Enhanced Mechanical Durability of Polymeric Nanowires via Carbyne-Enriched Plasma Coatings for Bactericidal Action
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Carbyne-Enriched Plasma-Deposited Films
2.3. PMMA Texturing
2.4. Hydrophobic Coating
2.5. Morphology Characterizaiton, Elemental Analysis, and Wetting Characterization
2.6. Durability Tests
2.7. Bacteria Culture and Viability Testing
3. Results and Discussion
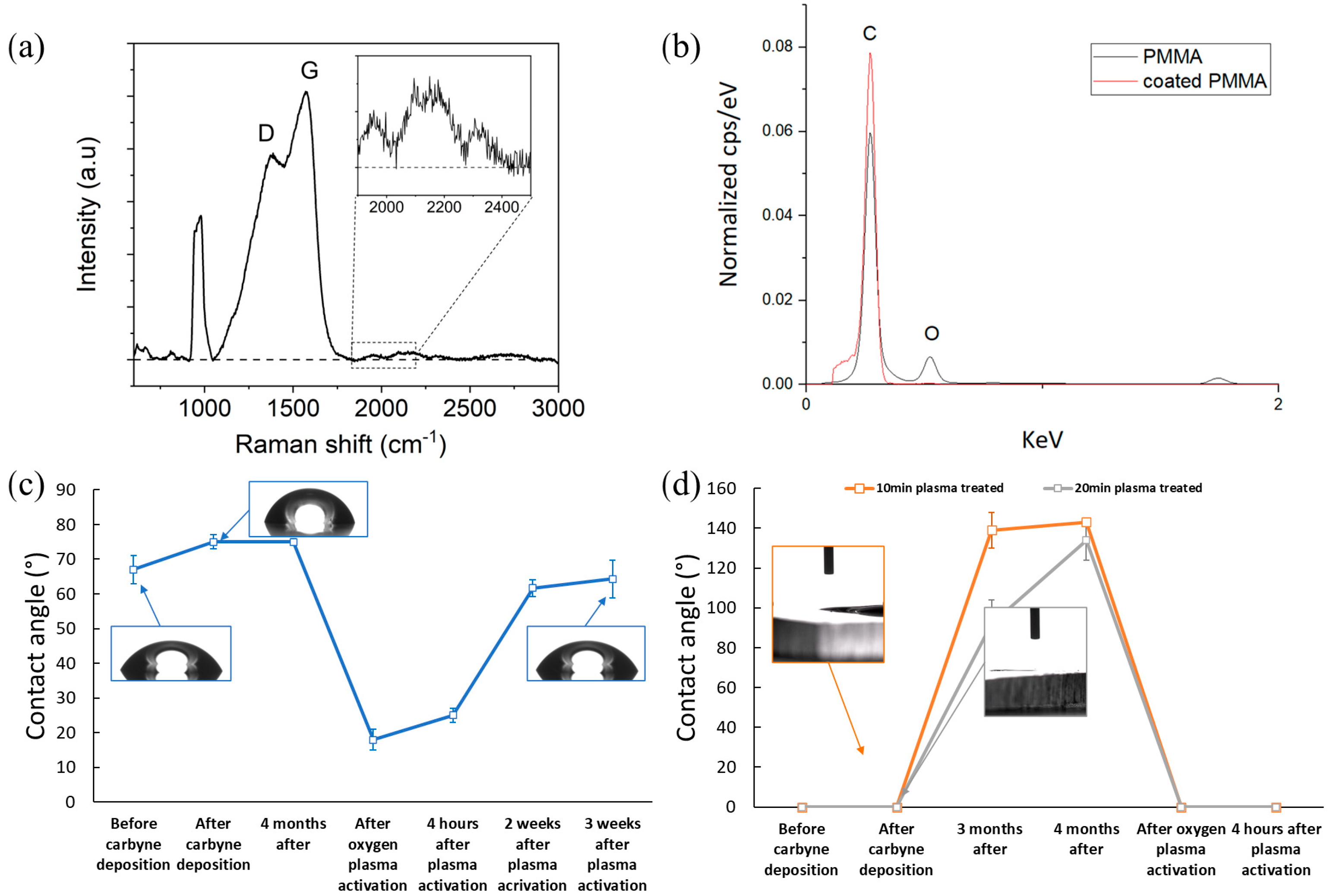
3.1. Film Characterization
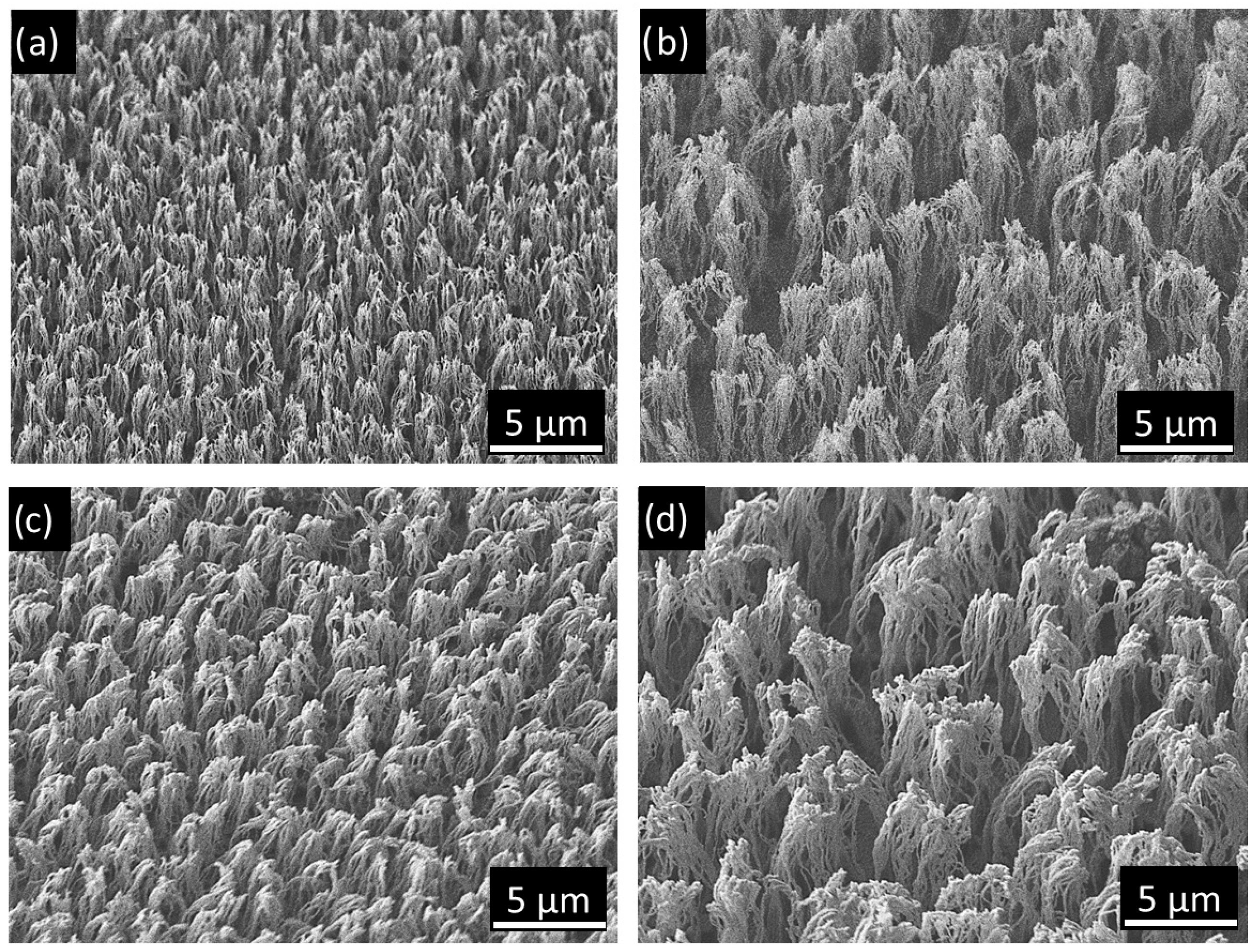
3.2. Topography Characterization of Carbyne-Enriched Coated Micro-Nanotextured Surfaces
3.3. Wetting Characterization
3.4. Surface Mechanical Durability
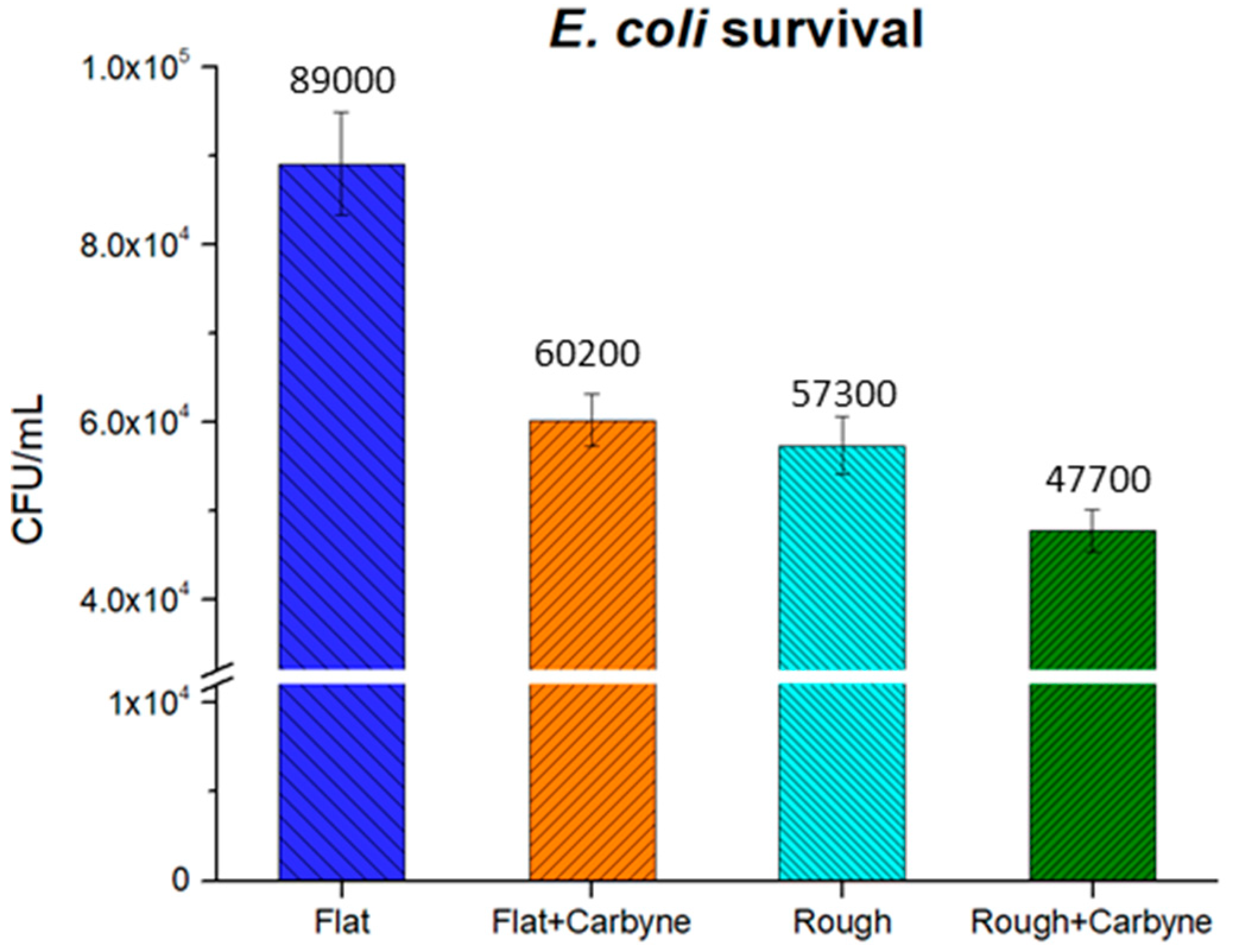
3.5. Bacteria Culture and Viability Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tapalski, D.V.; Nikolaev, N.S.; Ovsyankin, A.V.; Kochakov, V.D.; Golovina, E.A.; Matveenkov, M.V.; Sukhorukova, M.V.; Kozlov, R.S. Coatings Based on Two-Dimensionally Ordered Linear Chain Carbon for Protection of Titanium Implants from Microbial Colonization. Traumatol. Orthop. Russ. 2019, 25, 111–120. [Google Scholar] [CrossRef]
- Maliuchenko, L.I.; Nikolaev, N.S.; Pchelova, N.N.; Efimov, D.N.; Preobrazhenskaia, E.V.; Emelianov, V.U.; Longo, G. Linear-Chain Nanostructured Carbon with a Silver Film Plated on Metal Components Has a Promising Effect for the Treatment of Periprosthetic Joint Infection. Osteology 2021, 1, 238–246. [Google Scholar] [CrossRef]
- Sleptsov, V.V.; Elinson, V.M.; Simakina, N.V.; Laymin, A.N.; Tsygankov, I.V.; Kivaev, A.A.; Musina, A.D. Ophthalmological Application of Contact Lenses Modified by Means of Ion-Assisted Carbon Films. Diam. Relat. Mater. 1996, 5, 483–485. [Google Scholar] [CrossRef]
- Vlasenko, L.V.; Nechitailo, K.S. Bacterial Luminescent Biosensors in the System for Assessing the Mechanisms of Antibacterial Activity of Carbon-Based Nanomaterials. IOP Conf. Ser. Earth Environ. Sci. 2022, 979, 012056. [Google Scholar] [CrossRef]
