Coated Biodegradable Zinc Lithium Alloys: Development and Characterization of Co-Doped Strontium Copper Tricalcium Phosphate Coating for Antimicrobial Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Zn-Li Alloy Preparation
2.2. Synthesis of Bulk Target Material—Double Substituted Sr- and Cu- Tricalcium Phosphate (SrCu-TCP)
2.3. Pulsed Laser Deposition of Coatings
2.4. Physico-Chemical Characterization of Coatings
2.5. Microbiology Tests
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, Z.-Z.; Gao, X.-X.; Zhang, H.-J.; Liu, X.-F.; Li, H.-Y.; Zhou, C.; Yin, Y.-X.; Wang, L.-N. Design biodegradable Zn alloys: Second phases and their significant influences on alloy properties. Bioact. Mater. 2020, 5, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Young, J.; Reddy, R.G. Synthesis, mechanical properties, and in vitro corrosion behavior of biodegradable Zn–Li–Cu alloys. J. Alloys Compd. 2020, 844, 156257. [Google Scholar] [CrossRef]
- Zhao, S.; Seitz, J.-M.; Eifler, R.; Maier, H.J.; Guillory, R.J.; Earley, E.J.; Drelich, A.; Goldman, J.; Drelich, J.W. Zn-Li alloy after extrusion and drawing: Structural, mechanical characterization, and biodegradation in abdominal aorta of rat. Mater. Sci. Eng. C 2017, 76, 301–312. [Google Scholar] [CrossRef]
- Li, H.; Huang, Y.; Ji, X.; Wen, C.; Wang, L.-N. Fatigue and corrosion fatigue behaviors of biodegradable Zn-Li and Zn-Cu-Li under physiological conditions. J. Mater. Sci. Technol. 2022, 131, 48–59. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Farè, S.; Ge, Q.; Vedani, M.; Vimercati, G.; Gastaldi, D.; Migliavacca, F.; Petrini, L.; Trasatti, S. Evaluation of material properties and design requirements for biodegradable magnesium stents. Matéria 2010, 15, 96–103. [Google Scholar] [CrossRef]
- Li, H.; Wen, J.; Liu, Y.; He, J.; Shi, H.; Tian, P. Progress in Research on Biodegradable Magnesium Alloys: A Review. Adv. Eng. Mater. 2020, 22, 2000213. [Google Scholar] [CrossRef]
- Costantino, M.D.; Schuster, A.; Helmholz, H.; Meyer-Rachner, A.; Willumeit-Römer, R.; Luthringer-Feyerabend, B.J.C. Inflammatory response to magnesium-based biodegradable implant materials. Acta Biomater. 2020, 101, 598–608. [Google Scholar] [CrossRef]
- Li, M.; Ren, L.; Li, L.; He, P.; Lan, G.; Zhang, Y.; Yang, K. Cytotoxic Effect on Osteosarcoma MG-63 Cells by Degradation of Magnesium. J. Mater. Sci. Technol. 2014, 30, 888–893. [Google Scholar] [CrossRef]
