Facile Preparation of Composite Coatings with Incorporated 13X Zeolite and CeO2
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Morphology, Chemical and Phase Composition of Formed PEO Coatings
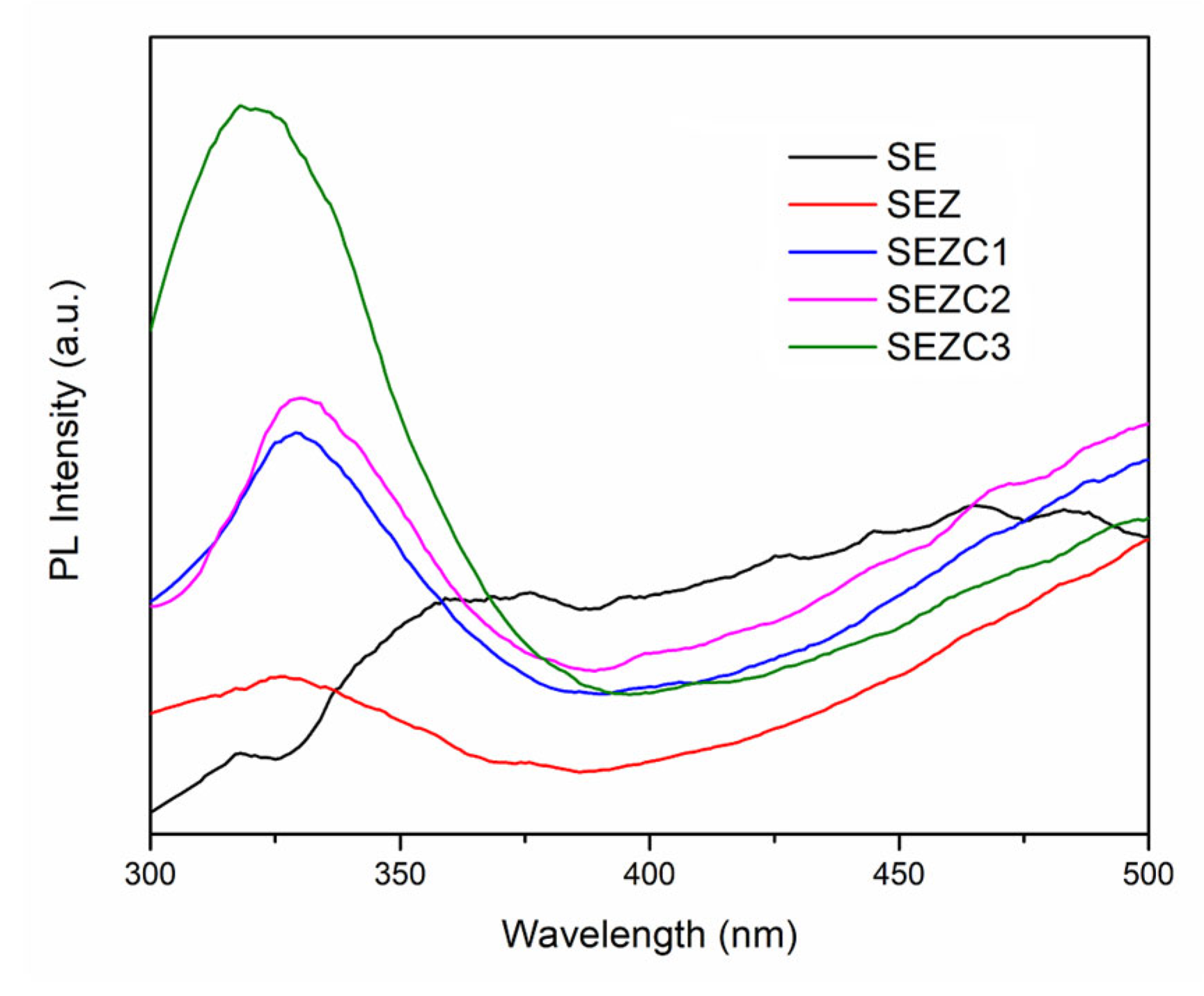
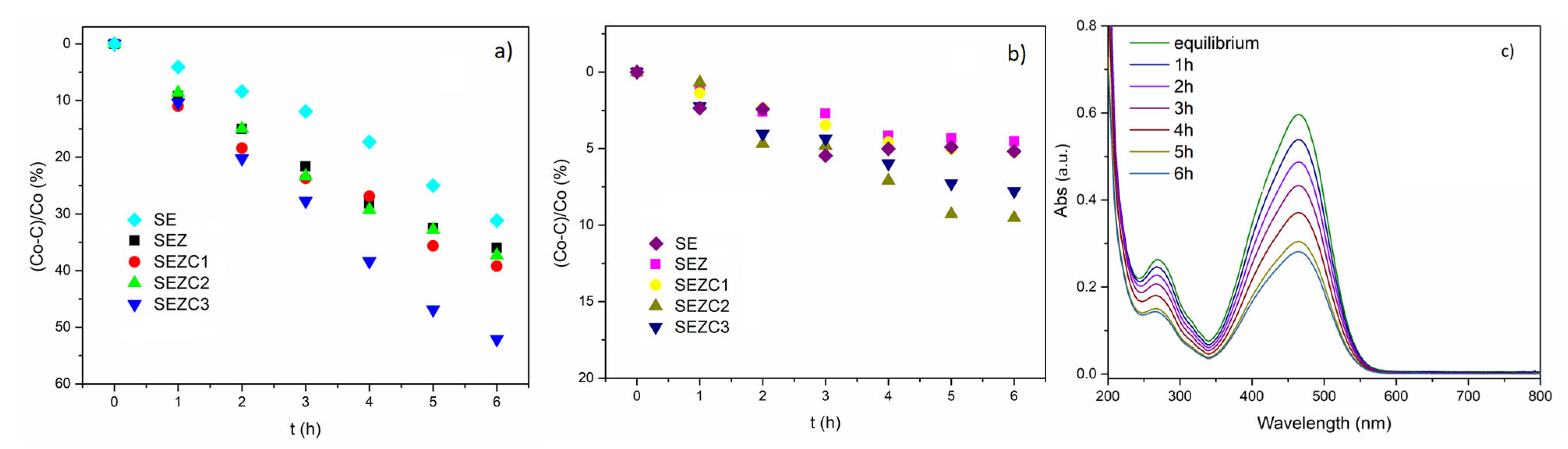
3.2. Photoluminescent and Photocatalytic Properties of Formed PEO Coatings
4. Discussion
5. Conclusions
- It is possible to form photoactive PEO coatings via co-deposition of 13X zeolite (1 g/L) and varying concentration of CeO2 (0.25 g/L, 0.5 g/L, and 1 g/L). Roughness of obtained coatings increases with the addition of 13X zeolite and CeO2 to solution, while their porosity decreases.
- Chemical composition of formed oxide coatings reveals the presence of species originating both from the substrate and from the electrolyte solution. Although Ce can be detected in oxide coatings, its concentration is near or below the detection limit of used EDS system.
- Semiquantitative detection of Ce is performed utilizing photoluminescence. PL emission spectra of coatings formed with addition of CeO2 to electrolyte feature luminescent peak corresponding to Ce3+. The concentration of Ce (observed as intensity of emission PL peak corresponding to Ce3+) increases with increased addition of CeO2 to electrolyte solution.
- Photodecomposition of MO registered for SEZC3 sample is found to be 50% of initial Mo concentration, which is comparable to photodecomposition value of 60% observed for oxide coatings with incorporated Ce-loaded 13X zeolite processed for 10 min under the same PEO conditions. However, the sample SEZC1 with chemical composition similar to chemical composition of PEO coatings with incorporated Ce-loaded 13X zeolite has photocatalytic activity which is 1.5 times lower.
