1. Introduction
Writing whiteboards bring a lot of convenience to our lives, but if they are not wiped clean within a short time after being written on, stains will be left on the whiteboard [
1,
2,
3,
4]. The surface of the whiteboard will become more and more dirty over time, which affects its daily use [
5,
6,
7,
8,
9]. If oily markers are accidentally used to write on a whiteboard, the marks are hard to wipe off, even after a short period of time. However, if a whiteboard is coated with anti-graffiti fluorocarbon polyurethane baking paint, this will allow people to write and draw freely. Even if their writing is kept for a long time, it still can be easily wiped clean with a paper towel [
10,
11,
12]. Using a graffiti-resistant baking paint on whiteboards is the key to solving this problem [
13].
Tang et al. used a waterborne two-component polyurethane emulsion and curing agent technology to develop a waterborne scrubbable paint with excellent graffiti resistance. Their paper investigates the influences that affect the effectiveness of graffiti resistance, including the ratio of emulsion/curing agents, the amount of diluent water, etc. The comprehensive performance of the paint was comparatively tested, and it was concluded that the paint possessed excellent graffiti removal performance, environmental protection performance, scrubbing resistance, etc. [
14]. Although it is easy to remove graffiti from the paint if it is caused by water-based whiteboard markers, watercolors, and chalks, it is harder to do anything about graffiti from oil-based markers.
Wu et al. used sodium allyloxy hydroxypropyl sulfonate, nonylphenol polyoxyethylene ether, and NP-10 as composite emulsifiers, and ammonium persulfate as an initiator to study fluorinated acrylate emulsion polymerization. Their paper studied the contact angle of paint films prepared with different fluorinated monomers and different cross-linking monomers [
15]. The results showed that with the increase of fluorinated monomer content, the water and cetane contact angles of paint films increased, and the stain resistance to graffiti of the paint films improved significantly. Although the resistance of the paint films to some graffiti improved significantly, they had almost no graffiti resistance to oil-based markers, because the room temperature self-drying non-crosslinking paint film was not dense enough.
Chen et al. used thermoset acrylic resins, synthetic fatty acid, hydroxyl-containing polydimethylsiloxane resins, and silane resins in combination with several additives to configure a solvent-borne alkyd-modified silicone resin [
16]. Paint was prepared by mixing the resin and HDI trimer N3390 as a cross-linking agent. The two-component paint has a dense paint film with graffiti resistance to water stains. Because there is no fluorocarbon material added to the paint, the effect of oil graffiti resistance is not very good.
Liu et al. synthesized polymethylsiloxane grafted by fluorocarbon side chains via a hydrosilylation reaction of polymethylhydrosiloxane (PMHS) with 2,2,3,4,4,4-hexafluorobutyl acrylate (HFBA) in the presence of Karstedt’s catalyst. The synthesized polymer was incorporated into two-component polyurethane coating formulations as an additive to prepare anti-graffiti coatings [
17]. Although the paint achieved a good anti-graffiti effect, the fluorosilicone additive is not chemically crosslinked with the main resin, and therefore does not have a long-lasting anti-graffiti effect. The fluorosilicone material is easily removed after repeated wiping, thus reducing the anti-graffiti effect of the coating.
Shen et al. dissolved solid PUA resin, methyltriethoxysilane, and perfluoropolyether (PFPE) into butyl acetate to make a paint, and then the liquid paint was coated and dried to form a solid paint film [
18]. PFPE plays a crucial role in the film’s composition, and the anti-icing, anti-graffiti, and anti-sticking properties of the film are derived from the low surface tension of the fluorocarbon material. However, since the main resin is a one-component PUA and does not have a cross-linking curing molecular structure, the performance of the paint film and the retention of PFPE on the paint film are unsatisfactory. A USA-based company has launched Nanoprotect AntiG, a water-based, odorless, two-component paint that provides multiple layers of anti-graffiti protection for concrete and stone surfaces, where graffiti can be simply removed with low-pressure water without the need to use chemical cleaners that can cause damage to the surface. There is one drawback to this product: when graffiti is removed, a new coat of Nanoprotect AntiG needs to be reapplied to prevent new graffiti.
Herein, in this paper, a fluorocarbon polyurethane amino baking paint for whiteboard surfaces was designed and evaluated which is not only suitable for children’s graffiti boards, but also for companies meeting presentations. The novelty of the paint is the preparation of fluorocarbon polyurethane hydroxyl resins and their modification with terminated hydroxyl silicone oil. The paint film exhibits graffiti resistance in addition to excellent hardness, flexibility, adhesion, salt spray resistance, etc. Due to a high degree of cross-linking with amino resin, the paint film is extremely dense. The synergy between the fluorocarbon branched chain and the terminal hydroxyl silicone oil makes it possible to easily erase the graffiti caused by oil-based markers, even after 24 h of retention. The various graffiti-resistant solutions are listed below, as shown in
Table 1.
2. Experiments
2.1. Materials
The raw materials used in the synthesis of fluorocarbon polyurethane hydroxy resins were as follows: Hexamethylene diisocyanate (HDI, 99%) was industrial grade and from Yantai Wanhua. Perfluorohexyl ethyl alcohol (TEOH6, 99%) was industrial grade and from Tianhe Chemical. Dibutyltin dilaurate (DBTDL, 99%), xylenes (XYL, 99.7%), dibutylamine (DNBA, 99.8%), and trimethylolpropane (TMP, 99.8%) were analytically pure and from Sinopharm Reagent. Dimethyl silicone oil (AK350, 97%) was industrial grade and from Shanghai Wenpu Chemical. Polyester diol (PE3020, 99%) was industrial grade and from Huafeng Chemical.
The raw materials used in the synthesis of fluorocarbon polyurethane amino baking paint were as follows: Hydroxy acrylic resin (FX-9221, 99%) was industrial grade and from Fangxin Resin. Terminal hydroxyl silicone oil (WS62M, 99%) was industrial grade and from Wacker Chemistry. Butyl acetate (BA, 99.8%) was industrial grade and from Fuqi Chemical. Amino resin (CYMEL 303 LF, 100%) was industrial grade and from Allnex Resin. The wetting agent (WET270, 99%) and leveling agent (TWIN 4000, 99%) were industrial grade and from Evonik Chemicals.
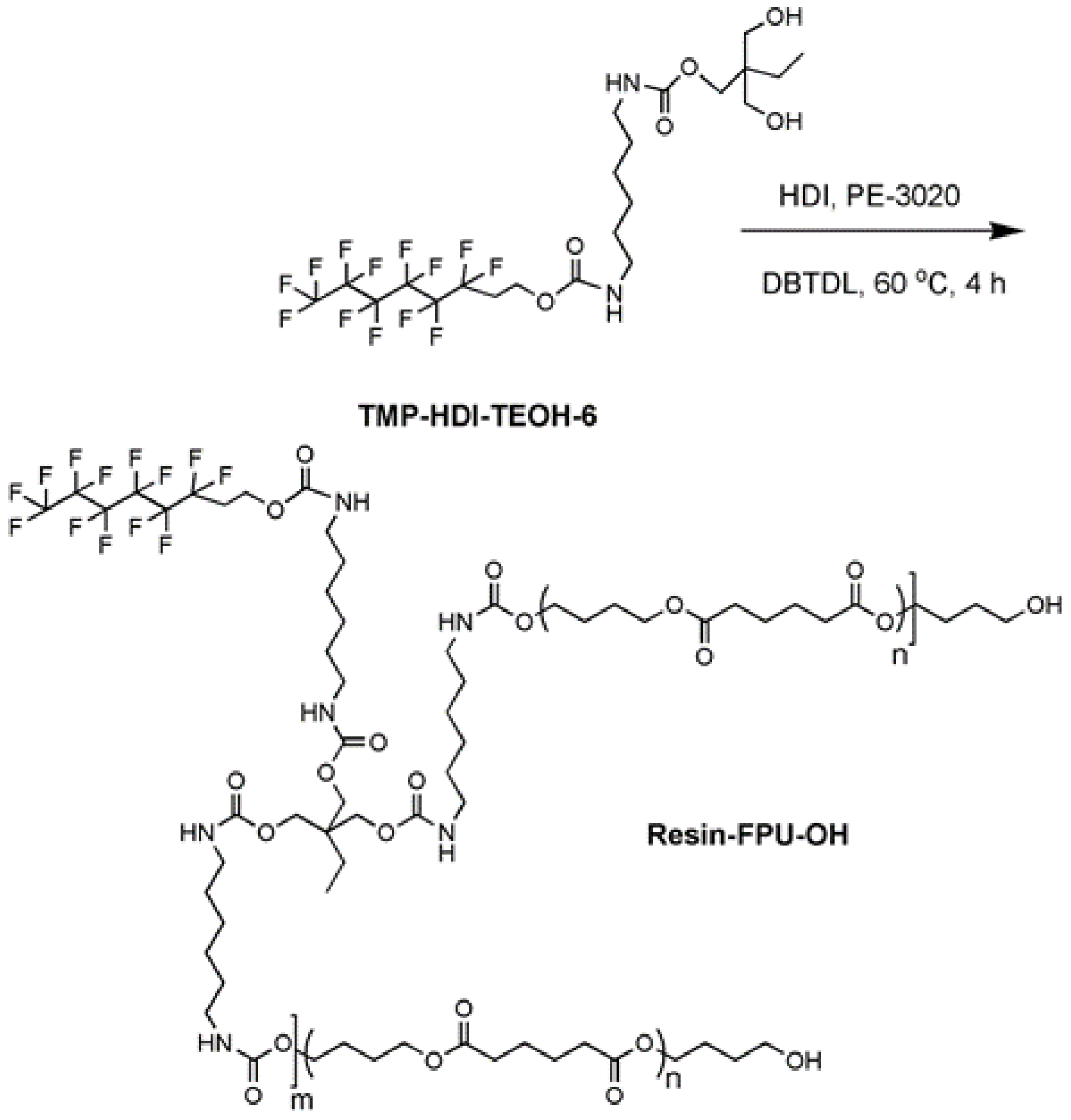
2.2. Synthesis of TMP-HDI-TEOH6
A four-necked flask containing a stirring rod, thermometer, constant pressure burette, and reflux condenser was placed into an electric heating oil bath with dimethyl silicone oil. Then, 1 mole of hexamethylene diisocyanate (HDI) was added and the flask was stirred while introducing nitrogen gas. The temperature was raised to 60 °C, 0.1 g of dibutyltin dilaurate (DBTDL) was added, and then 1 mole of perfluorohexyl TEOH6 was added by titration at constant pressure. During this process, XYL was added to reduce viscosity and promote reaction. After 4 h of continuous reaction, fluorocarbon monoisocyanate (HDI-TEOH6) was obtained [
19,
20,
21,
22,
23].
