derived Hard Carbon Materials for High-Performance Sodium-Ion Battery
Abstract
1. Introduction
2. Experiment
2.1. Laboratory Reagents and Equipment
2.2. Materials Preparation and Synthesis
2.3. Sample Characterization
2.4. Battery Assembly and Electrochemical Performance Testing
3. Results and Discussion

4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shao, W.; Shi, H.; Jian, X.; Wu, Z.-S.; Hu, F. Hard-carbon anodes for sodium-ion batteries: Recent status and challenging perspectives. Adv. Energy Sustain. Res. 2022, 3, 2200009. [Google Scholar] [CrossRef]
- Zhang, F.; Yao, Y.; Wan, J.; Henderson, D.; Zhang, X.; Hu, L. High temperature carbonized grass as a high performance sodium ion battery anode. ACS Appl. Mater. Interfaces 2017, 9, 391397. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.M.; Zhang, K.Y.; Yang, J.L.; Gu, Z.Y.; Wu, X.L. Differential bonding behaviors of sodium/potassium-ion storage in sawdust waste carbon derivatives. Chin. Chem. Lett. 2024, 35, 109304. [Google Scholar] [CrossRef]
- Chen, X.; Liu, C.; Fang, Y.; Ai, X.; Zhong, F.; Yang, H.; Cao, Y. Understanding of the sodium storage mechanism in hard carbon anoeds. Carbon Energy 2022, 4, 10031284. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, H.; He, X.; Chen, Q.; Zhao, J.; Wei, Y.; Wu, X.; Zhang, Z.; Jiang, Y.; Chou, S. Pre-oxidation strategy transforming waste foam to hard carbon anodes for boosting sodium storage performance. Small 2023, 20, 2307132. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Rojo, T.; Li, X. Hard carbon as sodium-ion battery anodes progress and challenges. ChemSusChem 2019, 12, 133144. [Google Scholar] [CrossRef]
- Deng, W.; Cao, Y.; Yuan, G.; Liu, G.; Zhang, X.; Xia, Y. Realizing improved sodium-ion storage by introducing carbonyl groups and closed micropores into a biomass derived hard carbon anode. ACS Appl. Mater. Interfaces 2021, 13, 4772847739. [Google Scholar] [CrossRef]