- Although co-deposition of 13X zeolite and CeO2 is a viable method for producing photocatalytic coatings, it requires higher concentration of CeO2 in electrolyte to achieve similar values of photocatalytic decomposition as in the case of PEO incorporation of Ce-loaded zeolites.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, J.A. Wastewater treatment and reuse for sustainable water resources management: A systematic literature review. Sustainability 2023, 15, 10940. [Google Scholar] [CrossRef]
- Zhang, L.; Xiong, Z.; Li, L.; Burt, R.; Zhao, X.S. Uptake and degradation of Orange 2 by zinc aluminum layered double oxides. J. Colloid Interface Sci. 2016, 469, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wen, W.; Xia, Y.; Wu, J.M. Photocatalytic activity of TiO2 nanorods, nanowires and nanoflowers filled with TiO2 nanoparticles. Thin Solid Film. 2018, 648, 103–107. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Anastasescu, C.; State, R.-N.; Vasile, A.; Papa, F.; Balint, I. Photocatalytic degradation of organic and inorganic pollutants to harmless end products: Assessment of practical application potential for water and air cleaning. Catalysts 2023, 13, 380. [Google Scholar] [CrossRef]
- Reddy, B.M.; Reddy, E.P.; Mehdi, S. Phase transformation study of titania in V2O5TiO2 and MoO3TiO2 catalysts by X-ray diffraction analysis. Mat. Chem. Phys. 1994, 36, 276–281. [Google Scholar] [CrossRef]