- Henderson, S.E.; Verdelis, K.; Maiti, S.; Pal, S.; Chung, W.L.; Chou, D.-T.; Kumta, P.N.; Almarza, A.J. Magnesium alloys as a biomaterial for degradable craniofacial screws. Acta Biomater. 2014, 10, 2323–2332. [Google Scholar] [CrossRef]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Zhang, Z.; Jia, Q.; Wu, X.; Cao, X.; Xu, C.; Zhang, J.; Yang, W. Study on microstructure and dynamic corrosion behavior of degradable Mg–Y–Zn–Zr alloy with different Sr contents. Mater. Res. Express 2019, 6, 125418. [Google Scholar] [CrossRef]
- Sanchez, A.H.M.; Luthringer, B.J.C.; Feyerabend, F.; Willumeit, R. Mg and Mg alloys: How comparable are in vitro and in vivo corrosion rates? A review. Acta Biomater. 2015, 13, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Waterman, J.; Staiger, M.; Woodfield, T. Controlling in vitro corrosion rate of pure Mg with rough surface texture via biomimetic coating systems. Corros. Eng. Sci. Technol. 2012, 47, 358–364. [Google Scholar] [CrossRef]
- Tian, Y.; Shao, Y.; Yu, Q.; Guo, S.; Dang, R.; Du, Y.; Zhu, G.; Lyu, S.; Chen, M. Investigating the corrosion behavior of as-cast Mg-Zn-Ca alloys with the same Zn/Ca atomic ratio by in situ observation. Mater. Corros. 2024. [Google Scholar] [CrossRef]
- Hara, T.; Takeda, T.-A.; Takagishi, T.; Fukue, K.; Kambe, T.; Fukada, T. Physiological roles of zinc transporters: Molecular and genetic importance in zinc homeostasis. J. Physiol. Sci. 2017, 67, 283–301. [Google Scholar] [CrossRef]
- Lowe, N.M.; Fraser, W.D.; Jackson, M.J. Is there a potential therapeutic value of copper and zinc for osteoporosis? Proc. Nutr. Soc. 2009, 61, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Komarova, E.G.; Sharkeev, Y.P.; Sedelnikova, M.B.; Prosolov, K.A.; Khlusov, I.A.; Prymak, O.; Epple, M. Zn- or Cu-Containing CaP-Based Coatings Formed by Micro-arc Oxidation on Titanium and Ti-40Nb Alloy: Part I—Microstructure, Composition and Properties. Materials 2020, 13, 4116. [Google Scholar] [CrossRef]
- Hernández-Escobar, D.; Champagne, S.; Yilmazer, H.; Dikici, B.; Boehlert, C.J.; Hermawan, H. Current status and perspectives of zinc-based absorbable alloys for biomedical applications. Acta Biomater. 2019, 97, 1–22. [Google Scholar] [CrossRef]
- O’Connor, J.P.; Kanjilal, D.; Teitelbaum, M.; Lin, S.S.; Cottrell, J.A. Zinc as a Therapeutic Agent in Bone Regeneration. Materials 2020, 13, 2211. [Google Scholar] [CrossRef]
- Molenda, M.; Kolmas, J. The Role of Zinc in Bone Tissue Health and Regeneration—A Review. Biol. Trace Elem. Res. 2023, 201, 5640–5651. [Google Scholar] [CrossRef]