- Ying, H.J.; Han, W.Q. Metallic Sn-Based Anode Materials: Application in High-Performance Lithium-Ion and Sodium-Ion Batteries. Adv. Sci. 2017, 11, 1700298. [Google Scholar] [CrossRef]
- Minakshi, M.; Barmi, M.; Mitchell, D.R.; Barlow, A.J.; Fichtner, M. Effect of oxidizer in the synthesis of NiMoO4 anchored nanostructure nickel molybdate for sodium-ion battery. Mater. Today Energy 2018, 10, 114. [Google Scholar]
- Ahmad, N.; Rinaldi, A.; Sidoli, M.; Magnani, G.; Vezzoni, V.; Scaravonati, S.; Pontiroli, D. Pre-treated biomass waste melon peels for high energy density semi solid-state supercapacitors. J. Power Sources 2024, 624, 235511. [Google Scholar] [CrossRef]
- Wickramaarachchi, W.A.M.K.P.; Minakshi, M.; Gao, X.; Dabare, R.; Wong, K.W. Hierarchical porous carbon from mango seed husk for electro chemical energy storage. Chem. Eng. J. Adv. 2021, 8, 100158. [Google Scholar] [CrossRef]
- Lei, X.; Zhang, L.; Guo, X.; Tian, Q.; Fan, X.; Tong, H.; Yang, Y. Regulation of composition, microstructure, and pore structure of biomass-based hard carbon to boost the sodium storage performance. J. Energy Storage 2024, 101, 113792. [Google Scholar] [CrossRef]
- Yan, B.; Han, C.; Dai, Y.; Li, M.; Wu, Z.; Gao, X. Biomass derived hard carbon materials for sodium ion battery anodes: Exploring the influence of carbon source on structure and sodium storage performance. Fuel 2024, 371, 132141. [Google Scholar] [CrossRef]
- Dahbi, M.; Kiso, M.; Kubota, K.; Horiba, T.; Chafik, T.; Hida, K.; Matsuyama, T.; Komaba, S. Synthesis of Hard Carbons from Argan Shell for Na-Ion Batteries. J. Mater. Chem. A 2017, 5, 99179928. [Google Scholar] [CrossRef]
- Kydyrbayeva, U.; Baltash, Y.; Mukhan, O.; Nurpeissova, A.; Kim, S.S.; Bakenov, Z.; Mukanova, A. The buckwheat-derived hard carbon as an anode material for sodium-ion energy storage system. J. Energy Storage 2024, 96, 112629. [Google Scholar] [CrossRef]
