Microstructure and Corrosion Resistance of Laser-Cladded FeCo1.5CrNi1.5Ti0.5 High-Entropy Alloy Coatings
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. XRD Analysis
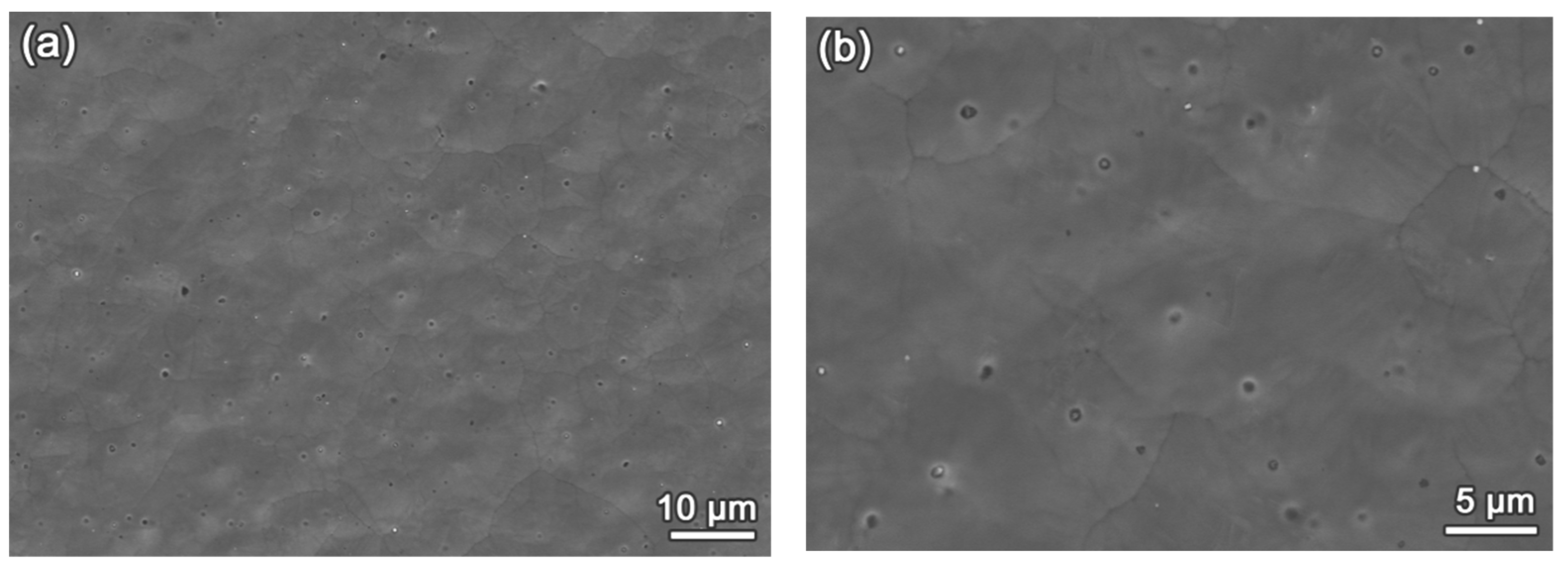
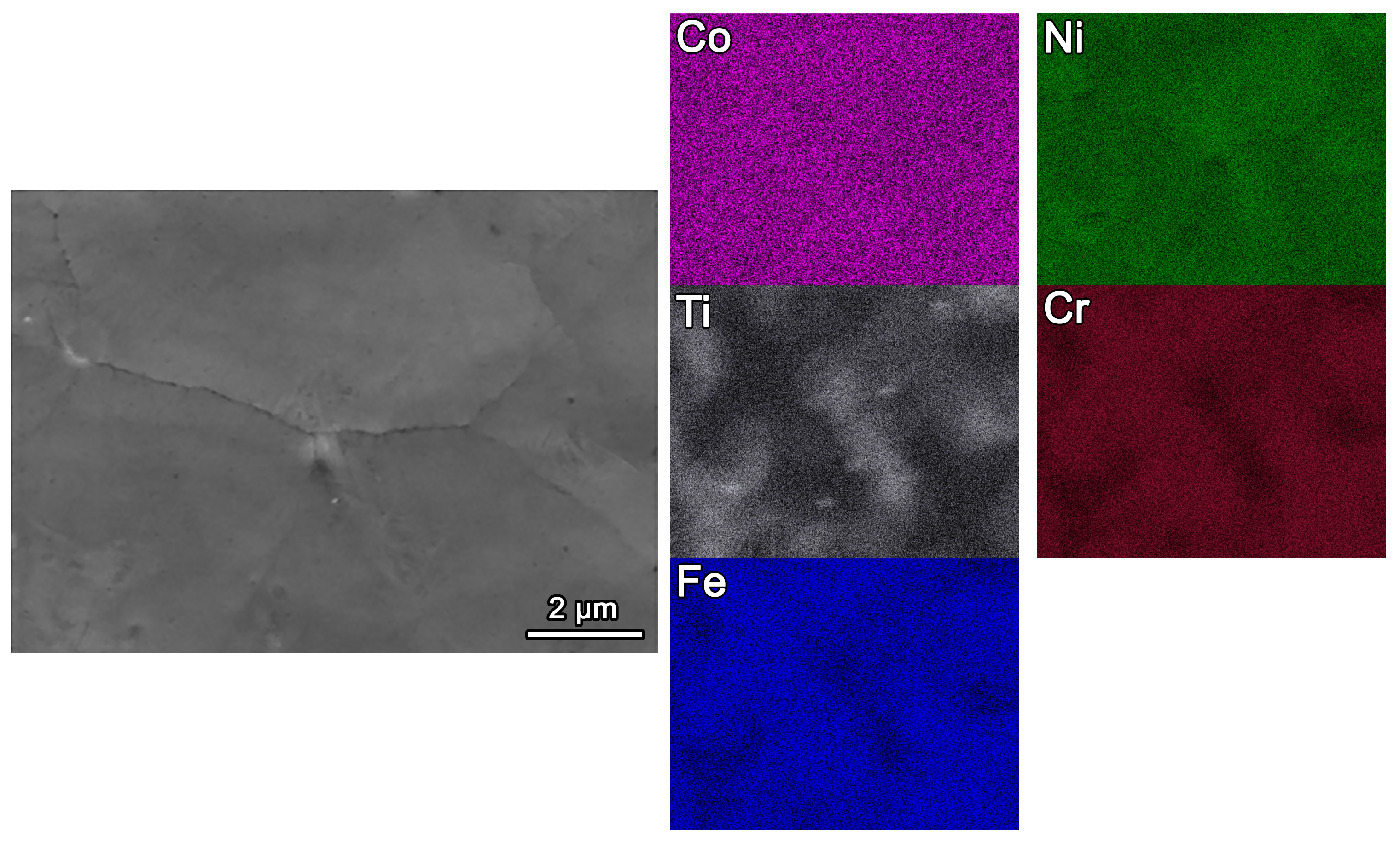
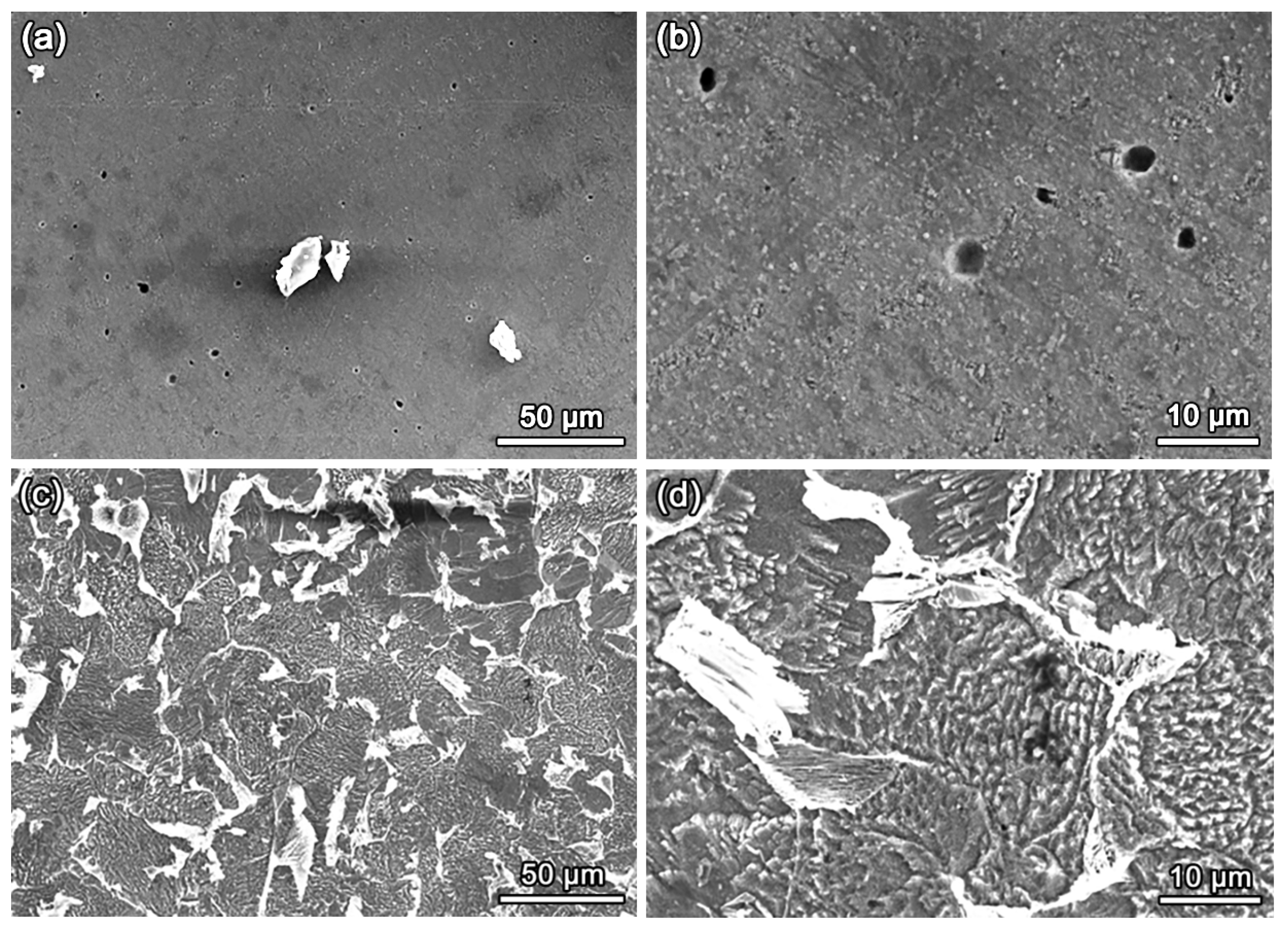
3.2. Microstructures of Fe-Based HEA Coatings
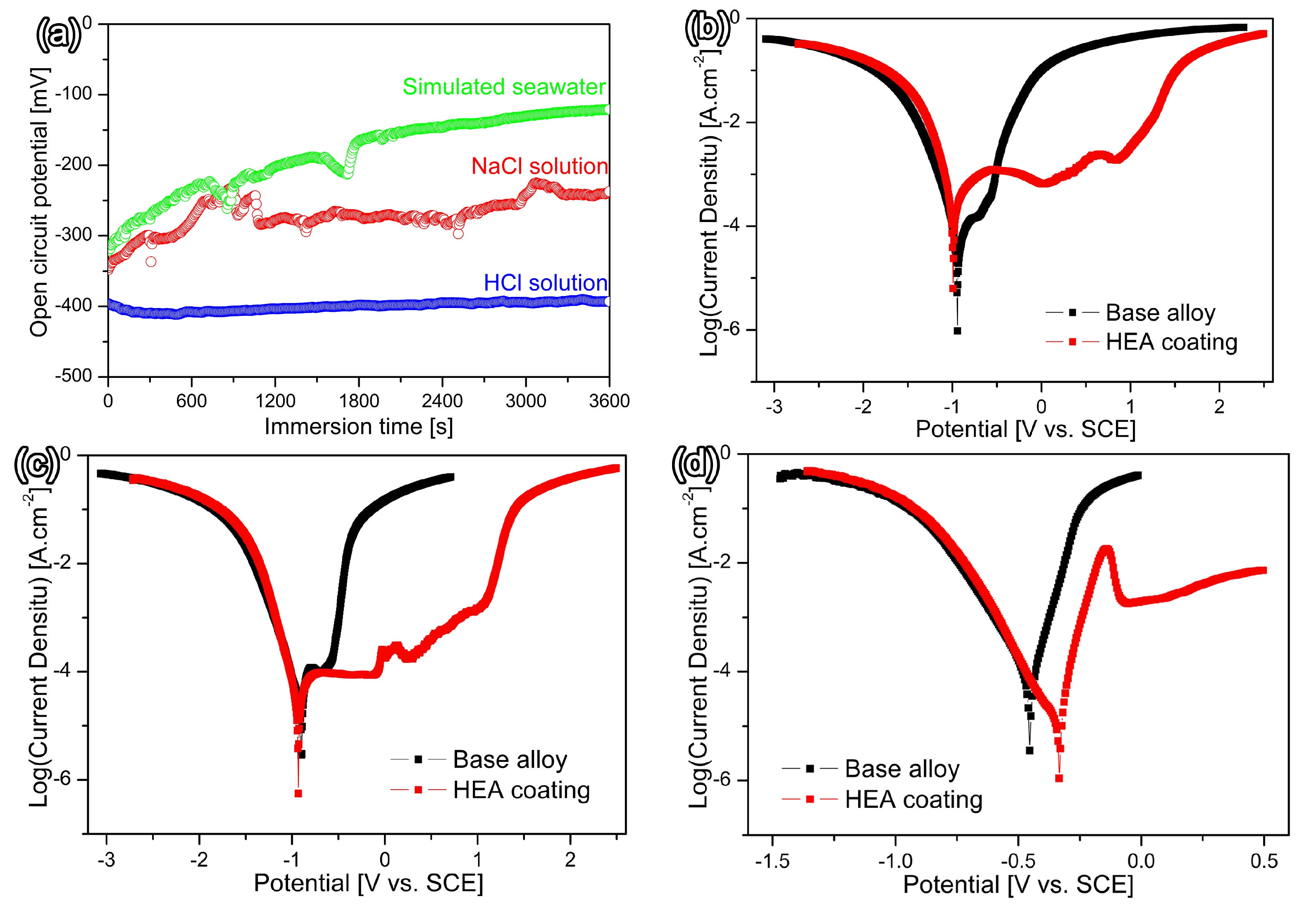
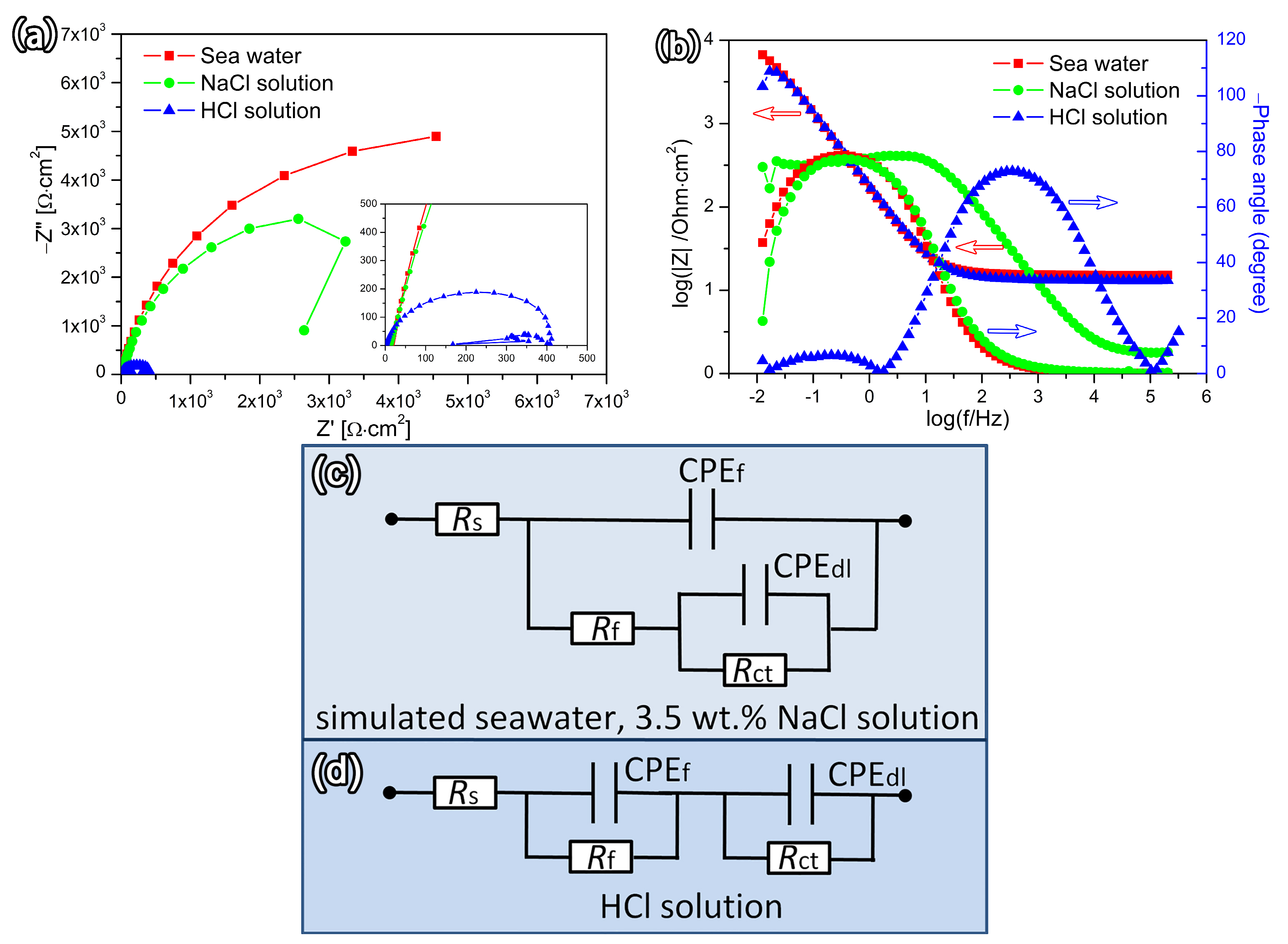
3.3. Corrosion Resistance
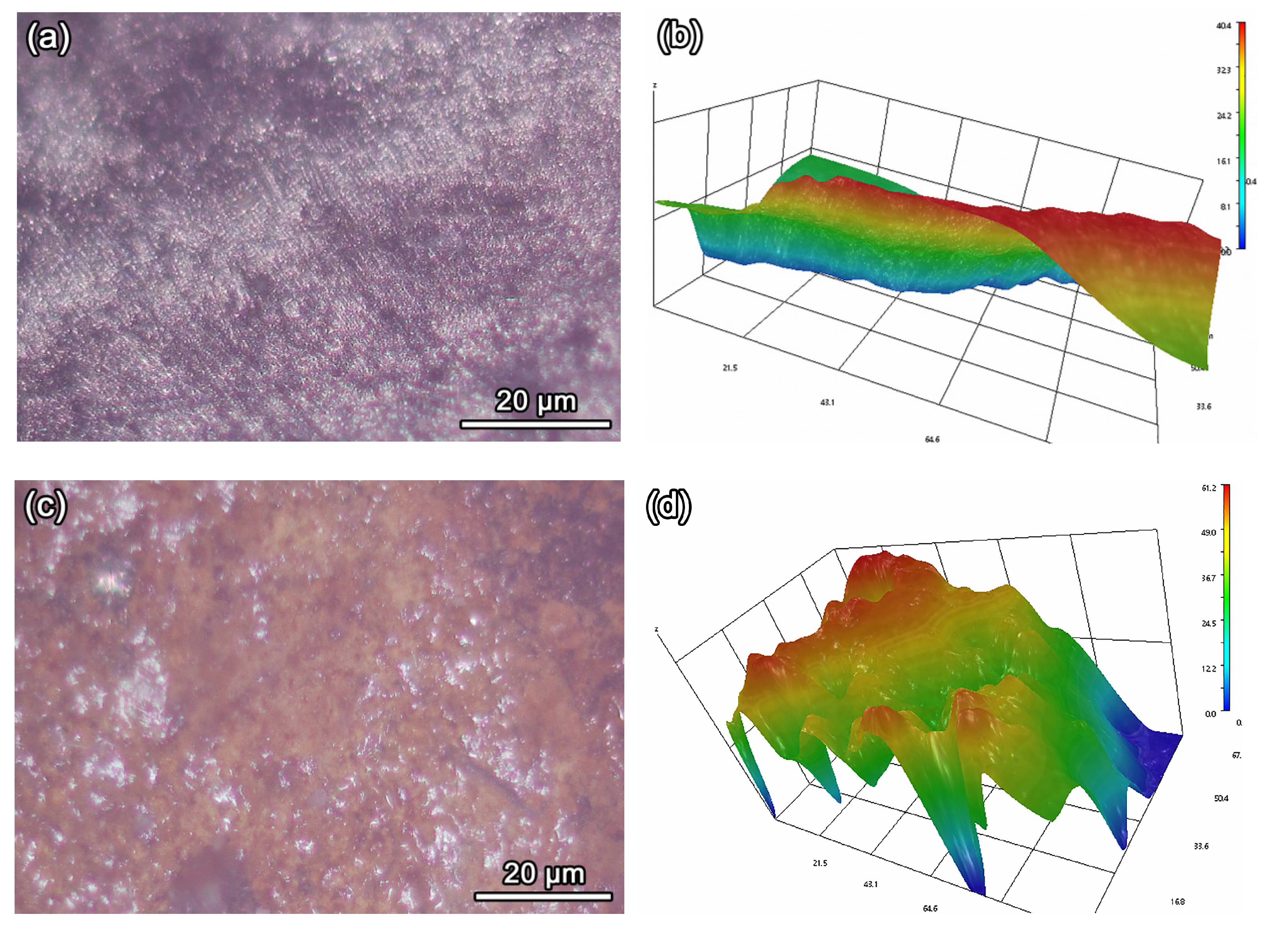
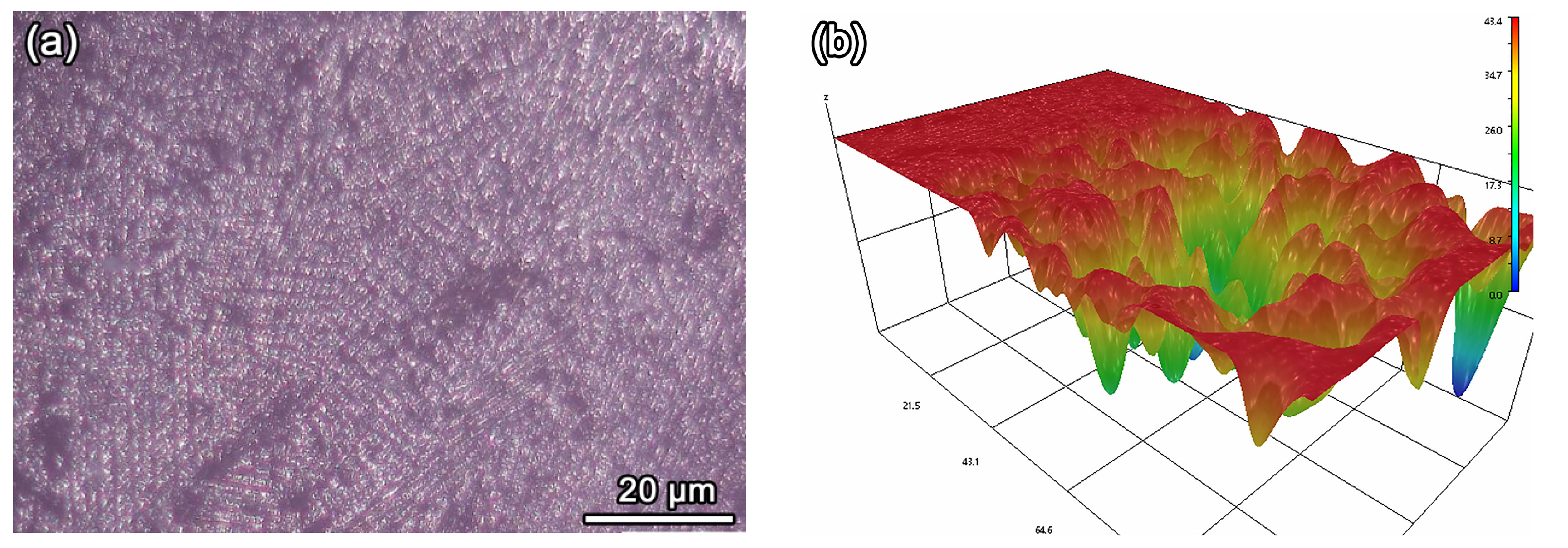
3.4. Analysis of Super Depth of Field and 3D Topological Morphology
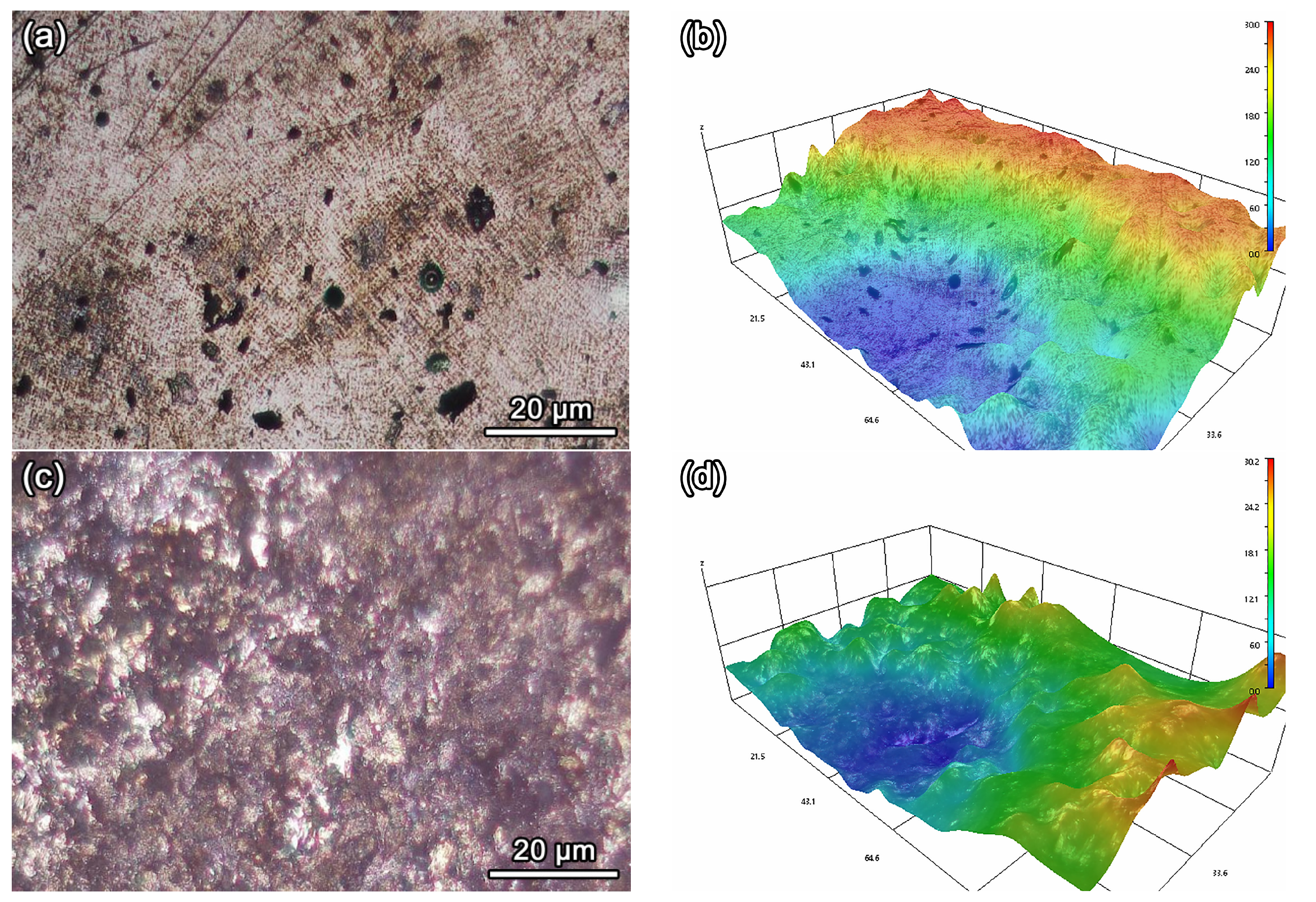
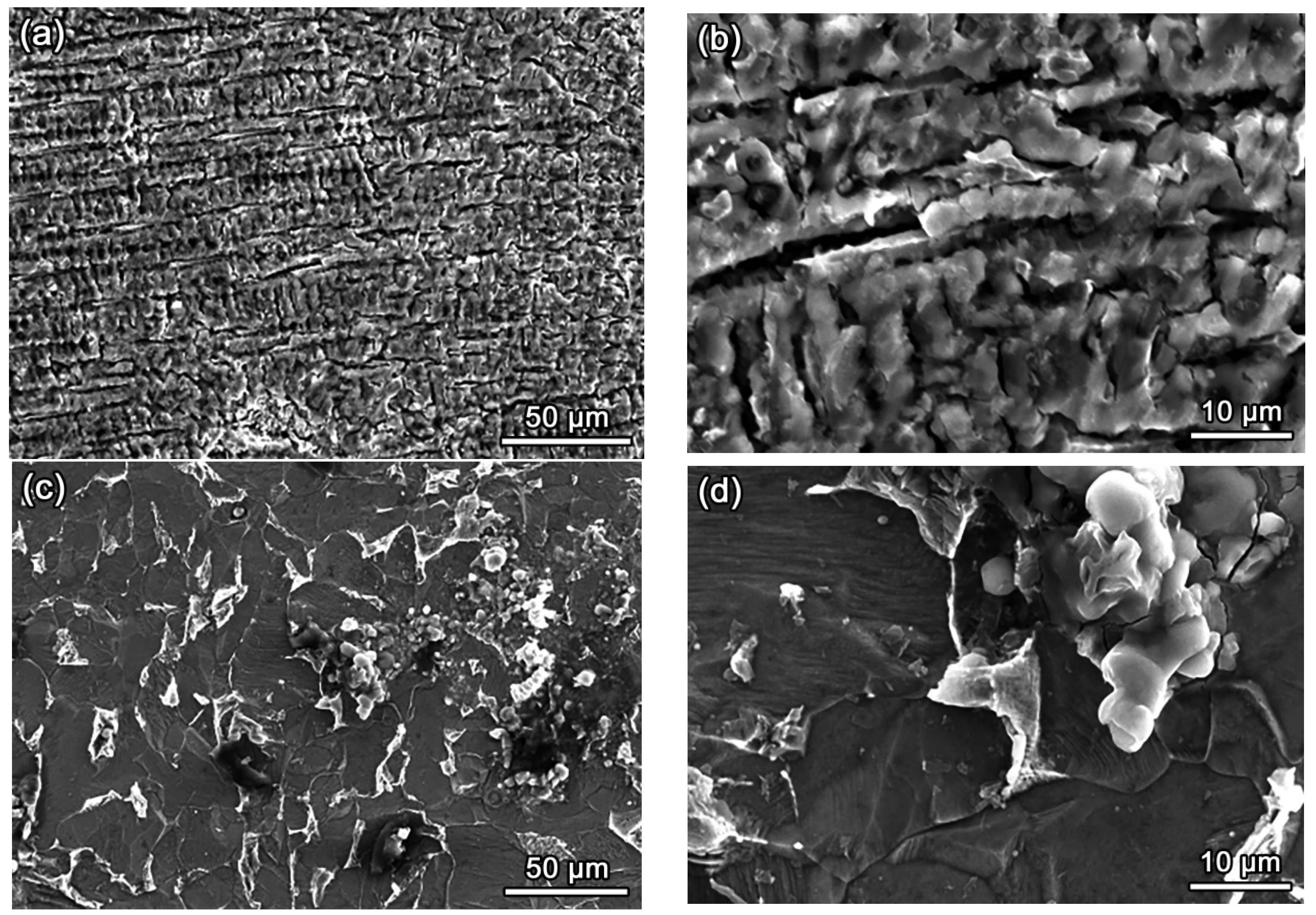
3.5. Analysis of Corrosion Morphology
4. Conclusions
- A single-phase face-centered cubic (FCC) structure HEA coating was successfully prepared using laser cladding technology.
- The studied Fe-based HEA coating has a small grain size, approximately 10.7 ± 0.25 by SEM observation.
- The Fe-based HEA coating exhibits superior corrosion resistance in simulated seawater and 3.5 wt.% NaCl solutions but presents lower resistance in a 5% HCl solution compared to the metal substrate.
- The surface of the current Fe-based HEA coating shows preferential interdendritic corrosion in simulated seawater and 3.5 wt.% NaCl solutions, whereas it displays a more pronounced pitting characteristic in a 5% HCl solution.