Abstract
The failure mechanism of the Pt-modified aluminide (Pt-Al) bond coating (β-(Ni, Pt)Al coating) in a simulated service environment has seldom been investigated. Based on a self-developed thermal barrier coating service environment simulator, a thermal shock experiment of single-phase Pt-Al bond coating on DD419 substrate at a temperature of 1170 °C was conducted combined with a real-time monitoring infrared thermal imager. The lifespan and failure mechanism of the coating are analyzed in detail. The results reveal that specimens of the Pt-Al bond coating, subjected to three repeated tests, exhibit failure after 650, 528, and 793 thermal shock cycles at 1170 °C, respectively. After failure, the contents of Pt and Al elements in the peeled region are lower than those in the unpeeled area, and a diffusion zone emerges in the bond coating. The failure mechanism of the Pt-Al bond coating during the thermal shock test can be attributed to three main aspects: (1) the diffusion and consumption of the Pt element reduced the oxidation resistance of the Pt-Al bond coating; (2) the diffusion and depletion of elemental Al causes a phase change in the coating, leading to the failure of the coating; (3) thermal stresses are generated in the Pt-Al bonded coating during the thermal shock test, which ultimately leads to wrinkling.
1. Introduction
Thermal barrier coating (TBC) has significantly enhanced the service temperature of turbine blades, which are mainly composed of a thermally insulating coating, an oxidation-resistant metal bond coating, and superalloy substrates [1,2,3,4]. Among these components, the bond coating is primarily an MCrAlY (M = Ni and/or Co) layer or Pt-modified aluminide (Pt-Al) bond coating [5,6], which is mainly deposited on nickel-based superalloys to bond the ceramic coating and the superalloy substrate. Currently, the Pt-Al bond coating is widely utilized in TBC systems for turbine components, including blades and nozzle guide vanes, due to its exceptional performance. The protection of Pt-Al bond coating is primarily due to the formation of a continuous α-Al2O3 regenerated layer on the surface [7], which offers comprehensive support against high-temperature oxidation and hot corrosion for the entire TBC [8,9]. Meanwhile, the Pt-Al bond coating exhibits superior mechanical properties and excellent oxidation and corrosion resistance [10,11].
In the actual service environment, aircraft undergo three primary stages: take off, flight, and landing, paralleling the corresponding stages of the engine turbine blades, which encompass heating, holding, and cooling. As a crucial element in safeguarding turbine blades, the Pt-Al bond coating is required to endure cyclic attacks of high-temperature, high-speed, and high-pressure gas. Due to the susceptibility of Pt-Al bond coatings to brittle fracture, the fatigue behavior of Pt-Al bond coating was evaluated by Mahesh et al. [12]. The effect of Pt-Al bond coatings on the fatigue fracture and deformation mechanism of a fourth-generation single-crystal high-temperature alloy under different temperature and stress conditions via a high-temperature cyclic fatigue test was investigated [13]. In thermal cycling exposure and mechanical property tests, the Pt-Al bond coating demonstrated effective oxidation protection. On the one hand, Pt can enhance the oxidation resistance by increasing the diffusion coefficient of Al in the alloy, and it enhances the adhesion of the protective layer to the substrate [14]. On the other hand, Pt can increase the modulus of elasticity of β-NiAl and also improve its creep resistance, preventing a significant decline in alloy ductility [7]. Researchers are also working on improvements to the wrinkle resistance and oxidation resistance of Pt-Al coatings [15,16,17,18]. The high-temperature oxidation resistance of β-(Ni, Pt)Al coatings can be significantly enhanced by controlling the Pt/Al ratio, and the precipitation behavior of topological close-packed (TCP) phases can be modulated [19]. Meanwhile, the addition of a higher content of Zr to replace Pt in (Ni, Pt)Al coatings provides more significant thermal corrosion resistance than pure (Ni, Pt)Al coatings [20]. However, current investigations on Pt-Al bond coatings primarily focus on the microscopic morphology, mechanical properties, high-temperature oxidation, and thermal cycling behavior in high-temperature furnaces. Furthermore, the Pt-Al bond coating can easily form metastable alumina crystal in the early oxidation stage [21], and two irreversible phases (γ′-Ni3Al and γ-Ni) are formed during high-temperature oxidation, depleting the Al content and diminishing the oxidation resistance of the coating [22]. Therefore, there is an urgent need to comprehensively characterize the high-temperature performance of Pt-Al bond coating and investigate the failure mechanism under the simulated service environments.