The temperature was maintained at 60 °C with agitation and nitrogen venting. One mole of trimethylolpropane (TMP) was added by titration at constant pressure. An appropriate amount of XYL was added to reduce viscosity. The reaction was maintained for 4 h and the reaction process was measured using the di-n-butylamine method (di-n-butylamine was dissolved in toluene to react with NCO, an excess of di-n-butylamine was titrated with a standard solution of HCl, and the NCO content in the sample was obtained). When the NCO content reached 0, the flask was lowered to room temperature and stirring and nitrogen gas were stopped, as a fluorocarbon diol monomer (TMP-HDI-TEOH6) with a solids content of 46%–50% [
24,
25,
26] had been obtained. The specific reaction steps are shown in
Figure 1.
2.3. Synthesis of Resin-FPU-OH
The temperature of the same four-necked flask was then increased to 60℃ while continuing the stirring and ventilation of nitrogen, then 1.8 mol of pre-dehydrated polyester polyol PE-3020 and xylene solvent was added to the above-synthesized TMP-HDI-TEOH6. Stirring continued for 30 min, then 1 mol of HDI was added by titration at constant pressure and stirring continued for 4 h [
27,
28,
29,
30]. Then, stirring and ventilation of nitrogen were stopped, as a 44%–46% solid content of fluorocarbon polyurethane hydroxy resin (Resin-FPU-OH) was obtained. The specific reactions are shown in
Figure 2.
2.4. Formulations of Fluorocarbon Polyurethane Amino Baking Paint
In addition to fluorocarbon polyurethane hydroxyl resin in the baking paint formulation, it was also necessary to add amino resins, Cymel 303LF, hydroxyl acrylic resins, and other ingredients like a wetting agent, leveling agent, butyl acetate, xylene, etc. [
31]. The specific formulations are shown in
Table 2.
The hardness, adhesion, flexibility, and salt spray resistance of the five starting formulations of fluorocarbon polyurethane baking paint were tested, and formulations F1 and F2, which did not meet the basic performance requirements of fluorocarbon polyurethane baking paint, were eliminated. The fluorine content of the surface of formulations F3 and F4 (F5 was not mixed with fluorocarbon polyurethane hydroxyl resins) was measured using XPS. The contact angles of water and cetane of the paint films of formulations F3, F4, and F5 were measured by using a contact angle tester. According to the test results of the above tests, formulation F3, with the best hydrophobicity and oleophobicity, was selected as the preferred formulation for further study [
32,
33,
34].
To obtain the upgraded formulations F6–F9, terminal hydroxyl silicone oil Wacker WS62M was added into formulation F3, and the butyl acetate (BA) was replaced with different ratios, as shown in
Table 3. The amount of terminal hydroxyl silicone oil added to the formulation should not be too high, due to the lower surface tension of terminal hydroxyl silicone oil resulting in alcohol-soluble markers not being able to write on the whiteboard, and handwriting shrinking to small droplets [
35].
2.5. Preparation of Paint Film
The preparation of fluorocarbon polyurethane amino baking paint for performance testing was as follows. The prepared paint was scraped onto an 8 cm × 16 cm white steel plate using a 25 μm wire rod film preparator. The steel plate was placed horizontally for 1 h to ensure good leveling and that the solvent completely evaporated. Then, the steel plate was placed into an oven and baked at a temperature of 180 °C for 30 min. A fully cured paint was obtained for further testing.
The preparation of fluorocarbon polyurethane amino baking paint for contact angle testing was as follows. The prepared paint was scraped onto a 15 cm × 15 cm glass plate with a 25 μm wire rod paint film preparer. The glass plate was placed horizontally for 1 h to ensure good leveling and that the solvent completely evaporated. Then, the glass plate was placed into an oven at 180 °C for 30 min. A fully cured paint was obtained for further testing.
2.6. Characterization
2.6.1. Fourier Transform Infrared Spectroscopy (FT-IR)
The Fourier transform infrared spectra of HDI-TEOH-6 and TMP-HDI-TEOH6 were measured using a Thermo Scientific Nicolet iS5 Fourier transform infrared spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA) at room temperature using the KBr compression method, with a spectral range of 500–4000 cm−1, and then compared with commonly used chemical bonding infrared absorption standard spectra. The Fourier transform infrared spectrum of Resin-FPU-OH was also measured using the same method.
2.6.2. Nuclear Magnetic Resonance (NMR)
The HNMR spectra of HDI-TEOH-6 and TMP-HDI-TEOH-6 were subjected to NMR analysis at room temperature using deuterated chloroform (CDCl3) as the solvent and a Brucker 400 M NMR instrument (Bruker Corporation, Billerica, MA, USA).
2.6.3. Gel Permeation Chromatography (GPC)
The molecular weight of polymer resins is an indicator that must be controlled when synthesizing. The molecular weight of polyurethane resins can be controlled by the R-value (NCO/OH). Gel permeation chromatography is a fast and simple separation and analysis technique used to separate polymers according to their molecular weight using volume exclusion chromatography. A gel permeation chromatography instrument, model Agilent PL-GPC50 (Agilent Corporation, Santa Clara, CA, USA), was used in this experiment to evaluate the molecular weight of fluorocarbon polyurethane hydroxy resin (Resin-FPU-OH).
2.6.4. X-ray Photoelectron Spectroscopy (XPS)
XPS testing can compare and analyze the composition of various elements on the surface of paint films, which helps to explain the hydrophobic and oleophobic properties of paint films. XPS testing was conducted on the F3 and F4 formulations. The instrument used was a model ESCALAB 250Xi (Thermo Fisher Scientific, Waltham, MA, USA) with an energy resolution of 0.6 eV. The surface fluorine content of the F3 and F4 fluorocarbon polyurethane amino baking paint formulations was analyzed using monochromatic dual anode photoelectron spectroscopy.
2.6.5. Scanning Electron Microscopy (SEM)
Paint film surfaces with more microstructures and nanostructures often exhibit better hydrophobic and oleophobic properties. Adding hydroxyl terminated silicone oil can cause changes in the microstructure of a paint film’s surface. A Gemini SEM300 scanning electron microscope (Zeiss AG, Oberkohen, Germany) with a magnification of 5 µm was used to capture the microstructure of the F3 and F6–F9 paint films.
2.6.6. Paint Film Basic Performance
The hardness of a paint film is one of the most important properties, representing the mechanical strength of the paint film, and its physical meaning can be understood as the resistance of the paint film’s surface to the action of another object with greater hardness. This experiment referred to GB/T6739-2006 [
36] by using an automotive pencil hardness tester (Guangzhou Biaogeda Precision Instrument Co., Ltd., Guangzhou, China) to test the hardness of the fluorocarbon polyurethane amino baking paint films.
The adhesion of a paint film refers to the ability of the paint film to bond with a substrate or other paint films. It is a prerequisite for the performance of paint films. This experiment referred to GB/T1720-1989 [
37] by using a pasteur knife (Guangzhou Biaogda Precision Instrument Co., Ltd., Guangzhou, China) to test the adhesion of the fluorocarbon polyurethane amino baking paint films.
