1. Introduction
Sodium-ion batteries have gradually become a research hotspot owing to their use of abundant sodium resources, low cost, and similar electrochemical mechanisms to lithium-ion batteries. They have the potential to make outstanding contributions to large-scale energy storage systems [
1,
2,
3,
4,
5,
6]. However, to meet the engineering and technical requirements of sodium-ion batteries, the available separators still need to go through a long process. As is well known, separators play an important role in preventing contact between the cathode and anode, as well as allowing ions to coordinate with the electrolyte within the battery, and have a significant impact on the performance of the battery, such as thermal safety, mechanical safety, rate performance, and cycle life [
7,
8,
9,
10,
11]. Therefore, the synergistic development of separator and electrode materials in sodium-ion batteries is extremely necessary [
12].
In recent years, with the gradual maturity of electrostatic spinning technology and the good heat resistance of some polymers, the method of using non-woven fabrics prepared from them as separators for lithium-ion batteries has become popular. Non-woven fabric membranes generally have good porosity, up to 80% or more, and larger pore sizes, which are very conducive to absorbing and storing electrolytes [
13,
14]. High safety diaphragms not only require a high heat-resistant shrinkage temperature to prevent direct contact between the positive and negative electrodes of the battery at high temperatures but also have good mechanical strength [
15,
16]. They can not only maintain stability during battery assembly and other operations but also effectively prevent direct contact between the positive and negative electrodes inside the battery at the pole end, which can lead to internal short circuits and further increases in temperature, causing greater danger [
17,
18].
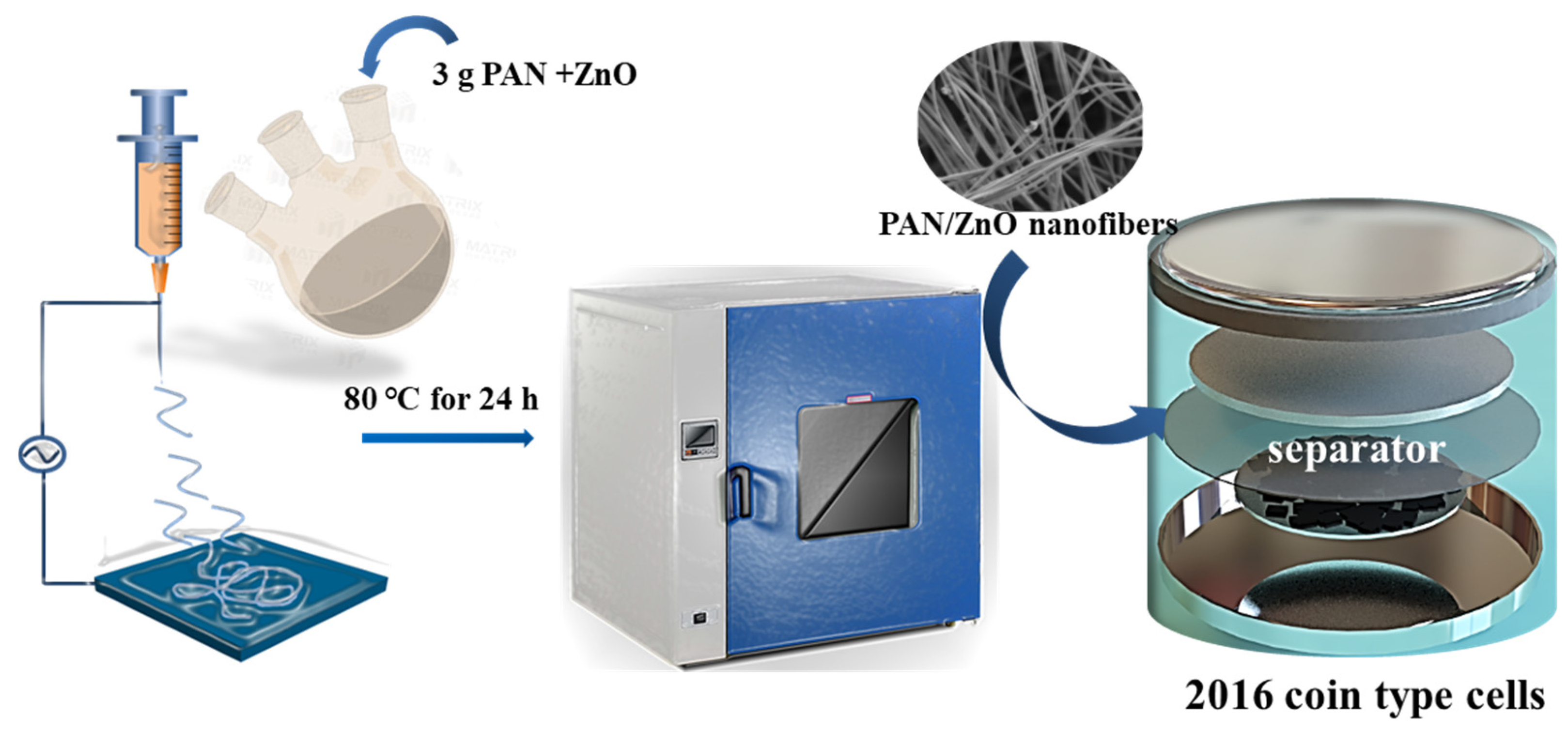
This article mainly explores the preparation and characterization of a novel separator for sodium-ion batteries. PAN/ZnO (polyacrylonitrile/ZnO nanofiber films) nanofiber thin films were prepared by electrostatic spinning technology, and the cyclic stability of the nanofiber separator was improved by changing the amount of ZnO nanoparticle doping. This article provides a novel flexible PAN composite separator for sodium-ion batteries, which has potential application prospects in flexible and high safety sodium-ion battery systems and has good commercial value. Moreover, it provides certain theoretical guidance for the preparation of high safety organic polymer separators.