- Seyed Dorraji, M.S.; Rasoulifard, M.H.; Daneshvar, H.; Vafa, A.; Amani-Ghadim, A.R. ZnS/ZnNiAl-LDH/GO nanocomposite as a visible-light photocatalyst: Preparation, characterization and modeling. J. Mater. Sci. Mater. Electron. 2019, 30, 12152–12162. [Google Scholar] [CrossRef]
- Lee, D.-E.; Kim, M.-K.; Danish, M.; Jo, W.-K. State-of-the-art review on photocatalysis for efficient wastewater treatment: Attractive approach in photocatalyst design and parameters affecting the photocatalytic degradation. Catal. Commun. 2023, 183, 106764. [Google Scholar] [CrossRef]
- Pansila, P.P.; Witit-Anun, N.; Chaiyakun, S. Effect of oxygen partial pressure on the morphological properties and the photocatalytic activities of titania thin films on unheated substrates by sputtering deposition method. Adv. Mater. Res. 2013, 770, 18–21. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Prog. Mater. Sci. 2021, 117, 100735. [Google Scholar] [CrossRef]
- Lu, X.; Mohedano, M.; Blawert, C.; Matykina, E.; Arrabal, R.; Kainer, K.U.; Zheludkevich, M.L. Plasma electrolytic oxidation coatings with particle additions—A review. Surf. Coat. Technol. 2016, 307, 1165–1182. [Google Scholar] [CrossRef]
- Lu, X.; Blawert, C.; Zheludkevich, M.L.; Kainer, K.U. Insights into plasma electrolytic oxidation treatment with particle addition. Corros. Sci. 2015, 101, 201–207. [Google Scholar] [CrossRef]
- Mojsilović, K.; Božović, N.; Stojanović, S.; Damjanović-Vasilić, L.; Serdechnova, M.; Blawert, C.; Zheludkevich, M.L.; Stojadinović, S.; Vasilić, R. Zeolite-containing photocatalysts immobilized on aluminum support by plasma electrolytic oxidation. Surf. Interfaces 2021, 26, 101307. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Zhao, S.; Serdechnova, M.; Blawert, C.; Wang, H.; Zheludkevich, M.L.; Chen, F. Double-ligand strategy to construct an inhibitor-loaded Zn-MOF and its corrosion protection ability for aluminum alloy 2A12. ACS Appl. Mater. Interfaces 2021, 13, 51685–51694. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Zhao, X.; Zhang, Z.; Yuan, Y.; Kong, W.; Zhang, Y. Duplex coating combining vanadate-intercalated layered double hydroxide and Ce-doped sol-gel layers on aluminum alloy for active corrosion protection. Materials 2023, 16, 775. [Google Scholar] [CrossRef] [PubMed]
- Dondur, V.; Dimitrijević, R.; Kremenović, A.; Damjanović, L.; Kićanović, M.; Cheong, H.M.; Macura, S. Phase transformations of hexacelsians doped with Li, Na and Ca. Mater. Sci. Forum 2005, 494, 107–112. [Google Scholar] [CrossRef]
- Galvão, T.L.P.; Bouali, A.C.; Serdechnova, M.; Yasakau, K.A.; Zheludkevich, M.L.; Tedim, J. Chapter 16—Anticorrosion thin film smart coatings for aluminum alloys. In Advances in Smart Coatings and Thin Films for Future Industrial and Biomedical Engineering Applications; Makhlouf, A.S.H., Abu-Thabit, N.Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 429–454. [Google Scholar]
- Alvarez-Aguiñaga, E.A.; Elizade-Gonzalez, M.; Sabinas-Hernandez, S.A. Unpredicted photocatalytic activity of clinoptilolite-mordenite natural zeolite. RSC Adv. 2020, 10, 39251–39260. [Google Scholar] [CrossRef]