Flexibility is very important for whiteboard paint. The T-bend method is widely used to evaluate flexibility. This experiment referred to GB/T 30791-2014 [
38], using a T-bending machine (Ruijin Instruments, Tianjin, China) to test the flexibility of the fluorocarbon polyurethane amino baking paint films.
Salt spray resistance refers to the ability of a paint film to protect steel from erosion. This experiment referred to GB/T 1771-2007 [
39] by using a JK-60A salt spray tester (Meiyu Instrument, Shanghai, China) to test the salt spray resistance of the fluorocarbon polyurethane amino baking paint films.
The acid and alkali resistance of a paint film refers to the degree to which the surface of the paint film can withstand erosion from acids, alkalis, and other chemicals. Acid and alkali resistance have a crucial impact on the quality and service life of the paint film. This experiment referred to GB 1763-1979 (Determination of chemical reagent resistance of paint film) [
40].
Paint weatherability refers to the stability of a coating film’s performance in the natural environment. For paint films, an appropriate weathering test method is to use an artificial climate aging test in which the yellowing performance after UV lamp illumination is the main metric used to evaluate the weathering resistance of the paint. This experiment referred to GB/T 23987-2009 (Paints and varnishes—Exposure of coatings to artificial weathering—Exposure to fluorescent UV lamps and water) by using an ASR-ZW-151A UV Tester (Guangdong Aisry Instrument Technology Co., Dongguan, China) [
41].
Thermogravimetric analysis (TGA) was conducted with a synchronous thermal analyzer STA 449C-Q600 (NETZSCH GmbH, Selb, Germany). The heating range was from room temperature to 600 °C, and the heating rate was 10 °C/min. The samples of the coating films were tested under nitrogen conditions.
2.6.7. Contact Angle Test
Fluorocarbon polyurethane amino baking paint that meets the basic requirements of amino baking paint needs to have high hydrophobicity and oleophobicity. The contact angle is the most intuitive indicator for obtaining the correct values. In order to study the hydrophobicity and oleophobicity of the paint films, water and hexadecane were selected as the testing liquids, and a glass plate was recommended as the substrate to test the contact angle. The initial contact angles of water and hexadecane were measured using an ASR705SB fully automatic water droplet angle tester (Guangdong Aisry Instrument Technology Co., Ltd., Dongguan, China).
In practical applications, a fluorocarbon polyurethane baking paint film cannot always maintain its initial state, and a whiteboard would be continuously brushed and then erased. Therefore, we needed to verify the water and cetane contact angle of the paint films after repeated wear. With an ASR339 reciprocating wear testing machine (Guangdong Aisry Instrument Technology Co., Ltd., Dongguan, China), the method used was to use a 500 g weight to press on a 3 M dishcloth and wipe the surface of the paint film back and forth 5000 times. Then, the same method as above was used to measure the water and cetane contact angle.
Parents, in order to make a writing whiteboard cleaner, sometimes use a cotton cloth with alcohol to wipe the whiteboard. Therefore, we needed to verify the water and cetane contact angle of each fluorocarbon polyurethane amino baking paint film after alcohol wiping. White fabric, with a 500 g gravity hammer inside and 99.7% alcohol wetting the fabric, was moved back and forth on the paint film surface 100 times at a speed of 1 cm/s. Then, the water and cetane contact angles were measured by the same method mentioned above.
The sliding angle test is a method used to evaluate the anti-graffiti properties of a paint film. By using a Sliding Angle Tester SDC-350 (Hill Instrument Technology Co., Dongguan, China), the sliding angle was tested with a tilt rate of 30°/min.
2.6.8. Test of Water and Oil Absorption of Paint Film
The water and oil absorption rates of a paint film can reflect its hydrophobic and oleophobic properties. Each paint films’ water absorption rate (W) was calculated according to HG/T 3344-2012 [
42] (Determination of water absorption of paint film). By changing the immersion medium to cetane with the same test method, the oil absorption rate of each paint film was also obtained.
The above water absorption rate and oil absorption rate experiments were repeated three times per paint, and the arithmetic mean value of the three experiments was taken to obtain the actual water absorption rate and oil absorption rate of each fluorocarbon polyurethane baking paint film.
2.6.9. Test of Graffiti Resistance of Paint Film
In addition to testing the contact angle, we also needed to test the graffiti resistance of the fluorocarbon polyurethane baking paints in real application scenarios by using oil-based markers and alcohol-soluble whiteboard markers, respectively. The most common black, red, and blue colors of oil-based markers and alcohol-soluble whiteboard pens were used for the tests. The markings were kept for 24 h and then wiped away with a dry cloth to observe the residue of the ink on the paint film. The lighter the marks on the paint film, the better its resistance to graffiti [
43].
3. Results and Discussion
3.1. Structural Characterization of TMP-HDI-TEOH6
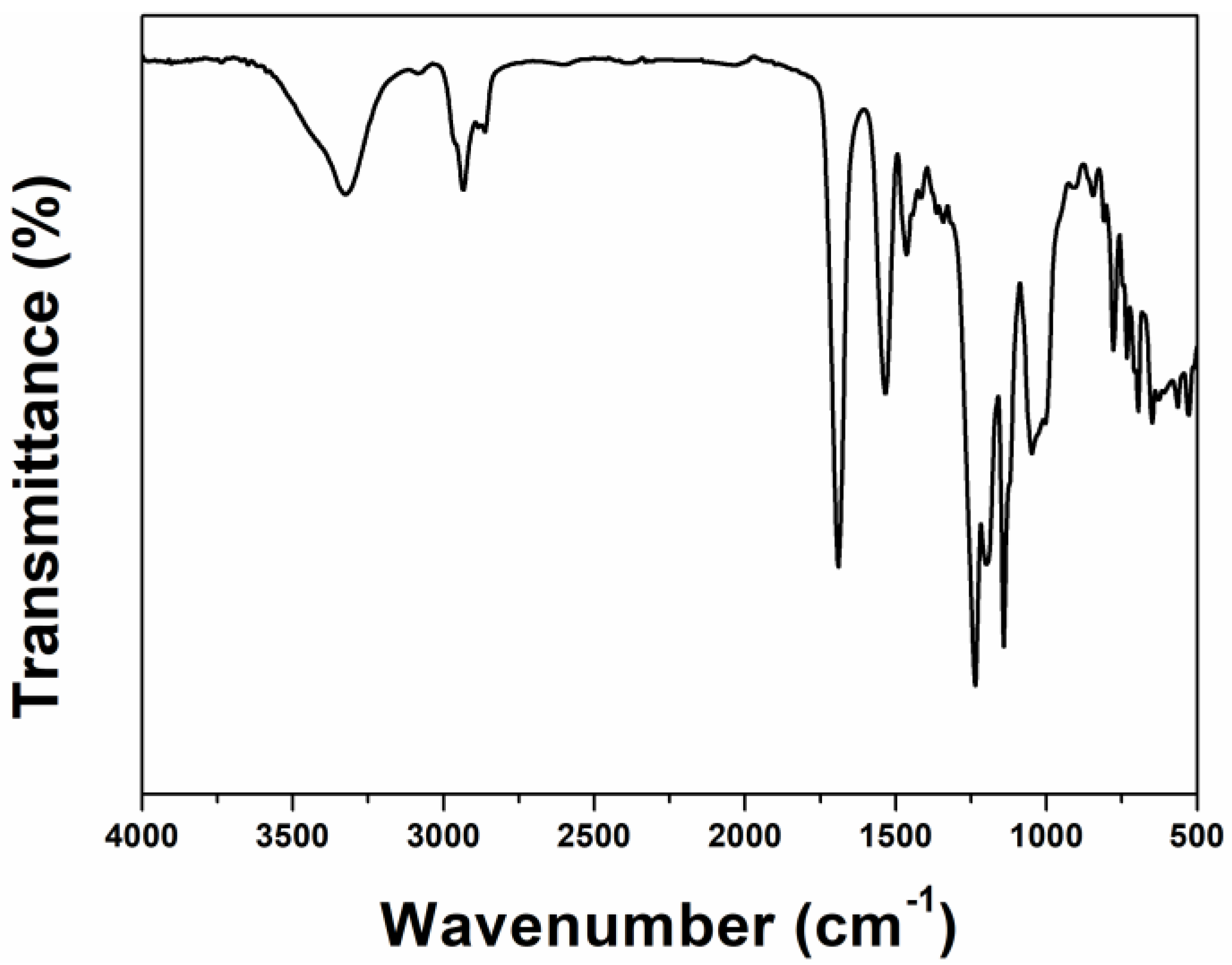
The IR spectrum of TMP-HDI-TEOH6 is shown in
Figure 3. We can clearly see the typical characteristic absorption bands of polyurethane. The peak located at 1719 cm
−1 is the stretching vibration absorption peak of C=O in urethane, the peak located at 1532 cm
−1 is the absorption peak of amide -NH-CO-, and the peak located at 3324 cm
−1 is the stretching vibration peak of N-H in urethane. The co-existence of the above characteristic absorption peaks indicates that a urethane group was generated. In addition, the strong characteristic absorption band at 1400–1000 cm
−1 is attributed to the C-F stretching vibration, indicating the presence of fluorocarbon groups. The addition of trimethylolpropane (TMP) introduces structures such as -CH2-CH3 and -OH, which can be seen on the infrared map. Absorption peaks of the stretching vibration of long-chain alkanes consisting of -CH2- CH3 appear near 2960–2850 cm
−1. The hydroxyl -OH expansion vibration peaks appear near 3376 cm
−1, which proves that the product contained diols. The asymmetric stretching vibration absorption peak located near 2273 cm
−1 corresponding to the -N=C=O group does not appear in the IR spectrum of TMP-HDI-TEOH6, which indicates that the isocyanate group has reacted completely. The above infrared analysis proves that TMP-HDI-TEOH6 was synthesized successfully [
44].