- Cerovic, A.; Miletic, I.; Sobajic, S.; Blagojevic, D.; Radusinovic, M.; El-Sohemy, A. Effects of zinc on the mineralization of bone nodules from human osteoblast-like cells. Biol. Trace Elem. Res. 2007, 116, 61–71. [Google Scholar] [CrossRef]
- Hadley, K.B.; Newman, S.M.; Hunt, J.R. Dietary zinc reduces osteoclast resorption activities and increases markers of osteoblast differentiation, matrix maturation, and mineralization in the long bones of growing rats. J. Nutr. Biochem. 2010, 21, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.-J.; Cho, Y.-E.; Kim, T.; Shin, H.-I.; Kwun, I.-S. Zinc may increase bone formation through stimulating cell proliferation, alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells. Nutr. Res. Pr. 2010, 4, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Moonga, B.S.; Dempster, D.W. Zinc is a potent inhibitor of osteoclastic bone resorption in vitro. J. Bone Miner. Res. 1995, 10, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhao, N.; Zhu, D. Endothelial Cellular Responses to Biodegradable Metal Zinc. ACS Biomater. Sci. Eng. 2015, 1, 1174–1182. [Google Scholar] [CrossRef]
- Fosca, M.; Streza, A.; Antoniac, I.V.; Vadalà, G.; Rau, J.V. Ion-Doped Calcium Phosphate-Based Coatings with Antibacterial Properties. J. Funct. Biomater. 2023, 14, 250. [Google Scholar] [CrossRef]
- Maleki-Ghaleh, H.; Siadati, M.H.; Fallah, A.; Koc, B.; Kavanlouei, M.; Khademi-Azandehi, P.; Moradpur-Tari, E.; Omidi, Y.; Barar, J.; Beygi-Khosrowshahi, Y.; et al. Antibacterial and Cellular Behaviors of Novel Zinc-Doped Hydroxyapatite/Graphene Nanocomposite for Bone Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 9564. [Google Scholar] [CrossRef]
- Laskus-Zakrzewska, A.; Zgadzaj, A.; Kolmas, J. Synthesis and physicochemical characterization of Zn-doped brushite. Ceram. Int. 2021, 47, 7798–7804. [Google Scholar] [CrossRef]
- Cruz, R.; Calasans-Maia, J.; Sartoretto, S.; Moraschini, V.; Rossi, A.M.; Louro, R.S.; Granjeiro, J.M.; Calasans-Maia, M.D. Does the incorporation of zinc into calcium phosphate improve bone repair? A systematic review. Ceram. Int. 2018, 44, 1240–1249. [Google Scholar] [CrossRef]
- Li, Z.; Shi, Z.-Z.; Hao, Y.; Li, H.-F.; Zhang, H.-J.; Liu, X.-F.; Wang, L.-N. Insight into role and mechanism of Li on the key aspects of biodegradable ZnLi alloys: Microstructure evolution, mechanical properties, corrosion behavior and cytotoxicity. Mater. Sci. Eng. C 2020, 114, 111049. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Wang, K.; Strich, R.; Zhou, J.G. In vitro biodegradation behavior, mechanical properties, and cytotoxicity of biodegradable Zn–Mg alloy. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, L.; Zhu, X.; Yang, L.; Shen, J.; Lu, T.; Liu, H.; Song, Z. Corrosion Mechanisms of a Biodegradable Zn-0.4Li Alloy in Simulated Gastrointestinal Environment. Coatings 2023, 13, 529. [Google Scholar] [CrossRef]