In this paper, a Pt-Al bond coating was successfully prepared on a nickel-based single-crystal superalloy substrate. The high-temperature thermal shock performance of the Pt-Al bond coating at 1170 °C using a self-developed TBC service environment simulator was investigated. The microstructure and phase composition of the Pt-Al bond coating after thermal shock were comprehensively examined. Additionally, the service life of the Pt-Al bond coating was assessed under the simulated service environment and the associated failure mechanisms were discussed.
2. Experimental Procedure
2.1. Preparation of the Single-Phase Pt-Al Coating
The second-generation nickel-based single-crystal superalloy DD419 with a diameter of 10 mm and a thickness of 5 mm was chosen as the substrate, and details of the components are shown in Table 1. To enhance the adhesion of the bond coating, the substrate underwent a process of roughening with sandpaper, followed by polishing with diamond polishing powder. This process ensured that the surface of the substrate became smooth and devoid of any noticeable scratches. The preparation of a single-phase β-(Ni, Pt)Al coating involved three steps: Pt electroplating on the substrate, vacuum annealing, and vapor aluminizing treatment [23]. A platinum plating layer with a thickness of 5 μm was electroplated on the substrate in an alkaline platinum plating solution. The chemical composition of the platinum plating solution is shown in Table 2. The plating current was 9 mA/cm2 and the deposition rate of the Pt layer was approximately 3 μm/h. Subsequently, annealing was conducted in a vacuum muffle furnace at 1040 °C for 1 h. These treatments not only enhanced the adhesion of the Pt-plated layer to the substrate but also facilitated inter-diffusion between the substrate alloy and Pt. Finally, the high-pressure gas aluminizing treatment was conducted at 1070 °C for 5 h to obtain the Pt-Al coating.

Table 1.
Normal chemical compositions (wt.%) of single-crystal superalloy DD419.

Table 2.
Chemical composition of the alkaline Pt-plating solution.
2.2. Thermal Shock Testing of the Pt-Al Bond Coating
Before conducting the thermal shock testing, an infrared calibration test should be executed on the Pt-Al coating surface to determine the emissivity of the bond coating at 1170 °C, which guarantees that the test temperature of the thermal shock attains a predetermined value [24]. The experimental setup for the infrared calibration is illustrated in Figure 1. Initially, the Pt-Al coating specimen was heated to 1170 °C using a high-temperature muffle furnace. Thermocouples A and B were positioned on two sides of the specimen, and the temperature discrepancy between the two thermocouples was maintained below 3 °C within a duration of 10 min. Subsequently, the temperature distribution on the surface of the specimen was observed by infrared thermal imaging (GF309, FLIR) after the Pt-Al coating specimen was retained at 1170 °C for 30 min. Once a uniform temperature distribution was achieved, the emissivity of the infrared thermometer (MR1SBSF, Raytak) was adjusted to ensure that the measured surface temperature reached 1170 °C. The average value of multiple measurements was considered, and the infrared emissivity calibration data are shown in Table 3. Ultimately, the emissivity of the Pt-Al coating at 1170 °C was determined to be 0.86.
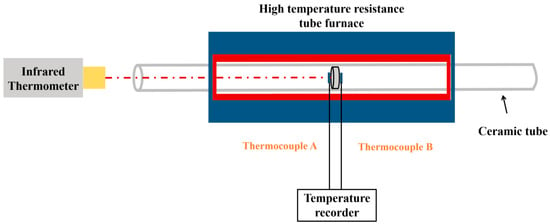
Figure 1.
Illustration of the determination of infrared emissivity.

Table 3.
The infrared emissivity calibration data of the Pt-Al coating.