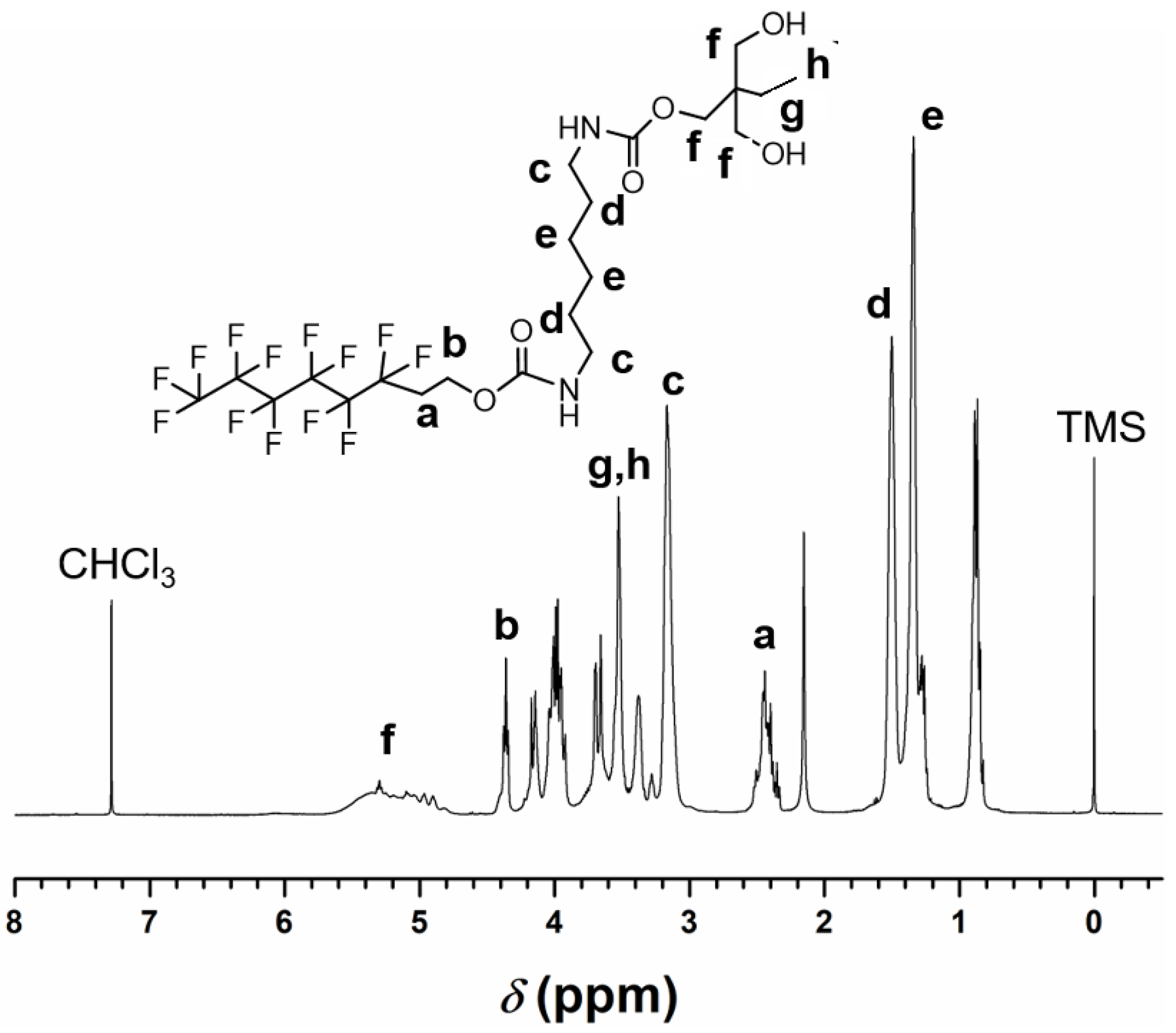
While analyzing the structure of the TMP-HDI-TEOH6 sample using FTIR, the synthesized TMP-HDI-TEOH6 sample was structurally characterized using HNMR chemical shifts. The HNMR plots of the samples are presented in
Figure 4. The following chemical shifts were detected at 2.39 ppm, 4.38 ppm, 3.20 ppm, 1.52 ppm, and 1.35 ppm, which corresponded to the protons at a, b, c, d, and e, respectively, in the molecular structure of TMP-HDI-TEOH6 in
Figure 4. HNMR spectra also showed that the chemical shifts at 4.72 ppm, 3.47 ppm, and 3.57 ppm corresponded to the protons at f, g, and h in the molecular structure of TMP-HDI-TEOH6, respectively. The above HNMR analysis proves that TMP-HDI-TEOH6 was successfully synthesized [
45].
3.2. Structural Characterization of Resin-FPU-OH
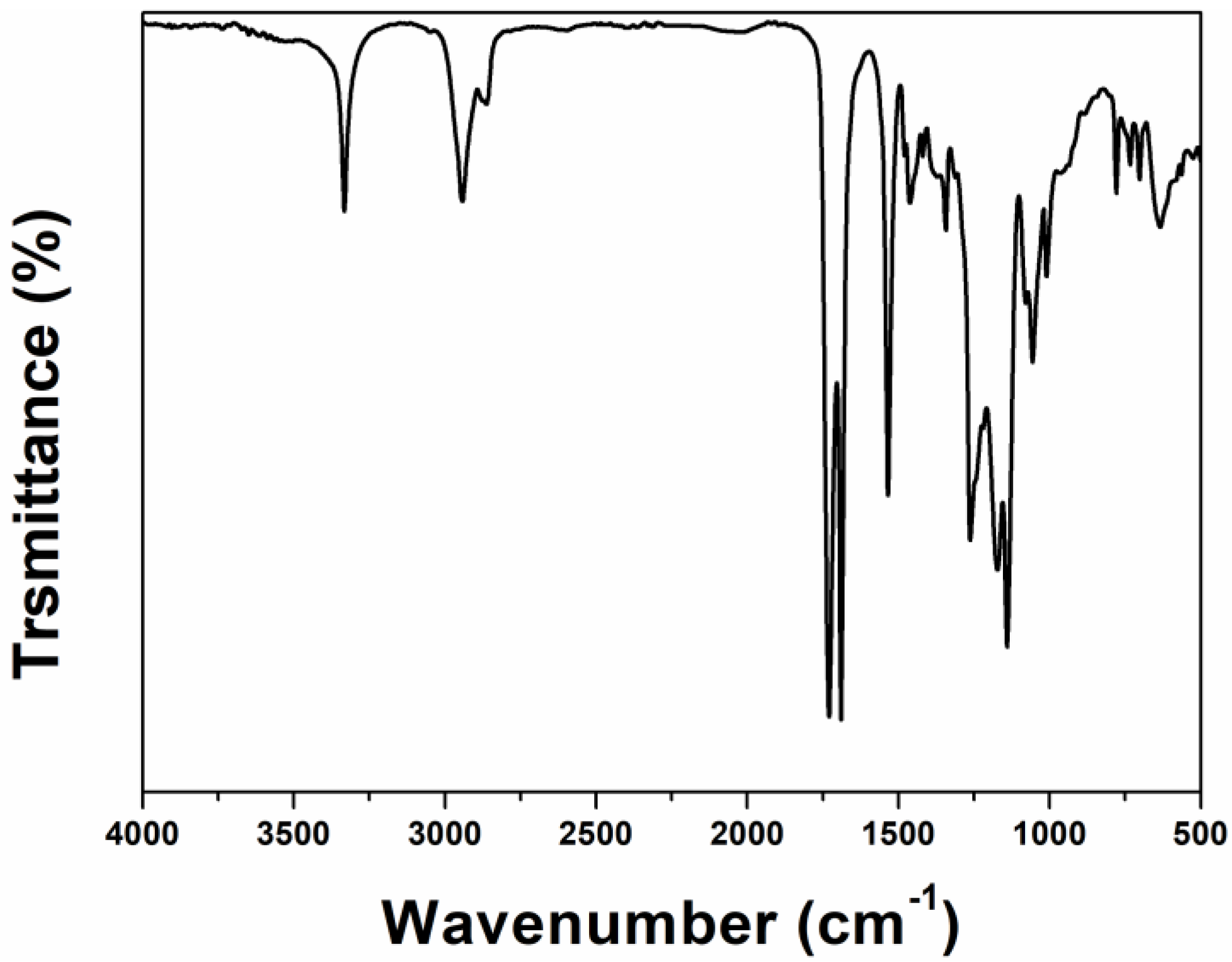
The infrared spectrum of the fluorocarbon polyurethane hydroxy resin (Resin-FPU-OH) is shown in
Figure 5. It can be seen that the stretching vibration absorption peak of C=O in ethyl carbamate appears at 1719 cm
−1, the absorption peak of amide in -NH-CO- appears at 1532 cm
−1, and the stretching vibration peak of N-H in ethyl carbamate appears at 3324 cm
−1, indicating the presence of ethyl carbamate -NH-CO-O-. The characteristic absorption peak at 1400–1000 cm
−1 indicates the presence of fluorocarbon functional groups in the monomer, 1730 cm
−1 shows the characteristic absorption peak of polyester C=O, and 1265 cm
−1 shows the stretching vibration characteristic peak of ester CO-O-C-.
