Silver Linings: Electrochemical Characterization of TiO2 Sol-Gel Coating on Ti6Al4V with AgNO3 for Antibacterial Excellence
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Physiological Solution
2.2. Substrate Pretreatment Process
2.3. Preparation of TiO2 Sols and Coatings
2.4. Electrochemical Characterization
2.5. Antimicrobial Evaluation
2.6. Coating Adhesion Determination
2.7. Coating Thickness Evaluation
3. Results and Discussions
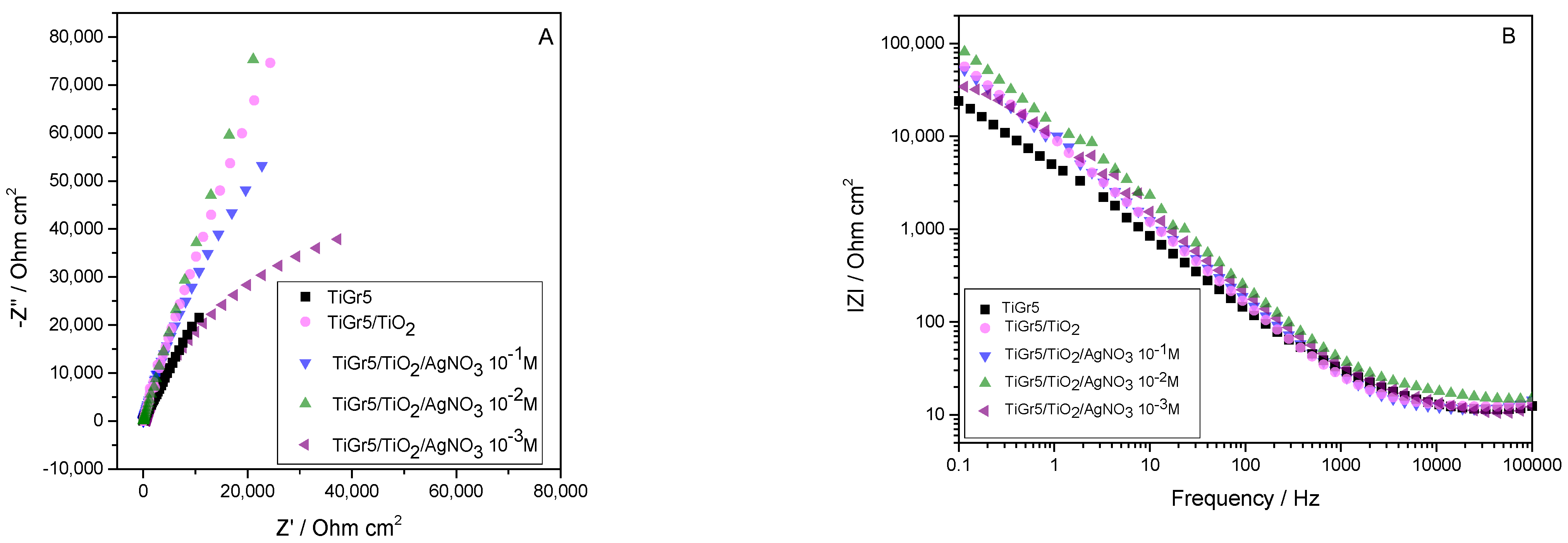
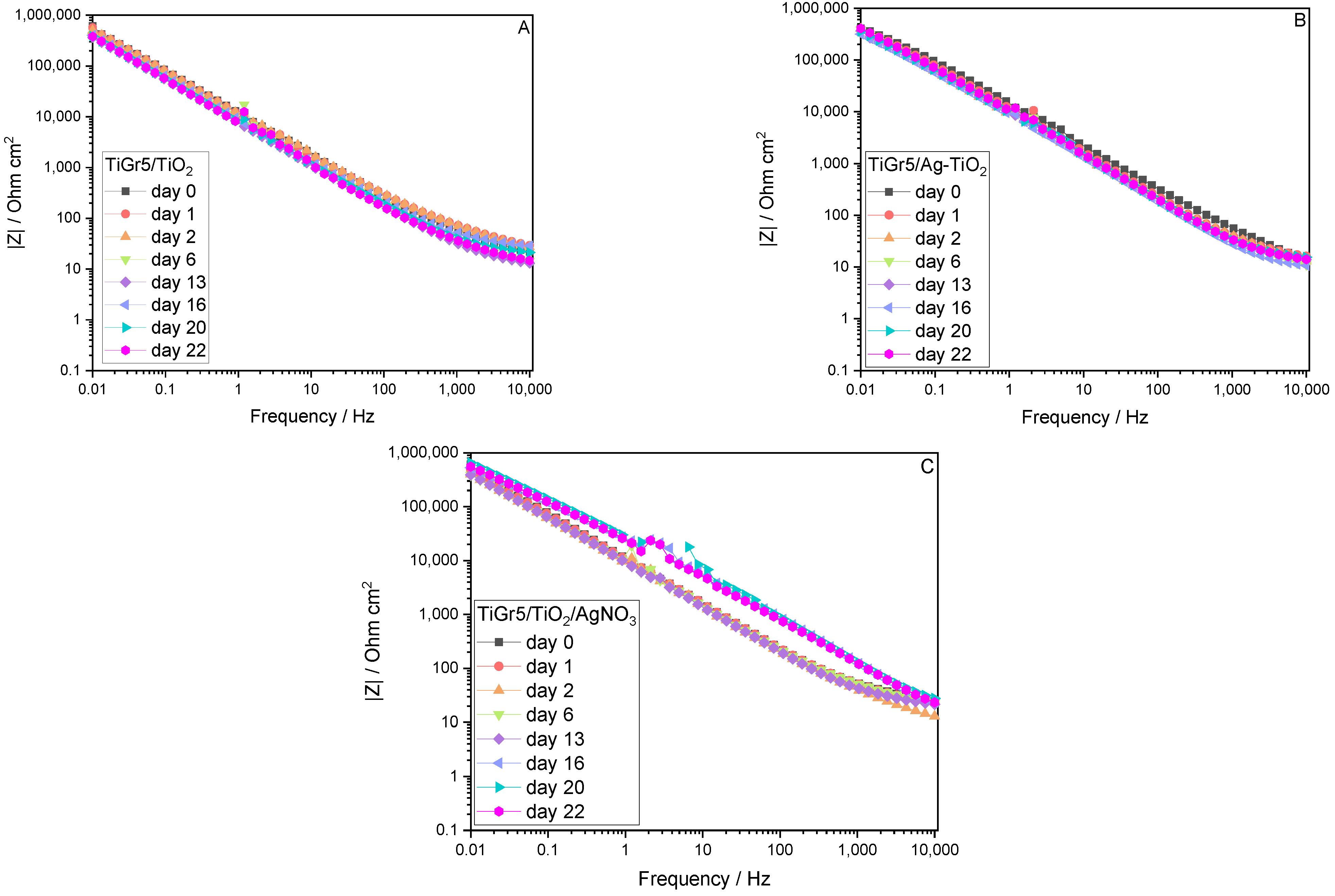
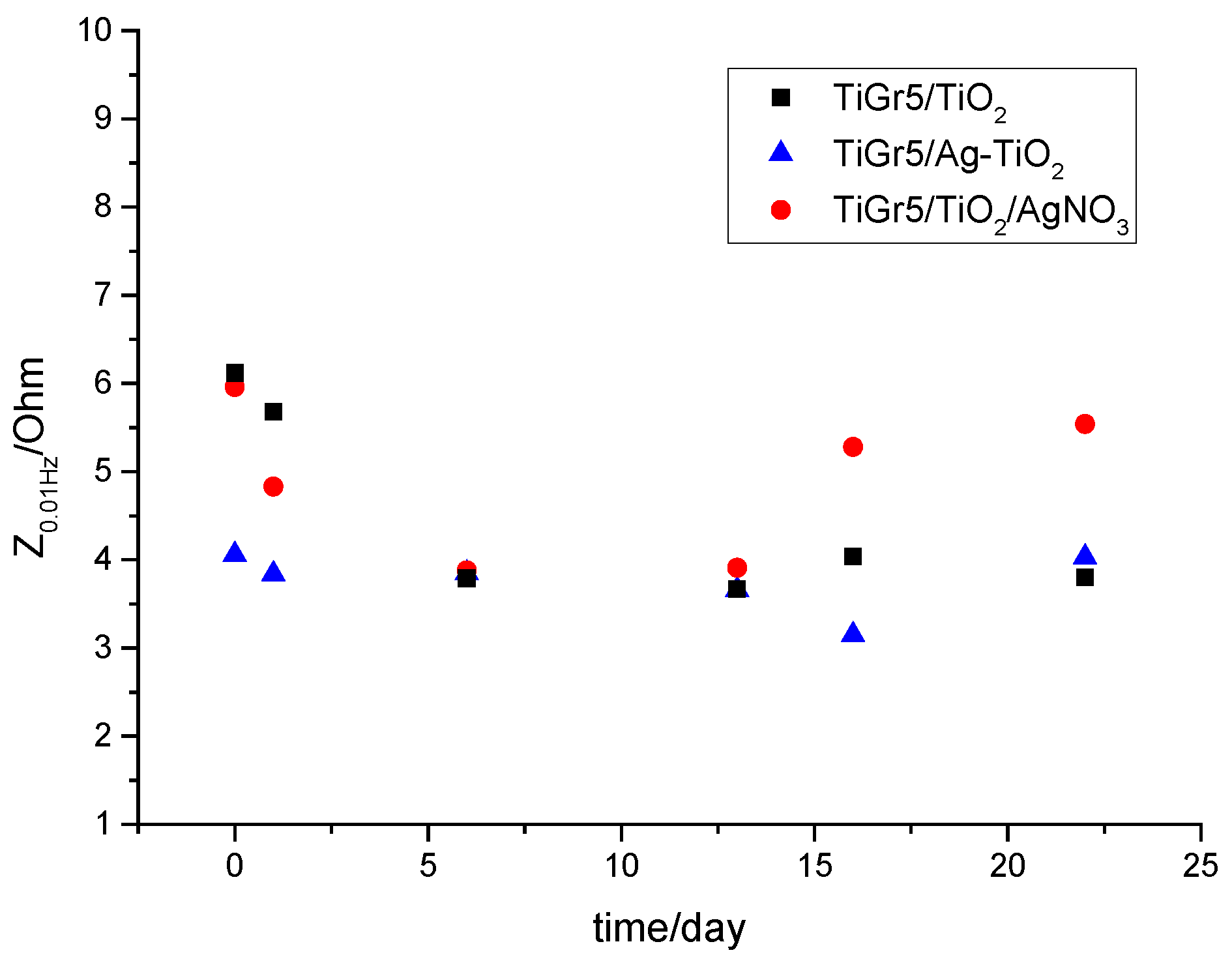
3.1. Electrochemical Evaluation
3.1.1. Electrochemical Impedance Spectroscopy
3.1.2. Polarization Curves
3.2. Coating Thickness
3.3. Coating Adhesion
3.4. Antimicrobial Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarmiento-González, A.; Marchante-Gayón, J.M.; Tejerina-Lobo, J.M.; Paz-Jiménez, J.; Sanz-Medel, A. High-resolution ICP–MS determination of Ti, V, Cr, Co, Ni, and Mo in human blood and urine of patients implanted with a hip or knee prosthesis. Anal. Bioanal. Chem. 2008, 391, 2583–2589. [Google Scholar] [CrossRef]
- Farooq, N.; Kallem, P.; ur Rehman, Z.; Imran Khan, M.; Kumar Gupta, R.; Tahseen, T.; Mushtaq, Z.; Ejaz, N.; Shanableh, A. Recent trends of titania (TiO2) based materials: A review on synthetic approaches and potential applications. J. King Saud. Univ. Sci. 2024, 36, 103210. [Google Scholar] [CrossRef]