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balaji, V.; Anthony, X.M. Development of high entropy alloys (HEAs): Current trends. Heliyon 2024, 10, 26464. [Google Scholar]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Takeuchi, A.; Wada, T.; Nagase, T.; Amiya, K. Ultra-high mixing entropy alloys with single bcc, hcp, or fcc structure in Co–Cr–V–Fe–X(X=Al, Ru, or Ni) systems designed with structure-dependent mixing entropy and mixing enthalpy of constituent binary equiatomic alloys. Mater. Trans. 2022, 63, 835–844. [Google Scholar] [CrossRef]
- Hu, Q.; Guo, S.; Wang, J.M.; Yan, Y.H.; Chen, S.S.; Lu, D.P.; Liu, K.M.; Zou, J.Z.; Zeng, X.R. Parametric study of amorphous high-Entropy alloys formation from two new perspectives: Atomic radius modification and crystalline structure of alloying elements. Sci. Rep. 2017, 7, 39917. [Google Scholar] [CrossRef]
- Li, T.X.; Wang, S.D.; Lu, Y.P.; Cao, Z.Q.; Wang, T.M.; Li, T.J. Research progress and prospect of high-entropy alloy materials. Strateg. Study CAE 2023, 25, 170–181. [Google Scholar] [CrossRef]
- George, E.P.; Curtin, W.A.; Tasan, C.C. High entropy alloys: A focused review of mechanical properties and deformation mechanisms. Acta Mater. 2020, 188, 435–474. [Google Scholar] [CrossRef]
- Fu, Y.; Li, J.; Luo, H.; Du, C.W.; Li, X.G. Recent advances on environmental corrosion behavior and mechanism of high-entropy alloys. J. Mater. Sci. Technol. 2021, 80, 217–233. [Google Scholar] [CrossRef]