- Latha, P.; Karuthapandian, S. Novel, facile and swift technique for synthesis of CeO2 nanocubes immobilized on zeolite for removal of CR and MO dye. J. Clust. Sci. 2017, 28, 3265–3280. [Google Scholar] [CrossRef]
- Chen, Q.; Lu, X.; Serdechnova, M.; Wang, C.; Lamaka, S.; Blawert, C.; Zheludkevich, M.L.; Wang, F. Formation of self-healing PEO coatings on AM50 Mg by in-situ incorporation of zeolite micro-container. Corros. Sci. 2022, 209, 110785. [Google Scholar] [CrossRef]
- Mojsilović, K.; Božović, N.; Stojanović, S.; Damjanović-Vasilić, L.J.; Serdechnova, M.; Blawert, C.; Zheludkevich, M.L.; Stojadinović, S.; Vasilić, R. Oxide coatings with immobilized Ce-ZSM5 as visible light photocatalysts. J. Serb. Chem. Soc. 2022, 87, 1035–1048. [Google Scholar]
- Al Abri, S.; Rogov, A.; Aliasghari, S.; Bendo, A.; Matthews, A.; Yerokhin, A.; Mingo, B. In-situ incorporation of Ce-zeolite during soft sparking plasma electrolytic oxidation. J. Mater. Res. Technol. 2024, 30, 2365–2376. [Google Scholar] [CrossRef]
- Clyne, T.W.; Troughton, S.C. A review of recent work on discharge characteristics during plasma electrolytic oxidation of various metals. Int. Mater. Rev. 2019, 64, 127–162. [Google Scholar] [CrossRef]
- Stojadinović, S.; Vasilić, R. Photoluminescence of Ce3+ and Ce3+/Tb3+ ions in Al2O3 host formed by plasma electrolytic oxidation. J. Lumin. 2018, 203, 576–581. [Google Scholar] [CrossRef]