3. Results and Discussion
Figure 2 shows the field emission scanning electron microscope figure of ZnO nanoparticles. From
Figure 2a, it can be seen that ZnO nanoparticles have a uniformly distributed nanosphere structure without large clusters. From
Figure 2b, it can be seen that the diameter of the ZnO nanoparticle is about 20 nm.
Figure 3a shows the carbon nanofiber applied to the negative electrode of the sodium-ion battery in this article. The diameter of the nanofiber is around 200 nm, and the surface is smooth without defects, with a uniform diameter distribution.
Figure 3b–f show the PAN/ZnO nanofiber applied to sodium-ion battery separators in this article.
Figure 3b,c show the PAN/ZnO nanofiber doped with 4%. From the figure, it can be seen that the diameter of the PAN/ZnO-4% nanofiber is about 500 nm, and the pore size distribution is disordered, with a pore size of about 7 μm. As shown in
Figure 3b,c, the PAN/ZnO nanofiber doped with 7% is shown. It can be seen from the images that the diameter of the PAN/ZnO-7% nanofiber is about 500 nm, and the pore size distribution is disordered, with a pore size of about 3 um. Compared to
Figure 3d,e, it can be observed that the pore size of PAN/ZnO-7% has decreased, and there are no obvious droplet or bead defects in the nanofiber, resulting in uniform fiber thickness. Shown in
Figure 3f is the 10% doped PAN/ZnO nanofiber, and it can be seen from the figure that there are obvious irregular small balls present, with uneven distribution of the fiber thickness.
Figure 4 shows the transmission electron microscopy figure of the PAN/ZnO-7% nanofiber. From
Figure 4a, it can be seen that the diameter of the PAN/ZnO-7% nanofiber is 500 nm, and the nanoparticles of ZnO can be clearly seen. From
Figure 4b, it can be seen that the diameter of the nanoparticles in the PAN/ZnO-7% nanofiber is approximately 20 nm, and they are uniformly distributed inside the composite nanofiber. The nanofiber structure is intact.