- Zhang, W.; Chabok, A.; Kooi, B.J.; Pei, Y.T. Additive manufactured high entropy alloys: A review of the microstructure and properties. Mater. Des. 2022, 220, 110875. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Rehman, E.U.; Ullah, S.; Atif, M.; Tariq, A. A review on laser cladding of high-entropy alloys, their recent trends and potential applications. J. Manuf. Process. 2021, 68, 225–273. [Google Scholar] [CrossRef]
- Yang, C.; Jing, C.N.; Fu, T.L.; Lin, T.; Guo, W.M.; Liu, N.N. Effect of CeO2 on the microstructure and properties of AlCoCrFeNi2.1 laser cladding coatings. J. Alloys Compd. 2024, 976, 172948. [Google Scholar] [CrossRef]
- Guo, Y.F.; Zhu, J.Q.; Cao, J.J.; Qiu, Z.G.; Chang, C.; Yan, X.C.; Yin, S.; Liu, M. Pitting corrosion mechanism of BCC+FCC dual-phase structured laser cladding FeCoCrNiAl0.5Ti0.5 HEAs coating. J. Alloys Compd. 2024, 980, 173643. [Google Scholar] [CrossRef]
- Ren, X.Y.; Sun, W.L.; Tian, S.; Zhu, C.J.; Qin, M.J.; Yang, Y.L.; Wu, W.N. Tribological and electrochemical behaviors of FeCoNiCrMox HEA coatings prepared by internal laser cladding on 316L steel tube. Mater. Charact. 2024, 211, 113906. [Google Scholar] [CrossRef]
- Wan, S.M.; Cui, X.F.; Liu, K.J.; Jin, G.; Wang, S.; Zhao, Y.; Li, J.; Yang, Y.Y.; Guan, Y.J. Preparation of AlTiVNiCu/Cu–Al gradient functional coating via laser cladding for enhanced wear and corrosion resistance of MgLi alloy. Surf. Coat. Technol. 2023, 475, 130137. [Google Scholar] [CrossRef]