The high-temperature thermal shock performance of the Pt-Al bond coating at 1170 °C was assessed using a self-developed TBC environment simulator. Notably, to emulate a more realistic service environment, aviation kerosene was utilized as fuel and nitrogen was employed to provide sufficient pressure. Liquid oxygen and compressed air were employed to facilitate the complete combustion of kerosene [25]. The detailed experimental parameters of the environmental simulator are outlined in Table 4. The temperature cycling curves (Figure 2a) show that the single thermal shock cycle, lasting 4 min, comprised three stages: heating, holding, and cooling. The Pt-Al coating surface was heated from room temperature to 1170 °C in 30 s, maintained at 1170 °C for 90 s, and then cooled for 120 s in an air environment. Figure 2b illustrates the temperature distribution of the bond coating during the holding stage exhibits uniformity at 1170 °C. The thermal shock procedure ceased every 10 cycles, and the surface morphology of the test specimens was recorded with a high-definition camera. Failure criteria are reached when the surface of the Pt-Al coating shows spallation (green area) and the degree of spallation reaches 10%. To minimize the potential impact of external factors, three sets of the same batch of Pt-Al coating specimens were examined, each labeled as #1, #2, and #3, respectively. To further validate the reliability of the thermal shock test, a corrosion solution was prepared by mixing 5 g of ferric chloride, 2 mL of hydrochloric acid, and 95 mL of alcohol. The cross-section of the failed specimens was corroded. Based on the micro-image threshold processing using Photoshop Software (Photoshop CC 2018), it was determined that the content of γ′ phase in the Pt-Al coating cross-section after corrosion was less than 50%. This proves that there were no instances of overheating during the thermal shock test. The specific procedure involved importing the image into Photoshop Software, converting the image into grayscale mode, and setting an appropriate threshold, usually 128. Pixels with brightness above 128 were rendered white, while those below 128 were rendered black. The percentage of black area denoted the proportion of γ′-phase content.

Table 4.
The main parameters of the environmental simulator during thermal shock test.

Figure 2.
(a) The temperature profile of the single-phase β-(Ni, Pt)Al coating specimen during thermal shock cycle; (b) the temperature distribution of the specimen during holding stage (the round shape is the specimen).
2.3. Microstructure Characterization
The phase composition of the as-deposited Pt-Al bond coating was determined using X-ray diffraction (XRD, CuKα, Ultimate IV, RIGAKU, Tokyo, Japan). The micro-morphology and element distribution of the coating surface and cross-section after failure were examined using a scanning electron microscope (SEM, TESCAN MIRA3 LMH) and an energy dispersive spectrometer (EDS, Oxford X MAX20).
3. Results and Discussion
3.1. Microstructure and Phase Composition of the As-Deposited Pt-Al Coating
The XRD pattern of the surface of the as-deposited Pt-Al bond coating is shown in Figure 3. The characteristic peak of the specimen is a typical structure of single-phase Pt-Al bond coating (β-(Ni, Pt)Al), showcasing the preferred orientation in the (110) plane and the identical intensity peak diffraction in the (100), (111), (200), (210), and (211) planes. Hence, it can be inferred that the presence of Pt element has no discernible impact on the diffusion rates of the Al and Ni elements.
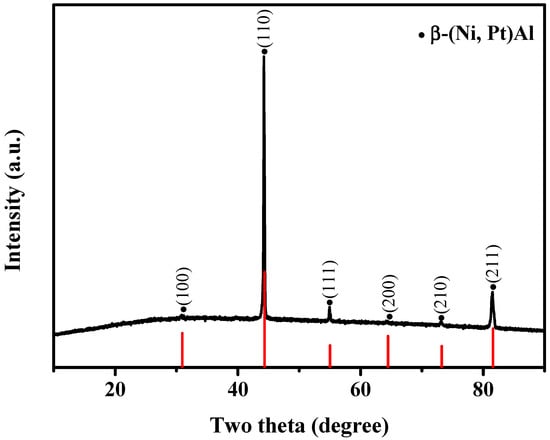
Figure 3.