- Cao, B.; Li, X.F. Recent Progress on Carbon-based Anode Materials for Na-ion Batteries. Acta Phys.-Chim. Sin. 2020, 36, 1905003. [Google Scholar]
- Wang, H.; Niu, H.; Shu, K.; Sun, L.; Wang, Y.; Du, Y.; Kang, Y.M. Regulating the “core-shell” microstructure of hard carbon through sodium hydroxide activation for achieving high-capacity SIBs anode. J. Mater. Sci. Technol. 2025, 209, 161170. [Google Scholar] [CrossRef]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-Ion Batteries. Adv. Funct. Mater. 2013, 23, 32553255. [Google Scholar] [CrossRef]
- Xie, F.; Xu, Z.; Guo, Z.; Titirici, M.-M. Hard carbons for sodium ion batteries and beyond. Prog. Energy 2020, 2, 042002. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Meng, Q.; Jensen, A.C.S.; Zhang, Q.; Zhang, Q.; Tong, Y.; Qi, Y.; Gu, L.; Titirici, M.; et al. Regulating pore structure of hierarchical porous waste cork derived hard carbon anode for enhanced Na storage performance. Adv. Energy Mater. 2019, 9, 1902852. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Lin, R.; Zhang, C.; Xiao, T.; Ma, Z.; Ma, X. Enhancing electrochemical performance in sodium ion batteries: Strategic modification of oxygen-containing functional groups in hard carbon. Fuel 2025, 381, 133397. [Google Scholar] [CrossRef]
- Stevens, D.A.; Dahn, J.R. The Mechanisms of Lithium and Sodium Insertion in Carbon Materials. J. Electrochem. Soc. 2001, 148, A803A811. [Google Scholar] [CrossRef]
- Fang, C.; Huang, Y.; Zhang, W.; Han, J.; Deng, Z.; Cao, Y.; Yang, H. Routes to High Energy Cathodes of Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1501727. [Google Scholar] [CrossRef]
- Ni, Q.; Bai, Y.; Wu, F.; Wu, C. Polyanion-Type Electrode Materials for Sodium-Ion Batteries. Adv. Sci. 2017, 4, 1600275. [Google Scholar] [CrossRef]