- Itzhaki, L.; Altus, E.; Basch, H.; Hoz, S. Harder than Diamond: Determining the Cross-Sectional Area and Young’s Modulus of Molecular Rods. Angew. Chem.-Int. Ed. 2005, 44, 7432–7435. [Google Scholar] [CrossRef]
- Kotrechko, S.; Mikhailovskij, I.; Mazilova, T.; Sadanov, E.; Timoshevskii, A.; Stetsenko, N.; Matviychuk, Y. Mechanical Properties of Carbyne: Experiment and Simulations. Nanoscale Res. Lett. 2015, 10, 2–7. [Google Scholar] [CrossRef]
- Yang, G. Synthesis, Properties, and Applications of Carbyne Nanocrystals. Mater. Sci. Eng. R Rep. 2022, 151, 100692. [Google Scholar] [CrossRef]
- Guseva, M.B.; Babaev, V.G.; Novikov, N.D.; Alexandrov, A.F.; Khvostov, V.V.; Savchenko, N.F.; Bystrova, N.A. New Carbon Materials for Medical and Ecological Applications. J. Wide Bandgap Mater. 2002, 9, 273–291. [Google Scholar] [CrossRef]
- Kirpatovsky, V.I. Medical Applications of Carbynoid Materials. In Carbyne and Carbynoid Structures; Heimann, R.B., Ed.; Springer: Dordrecht, The Netherlands, 1999; pp. 427–435. ISBN 978-94-011-4742-2. [Google Scholar]
- Kavan, L.; Heimann, R.B. Other Natural Carbynoid Structures. In Carbyne and Carbynoid Structures; Heimann, R.B., Ed.; Springer: Dordrecht, The Netherlands, 1999; pp. 31–38. ISBN 978-94-011-4742-2. [Google Scholar]
- Gilkes, K.W.R.; Pillinger, C.T. Carbon—How Many Allotropes Associated with Meteorites and Impact Phenomena? In Carbyne and Carbynoid Structures; Heimann, R.B., Ed.; Springer: Dordrecht, The Netherlands, 1999; pp. 17–30. ISBN 978-94-011-4742-2. [Google Scholar]
- Hardes, J.; Ahrens, H.; Gebert, C.; Streitbuerger, A.; Buerger, H.; Erren, M.; Gunsel, A.; Wedemeyer, C.; Saxler, G.; Winkelmann, W.; et al. Lack of Toxicological Side-Effects in Silver-Coated Megaprostheses in Humans. Biomaterials 2007, 28, 2869–2875. [Google Scholar] [CrossRef] [PubMed]