XRD pattern of the as-deposited Pt-Al bond coating (The black line represents the experimental value, and the red line is the XRD standard card of β-(Ni, Pt)Al).
The surface and cross-sectional morphologies of the Pt-Al bond coating are shown in Figure 4. Each specimen exhibits a typical β-(Ni, Pt)Al microstructure with the surface of the coating divided by grain boundary ridges (Figure 4a–c) [23]. The average grain sizes of specimens #1–3 were 27.57 μm, 28.11 μm, and 30.57 μm, respectively. The cross-sectional morphologies of the corresponding specimen indicate that Pt-Al bond coatings (Figure 4d–f) is continuous, uniform, and dense, with a thickness of about 50 μm. It comprises an outer zone of β-(Ni, Pt)Al and an inter-diffusion zone (IDZ) of refractory metal precipitation [26,27]. The differences in the surface and cross-section morphology of the specimens were comparatively small and satisfied the test requirements.

Figure 4.
The surface morphologies of the Pt-Al bond coating specimens: (a) specimen #1; (b) specimen #2; and (c) specimen #3. The cross-sectional morphologies of the Pt-Al bond coating specimens: (d) specimen #1; (e) specimen #2; and (f) specimen #3.
3.2. Macroscopic Morphology of the Pt-Al Bond Coating after Thermal Shock
Figure 5 shows the macroscopic morphology of three Pt-Al bond coating specimens after thermal shock testing. As the thermal shock cycles increase, a light gray-green morphology, discernible to the naked eye, begins to emerge at the edge of the coating surface, primarily indicating the trace of Pt-Al coating failure. Concurrently, the coating edge starts to peel, and the peeled area progressively expands. Eventually, specimens #1, #2, and #3 all reached the failure threshold after 650, 528, and 793 cycles, respectively.

Figure 5.
The macroscopic morphology of the Pt-Al bond coating surface after thermal shock cycles: (a) specimen #1; (b) specimen #2; and (c) specimen #3.
During the thermal shock testing, infrared thermal imaging technology was utilized to continuously monitor and record the temperature distribution and defects on the surface of the Pt-Al coating in real time. The temperature distribution of the Pt-Al coating specimens during the holding stage is shown in Figure 2b. At the holding stage, the temperature distribution of the surface of the Pt-Al bond coating remains uniform and is maintained at approximately 1170 °C. Subsequently, the surface temperature of the Pt-Al bond coating decreases to below 500 °C after cooling. This demonstrates that infrared thermal imaging can offer valuable assistance in temperature regulation, preventing overheating or insufficient temperature during the testing process.
3.3. Microstructure Degradation of the Failed Pt-Al Bond Coating
The cross-sectional morphologies of three failed Pt-Al bond coating specimens at different positions are shown in Figure 6. Regions A, B, and C correspond to the complete failure zone, the failed edge zone, and the non-failure zone, respectively. The top layer of both the complete failure region and the failure edge region of each Pt-Al coating specimen exhibits a wrinkled morphology. Due to the different thermal expansion coefficients of the coating and the superalloy substrate, the stress generated by the thermal shock process is considered to be one of the factors leading to the wrinkling of the coating surface [28]. During the thermal shock process, the EDS results (Table 5) for the different areas and different depths of specimen #1 show that the coating failed due to thermal shock. The Pt element content of the Pt-Al bond coatings on the regions A and B (point a1 and a2) is significantly lower than that of the non-failed region (point a3). It was also confirmed that the content of Pt element is also present on the substrate during the thermal shock test, indicating that the Pt element has diffused inward. It is worth noting that the EDS results at different depths in the unfailed region (point a3 and point a4) clearly suggest that the diffusion of the Al element to the top of the coating is evident, while the Ni, Co, and Cr element of the substrate clearly diffuses into the coating. The oxidation resistance of the Pt-Al bond coating decreases due to the continuous depletion of the Al element with increasing thermal shock cycling. Eventually, the Pt-Al bond coating specimens become ineffective at providing oxidation resistance when the loss of the Al element exceeds a certain degree of degradation [22].

Figure 6.