- Young, W. Review of Lithium Effects on Brain and Blood. Cell Transplant. 2009, 18, 951–975. [Google Scholar] [CrossRef] [PubMed]
- Aral, H.; Vecchio-Sadus, A. Toxicity of lithium to humans and the environment—A literature review. Ecotoxicol. Environ. Saf. 2008, 70, 349–356. [Google Scholar] [CrossRef]
- Tong, X.; Shen, T.; Zhou, X.; Zeng, J.; Tao, J.; Munir, K.; Li, Y.; Huang, S.; Wu, X.; Ma, J.; et al. Biodegradable Zn–Cu–Li alloys with ultrahigh strength, ductility, antibacterial ability, cytocompatibility, and suitable degradation rate for potential bone-implant applications. Smart Mater. Manuf. 2023, 1, 100012. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Y.; Xu, X.; Chen, L.; Xiao, T.; Luo, X.; Yan, Y.; Li, D.; Dai, Y.; Yu, K. Microstructure, Corrosion Behaviors in Different Simulated Body Fluids and Cytotoxicity of Zn–Li Alloy as Biodegradable Material. Mater. Trans. 2019, 60, 583–586. [Google Scholar] [CrossRef]
- Clément-Lacroix, P.; Ai, M.; Morvan, F.; Roman-Roman, S.; Vayssière, B.; Belleville, C.; Estrera, K.; Warman, M.L.; Baron, R.; Rawadi, G. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 17406–17411. [Google Scholar] [CrossRef]
- Zamani, A.; Omrani, G.R.; Nasab, M.M. Lithium’s effect on bone mineral density. Bone 2009, 44, 331–334. [Google Scholar] [CrossRef]
- Salahshoor, M.; Guo, Y. Biodegradable Orthopedic Magnesium-Calcium (MgCa) Alloys, Processing, and Corrosion Performance. Materials 2012, 5, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zeng, R.; Liu, C.; Gao, J. Comparison of calcium phosphate coatings on Mg–Al and Mg–Ca alloys and their corrosion behavior in Hank’s solution. Surf. Coat. Technol. 2010, 204, 3636–3640. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate coatings on magnesium and its biodegradable alloys. Acta Biomater. 2014, 10, 2919–2934. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.V.; De Bonis, A.; Teghil, R.; Curcio, M.; Fadeeva, I.V.; Barbaro, K.; Di Menno Di Bucchianico, M.; Fosca, M.; Zheng, Y. Double Substituted with Manganese and Strontium Tricalcium Phosphate Coatings on Zinc-Lithium Biodegradable Alloys for Biomedical Implant Applications. Coatings 2023, 13, 36. [Google Scholar] [CrossRef]