- Hauert, R. A Review of Modified DLC Coatings for Biological Applications. Diam. Relat. Mater. 2003, 12, 583–589. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- Tripathy, A.; Sen, P.; Su, B.; Briscoe, W.H. Natural and Bioinspired Nanostructured Bactericidal Surfaces. Adv. Colloid Interface Sci. 2017, 248, 85–104. [Google Scholar] [CrossRef]
- Xesfyngi, Y.; Georgoutsou-Spyridonos, M.; Tripathy, A.; Milionis, A.; Poulikakos, D.; Mastellos, D.C.; Tserepi, A. A High-Performance Antibacterial Nanostructured ZnO Microfluidic Device for Controlled Bacterial Lysis and DNA Release. Antibiotics 2023, 12, 1276. [Google Scholar] [CrossRef]
- Epstein, A.K.; Hochbaum, A.I.; Kim, P.; Aizenberg, J. Control of Bacterial Biofilm Growth on Surfaces by Nanostructural Mechanics and Geometry. Nanotechnology 2011, 22, 494007. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.F.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; et al. Bactericidal Activity of Black Silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef]
- Dimitrakellis, P.; Ellinas, K.; Kaprou, G.D.; Mastellos, D.C.; Tserepi, A.; Gogolides, E. Bactericidal Action of Smooth and Plasma Micro-Nanotextured Polymeric Surfaces with Varying Wettability, Enhanced by Incorporation of a Biocidal Agent. Macromol. Mater. Eng. 2021, 306, 2000694. [Google Scholar] [CrossRef]
- Kefallinou, D.; Ellinas, K.; Speliotis, T.; Stamatakis, K.; Gogolides, E.; Tserepi, A. Optimization of Antibacterial Properties of “Hybrid” Metal-Sputtered Superhydrophobic Surfaces. Coatings 2019, 10, 25. [Google Scholar] [CrossRef]
- Ellinas, K.; Kefallinou, D.; Stamatakis, K.; Gogolides, E.; Tserepi, A. Is There a Threshold in the Antibacterial Action of Superhydrophobic Surfaces? ACS Appl. Mater. Interfaces 2017, 9, 39781–39789. [Google Scholar] [CrossRef]
- Zeniou, A.; Ellinas, K.; Olziersky, A.; Gogolides, E. Ultra-High Aspect Ratio Si Nanowires Fabricated with Plasma Etching: Plasma Processing, Mechanical Stability Analysis against Adhesion and Capillary Forces and Oleophobicity. Nanotechnology 2014, 25, 035302. [Google Scholar] [CrossRef] [PubMed]