Cross-sectional SEM images of Pt-Al coating specimens after failure: (a–c) The cross-sectional morphologies of regions A–C, respectively. Regions A, B, and C correspond to the complete failure area, failure edge area, and non-failure area, respectively. The corresponding microstructures are shown in (a), (b), and (c), respectively.

Table 5.
Chemical composition of selected positions (point a1–a4) of specimen #1 in Figure 6 (in at.%).
To further investigate the failure mechanism of the Pt-Al bond coating after thermal shock, EDS scans of the cross-sections of regions A and C of specimen #3 are carried out, and the results are shown in Figure 7. The Al and O elements in the cross-sections of the regions A and C indicate that the Al element diffuses out to form the Al2O3 layer. This suggests that high temperature and oxygen lead to the formation of thermally grown oxide (TGO) on the surface of the Pt-Al bond coating. TGO, serving as a diffusion barrier for oxygen, plays a protective role against substrate oxidation [29]. Figure 7a shows that the Pt element of the completely failed Pt-Al coating has diffused and diluted. It is worth noting that Pt is a vital element for enhancing the performance of the coating, and its diffusion and dilution would directly lead to a decline in the coating performance. Meanwhile, Figure 7b indicates the presence of Pt element in region C, signifying that this area has not experienced failure, which aligns with the macro-morphology results.

Figure 7.
(a) and (b) show the corresponding EDS scans of the cross-sections of the regions A and C of specimen #3, respectively. Regions A and C correspond to the complete failure area and non-failure area, respectively.
Figure 8 shows the surface morphology of specimen #2 after thermal shock failure. The surface of region A exhibits pronounced pitting, and there are obvious spalled signs that the grain boundary ridges and the dense network structure have disappeared, indicating that the surface of the Pt-Al coating has undergone structural degradation. Conversely, the microstructure of the unfailed region C is similar to the original specimen. Moreover, the uneven distribution in failure region A indicates that the impact force and high temperature of the supersonic flame lead to the partial spallation of the oxidation-resistant Al2O3 layer, further accelerating the failure of the coating. This aligns with the findings from the cross-sectional results. The XRD pattern of the coating surface after thermal shock failure is shown in Figure 9. In addition to the β-(Ni, Pt)Al phase, the α-Al2O3 phase and γ′-Ni3Al phase are also observed. This is attributed to the oxygen combining with aluminum to form an α-Al2O3 oxidation-resistant layer during the thermal shock at 1170 °C. The diffusion and consumption of Al directly promotes the transformation of β → γ′ phase, resulting in volume reduction, and the merge and growth of pores [17], which accelerates the failure of the coating.

Figure 8.
(a) and (b) show the corresponding EDS scans of the surface of the regions A and C of specimen #2, respectively. Regions A and C correspond to the complete failure area and non-failure area, respectively.

Figure 9.
XRD pattern of the Pt-Al bond coating after thermal shock testing.
The depletion of Al element resulted in the formation of the γ′ phase in the subscale region of the investigated coatings [17]. Figure 10 shows the content of γ′ phase in a Pt-Al bond coating specimen after failure at 200 μm from the coating surface. The contents of γ′ phase in the Pt-Al bond coating specimens #1, #2, and #3 are 47.25%, 42.41%, and 47.65%, respectively. All three specimens show γ′-phase contents below 50% after thermal shock tests, confirming that none of them experienced overheating conditions and validating the reliability of the thermal shock test. The Pt-Al bond coating undergoes a β → γ′ phase transformation after long-term thermal shock, resulting in volume shrinkage. The induced internal stress might accelerate coating failure. Surprisingly, the depletion of Al element and the formation of a γ′ phase can improve the plasticity and fracture toughness [30], which may further increase the service life of the coating during thermal shock.

Figure 10.
The content of γ′ phase in Pt-Al bond coating specimen after failure: (a) specimen #1; (b) specimen #2; and (c) specimen #3. Microscopic image after threshold processing: (d) specimen #1; (e) specimen #2; and (f) specimen #3. The proportion of black area indicates the proportion of γ′-phase content.