- Chu, Y.; Zhang, J.; Zhang, Y.; Li, Q.; Jia, Y.; Dong, X.; Xiao, J.; Tao, Y.; Yang, Q. Reconfiguring hard carbons with emerging sodium ion batteries: A perspective. Adv. Mater. 2023, 35, 2212186. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Liu, Y.; Bai, Y.; Chen, G.; Li, Y.; Lu, J. Analysis of the Stable Interphase Responsible for the Excellent Electrochemical Performance of Graphite Electrodes in Sodium-Ion Batteries. Small 2020, 16, 2003268. [Google Scholar] [CrossRef]
- Sun, F.; Wang, H.; Qu, Z.; Wang, K.; Wang, L.; Gao, J.; Lu, Y. Carboxyl dominant oxygen rich carbon for improved sodium ion storage: Synergistic enhancement of adsorption and intercalation mechanisms. Adv. Energy Mater. 2021, 11, 2002981. [Google Scholar] [CrossRef]
- Arie, A.A.; Tekin, B.; Demir, E.; Demir-Cakan, R. Hard carbons derived from waste tea bag powder as anodes for sodium ion battery. Mater. Technol. 2019, 34, 515524. [Google Scholar] [CrossRef]
- El Moctar, I.; Ni, Q.; Bai, Y.; Wu, F.; Wu, C. Hard Carbon Anode Materials for Sodium-Ion Batteries. World Sci. 2018, 11, 1830003. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, J.; Zhang, P.; Li, Z.; Zhao, H. Abnormal preferential oxygen functionalization on the surface of soft/hard carbon for sodium storage. Appl. Surf. Sci. 2022, 602, 154336. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Wu, D.; Sun, F.; Gao, J.; Lai, X.; Zhao, G. Microcrystalline regulation of bituminous coal derived hard carbon by pre-oxidation strategy for improved sodium-ion storage. Fuel 2022, 310, 122072. [Google Scholar] [CrossRef]
- Qian, J.; Chen, Y.; Wu, L.; Cao, Y.; Ai, X.; Yang, H. High capacity Na-storage and superior cyclability of nanocomposite Sb/C anode for Na-ion batteries. Chem. Commun. 2012, 48, 70707072. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Lu, H.; Fang, Y.; Sushko, M.L.; Cao, Y.; Ai, X.; Yang, H.; Liu, J. Low-defect and low-porosity hard carbon with high coulombic efficiency and high capacity for practical sodium ion battery anode. Adv. Energy Mater. 2018, 8, 1703238. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Z.; Zhu, W.; Gariépy, V.; Gagnon, C.; Provencher, M.; Laul, D.; Veillette, R.; Trudeau, M.L.; Guerfi, A.; et al. High capacity and high efficiency maple tree-biomass-derived hard carbon as an anode material for sodium ion batteries. Materials 2018, 11, 1294. [Google Scholar] [CrossRef]