- Li, H.F.; Shi, Z.Z.; Wang, L.N. Opportunities and challenges of biodegradable Zn-based alloys. J. Mater. Sci. Technol. 2020, 46, 136–138. [Google Scholar] [CrossRef]
- Xing, H.; Tang, Y.; Fa, X.; Zhang, H.; Shi, Z.; Yao, S.; Wang, L. Enhanced Biocompatibility and Osteogenic Property of Biodegradable Zn-0.5Li Alloy through Calcium–Phosphorus Coating. Coatings 2024, 14, 350. [Google Scholar] [CrossRef]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Fadeeva, I.V.; Deyneko, D.V.; Forysenkova, A.A.; Morozov, V.A.; Akhmedova, S.A.; Kirsanova, V.A.; Sviridova, I.K.; Sergeeva, N.S.; Rodionov, S.A.; Udyanskaya, I.L.; et al. Strontium Substituted β-Tricalcium Phosphate Ceramics: Physiochemical Properties and Cytocompatibility. Molecules 2022, 27, 6085. [Google Scholar] [CrossRef]
- Fadeeva, I.V.; Lazoryak, B.I.; Davidova, G.A.; Murzakhanov, F.F.; Gabbasov, B.F.; Petrakova, N.V.; Fosca, M.; Barinov, S.M.; Vadalà, G.; Uskoković, V.; et al. Antibacterial and cell-friendly copper-substituted tricalcium phosphate ceramics for biomedical implant applications. Mater. Sci. Eng. C 2021, 129, 112410. [Google Scholar] [CrossRef]
- Kazakova, G.; Safronova, T.; Golubchikov, D.; Shevtsova, O.; Rau, J.V. Resorbable Mg2+-Containing Phosphates for Bone Tissue Repair. Materials 2021, 14, 4857. [Google Scholar] [CrossRef]
- Rau, J.V.; Fadeeva, I.V.; Fomin, A.S.; Barbaro, K.; Galvano, E.; Ryzhov, A.P.; Murzakhanov, F.; Gafurov, M.; Orlinskii, S.; Antoniac, I.; et al. Sic Parvis Magna: Manganese-Substituted Tricalcium Phosphate and Its Biophysical Properties. ACS Biomater. Sci. Eng. 2019, 5, 6632–6644. [Google Scholar] [CrossRef]
- Fadeeva, I.V.; Kalita, V.I.; Komlev, D.I.; Radiuk, A.A.; Fomin, A.S.; Davidova, G.A.; Fursova, N.K.; Murzakhanov, F.F.; Gafurov, M.R.; Fosca, M.; et al. In Vitro Properties of Manganese-Substituted Tricalcium Phosphate Coatings for Titanium Biomedical Implants Deposited by Arc Plasma. Materials 2020, 13, 4411. [Google Scholar] [CrossRef] [PubMed]
- Antoniac, I.V.; Filipescu, M.; Barbaro, K.; Bonciu, A.; Birjega, R.; Cotrut, C.M.; Galvano, E.; Fosca, M.; Fadeeva, I.V.; Vadalà, G.; et al. Iron Ion-Doped Tricalcium Phosphate Coatings Improve the Properties of Biodegradable Magnesium Alloys for Biomedical Implant Application. Adv. Mater. Interfaces 2020, 7, 2000531. [Google Scholar] [CrossRef]