The thermal shock results of the single-phase Pt-Al coatings demonstrate that the failure is mainly attributed to the degradation of the properties due to the diffusion of Pt elements and the formation of the γ′ phase in the subscale region due to the depletion of Al elements. In addition, the volume shrinkage and thermal expansion coefficient mismatch of the Pt-Al bonded coatings due to the β → γ′ phase transition accelerated the failure of the Pt-Al bond coating. In the future, important directions for the improvement of bond coating include reducing the diffusion and consumption of Pt elements through element doping, minimizing the thermal mismatch of the bonding layer, and enhancing the anti-wrinkle ability of the interface.
4. Conclusions
In this paper, a Pt-Al bond coating was prepared by Pt electroplating, vacuum annealing, and a vapor aluminizing treatment. A thermal shock experiment at 1170 °C was carried out on the single-phase Pt-Al bond coating, and its lifetime and failure mechanism were analyzed in detail. The main conclusions are presented as follows:
(1) A Pt-Al bond coating with a thickness of 50 μm is successfully prepared on the nickel-based single-crystal superalloy substrate. It comprises an outer zone of β-(Ni, Pt)Al and an inter-diffusion zone (IDZ) of refractory metal precipitation. The characteristic peak of the coating is a typical structure of single phase β-(Ni, Pt)Al with the preferred orientation in the (110) plane.
(2) The specimens #1, #2, and #3 of the Pt-Al bond coating fail after 650, 528, and 793 thermal shock cycles at 1170 °C, respectively. The contents of Pt and Al elements in the peeled region are lower than those in the unpeeled region. The Pt element of the completely failed Pt-Al coating has diffused and diluted, leading to a decline in the coating performance.
(3) The diffusion and consumption of the Pt element reduces the oxidation resistance of the Pt-Al bond coating. The depletion of Al element results in the formation of the γ′ phase in the subscale region. The Pt-Al bond coating undergoes a β → γ′ phase transformation after long-term thermal shock, resulting in volume shrinkage. Furthermore, the disparity in thermal expansion coefficients between the Pt-Al bond coating and the substrate generates stress within the coating during thermal shock testing, which leads to the coating wrinkling. The combined influence of these factors ultimately accelerate the failure of the Pt-Al bond coating.
Author Contributions
Conceptualization, Z.X., Q.L. and W.Z.; Methodology, Z.X., Q.L., X.H. and W.Z.; Validation, X.H. and J.G.; Formal analysis, Z.X., Q.L. and J.G.; Investigation, Z.X., Q.L. and X.H.; Resources, J.G. and W.Z.; Data curation, J.G.; Writing—original draft, Z.X. and Q.L.; Writing—review & editing, W.Z.; Supervision, W.Z.; Funding acquisition, W.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (Grant No. 12372102), the Science and Technology Innovation Program of Hunan Province (Grant No. 2022RC1082), the Scientific Research Foundation of Hunan Provincial Education Department (Grant No. 21A0120), and the Postgraduate Scientific Research Innovation Project of Hunan Province (Grant Nos. XDCX2021B139 and CX20230551).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schulz, U.; Leyens, C.; Fritscher, K.; Peters, M.; Saruhan-Brings, B.; Lavigne, O.; Dorvaux, J.-M.; Poulain, M.; Mévrel, R.; Caliez, M. Some recent trends in research and technology of advanced thermal barrier coatings. Aerosp. Sci. Technol. 2003, 7, 73–80. [Google Scholar] [CrossRef]