- Chen, X.; Tian, J.; Li, P.; Fang, Y.; Fang, Y.; Liang, X.; Cao, Y. An overall understanding of sodium storage behaviors in hard carbons by an “adsorption-intercalation/filling” hybrid mechanism. Adv. Energy Mater. 2022, 12, 2200886. [Google Scholar] [CrossRef]
- Li, Y.; Qian, J.; Zhang, M.; Wang, S.; Wang, Z.; Li, M.; Bai, Y.; An, Q.; Xu, H.; Wu, F.; et al. Co-Construction of Sulfur Vacancies and Heterojunctions in Tungsten Disulfide to Induce Fast Electronic/Ionic Diffusion Kinetics for Sodium-Ion Batteries. Adv. Mater. 2020, 32, 2005802. [Google Scholar] [CrossRef]
- Simone, V.; Boulineau, A.; de Geyer, A.; Rouchon, D.; Simonin, L.; Martinet, S. Hard carbon derived from cellulose as anode for sodium ion batteries: Dependence of electrochemical properties on structure. J. Energy Chem. 2016, 25, 761768. [Google Scholar] [CrossRef]
- Zhao, L.F.; Hu, Z.; Lai, W.H.; Tao, Y.; Peng, J.; Miao, Z.C.; Dou, S.X. Hard carbon anodes: Fundamental understanding and commercial perspectives for Na-ion batteries beyond Li-ion and K-ion counterparts. Adv. Energy Mater. 2021, 11, 2002704. [Google Scholar] [CrossRef]
- Sun, D.; Zhao, L.; Sun, P.; Zhao, K.; Sun, Y.; Zhang, Q.; Ma, X. Rationally Regulating Closed Pore Structures by Pitch Coating to Boost Sodium Storage Performance of Hard Carbon in Low-voltage Platforms. Adv. Funct. Mater. 2024, 34, 2403642. [Google Scholar] [CrossRef]
- Ma, R.; Chen, Y.; Li, Q.; Zhang, B.; Chen, F.; Leng, C.; Jia, D.; Guo, N.; Wang, L. Oxygen-driven closing pore formation in coal-based hard carbon for low-voltage rapid sodium storage. Chem. Eng. J. 2024, 493, 153389. [Google Scholar] [CrossRef]