- Fadeeva, I.V.; Goldberg, M.A.; Preobrazhensky, I.I.; Mamin, G.V.; Davidova, G.A.; Agafonova, N.V.; Fosca, M.; Russo, F.; Barinov, S.M.; Cavalu, S.; et al. Improved cytocompatibility and antibacterial properties of zinc-substituted brushite bone cement based on β-tricalcium phosphate. J. Mater. Sci. Mater. Med. 2021, 32, 99. [Google Scholar] [CrossRef] [PubMed]
- Božić Cvijan, B.; Korać Jačić, J.; Bajčetić, M. The Impact of Copper Ions on the Activity of Antibiotic Drugs. Molecules 2023, 28, 5133. [Google Scholar] [CrossRef] [PubMed]
- Salah, I.; Parkin, I.P.; Allan, E. Copper as an antimicrobial agent: Recent advances. RSC Adv. 2021, 11, 18179–18186. [Google Scholar] [CrossRef]
- Deyneko, D.V.; Fadeeva, I.V.; Borovikova, E.Y.; Dzhevakov, P.B.; Slukin, P.V.; Zheng, Y.; Xia, D.; Lazoryak, B.I.; Rau, J.V. Antimicrobial properties of co-doped tricalcium phosphates Ca3-2x(M′M″)x(PO4)2 (M = Zn2+, Cu2+, Mn2+ and Sr2+). Ceram. Int. 2022, 48, 29770–29781. [Google Scholar] [CrossRef]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef]
- Wang, M.; Tang, T. Surface treatment strategies to combat implant-related infection from the beginning. J. Orthop. Transl. 2019, 17, 42–54. [Google Scholar] [CrossRef]
- Aggarwal, V.K.; Bakhshi, H.; Ecker, N.U.; Parvizi, J.; Gehrke, T.; Kendoff, D. Organism Profile in Periprosthetic Joint Infection: Pathogens Differ at Two Arthroplasty Infection Referral Centers in Europe and in the United States. J. Knee Surg. 2014, 27, 399–406. [Google Scholar] [CrossRef]
- Miquel, S.; Lagrafeuille, R.; Souweine, B.; Forestier, C. Anti-biofilm Activity as a Health Issue. Front. Microbiol. 2016, 7, 592. [Google Scholar] [CrossRef]
- Cangui-Panchi, S.P.; Ñacato-Toapanta, A.L.; Enríquez-Martínez, L.J.; Reyes, J.; Garzon-Chavez, D.; Machado, A. Biofilm-forming microorganisms causing hospital-acquired infections from intravenous catheter: A systematic review. Curr. Res. Microb. Sci. 2022, 3, 100175. [Google Scholar] [CrossRef] [PubMed]
- Bonadonna, L.; Briancesco, R.; Coccia, A.M. Analysis of Microorganisms in Hospital Environments and Potential Risks. In Indoor Air Quality in Healthcare Facilities; Capolongo, S., Settimo, G., Gola, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 53–62. [Google Scholar] [CrossRef]
- INORGANIC STRUCTURE CRYSTAL DATABASE (ICSD). Available online: https://icsd.products.fiz-karlsruhe.de/ (accessed on 30 May 2024).