- Cotolan, N.; Rak, M.; Bele, M.; Cör, A.; Muresan, L.M.; Milošev, I. Sol-gel synthesis, characterization and properties of TiO2 and Ag-TiO2 coatings on titanium substrate. Surf. Coat. Technol. 2016, 307, 790–799. [Google Scholar] [CrossRef]
- Mai, L.; Wang, D.; Zhang, S.; Xie, Y.; Huang, C.; Zhang, Z. Synthesis and bactericidal ability of Ag/TiO2 composite films deposited on titanium plate. Appl. Surf. Sci. 2010, 257, 974–978. [Google Scholar] [CrossRef]
- Kravanja, K.A.; Finšgar, M. A review of techniques for the application of bioactive coatings on metal-based implants to achieve controlled release of active ingredients. Mater. Des. 2022, 217, 110653. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, Y.; Wang, H.; Lu, X.; Wang, Y.; Xu, M.; Tan, X.; Ju, J.; Shao, Y.; Liu, H.; et al. Preparation and antibacterial activity of Ag-doped TiO2 coating on the porous surface of aluminum. J. Mater. Res. Technol. 2024, 32, 1801–1808. [Google Scholar] [CrossRef]
- Wang, Y.; Ju, Y.; Wei, S.; Lu, W.; Yan, B.; Gao, W. Mechanical properties and microstructure of Au–Ni–TiO2 nano-composite coatings. Mater. Charact. 2015, 102, 189–194. [Google Scholar] [CrossRef]
- Hu, P.; Zhu, L.; Tian, C.; Xu, G.; Zhang, X.; Cai, G. Study of Anticorrosion and Antifouling Properties of a Cu-Doped TiO2 Coating Fabricated via Micro-Arc Oxidation. Materials 2023, 17, 217. [Google Scholar] [CrossRef]
- Gulati, K.; Maher, S.; Findlay, D.M.; Losic, D. Titania Nanotubes for Orchestrating Osteogenesis at the bone–implant Interface. Nanomedicine 2016, 11, 1847–1864. [Google Scholar] [CrossRef]