- Miller, R.A. Thermal barrier coatings for aircraft engines: History and directions. J. Therm. Spray Technol. 1997, 6, 35. [Google Scholar] [CrossRef]
- Padture, P.N. Thermal Barrier Coatings for Gas-Turbine Engine Applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.R.; Oechsner, M.; Padture, N.P. Thermal-barrier coatings for more efficient gas-turbine engines. MRS Bull. 2012, 37, 891–899. [Google Scholar] [CrossRef]
- Padture, N.P. Advanced structural ceramics in aerospace propulsion. Nat. Mater. 2016, 15, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.L.; Chen, M.H.; Wang, J.L.; Qiao, Y.X.; Guo, P.Y.; Zhu, S.L.; Wang, F.H. Microstructure and composition evolution of a single-crystal superalloy caused by elements interdiffusion with an overlay NiCrAlY coating on oxidation. J. Mater. Sci. Technol. 2020, 45, 49–58. [Google Scholar] [CrossRef]
- Alam, M.Z.; Hazari, N.; Varma, V.K.; Das, D.K. Effect of cyclic oxidation exposure on tensile properties of a Pt-aluminide bond-coated Ni-base superalloy. Metall. Mater. Trans. A 2011, 42, 4064–4074. [Google Scholar] [CrossRef]
- Tolpygo, V.K.; Clarke, D.R. Rumpling of CVD (Ni. Pt) Al diffusion coatings under intermediate temperature cycling. Surf. Coat. Technol. 2009, 203, 3278–3285. [Google Scholar] [CrossRef]
- Spitsberg, I.T.; Mumm, D.R.; Evans, A.G. On the failure mechanisms of thermal barrier coatings with diffusion aluminide bond coatings. Mater. Sci. Eng. A 2005, 394, 176–191. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, J.K.; Wu, L.L.; Wan, B.; Zhang, Y.K.; Gao, R.; Zhang, J.W.; Gou, H.Y. Mechanical properties and electronic structures of diverse PtAl intermetallics: First-principles calculations. Comput. Mater. Sci. 2016, 124, 273–281. [Google Scholar] [CrossRef]
- Liu, L.; He, J.; Wu, Y.T.; Li, J.C.; Wei, L.L.; Guo, H.B. Investigation on the tensile properties of PtAl and PtReAl coated Ni3Al-based single crystal superalloy. Mater. Sci. Eng. A 2023, 867, 144750. [Google Scholar] [CrossRef]
- Kumawat, M.K.; Sarkar, R.; Jayaram, V.; Alam, M.Z. Fatigue behavior of a freestanding Pt-aluminide (PtAl) bond coat at ambient temperature. Surf. Coat. Technol. 2021, 427, 127787. [Google Scholar] [CrossRef]
- Tao, X.P.; Tan, K.J.; Liang, J.J.; Wang, X.G.; Zhou, Y.Z.; Li, J.G.; Sun, X.F. Pt-Al bond coat dependence on the high-cycle fatigue rupture and deformation mechanisms of a fourth-generation single crystal superalloy at various temperatures. Mater. Des. 2023, 229, 111880. [Google Scholar] [CrossRef]
- Das, D.K. Microstructure and high temperature oxidation behavior of Pt-modified aluminide bond coats on Ni-base superalloys. Prog. Mater. Sci. 2013, 58, 151–182. [Google Scholar] [CrossRef]
- Qian, L.Y.; Xu, F.; Voisey, K.T.; Nekouie, V.; Zhou, Z.X.; Silberschmidt, V.V.; Hou, X.H. Incorporation and evolution of ZrO2 nano-particles in Pt-modified aluminide coating for high temperature applications. Surf. Coat. Technol. 2017, 311, 238–247. [Google Scholar] [CrossRef]
- Ye, L.; Chen, H.F.; Liu, B.; Yang, G.; Luo, H.J.; Gao, Y.F. Effect of Pt content on initial TGO formation and available Al reserve of Pt-Al coatings during thermal cycling. Surf. Coat. Technol. 2018, 337, 82–89. [Google Scholar] [CrossRef]