- He, R.; Wu, M.P.; Cui, C.; Jie, D.D.; Miao, X.J. Laser cladding manufacturing of TiC + TiBx reinforced FeNiCrTi0.3Al0.3 high entropy alloy composite coating: Effects on corrosion and tribology. J. Alloys Compd. 2024, 970, 172631. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, L.; Zhang, S.; Zhang, C.H.; Sun, X.Y.; Chen, H.T.; Bai, X.L.; Wu, C.L. Effect of synergistic cavitation erosion-corrosion on cavitation damage of CoCrFeNiMn high entropy alloy layer by laser cladding. Surf. Coat. Technol. 2023, 472, 129940. [Google Scholar] [CrossRef]
- Chou, Y.L.; Yeh, J.W.; Shih, H.C. The effect of molybdenum on the corrosion behaviour of the high-entropy alloys Co1.5CrFeNi1.5Ti0.5Mox in aqueous environments. Corros. Sci. 2010, 52, 2571–2581. [Google Scholar] [CrossRef]
- Moravcik, I.; Cizek, J.; Zapletal, J.; Kovacova, Z.; Vesely, J.; Minarik, P.; Kitzmantel, M.; Neubauer, E.; Dlouhy, I. Microstructure and mechanical properties of Ni1.5Co1.5CrFeTi0.5 high entropy alloy fabricated by mechanical alloying and spark plasma sintering. Mater. Des. 2017, 119, 141–150. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Liu, L.L.; Zhang, J.L.; Zhang, Q.; Zhai, C.S.; Zheng, H.X. Computational insights into gas atomization of FeCoNiCrMoBSi high-entropy alloy: From droplet formation to rapid solidification. Int. J. Heat Mass Transfer. 2024, 228, 125628. [Google Scholar] [CrossRef]
- Lentzaris, K.; Poulia, A.; Georgatis, E.; Lekatou, A.G.; Karantzalis, A.E. Analysis of microstructure and sliding wear behavior of Co1.5CrFeNi1.5Ti0.5 high-entropy alloy. J. Mater. Eng. Perform. 2018, 27, 5177–5186. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Zhao, D.D.; Wang, P.; Yan, M.; Yang, C.; Chen, Z.W.; Lu, J.; Lu, Z.P. Additive manufacturing of metals: Microstructure evolution and multistage control. J. Mater. Sci. Technol. 2022, 100, 224–236. [Google Scholar] [CrossRef]
- Ya, W.; Pathiraj, B.; Liu, S.J. 2D modelling of clad geometry and resulting thermal cycles during laser cladding. J. Mater. Process. Technol. 2016, 230, 217–232. [Google Scholar] [CrossRef]
- Shin, D.; Shyam, A.; Lee, S.; Yamamoto, Y.; Haynes, J.A. Solute segregation at the Al/θ′-Al2Cu interface in Al-Cu alloys. Acta Mater. 2017, 141, 327–340. [Google Scholar] [CrossRef]
- Jin, Z.J.; An, F.C.; Li, S.X.; Cui, Z.Y.; Lu, W.J.; Lu, S.Y. Effect of annealing temperature on the microstructure evolution and corrosion behavior of Carbon-interstitial FeMnCoCrNi high-entropy alloys. Corros. Sci. 2024, 228, 111813. [Google Scholar] [CrossRef]
- Luo, H.; Wang, X.Z.; Dong, C.F.; Xiao, K.; Li, X.G. Effect of cold deformation on the corrosion behaviour of UNS S31803 duplex stainless steel in simulated concrete pore solution. Corros. Sci. 2017, 124, 178–192. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Yao, J.Y.; Serrano, L.B.; Santos, S.F.; Cardoso, K.R. Microstructure and corrosion behavior of the Ti-V-Cr-Nb high-entropy alloys in 3.5wt% NaCl solution. Corros. Sci. 2023, 218, 111149. [Google Scholar] [CrossRef]