- Boanini, E.; Gazzano, M.; Nervi, C.; Chierotti, M.R.; Rubini, K.; Gobetto, R.; Bigi, A. Strontium and Zinc Substitution in β-Tricalcium Phosphate: An X-ray Diffraction, Solid State NMR and ATR-FTIR Study. J. Funct. Biomater. 2019, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Xidaki, D.; Agrafioti, P.; Diomatari, D.; Kaminari, A.; Tsalavoutas-Psarras, E.; Alexiou, P.; Psycharis, V.; Tsilibary, E.C.; Silvestros, S.; Sagnou, M. Synthesis of Hydroxyapatite, β-Tricalcium Phosphate and Biphasic Calcium Phosphate Particles to Act as Local Delivery Carriers of Curcumin: Loading, Release and In Vitro Studies. Materials 2018, 11, 595. [Google Scholar] [CrossRef]
- Habelitz, S.; Pascual, L.; Durán, A. Transformation of tricalcium phosphate into apatite by ammonia treatment. J. Mater. Sci. 2001, 36, 4131–4135. [Google Scholar] [CrossRef]

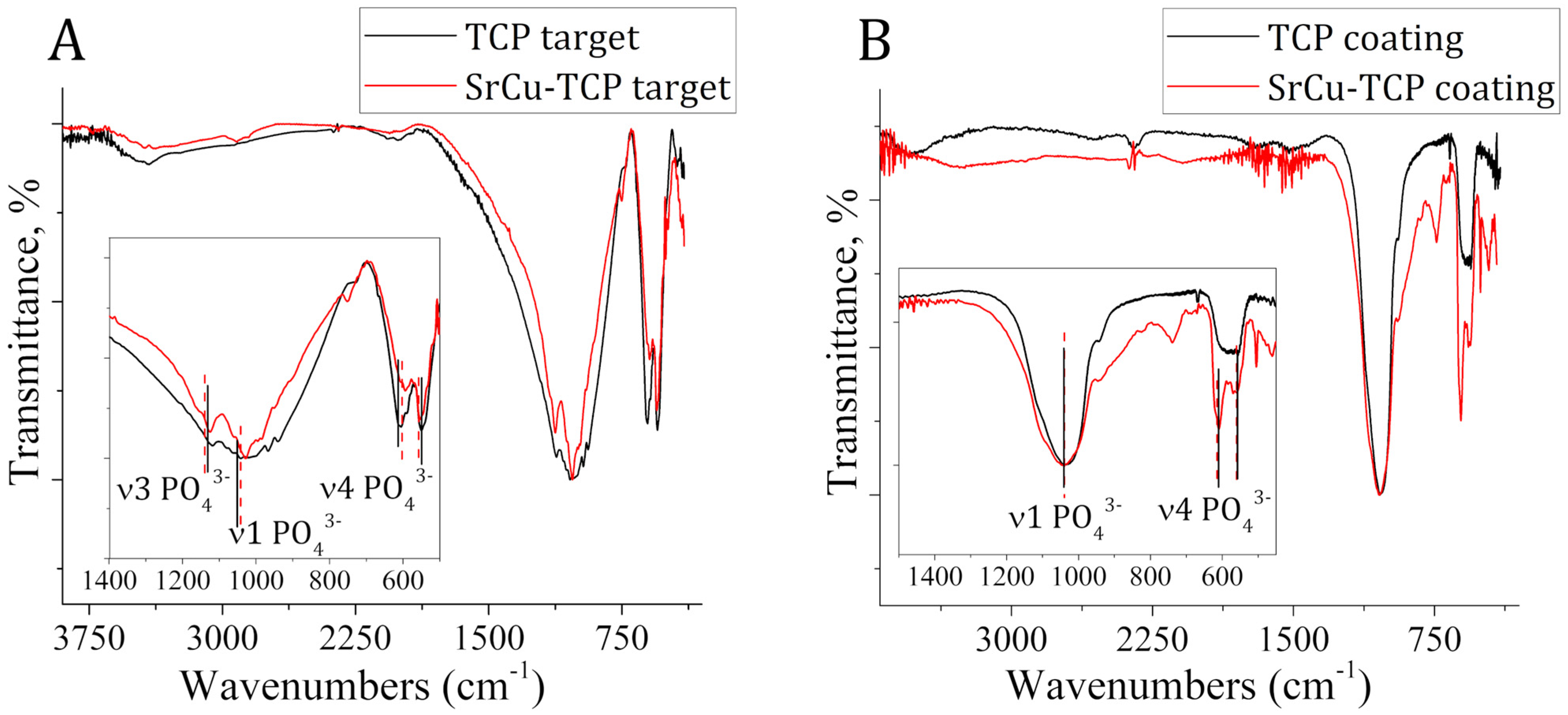
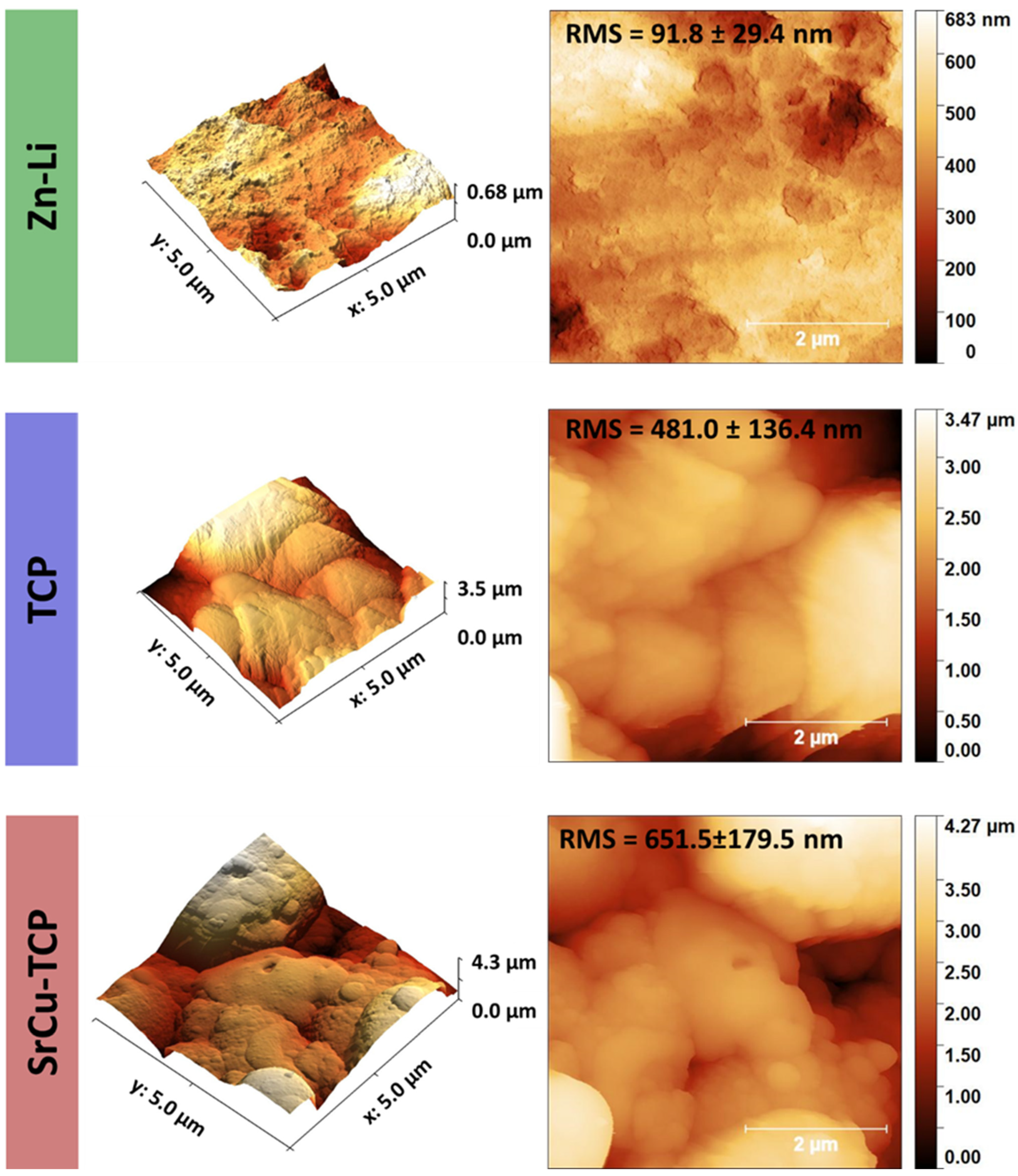

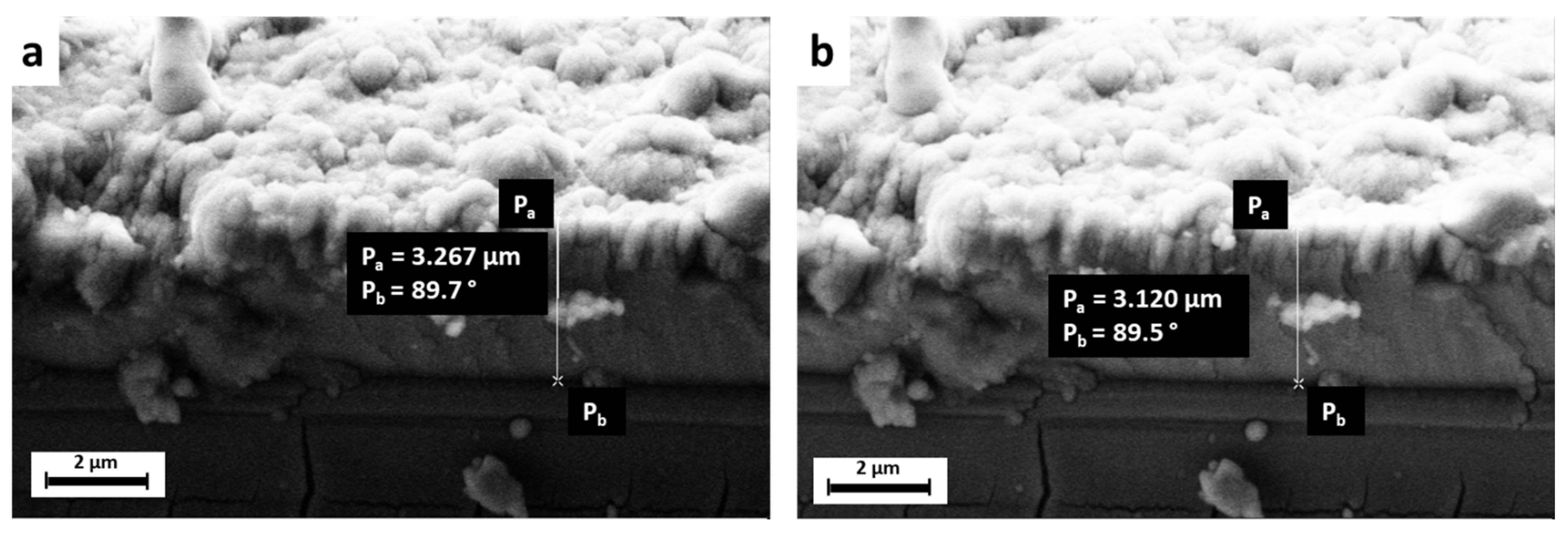
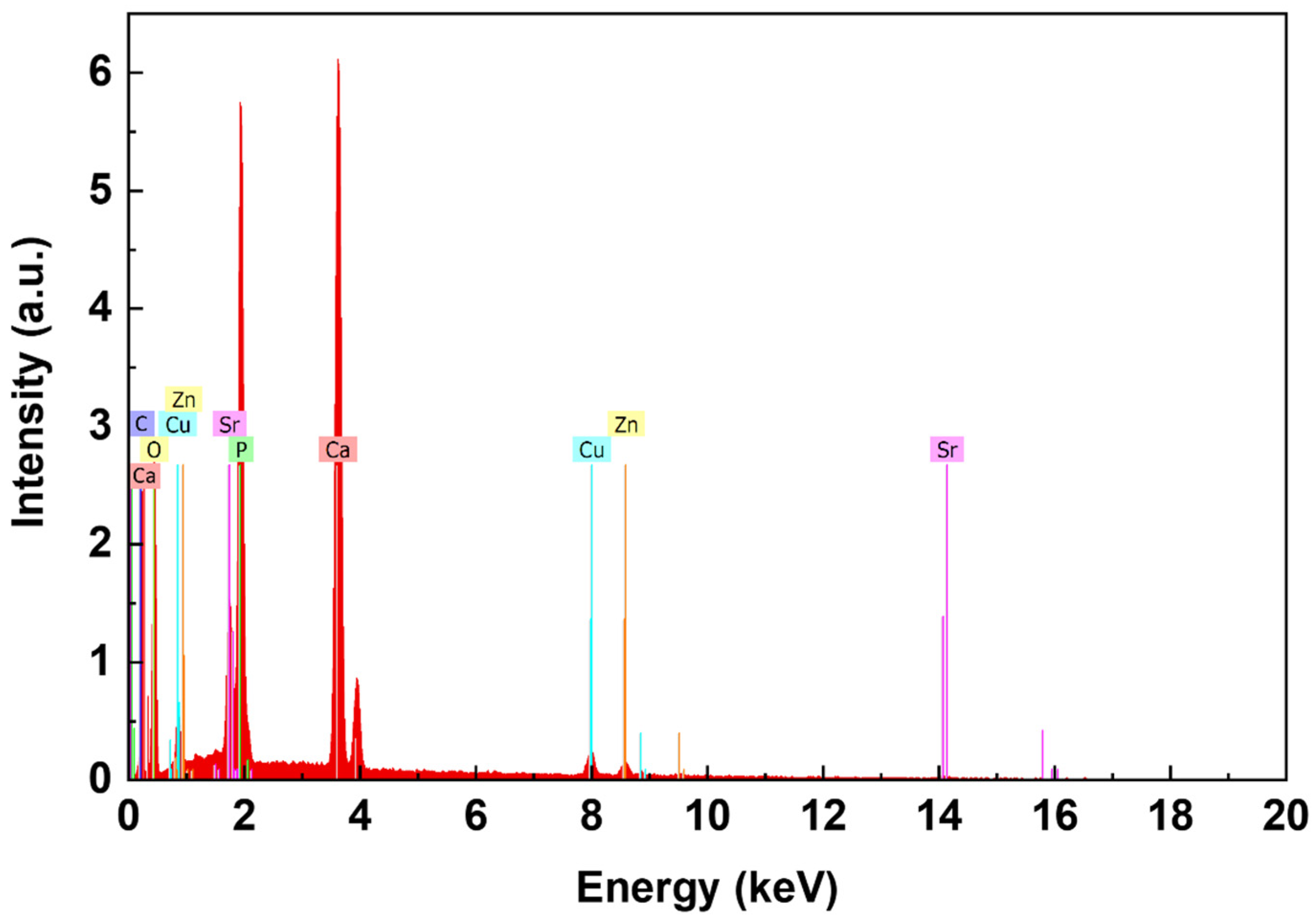
| Peak Number | β-TCP 2θ Peak Position (°) | SrCu-TCP 2θ Peak Position (°) | Δ2θ | hkl Miller Index [64] | R.I.% |
|---|---|---|---|---|---|
| 1 | 13.52 | 13.56 | 0.04 | 1 0 4 | 16 |
| 2 | 16.88 | 16.92 | 0.04 | 1 1 0 | 20 |
| 3 | 21.76 | 21.80 | 0.04 | 0 2 4 | 16 |
| 4 | 25.68 | 25.68 | 0.00 | 1 0 10 | 25 |
| 5 | 26.40 | 26.52 | 0.12 | 1 2 2 | 10 |
| 6 | 27.72 | 27.80 | 0.08 | 2 1 4 | 55 |
| 7 | 29.56 | 29.68 | 0.12 | 3 0 0 | 16 |
| 8 | 30.96 | 31.00 | 0.04 | 0 2 10 | 100 |
| 9 | 32.36 | 32.48 | 0.12 | 1 2 8 | 20 |
| 10 | 34.28 | 34.44 | 0.16 | 2 2 0 | 65 |
| 11 | 35.04 | 35.22 | 0.18 | 2 2 3 | 8 |
| 12 | 35.52 | 35.60 | 0.08 | 2 1 10 | 12 |
| 13 | 37.24 | 37.40 | 0.16 | 1 2 11 | 10 |
| 14 | 39.76 | 39.76 | 0.00 | 1 0 16 | 10 |
| 15 | 41.00 | 41.18 | 0.18 | 4 0 4 | 14 |
| 16 | 41.60 | 41.68 | 0.08 | 3 0 12 | 12 |
| 17 | 46.88 | 47.08 | 0.20 | 4 0 10 | 20 |
| 18 | 47.88 | 48.12 | 0.24 | 2 3 8 | 16 |
| 19 | 48.28 | 48.52 | 0.24 | 4 1 6 | 14 |
| 20 | 52.92 | 52.92 | 0.00 | 2 0 20 | 25 |
| 21 | 59.48 | 59.68 | 0.20 | 5 1 7 | 12 |
| Mode | TCP Target (cm−1) | SrCu-TCP Target (cm−1) | Band Shift (cm−1) |
|---|---|---|---|
| ν3 | 1120 | 1125 | 5 |
| ν1 | 1042 | 1027 | −15 |
| ν4 | 605 | 590 | 15 |
| ν4 | 549 | 554 | −6 |
| Sr, wt% | Cu, wt% | ||||
|---|---|---|---|---|---|
| EDX (Coatings) | AAS (Targets) | Theoretical Value, in Ca2.5Sr0.25Cu0.25(PO4)2 Powder | EDX (Coatings) | AAS (Targets) | Theoretical Value, in Ca2.5Sr0.25Cu0.25(PO4)2 Powder |
| 6.54 ± 0.33 | 6.68 ± 0.05 | 6.71 | 4.10 ± 0.18 | 4.78 ± 0.05 | 4.84 |
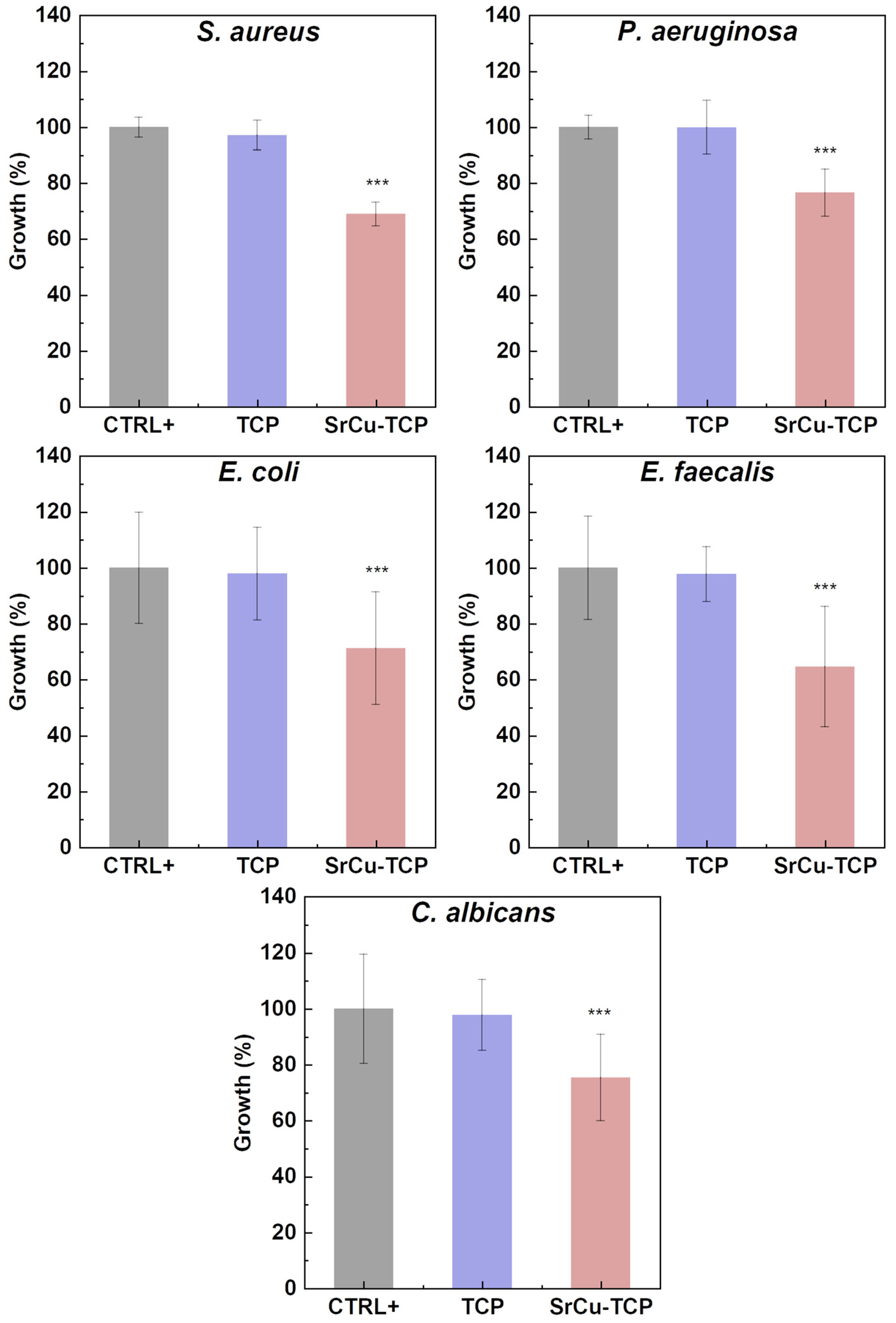
| OD 600 nm | SD | % Growth | % Inhibition | |
|---|---|---|---|---|
| S. aureus | ||||
| CTR+ | 1.009 | 0.03 | 100.0 | 0.0 |
| TCP | 0.980 | 0.05 | 97.1 | 2.9 |
| SrCu-TCP | 0.695 | 0.04 | 68.9 | 31.1 |
| P. aeruginosa | ||||