Figure 5a shows the XRD of a carbon nanofiber and PAN nanofiber. From
Figure 5a, it can be seen that there are two broad peaks of 16.3° and 22.3° for PAN; after heat treatment of the PAN nanofiber, the carbon nanofiber applied in this article was obtained. As shown in
Figure 5a, the carbon fibers disappeared with a broad wind of 16.3° and only had a broad peak of 22.6°, indicating complete carbonization and obtaining the complete carbon nanofiber.
Figure 5b shows the XRD patterns of the 4%, 7%, and 10% PAN/ZnO nanofibers. It can be seen from the images that there are two broad peaks of about 16.6° and 22.5° in the 4%, 7%, and 10% PAN/ZnO nanofibers, which are characteristic peaks of PAN and ZnO nanoparticles, as at 2θ, sharp characteristic diffraction peaks appear at 31.8°, 34.4°, 36.2°, 47.5°, 56.6°, 62.8°, 66.3°, 67.9°, and 69°, respectively. From
Figure 5b, it can be seen that, for the PAN/ZnO nanofiber with doping concentrations of 4%, 7%, and 10%, the higher the doping concentration of the ZnO nanoparticles in the XRD pattern, the stronger the diffraction peak of ZnO. The XRD pattern in
Figure 5b shows the diffraction peak structure of PAN and ZnO.
Figure 5c shows a physical image of the PAN/ZnO nanofiber, which has excellent flexibility.
Figure 6a,b show the cyclic voltammetry curves of the 4%, 7%, and 10% PAN/ZnO nanofibers as separator sodium-ion batteries. According to the CV curves in CNF and lithium metal half-cells, the operating voltage window of the half-cell is 0–2 V. From the figure, it can be seen that the cyclic voltammetry peaks of 4%, 7%, and 10% PAN/ZnO nanofibers as separators for sodium-ion batteries are similar, and the cyclic voltammetry curves of PAN/ZnO nanofiber-4% and PAN/ZnO nanofiber-7%, and PAN/ZnO nanofiber as the separator for sodium-ion batteries, have good repeatability for two to three cycles and excellent cycling performance.
Figure 7a shows the cycle curves of 4%, 7%, and 10% PAN/ZnO nanofibers as separators for sodium-ion batteries. It can be seen from
Figure 6a that the cycle stability of PAN/ZnO-4% nanofiber as a separator for sodium-ion batteries is poor. At a current density of 0.1 A/g after 40 cycles, the discharge-specific capacity of PAN/ZnO-4% nanofiber as a separator for sodium-ion batteries rapidly decreases, and it only has a specific capacity of 13.02 mAh/g after 120 cycles, accounting for 13.6% of the initial discharge-specific capacity. The cycling stability of PAN/ZnO-10% nanofiber as a separator for sodium-ion batteries is stronger than that of PAN/ZnO-4% nanofiber as a separator for sodium-ion batteries, but the capacity is lower. At a current density of 0.1 A/g after 20 cycles, it has a specific capacity of 17.3 mAh/g
−1, with a capacity retention rate of 54.8% for the initial discharge-specific capacity. PAN/ZnO-7% nanofiber as a separator for sodium-ion batteries has a better cycling performance. After 120 cycles at a current density of 0.1 A/g, the discharge-specific capacity of PAN/ZnO-7% nanofiber as a separator for sodium-ion batteries still remains at 92.22 mAh/g, accounting for 95.6% of the initial discharge-specific capacity, and its Coulomb effect remains around 97%. The PAN/ZnO-7% nanofiber has a better cycling performance and stability as a separator for sodium-ion batteries, attributed to their smaller pore size and more uniform nanofiber structure without small balls.
Figure 7b shows the rate plots of the 7% and 10% PAN/ZnO nanofibers used as separator sodium-ion batteries. At current densities of 0.1, 0.2, 0.3, and 0.5 A/g, the discharge-specific capacities of the PAN/ZnO-7% nanofiber used as a separator for sodium-ion batteries are 125.4, 32.3, 15.4, and 6.3 mAh/g, respectively. When the current density recovers to 0.1 C, it can be maintained at the reversible discharge-specific capacity of 92.1 mAh/g. Compared to PAN/ZnO-10% nanofiber membrane sodium-ion batteries, PAN/ZnO-7% nanofiber membrane sodium-ion batteries have a better specific capacity at different current densities.
