The Effect of La on the Surface Properties of Plasma Nitrided CoCrCuFeNi High-Entropy Alloys at 440 Degrees Celsius
Abstract
1. Introduction
2. Materials and Methods
3. Results
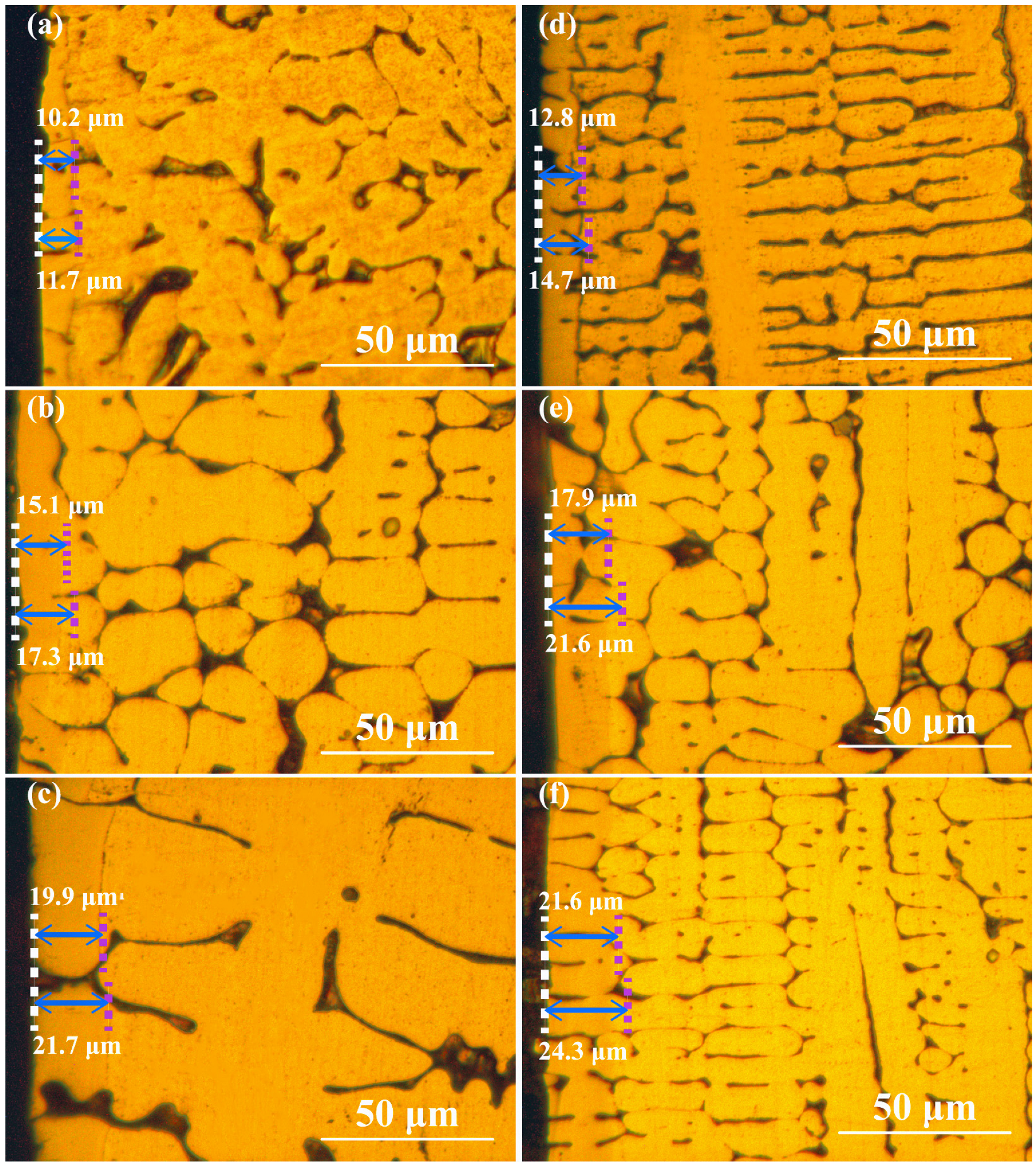
3.1. Microstructure and Microhardness of Modified Layer
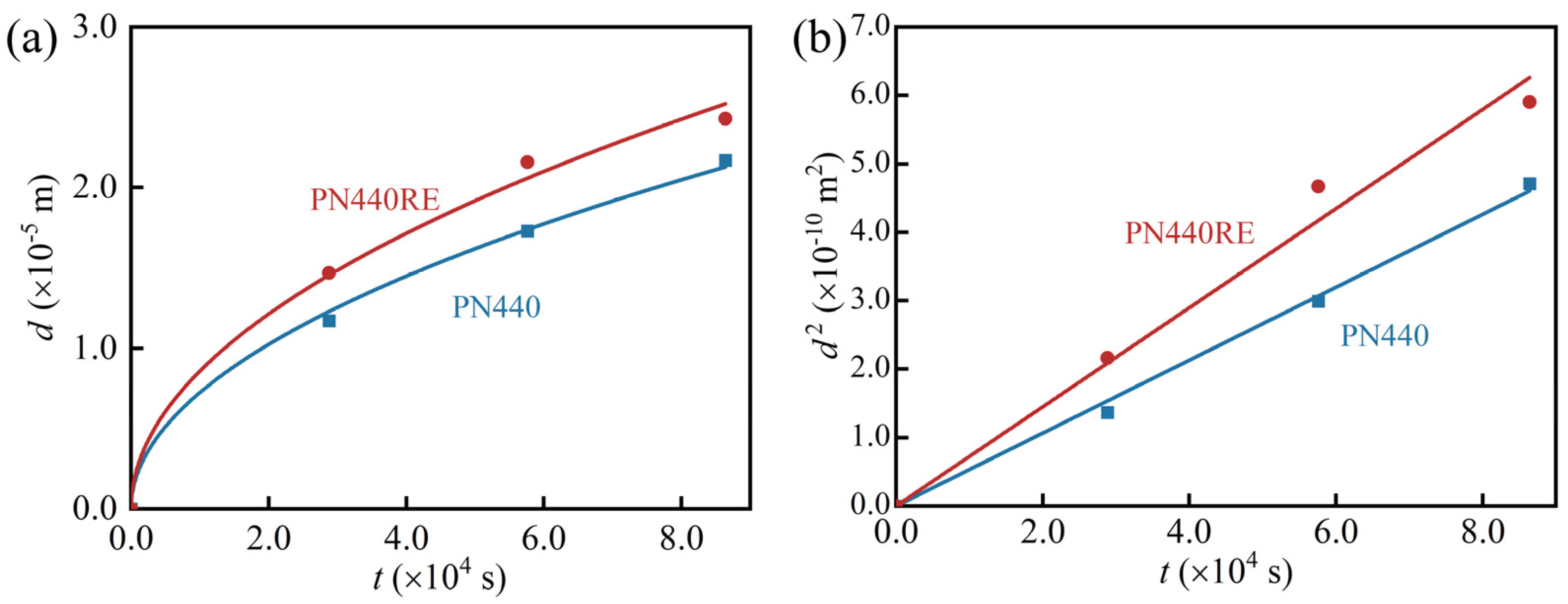
3.2. Growth Kinetic of the Nitrided Layer
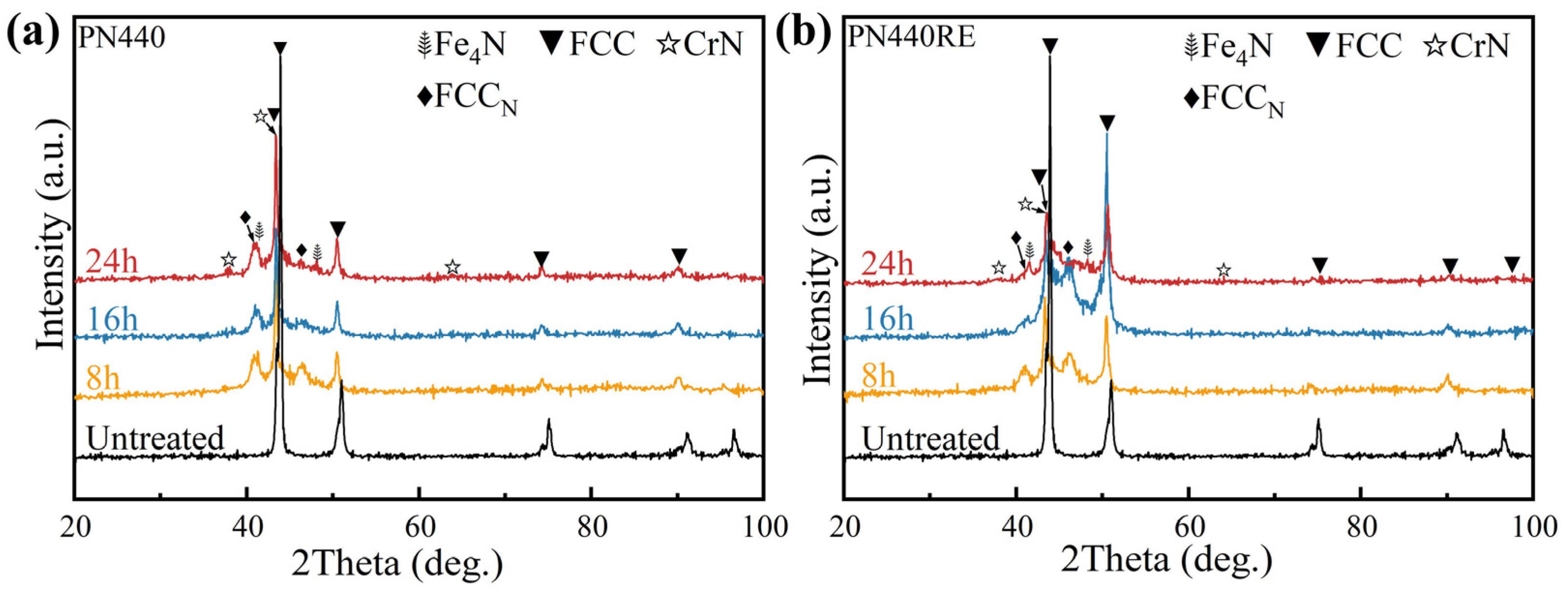
3.3. Phase Composition of the Modified Layer
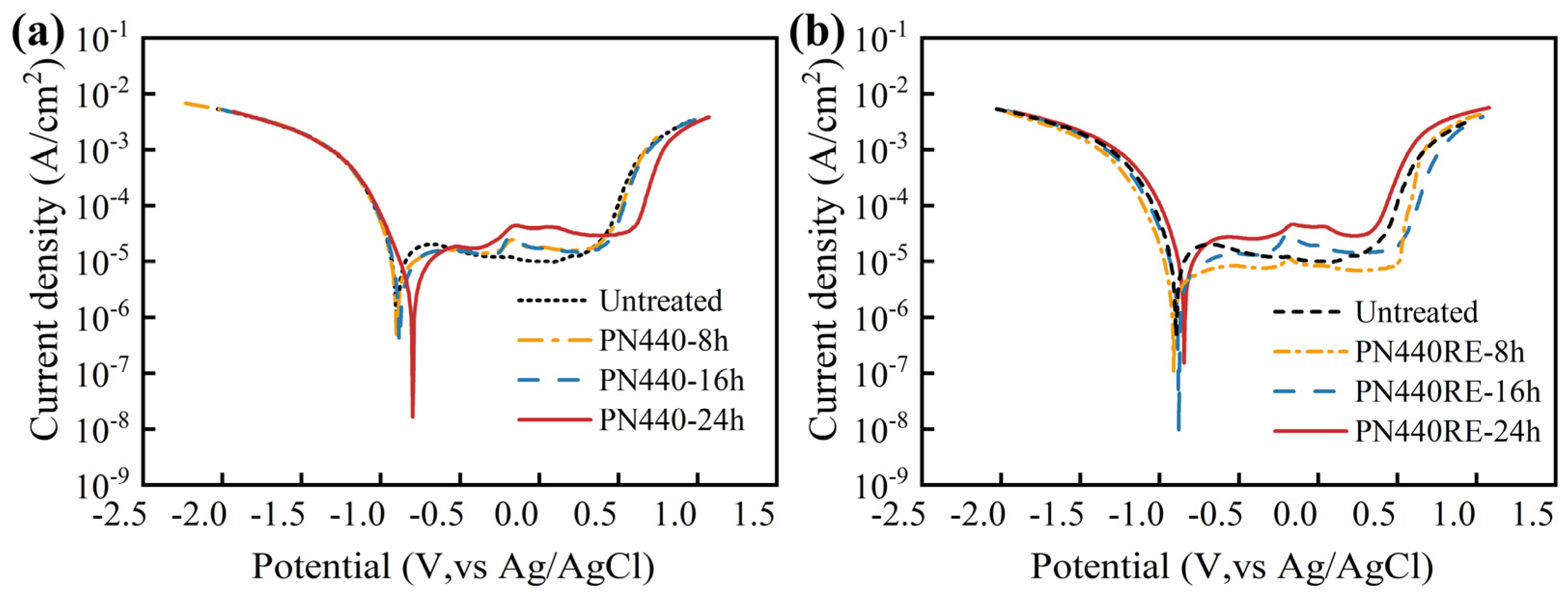
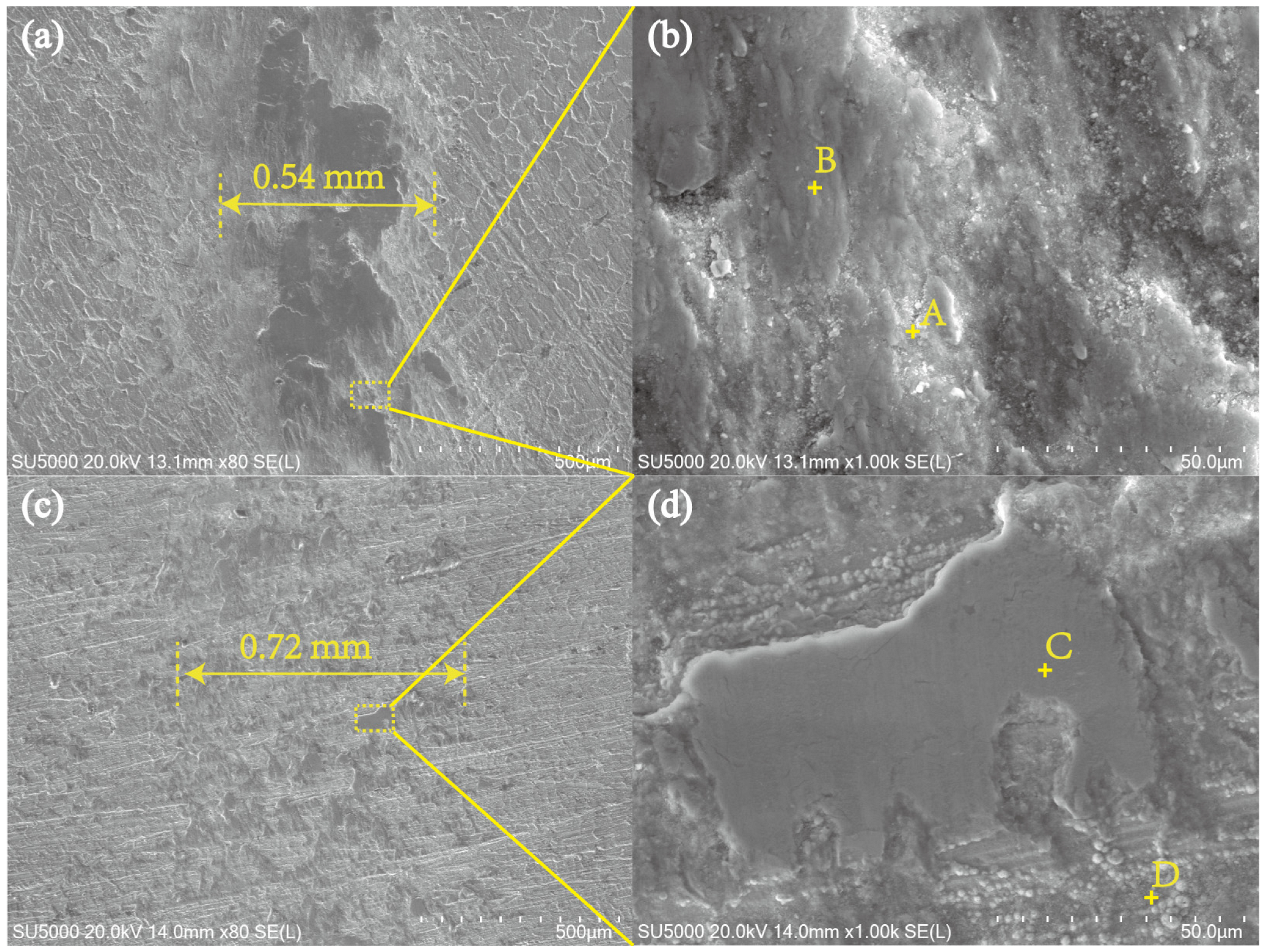