- Gu, Z.; Mao, P.; Gou, Y.F.; Chao, Y.; Xi, S.Q. Microstructure and properties of MgMoNbFeTi2Yx high entropy alloy coatings by laser cladding. Surf. Coat. Technol. 2020, 402, 126303. [Google Scholar] [CrossRef]
- Cao, H.B.; Hou, G.L.; Xu, T.C.; Ma, J.K.; Wan, H.Q.; An, Y.L.; Zhou, H.D.; Chen, J.M. Effect of seawater temperature on the corrosion and cavitation erosion-corrosion resistance of Al10Cr28Co28Ni34 high-entropy alloy coating. Corros. Sci. 2024, 228, 111822. [Google Scholar] [CrossRef]
- Cheng, J.B.; Liang, X.B.; Xu, B.S. Effect of Nb addition on the structure and mechanical behaviors of CoCrCuFeNi high-entropy alloy coatings. Surf. Coat. Technol. 2014, 240, 184–190. [Google Scholar] [CrossRef]
- Mao, A.Q.; Ding, P.P.; Quan, F.; Zhang, T.C.; Ran, X.Q.; Li, Y.B.; Jin, X.; Gu, X.L. Effect of aluminum element on microstructure evolution and properties of multicomponent Al-Co-Cr-Cu-Fe-Ni nanoparticles. J. Alloys Compd. 2018, 735, 1167–1175. [Google Scholar] [CrossRef]
- Feng, H.; Li, H.B.; Dai, J.; Han, Y.; Qu, J.D.; Jiang, Z.H.; Zhao, Y.; Zhang, T. Why CoCrFeMnNi HEA could not passivate in chloride solution?—A novel strategy to significantly improve corrosion resistance of CoCrFeMnNi HEA by N-alloying. Corros. Sci. 2022, 204, 110396. [Google Scholar]



| Chemical Reagent | NaCl | MgCl2 | Na2SO4 | CaCl2 | KCl | NaHCO3 | HBr | HBO3 | SrCl2 | NaF |
|---|---|---|---|---|---|---|---|---|---|---|
| Concentration | 24.530 | 11.110 | 4.090 | 1.160 | 0.685 | 0.201 | 0.101 | 0.027 | 0.028 | 0.003 |
| Corrosive Environments | Working Electrode | Corrosion Potential (V) | Current Density (A·cm−2) | Corrosion Rate (μm·Year−1) |