- Chandra, D.; Yang, S. Stability of High-Aspect-Ratio Micropillar Arrays against Adhesive and Capillary Forces. Acc. Chem. Res. 2010, 43, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, M.; Kolev, G.; Dobrikov, G.; Brigadin, A.; Lukin, A. Unlocking the Carbyne-Enriched Nanocoating Sensitivity to Volatile Organic Vapors with Plasma-Driven Deposition onto Bulk Micromachined Silicon Membranes. Nanomaterials 2022, 12, 2066. [Google Scholar] [CrossRef]
- Smyrnakis, A.; Ioannou, D.; Ellinas, K.; Tserepi, A.; Gogolides, E. Real-Time Monitoring and Quantification of Underwater Superhydrophobicity. Adv. Mater. Interfaces 2022, 9, 2101393. [Google Scholar] [CrossRef]
- Tallant, D.R.; Friedmann, T.A.; Missert, N.A.; Siegal, M.P.; Sullivan, J.P. Raman Spectroscopy of Amorphous Carbon. Mater. Res. Soc. Symp.-Proc. 1997, 498, 37–48. [Google Scholar] [CrossRef]
- Khanna, R.; Ikram-Ul-Haq, M.; Rawal, A.; Rajarao, R.; Sahajwalla, V.; Cayumil, R.; Mukherjee, P.S. Formation of Carbyne-like Materials during Low Temperature Pyrolysis of Lignocellulosic Biomass: A Natural Resource of Linear Sp Carbons. Sci. Rep. 2017, 7, 16832. [Google Scholar] [CrossRef]
- Casari, C.S.; Milani, A. Carbyne: From the Elusive Allotrope to Stable Carbon Atom Wires. MRS Commun. 2018, 8, 207–219. [Google Scholar] [CrossRef]
- Ravagnan, L.; Siviero, F.; Lenardi, C.; Piseri, P.; Barborini, E.; Milani, P.; Casari, C.S.; Li Bassi, A.; Bottani, C.E. Cluster-Beam Deposition and in Situ Characterization of Carbyne-Rich Carbon Films. Phys. Rev. Lett. 2002, 89, 285506. [Google Scholar] [CrossRef]
- Casari, C.S.; Bassi, A.L.; Ravagnan, L.; Siviero, F.; Lenardi, C.; Piseri, P.; Bongiorno, G.; Bottani, C.E.; Milani, P. Chemical and Thermal Stability of Carbyne-like Structures in Cluster-Assembled Carbon Films. Phys. Rev. B-Condens. Matter Mater. Phys. 2004, 69, 075422. [Google Scholar] [CrossRef]