- Murr, L.E. Strategies for creating living, additively manufactured, open-cellular metal and alloy implants by promoting osseointegration, osteoinduction and vascularization: An overview. J. Mater. Sci. Technol. 2019, 35, 231–241. [Google Scholar] [CrossRef]
- Folorunso, A.; Akintelu, S.; Oyebamiji, A.K.; Ajayi, S.; Abiola, B.; Abdusalam, I.; Morakinyo, A. Biosynthesis, characterization and antimicrobial activity of gold nanoparticles from leaf extracts of Annona muricata. J. Nanostructure Chem. 2019, 9, 111–117. [Google Scholar] [CrossRef]
- Sankareswari, M.; Amutha, C.; Vasantha, V.S.; Hwan Oh, T.; Arunpandian, M.; Selvakumar, K. Biosynthesis of bimetallic Au-Ag nanoparticles using Abrus precatorius seed Extract: Analysis of photocatalytic, cytotoxic, and antibacterial activities. Inorg. Chem. Commun. 2024, 169, 113134. [Google Scholar] [CrossRef]
- Skiba, M.; Khrokalo, L.; Linyuchev, A.; Vorobyova, V. State of the art on plasma-liquid route synthesis monometallic Au, bimetallic Au/Ag core–shell and alloy nanoparticles: Toxicological, canalytical, antimicrobial activity for environmental control. J. Mol. Struct. 2024, 1318, 139293. [Google Scholar] [CrossRef]
- Daimari, J.; Basumatary, S.; Deka, A.K. Bimetallic nanoparticles from coinage metals (Cu, Ag, Au) and its biomedical applications: A Review. Nano-Struct. Nano-Objects 2024, 39, 101247. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Herdade, A.M.; Guerra, C.; Peixoto, D.; Pereira-Silva, M.; Zeinali, M.; Mascarenhas-Melo, F.; Paranhos, A.; Veiga, F. Plant-mediated green synthesis of metal-based nanoparticles for dermopharmaceutical and cosmetic applications. Int. J. Pharm. 