3.4. Corrosion Properties of the Nitrided Layer
3.5. Friction Performance Analysis
4. Conclusions
- The thickness of the nitrided layer of PN440RE increased by 25.49%, 18.54%, and 8.54% compared to PN440, suggesting that La may contribute to a thicker nitrided layer for the same nitriding time. The hardness curve indicates a more homogeneous distribution of nitrogen atoms in the nitrided layer of PN440RE.
- The effective hardening layer versus time curve shows that the growth of the effective hardening layer of PN440 and PN440RE follows a parabolic law. This means that the plasma nitriding of CoCrCuFeNi high-entropy alloys is diffusion-controlled, and the effective hardening layer thickness growth is limited after 16 h of nitriding treatment with La. The results indicate that the addition of La can produce a higher quality effective hardening layer in a shorter time.
- The phase composition of the nitrided layer is affected by the nitriding time. Specifically, as the nitriding time increases, the diffraction peak angle of FCCN changes differently in PN440 and PN440RE. After the PN440 treatment, the diffraction peak angle of FCCN shifts toward a smaller angle, while the opposite is true for PN440RE.
- Corrosion resistance is determined by the concentration of interstitial nitrogen atoms in the FCCN lattice of the nitrided layer. The angle of the diffraction peak of FCCN can determine the nitrogen concentration. A smaller diffraction peak angle indicates a larger interplanar distance, higher nitrogen concentration, and better corrosion resistance.