- Oskay, C.; Galetz, M.C.; Murakami, H. Oxide scale formation and microstructural degradation of conventional. Pt-and Pt/Ir-modified NiAl diffusion coatings during thermocyclic exposure at 1100 °C. Corros. Sci. 2018, 144, 313–327. [Google Scholar] [CrossRef]
- Yang, S.S.; Li, S.; Wang, J.L.; Bao, Z.B.; Yang, L.L.; Chen, M.H.; Zhu, S.L.; Wang, F.H. A novel single-phase γ′ nanocrystalline coating with high resistance to oxidation and scale rumpling yet mitigated interdiffusion with the alloy substrate. Corros. Sci. 2021, 192, 109866. [Google Scholar] [CrossRef]
- Zou, H.Y.; Yin, B.; Hu, T.Y.; Deng, P.; Mao, J.; Cai, J.; Hua, Y.Q.; Zhang, X.F. Impact of Pt/Al ratios on the cyclic oxidation and TCP precipitation of β-(Ni. Pt) Al coated superalloy at 1150 °C. J. Mater. Res. Technol. 2024, 29, 2227–2238. [Google Scholar] [CrossRef]
- Hu, T.Y.; Yin, B.; Zhang, X.F.; Li, W.Z.; Mao, J.; Zhang, X.H.; Deng, C.M.; Liu, M. Increasing hot corrosion resistance of β-(Ni. Pt) Al coating by replacing Pt partially with Zr. Corros. Sci. 2023, 220, 111290. [Google Scholar] [CrossRef]
- Li, S.; Xu, M.M.; Zhang, C.Y.; Bao, Z.B.; Yang, Y.F.; Zhu, S.L.; Wang, F.H. Effect of pre-oxidation on the failure mechanisms of EB-PVD thermal barrier coatings with (Ni. Pt) Al bond coats. Corros. Sci. 2021, 193, 109873. [Google Scholar] [CrossRef]
- Alam, M.Z.; Sarkar, S.B.; Das, D.K. Refurbishment of thermally degraded diffusion Pt-aluminide (PtAl) bond coat on a Ni-base superalloy. Surf. Coat. Technol. 2018, 354, 101–111. [Google Scholar] [CrossRef]
- Li, S.; Xu, M.M.; Zhang, C.Y.; Niu, Y.S.; Bao, Z.B.; Zhu, S.L.; Wang, F.H. Co-doping effect of Hf and Y on improving cyclic oxidation behavior of (Ni. Pt) Al coating at 1150 °C. Corros. Sci. 2021, 178, 109093. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, X.P.; Zhu, W.; Liu, G.L.; Guo, J.W.; Bin, J. Thermal shock performance and failure behavior of Zr6Ta2O17-8YSZ double-ceramic-layer thermal barrier coatings prepared by atmospheric plasma spraying. Ceram. Int. 2022, 48, 24402–24410. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, C.X.; Yang, L.; Zhou, Y.C.; Liu, Z.Y. Real-time detection of damage evolution and fracture of EB-PVD thermal barrier coatings under thermal shock: An acoustic emission combined with digital image correlation method. Surf. Coat. Technol. 2020, 399, 126151. [Google Scholar] [CrossRef]
- Parlikar, C.; Alam, M.Z.; Chatterjee, D.; Das, D.K. Oxidation and concomitant effects on the microstructure and high temperature tensile properties of a DS Ni-base superalloy applied with different thicknesses of Pt-aluminide (PtAl) bond coat. Surf. Coat. Technol. 2019, 373, 25–37. [Google Scholar] [CrossRef]
- Chen, H.F.; Zhang, C.; Xuan, J.H.; Liu, B.; Yang, G.; Gao, Y.F.; Luo, H.J. Effect of TGO evolution and element diffusion on the life span of YSZ/Pt–Al and YSZ/NiCrAlY coatings at high temperature. Ceram. Int. 2020, 46, 813–823. [Google Scholar] [CrossRef]
- Pennefather, R.; Boone, D. Mechanical degradation of coating systems in high-temperature cyclic oxidation. Surf. Coat. Technol. 1995, 76, 47–52. [Google Scholar] [CrossRef]
- Jackson, R.W.; Lipkin, D.M.; Pollock, T.M. Thermal barrier coating adherence to Hf-modified B2 NiAl bond coatings. Acta Mater. 2014, 80, 39–47. [Google Scholar] [CrossRef]
- Oskay, C.; Galetz, M.C.; Murakami, H. Mechanical behaviour of conventional. Pt-and Pt/Ir-modified NiAl diffusion coatings after thermocyclic exposure at 1100 °C. Mater. High Temp. 2019, 36, 404–416. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).