2021, 597, 120311. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, T.; Zhang, X.; Fu, S.; Yang, L.; Dong, Z.; Ma, Y.; Zhang, E. The synergistic effect of Ag and ZnO on the microstructure, corrosion resistance and in vitro biological performance of titania coating. Surf. Coat. Technol. 2021, 426, 127798. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, H.; Geng, Z.; Huang, X.; Hang, R.; Ma, Y.; Yao, X.; Tang, B. Microstructure and cytotoxicity evaluation of duplex-treated silver-containing antibacterial TiO2 coatings. Mater. Sci. Eng. C 2014, 45, 402–410. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, H.; Zhang, E.; You, J.; Ma, X.; Bai, X. Antibacterial activities and biocompatibilities of Ti-Ag alloys prepared by spark plasma sintering and acid etching. Mater. Sci. Eng. C 2018, 92, 121–131. [Google Scholar] [CrossRef]
- Lv, Y.; Wu, Y.; Lu, X.; Yu, Y.; Fu, S.; Yang, L.; Dong, Z.; Zhang, X. Microstructure, bio-corrosion and biological property of Ag-incorporated TiO2 coatings: Influence of Ag2O contents. Ceram. Int. 2019, 45, 22357–22367. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Folorunso, A.S.; Oyebamiji, A.K.; Erazua, E.A. Antibacterial potency of silver nanoparticles synthetized using Boerhaavia diffusa leaf extract as reductive and stabilizing agent. Int. J. Pharma Sci. Res. 2019, 10, 374–380. [Google Scholar]