- Both PN440-24 and PN440RE-24 experience oxidative wear accompanied by adhesive wear. However, due to the higher level of adhesive wear in PN440-24, the wear rate of PN440RE-24 is 86.0% lower than that of PN440-24.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.S.; Shun, T.-T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Kumar, D.; Seetharam, R.; Ponappa, K. A Review on Microstructures, Mechanical Properties and Processing of High Entropy Alloys Reinforced Composite Materials. J. Alloys Compd. 2024, 972, 172732. [Google Scholar] [CrossRef]
- Wan, W.; Liang, K.; Zhu, P.; He, P.; Zhang, S. Recent Advances in the Synthesis and Fabrication Methods of High-Entropy Alloy Nanoparticles. J. Mater. Sci. Technol. 2024, 178, 226–246. [Google Scholar] [CrossRef]
- Yeh, J.W. Alloy Design Strategies and Future Trends in High-Entropy Alloys. JOM 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiao, J.W.; Liaw, P.K. A Brief Review of High Entropy Alloys and Serration Behavior and Flow Units. J. Iron Steel Res. Int. 2016, 23, 2–6. [Google Scholar] [CrossRef]
- Liu, L.Y.; Zhang, Y.; Han, J.H.; Wang, X.Y.; Jiang, W.Q.; Liu, C.T.; Zhang, Z.W.; Liaw, P.K. Nanoprecipitate-Strengthened High-Entropy Alloys. Adv. Sci. 2021, 8, 2100870. [Google Scholar] [CrossRef]
- Chaudhary, V.; Soni, V.; Gwalani, B.; Ramanujan, R.V.; Banerjee, R. Influence of Non-Magnetic Cu on Enhancing the Low Temperature Magnetic Properties and Curie Temperature of Feconicrcu(X) High Entropy Alloys. Scr. Mater. 2020, 182, 99–103. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Fang, W.; Chang, R.-B.; Ji, P.-G.; Wang, Q.-Z. Modifying Element Diffusion Pathway by Transition Layer Structure in High-Entropy Alloy Particle Reinforced Cu Matrix Composites. Trans. Nonferrous Met. Soc. China 2019, 29, 2331–2339. [Google Scholar] [CrossRef]
- Hemphill, M.A.; Yuan, T.; Wang, G.Y.; Yeh, J.W.; Tsai, C.W.; Chuang, A.; Liaw, P.K. Fatigue Behavior of Al0.5cocrcufeni High Entropy Alloys. Acta Mater. 2012, 60, 5723–5734. [Google Scholar] [CrossRef]
- Huang, G.Q.; Li, B.; Chen, Y.N.; Xuan, F.Z. Nanotwining Induced by Tensile Fatigue and Dynamic Impact of Laser Powder B E D Fusion Additively Manufactured Cocrfeni High-Entropy Alloy. J. Mater. Sci. Technol. 2024, 183, 241–257. [Google Scholar] [CrossRef]
- Feng, R.; Feng, B.J.; Gao, M.C.; Zhang, C.; Neuefeind, J.C.; Poplawsky, J.D.; Ren, Y.; An, K.; Widom, M.; Liaw, P.K. Superior High-Temperature Strength in a Supersaturated Refractory High-Entropy Alloy. Adv. Mater. 2021, 33, 2102401. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.J.; Yeh, A.C. The Evolution of Microstructures and High Temperature Properties of AlXCo1.5Crfeni1.5TiY High Entropy Alloys. J. Alloys Compd. 2015, 653, 379–385. [Google Scholar] [CrossRef]
- Zhang, H.T.; Wang, C.L.; Miao, J.W.; Shi, S.Y.; Li, T.J.; Yan, H.W.; Zhang, Y.A.; Lu, Y.P. Effect of Microstructure Evolution on Wear Resistance of Equal Molar Cocrfeni High-Entropy Alloy. Rare Metals 2023, 42, 3797–3805. [Google Scholar] [CrossRef]
- Wang, K.; Huang, J.; Chen, H.; Wang, Y.; Yan, W.; Yuan, X.; Song, S.; Zhang, J.; Sun, X. Recent Progress in High Entropy Alloys for Electrocatalysts. Electrochem. Energy Rev. 2022, 5, 17. [Google Scholar] [CrossRef]
- Pickering, E.J.; Jones, N.G. High-Entropy Alloys: A Critical Assessment of Their Founding Principles and Future Prospects. Int. Mater. Rev. 2016, 61, 183–202. [Google Scholar] [CrossRef]