| CTR+ | 0.984 | 0.03 | 100.0 | 0.0 |
| TCP | 0.983 | 0.09 | 99.9 | 0.1 |
| SrCu-TCP | 0.753 | 0.08 | 76.5 | 23.5 |
| E. coli | ||||
| CTR+ | 1.204 | 0.17 | 100.0 | 0.0 |
| TCP | 1.179 | 0.11 | 97.9 | 2.1 |
| SrCu-TCP | 0.858 | 0.21 | 71.3 | 28.7 |
| E. faecalis | ||||
| CTR+ | 0.995 | 0.17 | 100.0 | 0.0 |
| TCP | 0.973 | 0.07 | 97.8 | 2.2 |
| SrCu-TCP | 0.643 | 0.21 | 64.7 | 35.3 |
| C. albicans | ||||
| CTR+ | 0.810 | 0.15 | 100.0 | 0.0 |
| TCP | 0.792 | 0.09 | 97.8 | 2.2 |
| SrCu-TCP | 0.611 | 0.12 | 75.4 | 24.6 |
| % Inhibition | ||||||
|---|---|---|---|---|---|---|
| Sample | S. aureus | P. aeruginosa | E. coli | E. faecalis | C. albicans | Reference |
| SrCu-TCP (coating) | 31.1 | 23.5 | 28.7 | 35.3 | 24.6 | present study |
| SrCu-TCP (powder) | 92.0 | 95.5 | 64.9 | 96.3 | 70.9 | [57] |
| SrMn-TCP (coating) | 12.2 | 9.4 | 9.0 | 9.7 | 50.0 | [44] |
| SrMn-TCP (powder) | 57.8 | 92.9 | 19.0 | 67.4 | 41.9 | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rau, J.V.; De Bonis, A.; Curcio, M.; Barbaro, K.; Fosca, M.; Fadeeva, I.V.; Cardoso, G.C.; Teghil, R.; Slonskaya, T.K.; Zheng, Y. Coated Biodegradable Zinc Lithium Alloys: Development and Characterization of Co-Doped Strontium Copper Tricalcium Phosphate Coating for Antimicrobial Applications. Coatings 2024, 14, 1073. https://doi.org/10.3390/coatings14081073
Rau JV, De Bonis A, Curcio M, Barbaro K, Fosca M, Fadeeva IV, Cardoso GC, Teghil R, Slonskaya TK, Zheng Y. Coated Biodegradable Zinc Lithium Alloys: Development and Characterization of Co-Doped Strontium Copper Tricalcium Phosphate Coating for Antimicrobial Applications. Coatings. 2024; 14(8):1073. https://doi.org/10.3390/coatings14081073
Chicago/Turabian StyleRau, Julietta V., Angela De Bonis, Mariangela Curcio, Katia Barbaro, Marco Fosca, Inna V. Fadeeva, Giovana Collombaro Cardoso, Roberto Teghil, Tatiana K. Slonskaya, and Yufeng Zheng. 2024. "Coated Biodegradable Zinc Lithium Alloys: Development and Characterization of Co-Doped Strontium Copper Tricalcium Phosphate Coating for Antimicrobial Applications" Coatings 14, no. 8: 1073. https://doi.org/10.3390/coatings14081073
APA StyleRau, J. V., De Bonis, A., Curcio, M., Barbaro, K., Fosca, M., Fadeeva, I. V., Cardoso, G. C., Teghil, R., Slonskaya, T. K., & Zheng, Y. (2024). Coated Biodegradable Zinc Lithium Alloys: Development and Characterization of Co-Doped Strontium Copper Tricalcium Phosphate Coating for Antimicrobial Applications. Coatings, 14(8), 1073. https://doi.org/10.3390/coatings14081073