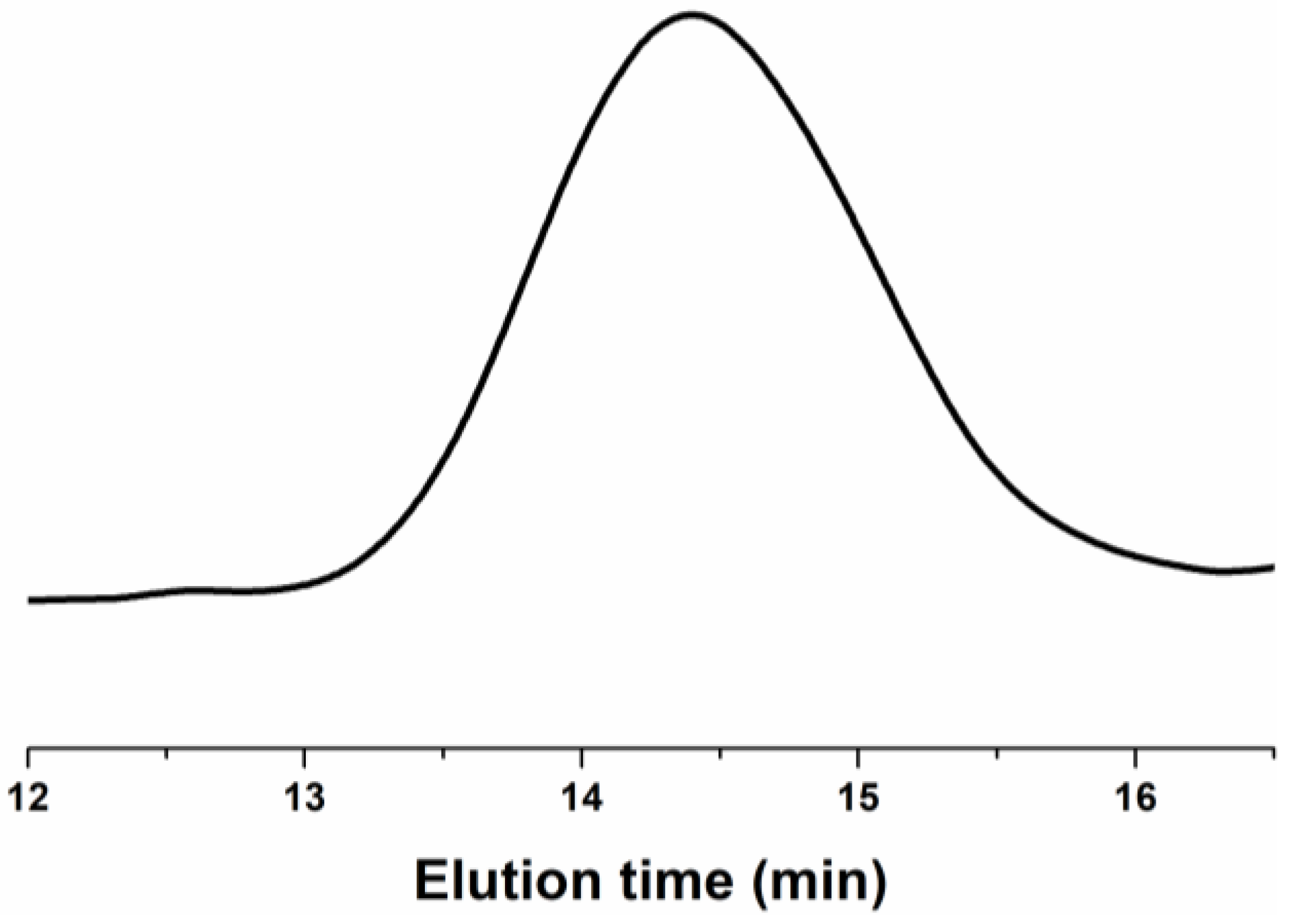
Gel permeation chromatography (GPC) analysis was performed on the fluorocarbon polyurethane hydroxyl resin (Resin-FPU-OH), and the obtained profiles are shown in
Figure 6. From gel permeation chromatography analysis, the number average molecular weight (Mn) was 5.744 × 10
3 (±17.081%), the viscosity average molecular weight (Mv) was 6.014 × 10
3 (±2.025%), the weight average molecular weight (Mw) was 6.096 × 10
3 (±22.373%), and the higher average molecular weight (Mz) was 6.908 × 10
3 (±60.591%). The polydispersity index (Polydispersity in Mw/Mn) was 1.061 (±28.148%) and Mz/Mn was 1.203 (±62.953%). From the data, it can be seen that the polydispersity of Resin-FPU-OH is relatively wide and the average relative molecular mass of the product is relatively concentrated [
46].
3.3. Basic Performance of Paint Film
The basic performance test results of the fluorocarbon polyurethane amino baking paint films are shown in
Table 4.
Fluorocarbon polyurethane hydroxyl resins have smaller molecular weights and higher proportions of terminal hydroxyl groups, so formulations with more fluorocarbon polyurethane hydroxyl resins, instead of hydroxy acrylic resins with higher molecular weights, have higher crosslink densities of their paint films and more fluorocarbon chains. From
Table 4, as formulations F5 to F1 gradually contain more Resin-FPU-OH, their paint films gradually exhibit higher hardness and better salt spray resistance, but less flexibility and poorer adhesion [
47]. All five paints passed the tests of acid resistance, alkali resistance, and UV resistance.
Amino baking paint films for whiteboards need to exceed 2H hardness, Class 0 adhesion, 1T flexibility, and 360 h of salt spray resistance. The films of F1 and F2 showed good hardness and salt spray resistance, but poor adhesion and flexibility, so these two formulations were excluded. For F3, F4, and F5, their hardness, adhesion, flexibility, and salt spray resistance mostly met these requirements, so more hydrophobic and oleophobic performance tests could be conducted.