Figure 7c shows the first cycle charge discharge curves of the 4%, 7%, and 10% PAN/ZnO nanofiber membrane sodium-ion batteries. The PAN/ZnO-7% nanofiber membrane for sodium-ion batteries has a higher specific capacity and Coulombic efficiency.
Figure 7d shows the charge–discharge curves of a PAN/ZnO-7% nanofiber membrane sodium-ion battery at different current densities. It can be seen from the figure that the specific capacity changes significantly when the current density increases to 0.2 A/g. However, as the current density increases, the trend of the specific capacity changes gradually decreases and tends to stabilize after reaching 0.3 A/g.
Figure 8 shows the infiltration angle of the PAN/ZnO-7% nanofiber in an electrolyte, which is lost instantaneously after dropping. The PAN/ZnO-7% nanofiber has excellent electrolysate storage properties, which may be due to the structure of the nanofiber membrane, which increases the infiltration of the electrolysate. In general, the lower contact angle helps to promote the transmission of the ionizer, thereby improving the cycle performance for sodium-ion batteries.
Shown in
Figure 9a is the effect diagram of clamping at the beginning, stretching until pulling it off.
This material has good mechanical properties and high ductility. Now, the mechanical properties of each component are tested, and the scores of each group are obtained, as shown in the figure above. The blue line, the red line, and the black line represent PAN/ZnO-4%, PAN/ZnO-7%, and PAN/ZnO-10%. With the increase in pressure, the stress of the three color lines changes significantly.
First, when the tensible strength of PAN/ZnO-4% is close to 2 MPa, there is a clear peak, and the elongation reaches 8% before it breaks. Secondly, when the tensible strength of PAN/ZnO-7% is close to 5.5 MPa, the elongation reaches 70% before it will break. Finally, when the tensible strength of PAN/ZnO-10% is close to 4 MPa, there is an obvious peak, and the elongation is about 55% before it breaks. As shown in
Figure 9b, we know that the stress and pressure obtained are different depending on the amount of PAN/ZnO doped.
As shown in
Figure 9d, the amount of PAN/ZnO-4% breaks when the extendable force is close to 2 MPa, the amount of PAN/ZnO-7% breaks when the extendable force is close to 5.5 MPa, and the amount of PAN/ZnO-10% breaks when the extendable force is close to 3.5 MPa.
In conclusion, we may infer that the stability test of each component’s ductility and tensile stability is determined by comparing the quantity of PAN/ZnO doped in each component. Therefore, it will have good mechanical qualities, depending on how much PAN/ZnO is applied. When utilized as a sodium-ion diaphragm, PAN/ZnO-7%exhibits the highest stability and the best mechanical characteristics throughout the charge and discharge cycles, as shown in
Figure 10.
PAN/ZnO nanofiber films were prepared by electrostatic spinning technology, and the cyclic stability of the nanofiber separator was improved by changing the amount of ZnO nanoparticle doping. The nanoparticles in the PAN/ZnO-7% nanofiber are uniformly distributed inside the composite nanofiber. The PAN/ZnO-7% nanofiber has excellent electrolysate storage properties, and the lower contact angle helps to promote the transmission of the ionizer, thereby improving the cycle performance for sodium-ion batteries. Using electrodes with excellent electrolyte wettability, the assembled sodium-ion battery has high energy density and power density, a stable cycling performance, and good safety performance. When utilized as a sodium-ion diaphragm, PAN/ZnO-7% has the best mechanical properties and the strongest stability during the charge and discharge cycles. Compared to 4% and 10% PAN/ZnO nanofibers as separators for sodium-ion batteries, PAN/ZnO-7% nanofiber as a separator for sodium-ion batteries has a better specific capacity at different current densities. This work provides new insights into improved diaphragm strategies that are expected to be widely applied to sodium-ion batteries and large-scale clean energy storage technologies.