- Albert, E.; Albouy, P.A.; Ayral, A.; Basa, P.; Csík, G.; Nagy, N.; Roualdes, S.; Rouessac, V.; Sáfrán, G.; Suhajda, Á.; et al. Antibacterial properties of Ag–TiO2 composite sol–gel coatings. RSC Adv. 2015, 5, 59070–59081. [Google Scholar] [CrossRef]
- Szabó, G.S.; Volentiru, E.; Hórvölgyi, Z.; Mureşan, L.M. Protective TiO2 coatings prepared by sol-gel method on zinc. Stud. UBB Chem. 2015, 60, 225–235. [Google Scholar]
- Both, J.; Fülöp, A.P.; Szabó, G.S.; Katona, G.; Ciorîță, A.; Mureșan, L.M. Effect of the Preparation Method on the Properties of Eugenol-Doped Titanium Dioxide (TiO2) Sol-Gel Coating on Titanium (Ti) Substrates. Gels 2023, 9, 668. [Google Scholar] [CrossRef] [PubMed]
- Behera, A.N.; Paul, B.; Chaudhary, R.K.; Mishra, P.; Hubli, R.C.; Chakravartty, J.K. Electrochemical and Hot Deformation Behaviour of Co–Cr–Mo Alloys. Mater. Today Proc. 2016, 3, 3162–3171. [Google Scholar] [CrossRef]
- Trapalis, C.; Todorova, N.; Anastasescu, M.; Anastasescu, C.; Stoica, M.; Gartner, M.; Zaharescu, M.; Stoica, T. Atomic force microscopy study of TiO2 sol–gel films thermally treated under NH3 atmosphere. Thin Solid Film. 2009, 517, 6243–6247. [Google Scholar] [CrossRef]
- Niazmand, M.; Maghsoudipour, A.; Alizadeh, M.; Khakpour, Z.; Kariminejad, A. Effect of dip coating parameters on microstructure and thickness of 8YSZ electrolyte coated on NiO-YSZ by sol-gel process for SOFCs applications. Ceram. Int. 2022, 48, 16091–16098. [Google Scholar] [CrossRef]
- ASTM D3359; Standard Test Methods for Measuring Adhesion by Tape Test. ASTM International: West Conshohocken, PA, USA, 2012.