- Meng, F.; Baker, I. Nitriding of a High Entropy Fenimnalcr Alloy. J. Alloys Compd. 2015, 645, 376–381. [Google Scholar] [CrossRef]
- Chen, R.; Cai, Z.; Pu, J.; Lu, Z.; Chen, S.; Zheng, S.; Zeng, C. Effects of Nitriding on the Microstructure and Properties of Valticrmo High-Entropy Alloy Coatings by Sputtering Technique. J. Alloys Compd. 2020, 827, 153836. [Google Scholar] [CrossRef]
- Tang, W.-Y.; Chuang, M.-H.; Lin, S.-J.; Yeh, J.-W. Microstructures and Mechanical Performance of Plasma-Nitrided Al0.3crfe1.5mnni0.5 High-Entropy Alloys. Metall. Mater. Trans. A 2012, 43, 2390–2400. [Google Scholar] [CrossRef]
- Tang, W.-Y.; Chuang, M.-H.; Chen, H.-Y.; Yeh, J.-W. Microstructure and Mechanical Performance of New Al0.5crfe1.5mnni0.5 High-Entropy Alloys Improved by Plasma Nitriding. Surf. Coat. Technol. 2010, 204, 3118–3124. [Google Scholar] [CrossRef]
- Tang, W.-Y.; Yeh, J.-W. Effect of Aluminum Content on Plasma-Nitrided Alxcocrcufeni High-Entropy Alloys. Metall. Mater. Trans. A 2009, 40, 1479–1486. [Google Scholar] [CrossRef]
- Lan, L.W.; Wang, X.J.; Guo, R.P.; Yang, H.J.; Qiao, J.W. Effect of Environments and Normal Loads on Tribological Properties of Nitrided Ni45(Fecocr)40(Alti)15 High-Entropy Alloys. J. Mater. Sci. Technol. 2020, 42, 85–96. [Google Scholar] [CrossRef]
- Karimoto, T.; Nishimoto, A. Plasma-Nitriding Properties of Cocrfemnni High-Entropy Alloys Produced by Spark Plasma Sintering. Metals 2020, 10, 761. [Google Scholar] [CrossRef]
- Nishimoto, A.; Fukube, T.; Maruyama, T. Microstructural, Mechanical, and Corrosion Properties of Plasma-Nitrided Cocrfemnni High-Entropy Alloys. Surf. Coat. Technol. 2019, 376, 52–58. [Google Scholar] [CrossRef]
- Peng, J.; Dong, H.; Bell, T.; Chen, F.; Mo, Z.; Wang, C.; Peng, Q. Effect of Rare Earth Elements on Plasma Nitriding of 38crmoai Steel. Surf. Eng. 1996, 12, 147–151. [Google Scholar] [CrossRef]
- Bell, T.; Sun, Y.; Lui, Z.; Yan, M. Rare-Earth Surface Engineering. Heat Treat. Met. 2000, 27, 1–8. [Google Scholar]
- You, Y.; Yan, J.; Yan, M. Atomistic Diffusion Mechanism of Rare Earth Carburizing/Nitriding on Iron-Based Alloy. Appl. Surf. Sci. 2019, 484, 710–715. [Google Scholar] [CrossRef]
- Liu, D.J.; You, Y.; Yan, M.F.; Chen, H.T.; Li, R.; Hong, L.; Han, T.J. Acceleration of Plasma Nitriding at 550 °C with Rare Earth on the Surface of 38crmoal Steel. Coatings 2021, 11, 1122. [Google Scholar] [CrossRef]
- Tang, L.N.; Yan, M.F. Microstructure and Corrosion Resistance of Quenched Aisi 4140 Steel Plasma Nitrided and Nitrocarburised with and without Rare Earths. Mater. Sci. Technol. 2013, 29, 610–615. [Google Scholar] [CrossRef]
- You, Y.; Li, R.; Yan, M.; Yan, J.; Chen, H.; Wang, C.; Liu, D.; Hong, L.; Han, T. Low-Temperature Plasma Nitriding of 3cr13 Steel Accelerated by Rare-Earth Block. Coatings 2021, 11, 1050. [Google Scholar] [CrossRef]
- Xie, W.; Chen, Y.; Chen, D.; Yang, Y.; Zhang, C.; Cui, G.; Wang, Y. Low-Pressure Gas Nitriding of Aisi 304 Austenitic Stainless Steel: Reducing the Precipitation of Chromium Nitrides. Mater. Res. Express 2020, 7, 066406. [Google Scholar] [CrossRef]
- Liu, R.L.; Yan, M.F.; Wu, Y.Q.; Zhao, C.Z. Microstructure and Properties of 17-4ph Steel Plasma Nitrocarburized with a Carrier Gas Containing Rare Earth Elements. Mater. Charact. 2010, 61, 19–24. [Google Scholar] [CrossRef]
- Chen, F.-S.; Wang, K.-L. The Kinetics and Mechanism of Multi-Component Diffusion on Aisi 1045 Steel. Surf. Coat. Technol. 1999, 115, 239–248. [Google Scholar] [CrossRef]