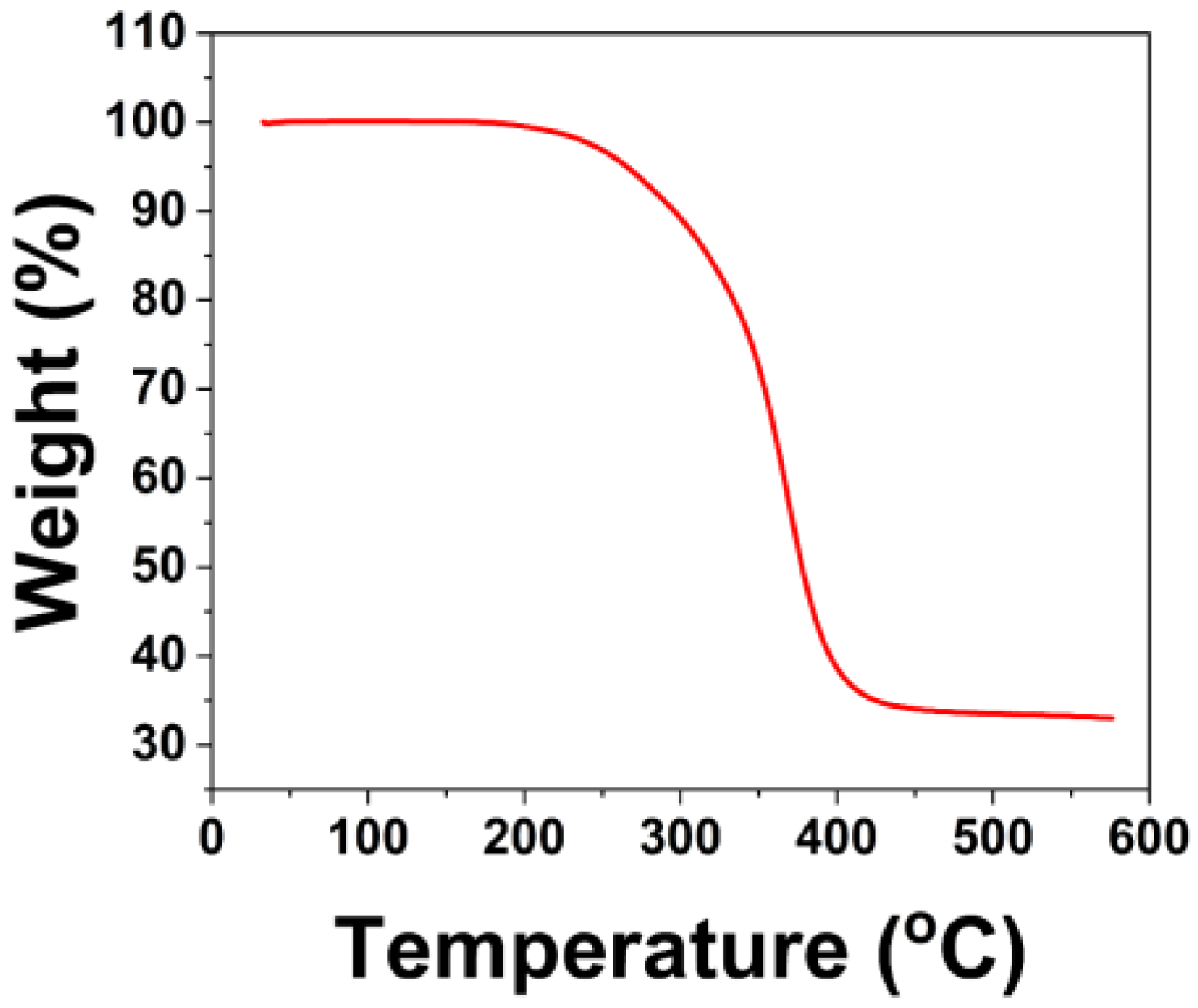
After the above tests, F3 was able to meet the basic performance requirements for paint films. In order to further verify the thermal stability of the paint film, TGA testing was conducted on F3, and the results are shown in
Figure 7.
3.4. Preliminary Evaluation of Hydrophobic and Oleophobic Properties
Further testing was conducted to find the formulation with the best hydrophobic and oleophobic properties from F3, F4, and F5, which met the basic characteristics of baking paint. Due to the fact that all the formulations have the same total weight and elements, the integrated area of F can be considered as the result of the relative content of F in different formulations. From
Figure 8, it can be seen that the integrated area under the XPS curve of formula F3 (integrated area 403,095.815) is greater than that of formula F4 (integrated area 269,773.235). Therefore, it can be concluded that the surface fluorine content of formula F3 is higher than that of F4, which also means that formula F3 has better hydrophobic and oleophobic properties than F4. However, it was necessary to further verify whether the F3 formula has better hydrophobic and oleophobic properties through measuring the water and cetane contact angles on the surface of the paint films [
48].
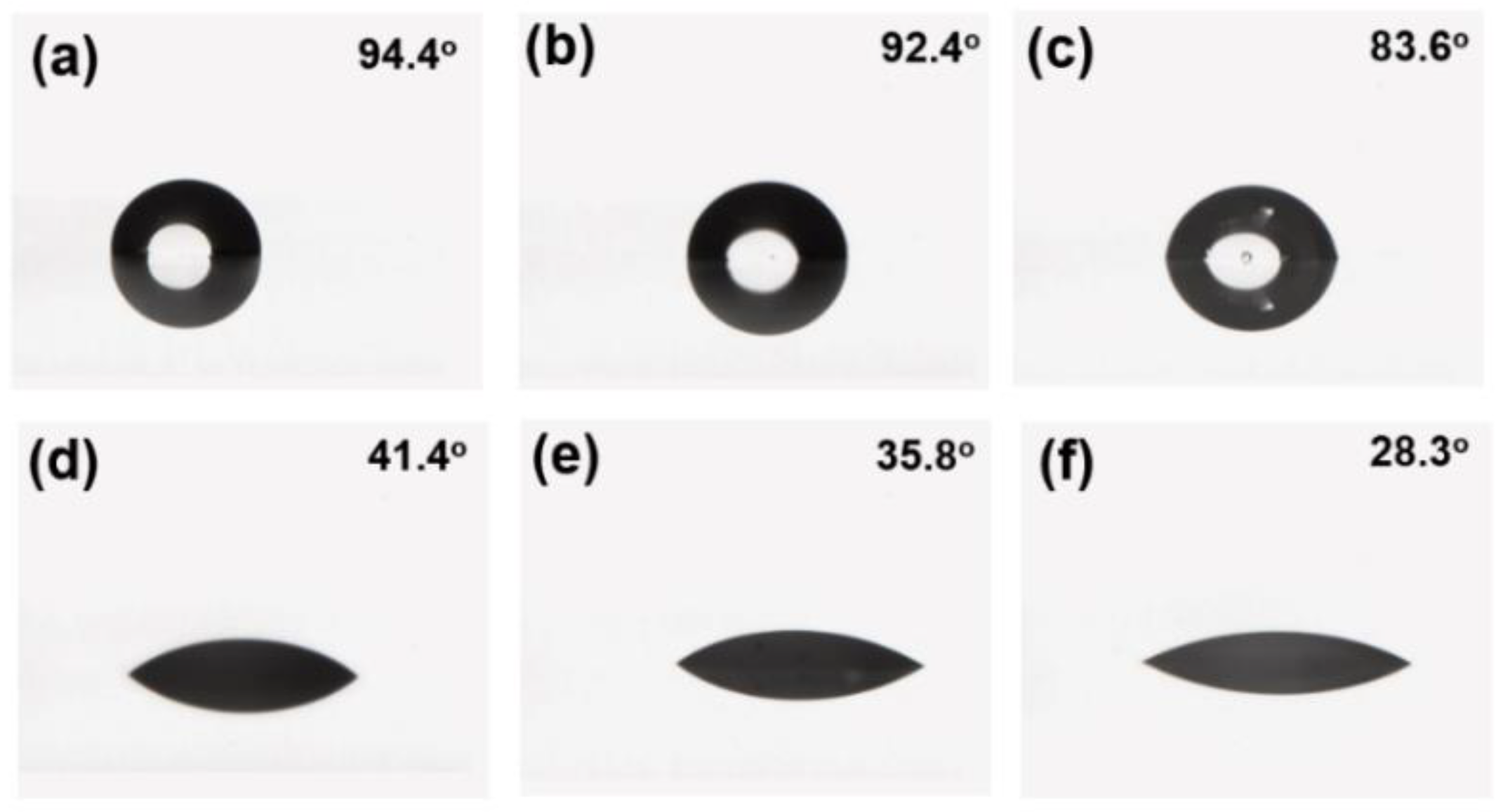
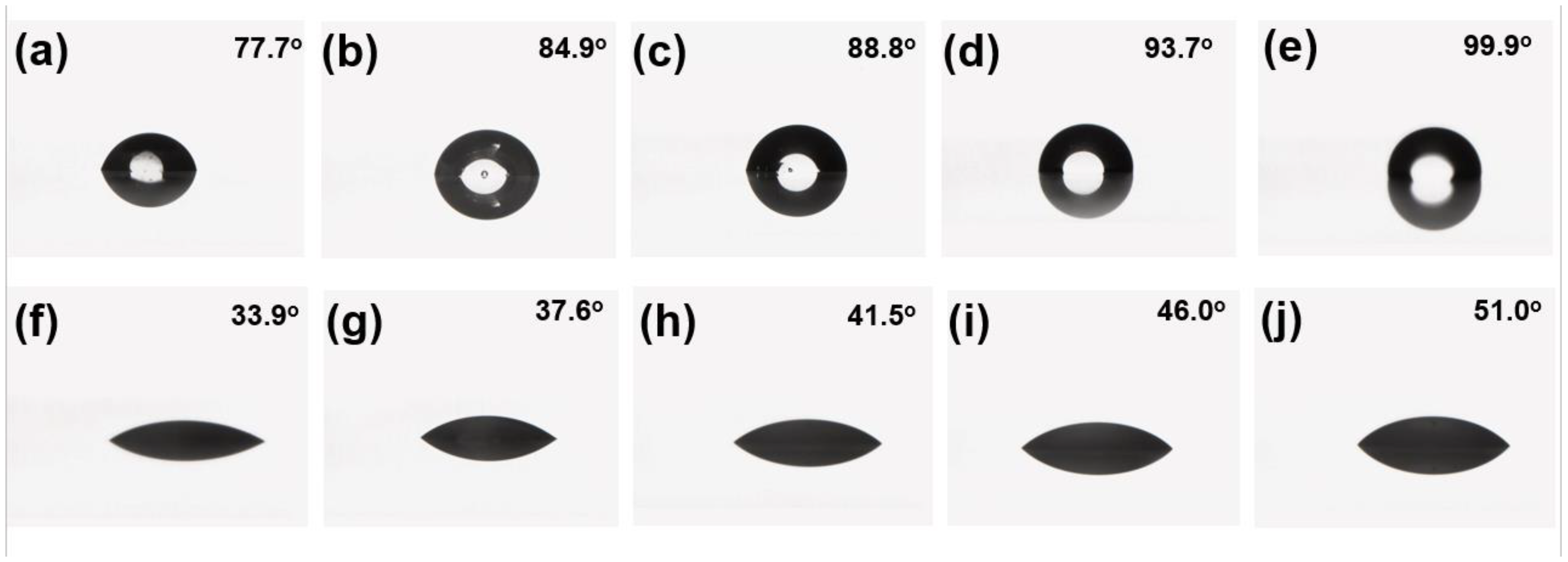
Typically, the fluorine content on the surface of a paint film is positively correlated with the contact angle of the liquid on its surface, but this inference is not always correct. When the proportion of fluorine in the main chain is high, the liquid contact angle on the surface of the paint film is not large. Therefore, in addition to testing the fluorine content on the surface of the paint film using XPS, further testing of the water and cetane contact angles was needed to visually verify the hydrophobicity and oleophobic of each paint film. The test results of the water and cetane contact angles of F3, F4, and F5 are shown in
Figure 9.