- Xu, T.; Qiu, X.; Zhang, X.; Xia, Y. Regulation of surface oxygen functional groups and pore structure of bamboo-derived hard carbon for enhanced sodium storage performance. Chem. Eng. J. 2023, 452, 139514. [Google Scholar] [CrossRef]
- Wahid, M.; Puthusseri, D.; Gawli, Y.; Sharma, N.; Ogale, S. Hard carbons for sodium ion battery anodes: Synthetic strategies, material properties, and storage mechanisms. ChemSusChem 2018, 11, 506526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, L.; Ren, Q.; Yan, L.; Zhang, F.; Lv, W.; Shi, Z. Tailoring a Phenolic Resin Precursor by Facile Pre-oxidation Tactics to Realize a High-Initial-Coulombic-Efficiency Hard Carbon Anode for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 3165031659. [Google Scholar] [CrossRef] [PubMed]

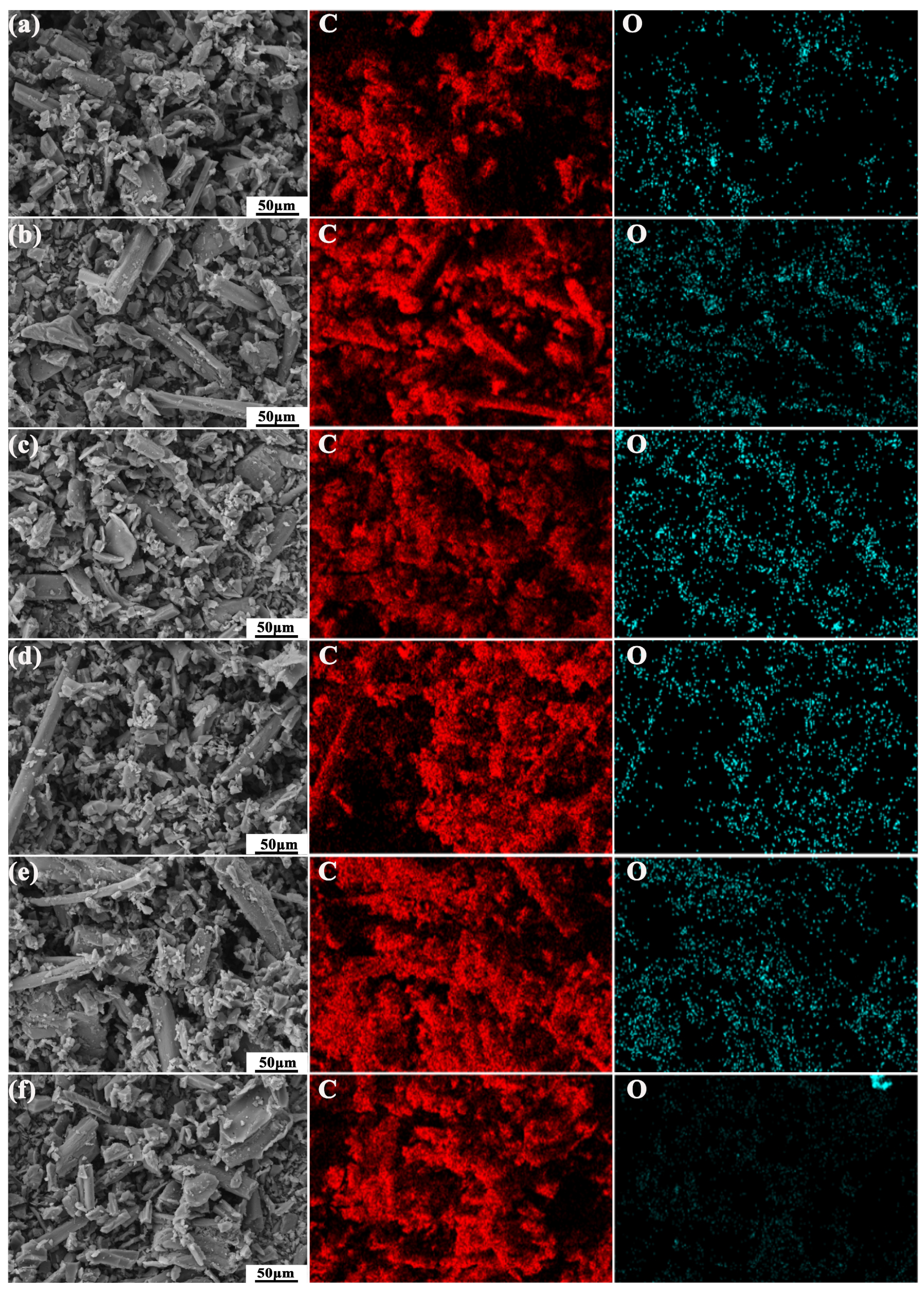
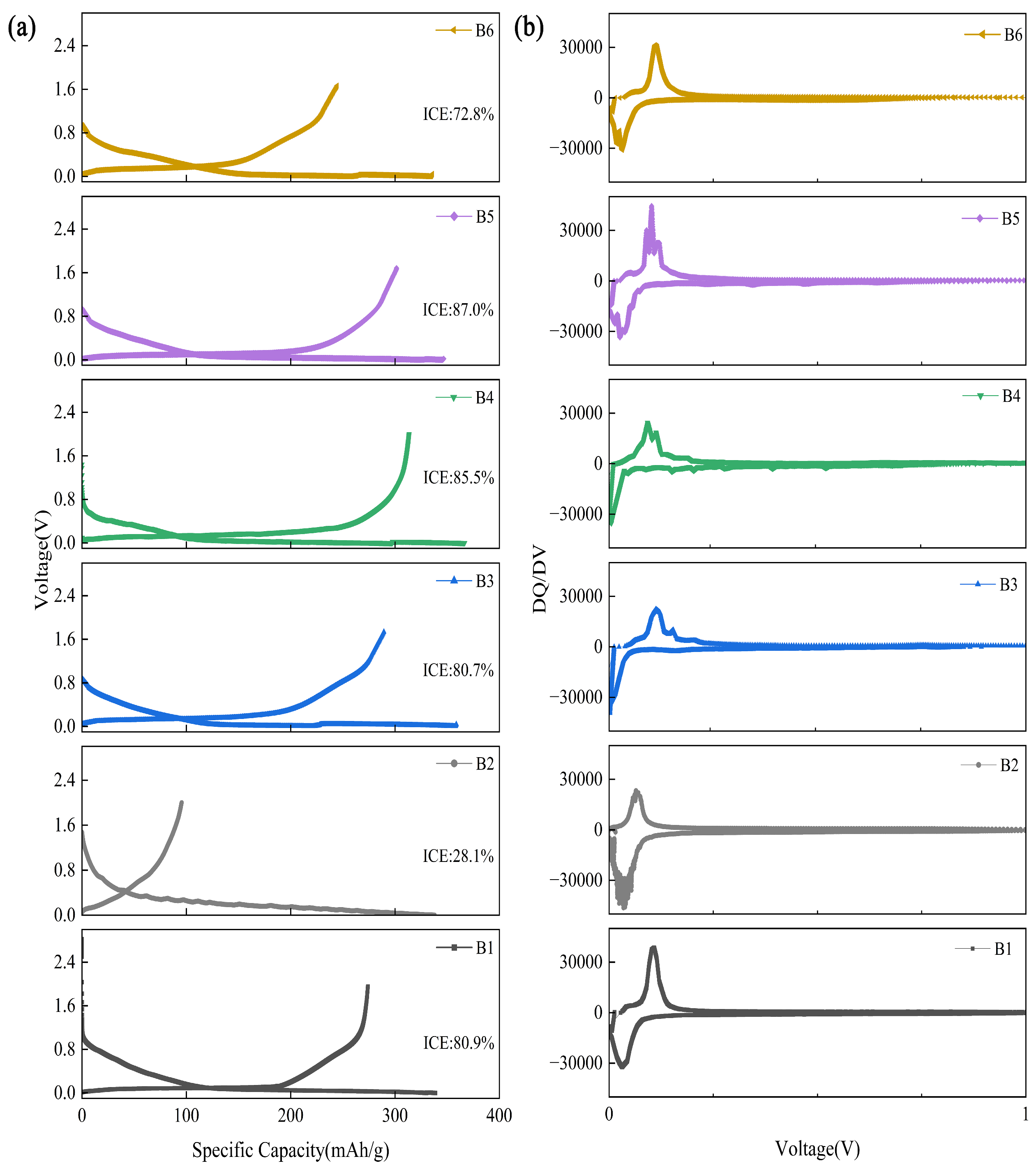
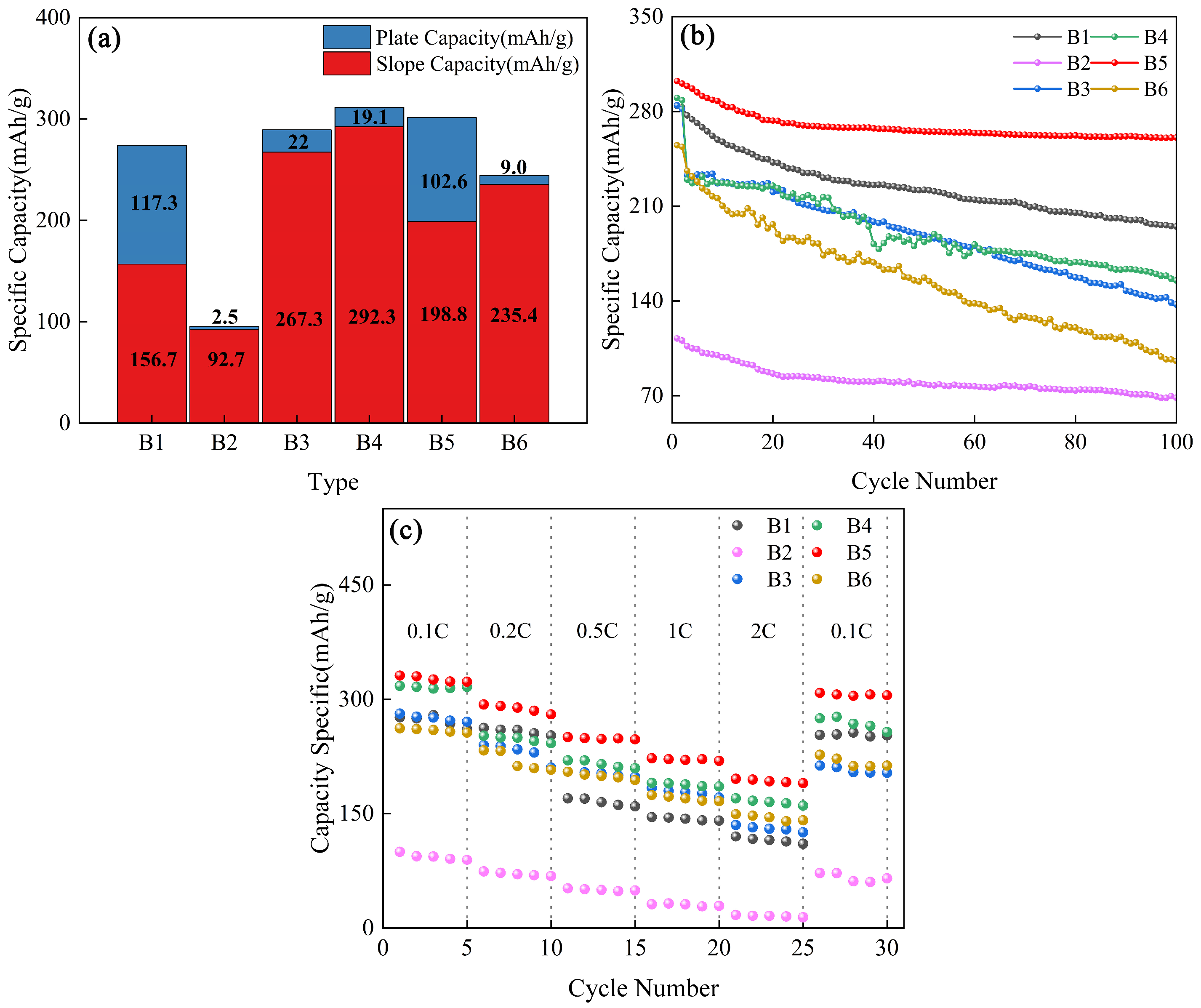
| Type | C (wt%) | O (wt%) | SBET (m2/g) |
|---|---|---|---|
| B1 | 97.93 | 2.07 | 3.8 |
| B2 | 97.63 | 2.37 | 3.9 |
| B3 | 97.40 | 2.60 | 4.1 |
| B4 | 96.66 | 3.34 | 4.6 |
| B5 | 96.29 | 3.71 | 4.9 |
| B6 | 96.23 | 3.77 | 5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Cui, J.; Wang, S.; Xu, W.; Guo, R. Biomass-Derived Hard Carbon Materials for High-Performance Sodium-Ion Battery. Coatings 2025, 15, 156. https://doi.org/10.3390/coatings15020156
Chen Y, Cui J, Wang S, Xu W, Guo R. Biomass-Derived Hard Carbon Materials for High-Performance Sodium-Ion Battery. Coatings. 2025; 15(2):156. https://doi.org/10.3390/coatings15020156
Chicago/Turabian StyleChen, Yixing, Jiaming Cui, Sheng Wang, Wentao Xu, and Ruoqi Guo. 2025. "Biomass-Derived Hard Carbon Materials for High-Performance Sodium-Ion Battery" Coatings 15, no. 2: 156. https://doi.org/10.3390/coatings15020156
APA StyleChen, Y., Cui, J., Wang, S., Xu, W., & Guo, R. (2025). Biomass-Derived Hard Carbon Materials for High-Performance Sodium-Ion Battery. Coatings, 15(2), 156. https://doi.org/10.3390/coatings15020156