- Liu, R.L.; Qiao, Y.J.; Yan, M.F.; Fu, Y.D. Layer Growth Kinetics and Wear Resistance of Martensitic Precipitation Hardening Stainless Steel Plasma Nitrocarburized at 460 °C with Rare Earth Addition. Met. Mater. Int. 2013, 19, 1151–1157. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Chai, Y.; Wei, W.; Hu, J. Kinetics and Enhancement Mechanism of Plasma Oxynitriding for Aisi 1045 Steel. Surf. Coat. Technol. 2016, 302, 22–26. [Google Scholar] [CrossRef]
- Li, C.X.; Bell, T. Corrosion Properties of Active Screen Plasma Nitrided 316 Austenitic Stainless Steel. Corros. Sci. 2004, 46, 1527–1547. [Google Scholar] [CrossRef]
- Fossati, A.; Borgioli, F.; Galvanetto, E.; Bacci, T. Corrosion Resistance Properties of Glow-Discharge Nitrided Aisi 316l Austenitic Stainless Steel in Nacl Solutions. Corros. Sci. 2006, 48, 1513–1527. [Google Scholar] [CrossRef]
- Borgioli, F.; Galvanetto, E.; Bacci, T. Low Temperature Nitriding of Aisi 300 and 200 Series Austenitic Stainless Steels. Vacuum 2016, 127, 51–60. [Google Scholar] [CrossRef]
- Xie, Y.L.; Miyamoto, G.; Furuhara, T. Nanosized Cr-N Clustering in Expanded Austenite Layer of Low Temperature Plasma-Nitrided Fe-35ni-10cr Alloy. Scr. Mater. 2022, 213, 114637. [Google Scholar] [CrossRef]
- Tao, X.; Yang, Y.; Qi, J.; Cai, B.; Rainforth, W.M.; Li, X.; Dong, H. An Investigation on Nitrogen Uptake and Microstructure of Equimolar Quaternary Feconicr High Entropy Alloy after Active-Screen Plasma Nitriding. Mater. Charact. 2024, 208, 113593. [Google Scholar] [CrossRef]
- Tao, X.; Yang, Y.P.; Qi, J.H.; Cai, B.; Rainforth, W.M.; Li, X.Y.; Dong, H.S. Active Screen Plasma Nitriding of a Si-Alloyed Fecrni Medium Entropy Alloy: High Interstitial Absorption and an Anomalous Si-Induced Decomposition Mechanism in N-Expanded Austenite. Appl. Surf. Sci. 2023, 624, 157137. [Google Scholar] [CrossRef]
- Qin, X.; Guo, X.; Lu, J.; Chen, L.; Qin, J.; Lu, W. Erosion-Wear and Intergranular Corrosion Resistance Properties of Aisi 304l Austenitic Stainless Steel after Low-Temperature Plasma Nitriding. J. Alloys Compd. 2017, 698, 1094–1101. [Google Scholar] [CrossRef]
- Baba, H.; Kodama, T.; Katada, Y. Role of Nitrogen on the Corrosion Behavior of Austenitic Stainless Steels. Corros. Sci. 2002, 44, 2393–2407. [Google Scholar] [CrossRef]
- Dong, H. S-Phase Surface Engineering of Fe-Cr, Co-Cr and Ni-Cr Alloys. Int. Mater. Rev. 2010, 55, 65–98. [Google Scholar] [CrossRef]
- Stott, F.H. The Role of Oxidation in the Wear of Alloys. Tribol. Int. 1998, 31, 61–71. [Google Scholar] [CrossRef]
- Cui, X.H.; Wang, S.Q.; Wang, F.; Chen, K.M. Research on Oxidation Wear Mechanism of the Cast Steels. Wear 2008, 265, 468–476. [Google Scholar] [CrossRef]
- Zhang, C.S.; Yan, M.F.; Sun, Z.; Wang, Y.X.; You, Y.; Bai, B.; Chen, L.; Long, Z.; Li, R.W. Optimizing the Mechanical Properties of M50nil Steel by Plasma Nitrocarburizing. Appl. Surf. Sci. 2014, 315, 28–35. [Google Scholar] [CrossRef]
- Liu, L.; Shen, H.H.; Liu, X.Z.; Guo, Q.; Meng, T.X.; Wang, Z.X.; Yang, H.J.; Liu, X.P. Wear Resistance of Tin(Ti2N)/Ti Composite Layer Formed on C17200 Alloy by Plasma Surface Ti-Alloying and Nitriding. Appl. Surf. Sci. 2016, 388, 103–108. [Google Scholar] [CrossRef]
- Naeem, M.; Díaz-Guillén, J.C.; Khalid, A.; Guzman-Flores, I.; Muñoz-Arroyo, R.; Iqbal, J.; Sousa, R.R.M. Improved Wear Resistance of Aisi-1045 Steel by Hybrid Treatment of Plasma Nitriding and Post-Oxidation. Tribol. Int. 2022, 175, 107869. [Google Scholar] [CrossRef]



| Composition | Co | Cr | Cu | Fe | Ni |
|---|---|---|---|---|---|
| Nominally | 20 | 20 | 20 | 20 | 20 |