|---|---|---|---|---|
| Simulated seawater | FeCo1.5CrNi1.5Ti0.5 HEA coating | −0.990 | 7.94 × 10−5 | 925 |
| Substrate | −0.942 | 2.12 × 10−5 | 246 | |
| 3.5 wt% NaCl solution | FeCo1.5CrNi1.5Ti0.5 HEA coating | −0.893 | 1.98 × 10−5 | 936 |
| Substrate | −0.902 | 1.47 × 10−5 | 171 | |
| 5% HCl solution | FeCo1.5CrNi1.5Ti0.5 HEA coating | −0.331 | 0.35 × 10−5 | 50.3 |
| Substrate | −0.452 | 3.31 × 10−5 | 385 | |
| Simulated seawater | MgMoNbFeTi2 HEA coating [29] | −1.252 | 7.23 × 10−5 | - |
| Simulated seawater | Al10Cr28Co28Ni34 HEA coating [30] | −0.862 | 10.21 × 10−5 | - |
| 3.5 wt% NaCl solution | FeCoCrNiAl0.5Ti0.5 HEA coating [11] | −0.758 | 4.39 × 10−5 | 960 |
| 3.5 wt% NaCl solution | FeCoNiCrMo0.4 HEA coating [12] | −0.966 | 5.53 × 10−5 | - |
| 6M HCl solution | CoCrCuFeNiNb HEA coating [31] | −0.397 | 8.93 × 10−5 | - |
| 10% HCl solution | AlCoCrCuFeNi HEA coating [32] | −0.657 | 0.315 | - |
| Specimen | Solution | Rs (Ω⋅cm2) | Rf (Ω⋅cm2) | CPEf Y0(Ω−1sncm−2) | n1 | Rct (Ω⋅cm2) | CPEdl Y0(Ω−1sncm−2) | n2 | Rp (Ω⋅cm2) |
|---|---|---|---|---|---|---|---|---|---|
| FeCo1.5CrNi1.5Ti0.5 HEA coating | simulated seawater | 15.30 | 20.80 | 8.47 × 10−4 | 0.92 | 12.4 × 103 | 2.58 × 10−4 | 0.91 | 12,420.80 |
| 3.5 wt.% NaCl solution | 13.40 | 42.20 | 8.30 × 10−4 | 0.92 | 9.23 × 103 | 2.58 × 10−4 | 0.87 | 9272.20 | |
| 5% HCl solution | 1.79 | 134.00 | 1.28 × 10−4 | 1.13 | 0.283 × 103 | 0.15 × 10−4 | 0.86 | 417.00 |