As can be seen from
Figure 9, the comparison of water and cetane contact angles on the paint film is F3 > F4 > F5, and the amount of fluorocarbon polyurethane hydroxyl resins added in the formulation is also F3 > F4 > F5. F5 does not have fluorocarbon polyurethane hydroxyl resins, but is used as a comparative formulation. The contact angle test shows that the water and cetane contact angles are positively correlated with the amount of fluorocarbon polyurethane hydroxyl resin added in the paint formulation.
3.5. Initial Hydrophobic and Oleophobic Properties of Paint Films
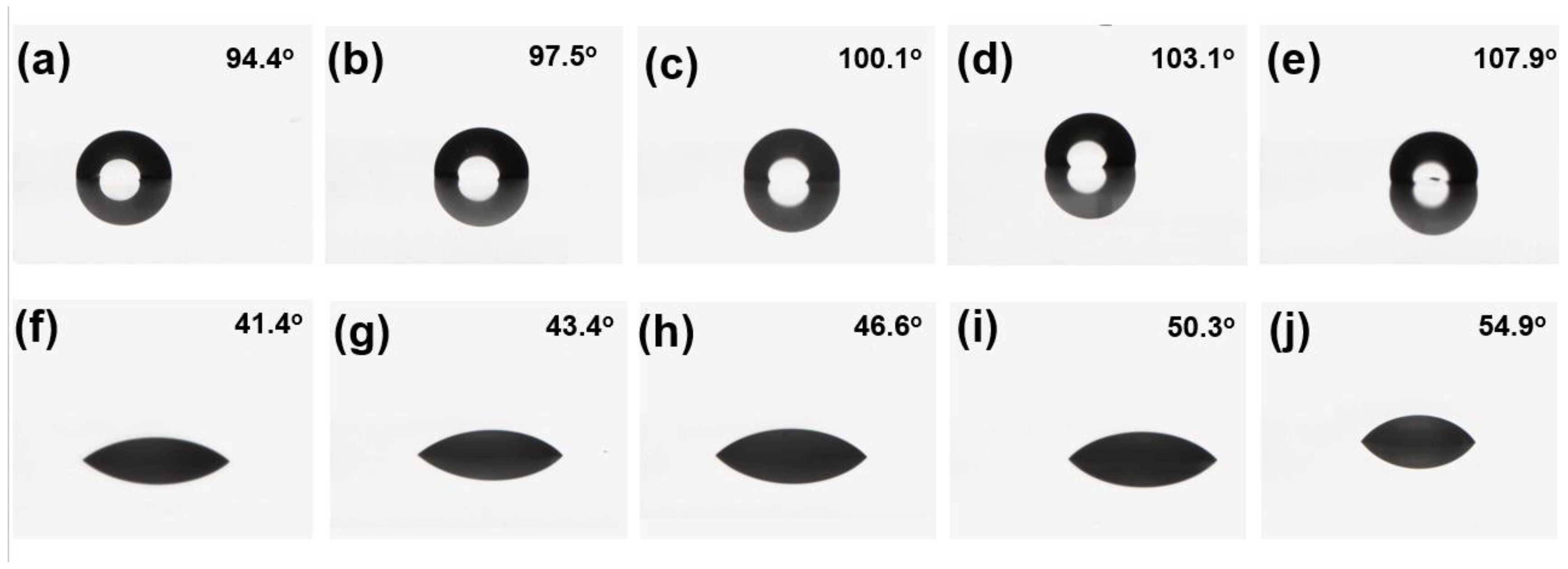
After selecting the preferred formulation, F3, upgraded formulations F6–F9 were obtained by adding different proportions of Wacker WS62M terminal hydroxyl silicone oil. The effects of adding terminal hydroxyl silicone oil on the hydrophobic and oleophobic performance of the paint films were measured by the contact angles of water and cetane, and the test results are shown in
Figure 10.
All five formulations demonstrated good hydrophobic and oleophobic properties, and the contact angles of water and hexadecane increased with the increase of the proportion of hydroxyl terminated silicone oil WS62M. This confirms that the addition of WS62M can improve the hydrophobicity and oleophobicity of paint films. The degree of improvement is positively correlated with the proportion of hydroxyl terminated silicone oil WS62M, because hydroxyl terminated silicone oil WS62M can lower surface tension on the surface of the paint film and synergistically interact with the fluorocarbon polyurethane hydroxyl resin, making the paint film more hydrophobic and oleophobic [
49].
Measurement of the sliding angle helps to compare the hydrophobicity and oleophobicity of different paint films, which is also closely related to the graffiti resistance of paint films. The results of the water and cetane sliding angle tests of the five formulations are shown in
Table 5. It can be seen that F9, with more hydroxyl terminated silicone oil, has minimal water and cetane sliding angles. This is due to the presence of more hydroxyl silicone oils and fluorocarbon functional groups giving the film a lower coefficient of friction.
3.6. Microstructure and Morphologies of Paint Film
The SEM pictures of the five formulations are displayed in
Figure 11.
It can be observed that more hydroxyl terminated silicone oil WS62M in the formulation can cause a gradual increase in texture, seemingly with better micro-roughness. This may be one of the reasons why the paint film is more hydrophobic and oil repellent [
50].
3.7. Hydrophobic and Oleophobic Properties of Paint Film after Abrasion
The pictures of water and cetane contact angles of the paint films after 5000 abrasions are displayed in
Figure 12.
The diamond grit contained in the dishcloth removed some of the substances on the surface of the paint film during repeated friction. Compared with their initial status, the water and cetane contact angles for the same formula paint film after abrasion decreased significantly, indicating that physical friction has a greater effect on the contact angle of the paint film, which affects the hydrophobicity and oleophobicity of the paint film.
After abrasion with the dishcloth, for the same paint film, the decrease of water contact angle was larger than the decrease of cetane contact angle. On the one hand, the initial water contact angle was about twice the cetane contact angle, so the water contact angle decreased more than the cetane contact angle. On the other hand, the fluorocarbon material on the surface of the paint film is difficult to sand off due to its low friction coefficient, while the ordinary material on the surface of the paint film is easier to sand off due to its high friction coefficient.
We find that for different paint films, the higher the amount of terminal hydroxyl silicone oil WS62M added, the less the change in contact angle is affected by abrasion. This is due to the lubricating effect of the terminal hydroxyl silicone oil on the surface of the paint film, which further reduces the coefficient of friction of the paint film and makes the paint film more resistant to repeated sanding with a dishcloth.
3.8. Hydrophobic and Oleophobic Properties of Paint Film after Alcohol Wiping
The five paint films’ water and cetane contact angles were tested after 100 reciprocal wipes with a fabric moistened with alcohol on the surface of each fluorocarbon polyurethane amino baking paint film. The test results are shown in
Figure 13.
After wiping with alcohol-moistened fabric, the water contact angle and cetane contact angle of the five paint films decreased significantly, indicating that alcohol has an effect on their hydrophobic and oleophobic properties. Because alcohol is an organic solvent, the polymer resin on the surface of the paint films was dissolved and destroyed during repeated rubbing with alcohol, thus affecting the contact angle.