| Actual | 18.88 | 21.75 | 18.82 | 20.93 | 19.62 |
| Identified Name | Temperature, °C | Time, h | Atmosphere | Gas Pressure, Pa |
|---|---|---|---|---|
| PN440-8 | 440 | 8 | NH3 | 260 |
| PN440-16 | 440 | 16 | NH3 | 260 |
| PN440-24 | 440 | 24 | NH3 | 260 |
| PN440RE-8 | 440 | 8 | NH3 | 260 |
| PN440RE-16 | 440 | 16 | NH3 | 260 |
| PN440RE-24 | 440 | 24 | NH3 | 260 |
| 2θ (°) | Intensity (a.u.) | Phase | Plane (hkl) | |
|---|---|---|---|---|
| untreated | 43.90 | 828.30 | FCC | 111 |
| 51.02 | 156.20 | FCC | 200 | |
| 75.08 | 76.30 | FCC | 220 | |
| PN440-8 | 40.86 | 63.80 | FCCN | 111 |
| 46.46 | 46.60 | FCCN | 200 | |
| 41.26 | 67.70 | Fe4N | 111 | |
| 48.06 | 27.00 | Fe4N | 200 | |
| PN440-16 | 40.90 | 40.50 | FCCN | 111 |
| 46.36 | 27.50 | FCCN | 200 | |
| 41.26 | 44.60 | Fe4N | 111 | |
| 48.06 | 12.80 | Fe4N | 200 | |
| PN440-24 | 40.94 | 58.20 | FCCN | 111 |
| 46.28 | 36.50 | FCCN | 200 | |
| 41.40 | 49.70 | Fe4N | 111 | |
| 48.12 | 36.00 | Fe4N | 200 | |
| PN440RE-8 | 40.86 | 41.70 | FCCN | 111 |
| 46.04 | 63.10 | FCCN | 200 | |
| 41.32 | 31.40 | Fe4N | 111 | |
| 48.22 | 26.90 | Fe4N | 200 | |
| PN440RE-16 | 41.02 | 32.40 | FCCN | 111 |
| 46.18 | 120.10 | FCCN | 200 | |
| 41.38 | 31.70 | Fe4N | 111 | |
| 48.12 | 54.20 | Fe4N | 200 | |
| PN440RE-24 | 41.04 | 25.60 | FCCN | 111 |
| 46.70 | 35.80 | FCCN | 200 | |
| 41.52 | 32.20 | Fe4N | 111 | |
| 48.14 | 30.30 | Fe4N | 200 |
| Untreated | 8 h | 16 h | 24 h | |
|---|---|---|---|---|
| Corrosion rate (×10−2 mm/a) | 6.89 | 6.05 | 4.92 | 3.45 |
| Io (×10−6 A/cm2) | 5.92 | 5.20 | 4.24 | 2.96 |
| Eo/V | −0.88 | −0.89 | −0.87 | −0.79 |
| Passivation zone width/V | 1.17 | 1.27 | 1.30 | 1.36 |
| Untreated | 8 h | 16 h | 24 h | |
|---|---|---|---|---|
| Corrosion rate (×10−2 mm/a) | 6.89 | 2.83 | 2.50 | 5.21 |
| Io (×10−6 A/cm2) | 5.92 | 2.43 | 2.13 | 4.48 |
| Eo/V | −0.88 | −0.91 | −0.87 | −0.84 |
| Passivation zone width/V | 1.17 | 1.36 | 1.39 | 1.16 |
| Elements | A | B | C | D |
|---|---|---|---|---|
| N | 0 | 0 | 0 | 0.91 |
| O | 25.26 | 25.19 | 30.02 | 15.25 |
| Cr | 2.68 | 2.75 | 23.64 | 14.34 |
| Fe | 67.35 | 67.26 | 64.18 | 36.70 |
| Co | 1.31 | 1.33 | 0.93 | 13.53 |
| Ni | 1.14 | 1.13 | 0.61 | 10.57 |
| Cu | 2.25 | 2.34 | 1.02 | 7.97 |
| W | 0 | 0 | 0.61 | 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; You, Y.; Yan, M.; Zhang, Y.; Sheng, W.; Wang, Y.; Xu, S.; Gu, F.; Wang, C.; Cheng, W. The Effect of La on the Surface Properties of Plasma Nitrided CoCrCuFeNi High-Entropy Alloys at 440 Degrees Celsius. Coatings 2024, 14, 303. https://doi.org/10.3390/coatings14030303
Wang Y, You Y, Yan M, Zhang Y, Sheng W, Wang Y, Xu S, Gu F, Wang C, Cheng W. The Effect of La on the Surface Properties of Plasma Nitrided CoCrCuFeNi High-Entropy Alloys at 440 Degrees Celsius. Coatings. 2024; 14(3):303. https://doi.org/10.3390/coatings14030303
Chicago/Turabian StyleWang, Yifan, Yuan You, Mufu Yan, Yanxiang Zhang, Wenping Sheng, Yan Wang, Shimiao Xu, Feng Gu, Chaohui Wang, and Weidong Cheng. 2024. "The Effect of La on the Surface Properties of Plasma Nitrided CoCrCuFeNi High-Entropy Alloys at 440 Degrees Celsius" Coatings 14, no. 3: 303. https://doi.org/10.3390/coatings14030303
APA StyleWang, Y., You, Y., Yan, M., Zhang, Y., Sheng, W., Wang, Y., Xu, S., Gu, F., Wang, C., & Cheng, W. (2024). The Effect of La on the Surface Properties of Plasma Nitrided CoCrCuFeNi High-Entropy Alloys at 440 Degrees Celsius. Coatings, 14(3), 303. https://doi.org/10.3390/coatings14030303