- Tsounidi, D.; Petrou, P.; Aleksandrova, M.; Tsanev, T.; Tserepi, A.; Gogolides, E.; Bernasik, A.; Awsiuk, K.; Janiszewska, N.; Budkowski, A.; et al. Carbyne-Enriched Carbon Coatings on Silicon Chips as Biosensing Surfaces with Stable-over-Time Biomolecule Binding Capacity. Nanomaterials 2025, 15, 1384. [Google Scholar] [CrossRef] [PubMed]
- El-Newehy, M.H.; Kim, H.Y.; Khattab, T.A.; El-Naggar, M.E. Production of Photoluminescent Transparent Poly (Methyl Methacrylate) for Smart Windows. Luminescence 2022, 37, 97–107. [Google Scholar] [CrossRef]
- Riau, A.K.; Mondal, D.; Yam, G.H.F.; Setiawan, M.; Liedberg, B.; Venkatraman, S.S.; Mehta, J.S. Surface Modification of PMMA to Improve Adhesion to Corneal Substitutes in a Synthetic Core–Skirt Keratoprosthesis. ACS Appl. Mater. Interfaces 2015, 7, 21690–21702. [Google Scholar] [CrossRef]
- Tsougeni, K.; Vourdas, N.; Tserepi, A.; Gogolides, E.; Cardinaud, C. Mechanisms of Oxygen Plasma Nanotexturing of Organic Polymer Surfaces: From Stable Super Hydrophilic to Super Hydrophobic Surfaces. Langmuir 2009, 25, 11748–11759. [Google Scholar] [CrossRef]
- Faria, B.; Silvestre, N.; Bernardes, C.; Lopes, N. Towards the Development of Nanosprings from Confined Carbyne Chains. Physical E 2020, 117, 113831. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [PubMed]
- Gogolides, E.; Constantoudis, V.; Kokkoris, G.; Kontziampasis, D.; Tsougeni, K.; Boulousis, G.; Vlachopoulou, M.; Tserepi, A. Controlling Roughness: From Etching to Nanotexturing and Plasma-Directed Organization on Organic and Inorganic Materials. J. Phys. D Appl. Phys. 2011, 44, 174021. [Google Scholar] [CrossRef]
- Nioras, D.; Ellinas, K.; Constantoudis, V.; Gogolides, E. How Different Are Fog Collection and Dew Water Harvesting on Surfaces with Different Wetting Behaviors? ACS Appl. Mater. Interfaces 2021, 13, 48322–48332. [Google Scholar] [CrossRef]