After wiping with alcohol-moistened fabric, the decrease of water contact angle was larger than the decrease of cetane contact angle for the same paint film. On the one hand, because the water contact angle in the initial state was about twice the cetane contact angle, the absolute value of the water contact angle decreased more. On the other hand, alcohol as a solvent has no effect on the fluorocarbon material but, according to the principle of similar solubility, alcohol has a solubilizing effect on the paint film. As such, the hydrophobic material was more degraded, so the water contact angle dropped more than the cetane contact angle.
After wiping with alcohol-moistened fabric, the water and cetane contact angles of the paint films with a higher dosage of the terminal hydroxyl silicone oil WS62M were less affected, because the addition of more terminal hydroxyl silicone oil WS62M cross-links with the amino resin, preventing the paint film from being further damaged by solvation and making the film more resistant to repeated rubbing with alcohol [
51].
3.9. Water and Oil Absorption of Paint Film
The water and oil absorption rates of the paint films were used to evaluate the hydrophobicity and oleophobicity of the fluorocarbon polyurethane amino baking paints, and the test results are shown in
Table 6.
Compared with oil absorption rates, water absorption rates were much lower for the same paint films after 24 h of water and cetane immersion, respectively. According to the principle of similarity and solubility, the paint films are lipophilic, so it was easier for cetane molecules to enter the interior of the paint film, resulting in a higher value of oil absorption than water absorption.
From the table, it can be found that the water and oil absorption of each paint film decreased with the increase in the amount of terminal hydroxyl silicone oil added. This is because more terminal hydroxyl silicone oil crosslinks with the amino resin to form a denser paint film and this reduces the surface tension of the film at the same time, making it more difficult for water or cetane to penetrate into the interior of the film [
52].
3.10. Graffiti Resistance of Paint Film
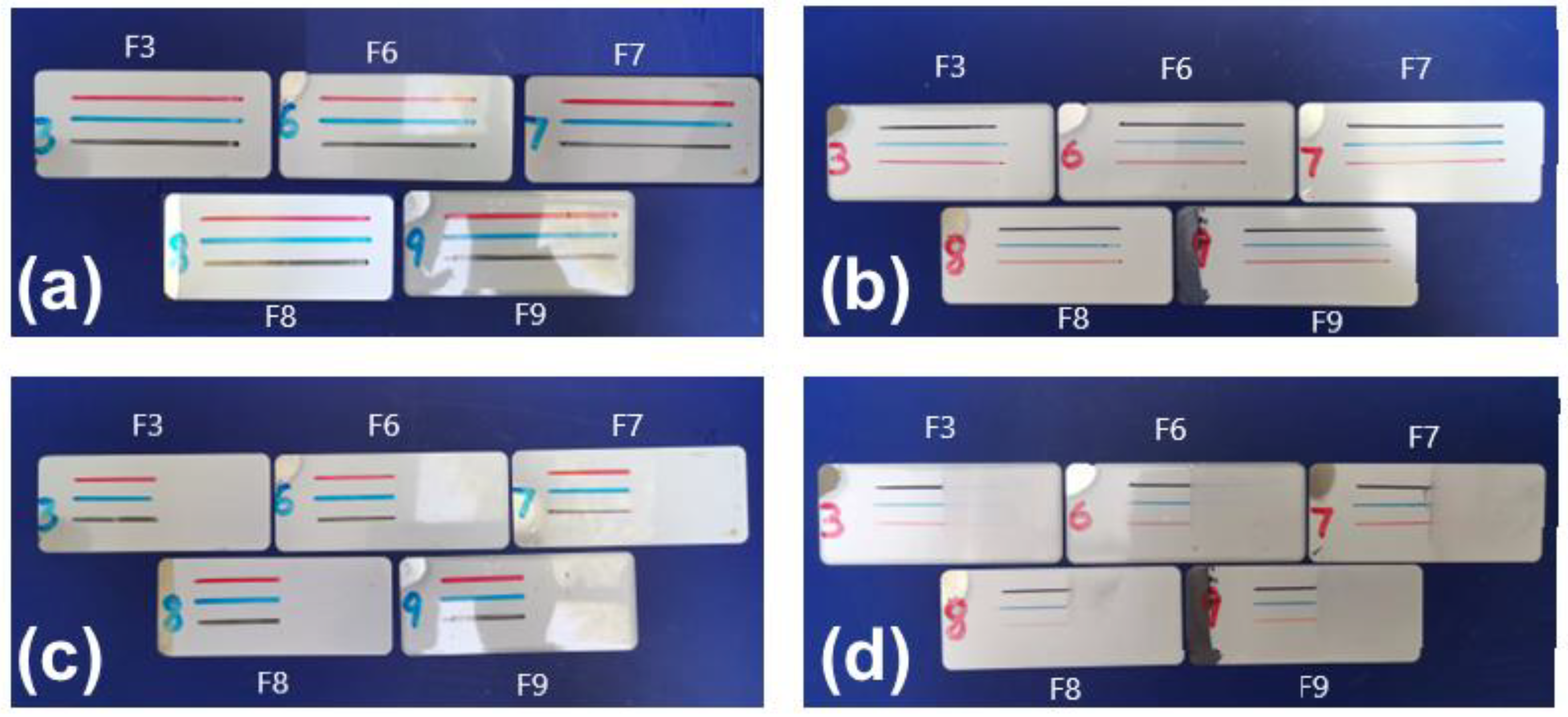
In addition to testing the contact angles, we also need to simulate actual application scenarios to test the graffiti resistance performance of the fluorocarbon polyurethane baking paints by using the most common black, red, and blue oil-based markers and alcohol-soluble whiteboard pens to draw a line on each fluorocarbon polyurethane baking paint film. Then, the lines were kept for 24 h and wiped away with a dry cloth. The test results are shown in
Figure 14.
From
Figure 14, we find that all five paint films have good resistance to graffiti caused by alcohol-based whiteboard markers. However, for graffiti caused by oil-based markers, paint films F3, F6, and F7 showed more residue, while F8 and F9 showed smaller amounts of residue and F9 showed even less residue (darker light on the right side of the picture). In addition, there was a difference in graffiti resistance for the three colors of oil markers. Much more black residue was left on the paint films than red and blue. This result was related to the stronger coloring power of carbon black than the red and blue pigments.
Therefore, it can be concluded that paint films with more terminal hydroxyl silicone oils have better graffiti resistance, especially for graffiti caused by oil-based markers. This is because the terminal hydroxyl silicone oil crosslinked with the amino resin forms a dense isolation film on the surface of the paint film, preventing the ink from penetrating into the paint film. The low surface tension of the terminal hydroxyl silicone oil greatly reduces the adhesion of the ink to the paint film. We can therefore conclude that paint films with more terminal hydroxyl silicone oil possess excellent anti-graffiti performance [
53].
4. Summary
This article investigated fluorocarbon polyurethane amino baking paints used for writing whiteboards, with the aim of making graffiti caused by oil-based marker pens and alcohol-soluble pens more easily wiped clean after prolonged retention.
After several steps of reactions, fluorocarbon polyurethane hydroxyl resin (Resin-FPU-OH) was obtained, which was characterized by using infrared analysis, nuclear magnetic resonance, and gel permeation chromatography. Amino baking paints were prepared using this core material.
The basic performance of five starting formulations with different proportions of fluorocarbon polyurethane hydroxyl resins were tested. Formulations that did not meet the basic property requirements for whiteboard paint films were screened out, and F3 was selected as the preferred formulation by testing the fluorine content, the sliding angle, and the water and cetane contact angles of each paint film. The upgraded formulations F6–F9 were prepared by adding different proportions of the terminal hydroxylated silicone oil to the preferred formulation F3 for further evaluation of hydrophobic and oleophobic properties.
The microstructures and morphologies of these paint films were observed by SEM. The contact angles of water and cetane of each paint film were tested on initial status and other statuses, such as after dishcloth abrasion and alcohol wiping. The water and oil absorption rates of the paint films were calculated. The anti-graffiti resistance of the paint films was also tested by observing the residue of lines caused by several colors of oil-based markers and alcohol-soluble whiteboard pens after they were retained for 24 h. All of these tests verified that fluorocarbon polyurethane baking paint films with more terminal hydroxyl silicone oil added had denser surfaces, bigger contact angles of water and cetane, lower water and oil absorption rates, and better graffiti resistance.
Compared with traditional whiteboard baking paints, fluorocarbon polyurethane baking paints have excellent hydrophobic, oleophobic, and graffiti-resistance properties due to the fluorocarbon material on their surfaces. Moreover, the addition of terminal hydroxyl silicone oil can further enhance the hydrophobicity and oleophobicity of paint films, even after abrasion with a dishcloth and rubbing with alcohol. Fluorocarbon chains and silicone oils synergize with each other to provide better graffiti resistance to whiteboard baking paints. In particular, it should be emphasized that both fluorocarbon and silicone functional groups are chemically grafted onto the main chain of the coating resin and are highly cross-linked with the amino resin to ensure that the paint film is highly dense and resistant to repeated rubbing, achieving a long-lasting graffiti-resistant effect.