- Behnami Far, V.; Jafarzadeh, K.; Shooshtari Gugtapeh, H.; Mirali, S.M. A study on electrical properties of thermally grown TiO2 film at the interface of Ti/RuO2–IrO2–TiO2 anode using Mott-Schottky and electrochemical impedance spectroscopy techniques. Mater. Chem. Phys. 2020, 256, 123756. [Google Scholar] [CrossRef]
- Vidovich, G.L.; Leykis, D.I.; Kabanov, B.N. The Anodic Passivation of Silver in Alkali Solutions. Dokl. Akad. Nauk. SSSR 1960, 132, 868–871. [Google Scholar]
- Muresan, L.M. Corrosion Protective Coatings for Ti and Ti Alloys Used for Biomedical Implants. In Intelligent Coatings for Corrosion Control; Elsevier: Amsterdam, The Netherlands, 2015; pp. 585–602. [Google Scholar]
- Bapat, L.; Ramanan, J. Chemical Corrosion of Silver in Silver Nitrate Containing TiO2 and Alkaline Earth Metal Ions. Key Eng. Mater. 1991, 20–28, 489–499. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhong, X.; Li, L.; Hu, J.; Peng, Z. Unmasking the delamination mechanisms of a defective coating under the co-existence of alternating stress and corrosion. Prog. Org. Coat. 2023, 180, 107560. [Google Scholar] [CrossRef]
- Both, J.; Szabó, G.; Katona, G.; Muresan, L.M. Tannic acid reinforced sol-gel silica coatings for corrosion protection of zinc substrates. Mater. Chem. Phys. 2022, 282, 125912. [Google Scholar] [CrossRef]
- Mohamed, D.S.; Abd El-Baky, R.M.; Sandle, T.; Mandour, S.A.; Ahmed, E.F. Antimicrobial Activity of Silver-Treated Bacteria against other Multi-Drug Resistant Pathogens in Their Environment. Antibiotics 2020, 9, 181. [Google Scholar] [CrossRef]
- Aronson, J.K. MA DPhil MBChB FRCP FBPharmacolS FFPM(Hon). In Silver Salts and Derivatives, Meyler’s Side Effects of Drugs, 16th ed.; Elsevier Science: Amsterdam, The Netherlands, 2015; pp. 382–388. [Google Scholar]



| Sample | E (V) | 108 × icorr (µA/cm2) | bc V/dec | ba V/dec |
|---|---|---|---|---|
| TiGr5 | −0.379 | 1.32 | 0.082 | 0.068 |
| TiGr5/TiO2 | 0.083 | 0.32 | 0.152 | 0.126 |
| TiGr5/Ag-TiO2 10−2 M | 0.295 | 4.25 | 0.079 | 0.068 |
| TiGr5/TiO2/AgNO3 10−1 M | 0.078 | 1.99 | 0.095 | 0.058 |
| TiGr5/TiO2/AgNO3 10−2 M | 0.203 | 1.17 | 0.119 | 0.245 |
| TiGr5/TiO2/AgNO3 10−3 M | 0.081 | 0.977 | 0.185 | 0.313 |
| Sample | Coating Thickness (µm) |
|---|---|
| TiGr5/TiO2 | 96 |
| TiGr5/Ag-TiO2 | 96 |
| TiGr5/TiO2/AgNO3 | 122 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Both, J.; Szabó, G.S.; Ciorîță, A.; Mureșan, L.M. Silver Linings: Electrochemical Characterization of TiO2 Sol-Gel Coating on Ti6Al4V with AgNO3 for Antibacterial Excellence. Coatings 2024, 14, 1532. https://doi.org/10.3390/coatings14121532
Both J, Szabó GS, Ciorîță A, Mureșan LM. Silver Linings: Electrochemical Characterization of TiO2 Sol-Gel Coating on Ti6Al4V with AgNO3 for Antibacterial Excellence. Coatings. 2024; 14(12):1532. https://doi.org/10.3390/coatings14121532
Chicago/Turabian StyleBoth, Julia, Gabriella Stefania Szabó, Alexandra Ciorîță, and Liana Maria Mureșan. 2024. "Silver Linings: Electrochemical Characterization of TiO2 Sol-Gel Coating on Ti6Al4V with AgNO3 for Antibacterial Excellence" Coatings 14, no. 12: 1532. https://doi.org/10.3390/coatings14121532
APA StyleBoth, J., Szabó, G. S., Ciorîță, A., & Mureșan, L. M. (2024). Silver Linings: Electrochemical Characterization of TiO2 Sol-Gel Coating on Ti6Al4V with AgNO3 for Antibacterial Excellence. Coatings, 14(12), 1532. https://doi.org/10.3390/coatings14121532