- Ioannou, D.; Shah, P.; Ellinas, K.; Kappl, M.; Sapalidis, A.; Butt, H.-J.; Gogolides, E. Antifouling Plasma-Treated Membranes with Stable Superhydrophobic Properties for Membrane Distillation. ACS Appl. Polym. Mater. 2023, 5, 9785–9795. [Google Scholar] [CrossRef]

| 40 nm Thickness | 100 nm Thickness | |
|---|---|---|
| Ion Plasma Voltage/Current (V/mA) | 2000/115 | 750/70 |
| Carbon Plasma Pulses (#) | 3000 | 7000 |
| Carbon Plasma Voltage/Frequency (V/Hz) | 300/3 | 300/3 |
| Pressure (Pa) | 0.128 | 0.128 |
| Duration (min) | 5 | 5 |
| PMMA Surface | I (%) | δΙ (%) |
|---|---|---|
| Flat + Carbyne | 32 | 5 |
| Rough | 36 | 6 |
| Rough + Carbyne | 46 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nioras, D.; Kefallinou, D.; Ioannou, D.; Panes-Ruiz, L.A.; Ibarlucea, B.; Cuniberti, G.; Lan, T.; Tserepi, A.; Gogolides, E. Enhanced Mechanical Durability of Polymeric Nanowires via Carbyne-Enriched Plasma Coatings for Bactericidal Action. Coatings 2025, 15, 1247. https://doi.org/10.3390/coatings15111247
Nioras D, Kefallinou D, Ioannou D, Panes-Ruiz LA, Ibarlucea B, Cuniberti G, Lan T, Tserepi A, Gogolides E. Enhanced Mechanical Durability of Polymeric Nanowires via Carbyne-Enriched Plasma Coatings for Bactericidal Action. Coatings. 2025; 15(11):1247. https://doi.org/10.3390/coatings15111247
Chicago/Turabian StyleNioras, Dimitrios, Dionysia Kefallinou, Dimosthenis Ioannou, Luis Antonio Panes-Ruiz, Bergoi Ibarlucea, Gianaurelio Cuniberti, Tianshu Lan, Angeliki Tserepi, and Evangelos Gogolides. 2025. "Enhanced Mechanical Durability of Polymeric Nanowires via Carbyne-Enriched Plasma Coatings for Bactericidal Action" Coatings 15, no. 11: 1247. https://doi.org/10.3390/coatings15111247
APA StyleNioras, D., Kefallinou, D., Ioannou, D., Panes-Ruiz, L. A., Ibarlucea, B., Cuniberti, G., Lan, T., Tserepi, A., & Gogolides, E. (2025). Enhanced Mechanical Durability of Polymeric Nanowires via Carbyne-Enriched Plasma Coatings for Bactericidal Action. Coatings, 15(11), 1247. https://doi.org/10.3390/coatings15111247