| Corrosive Environments | Fe (at%) | Co (at%) | Ni (at%) | Cr (at%) | Ti (at%) | Cl− (at%) | O2− (at%) |
|---|---|---|---|---|---|---|---|
| Simulated seawater | 11.25 | 17.03 | 21.12 | 10.27 | 10.95 | 4.40 | 24.97 |
| 3.5 wt% NaCl solution | 10.73 | 17.84 | 21.77 | 9.26 | 11.83 | 3.30 | 25.54 |
| 5% HCl solution | 10.53 | 18.08 | 18.09 | 10.71 | 7.40 | 3.82 | 28.36 |
| Theoretical atomic ratio | 20 | 30 | 20 | 30 | 10 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Tian, S.; Liu, R.; Chen, D.; Wang, C.; Li, J.; Yang, S. Microstructure and Corrosion Resistance of Laser-Cladded FeCo1.5CrNi1.5Ti0.5 High-Entropy Alloy Coatings. Coatings 2024, 14, 1608. https://doi.org/10.3390/coatings14121608
Wang S, Tian S, Liu R, Chen D, Wang C, Li J, Yang S. Microstructure and Corrosion Resistance of Laser-Cladded FeCo1.5CrNi1.5Ti0.5 High-Entropy Alloy Coatings. Coatings. 2024; 14(12):1608. https://doi.org/10.3390/coatings14121608
Chicago/Turabian StyleWang, Sui, Siqi Tian, Renjie Liu, Dengya Chen, Chao Wang, Jing Li, and Sen Yang. 2024. "Microstructure and Corrosion Resistance of Laser-Cladded FeCo1.5CrNi1.5Ti0.5 High-Entropy Alloy Coatings" Coatings 14, no. 12: 1608. https://doi.org/10.3390/coatings14121608
APA StyleWang, S., Tian, S., Liu, R., Chen, D., Wang, C., Li, J., & Yang, S. (2024). Microstructure and Corrosion Resistance of Laser-Cladded FeCo1.5CrNi1.5Ti0.5 High-Entropy Alloy Coatings. Coatings, 14(12), 1608. https://doi.org/10.3390/coatings14121608