- Mojsilović, K.; Lačnjevac, U.; Stojanović, S.; Damjanović-Vasilić, L.J.; Stojadinović, S.; Vasilić, R. Formation and properties of oxide coatings with immobilized zeolites obtained by plasma electrolytic oxidation of aluminum. Metals 2021, 11, 1241. [Google Scholar] [CrossRef]
- Akhtar, F.; Bergstro, L. Colloidal Processing and Thermal Treatment of Binderless Hierarchically Porous Zeolite 13X Monoliths for CO2 Capture. J. Am. Ceram. Soc. 2011, 94, 92–98. [Google Scholar] [CrossRef]
- Kosmulski, M. The pH dependent surface charging and points of zero charge. X. Update. Adv. Colloid Interface Sci. 2023, 319, 102973. [Google Scholar] [CrossRef] [PubMed]
- Arrabal, R.; Mohedano, M.; Matykina, E.; Pardo, A.; Mingo, B.; Merino, M.C. Characterization and wear beahviour of PEO coatings on 6082-T6 aluminium alloy with incorporated α-Al2O3 particles. Surf. Coat. Technol. 2015, 269, 64–73. [Google Scholar] [CrossRef]
- Latha, P.; Prakash, K.; Karuthapandian, S. Enhanced visible light photocatalytic activity of CeO2/alumina nanocomposite: Synthesized via facile mixing-calcination method for dye degradation. Adv. Powder Technol. 2017, 28, 2903–2913. [Google Scholar] [CrossRef]
- Sridharan, M.; Maiyalagan, T. Synergistically enhanced electrocatalytic activity of cerium oxide/manganese tungstate composite for oxygen reduction reaction. J. Mater. Sci. Mater. Electron. 2022, 33, 9538–9548. [Google Scholar] [CrossRef]
- Manojkumar, P.; Lokeshkumar, E.; Premchand, C.; Saikiran, A.; Rama Krishna, L.; Rameshbabu, N. Facile preparation of immobilized visible light active W-TiO2/rGO composite photocatalyst by plasma electrolytic oxidation process. Phys. B Condens. Matter 2022, 632, 413680. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.; Byeon, S.-H. Ce4+/Ce3+ redox-controlled luminescence ‘on/off’ switching of highly oriented Ce(OH)2Cl and Tb-doped Ce(OH)2 Cl films. J. Mater. Chem. C 2017, 5, 444–451. [Google Scholar] [CrossRef]
- Berwal, U.; Singh, V.; Sharma, R. Effect of Ce4+→Ce3+ conversion on the structural and luminescence properties of Ce4+ doped Gd2Ti2O7 pyrochlore oxide. J. Lumin. 2023, 257, 119687. [Google Scholar] [CrossRef]
- Stojadinović, S.; Radić, N.; Tadić, N.; Vasilić, R.; Grbić, B. Enhanced ultraviolet light driven photocatalytic activity of ZnO particles incorporated by plasma electrolytic oxidation into Al2O3 coatings co-doped with Ce. Opt. Mater. 2020, 101, 109768. [Google Scholar] [CrossRef]
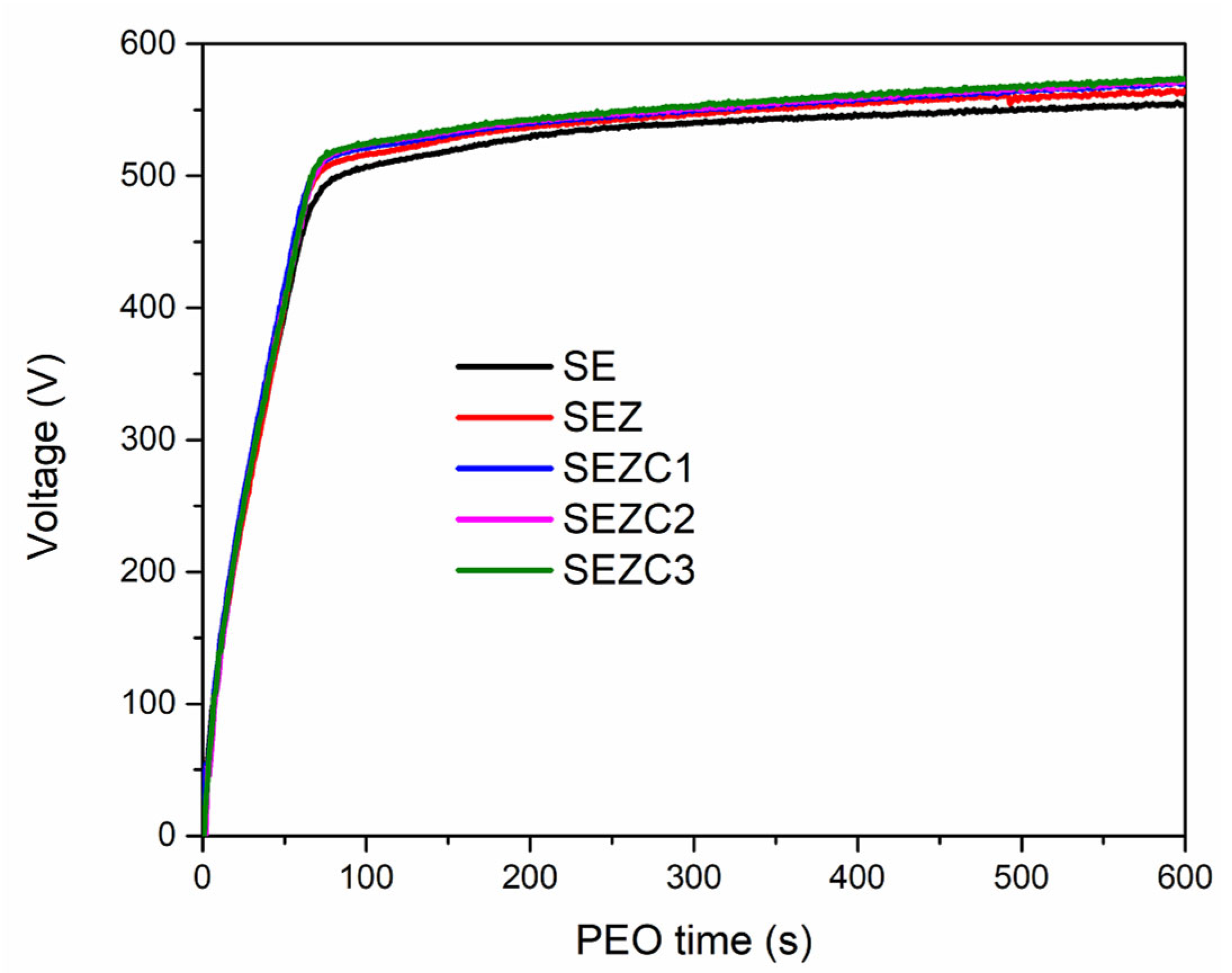
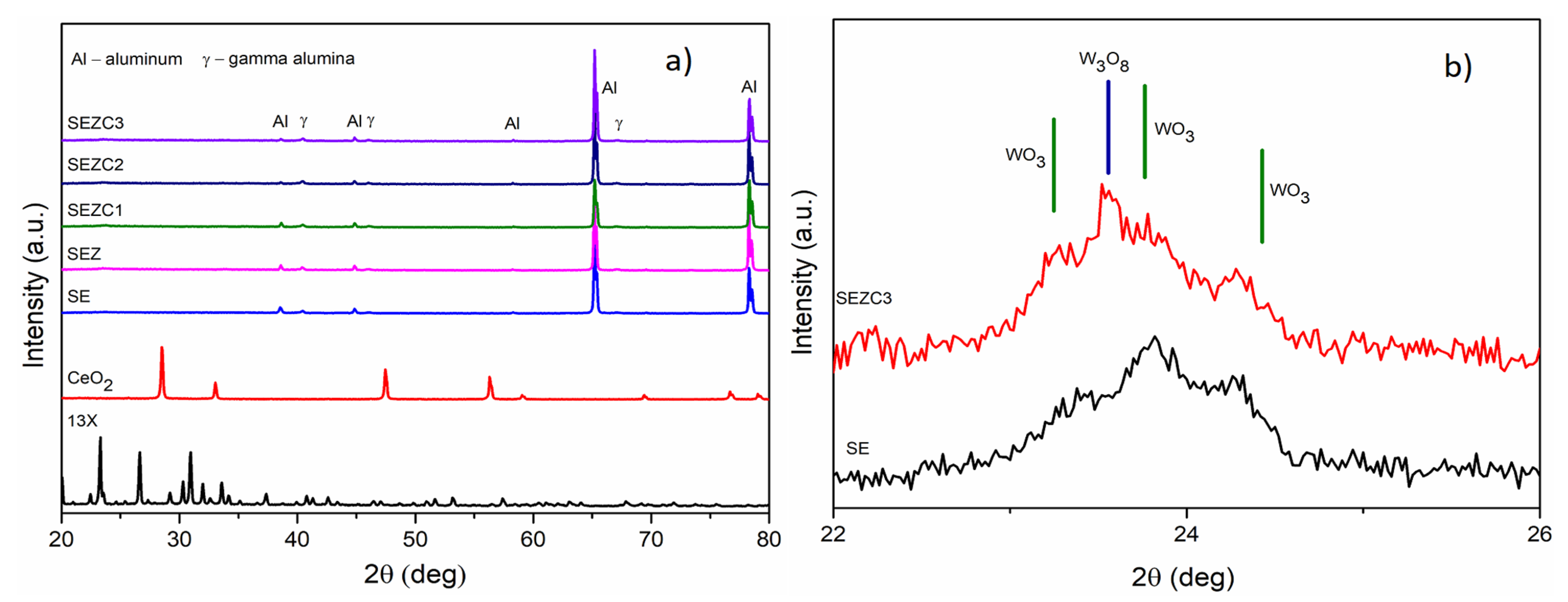

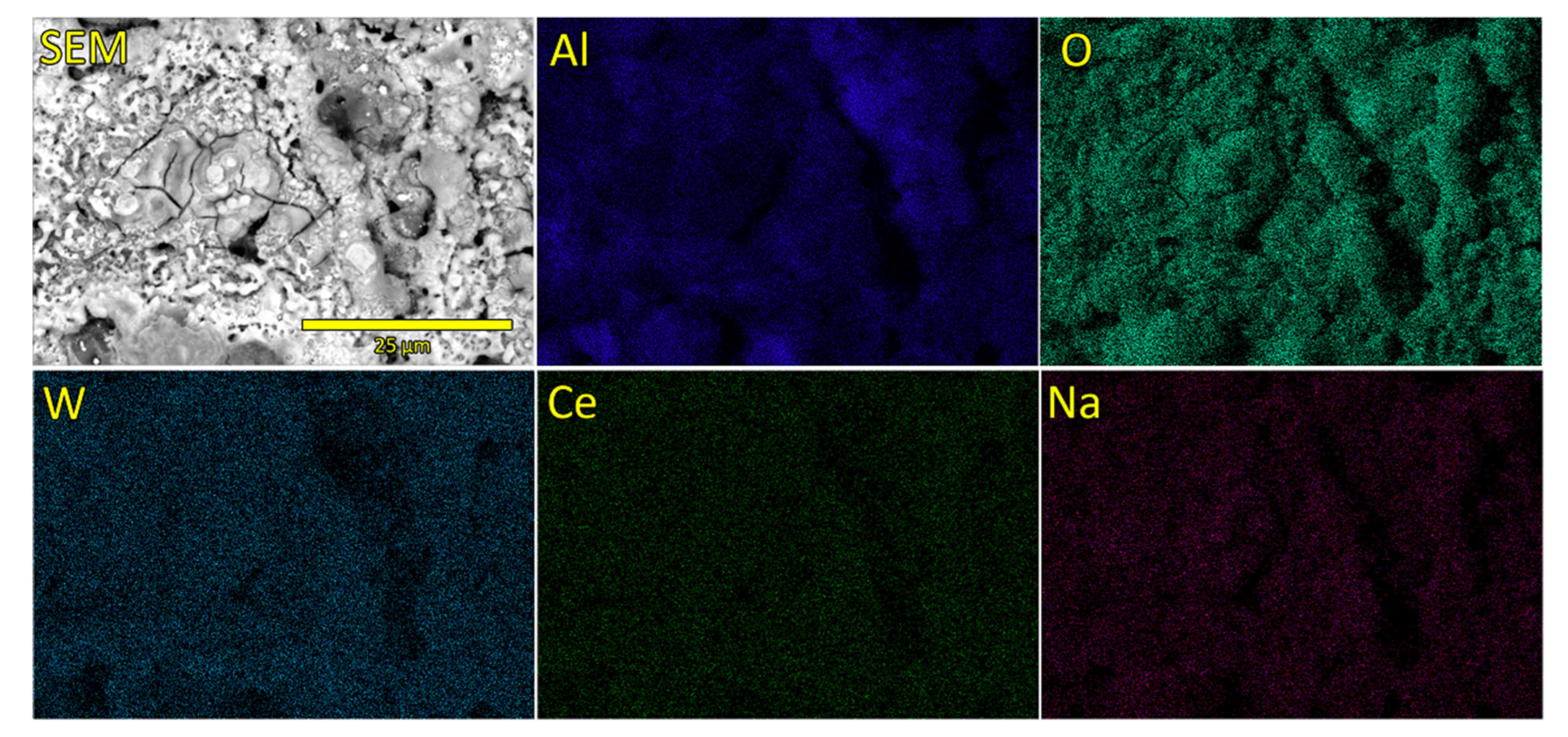

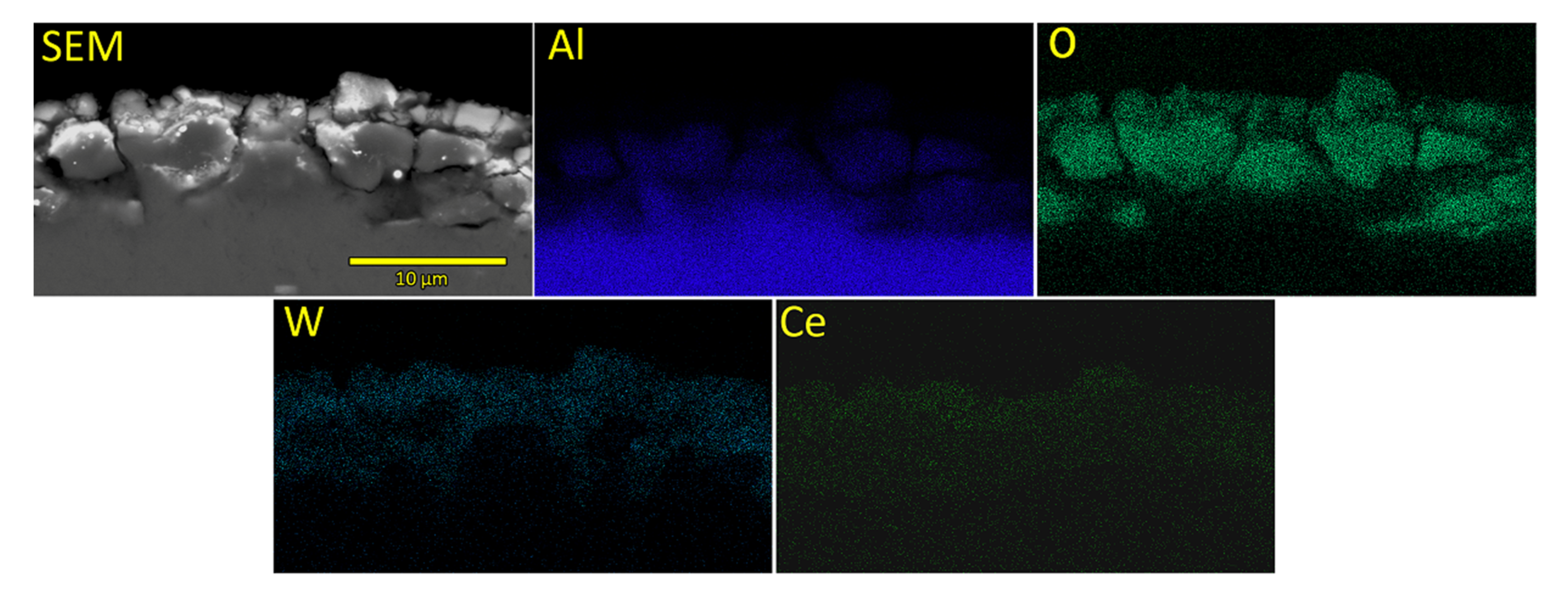
| Sample Name | Electrolyte | pH | Conductivity (mS) |
|---|---|---|---|
| SE | 0.01 M Na2WO4∙2H2O | 7.30 | 2.04 |
| SEZ | SE + 1 g/L 13X | 7.05 | 2.08 |
| SEZC1 | SE + 1 g/L 13X + 0.25 g/L CeO2 | 7.04 | 2.07 |
| SEZC2 | SE + 1 g/L 13X + 0.5 g/L CeO2 | 7.05 | 2.08 |
| SEZC3 | SE + 1 g/L 13X + 1 g/L CeO2 | 7.06 | 2.07 |
| Sample Name | Composition (wt%) | |||||
|---|---|---|---|---|---|---|
| O | Na | Al | W | Si | Ce | |
| SE | 32.24 | 0.61 | 24.57 | 38.65 | 3.93 | - |
| SEZ | 32.34 | 0.51 | 17.85 | 45.19 | 4.11 | - |
| SEZC1 | 35.85 | 0.35 | 19.36 | 40.43 | 4.01 | - |
| SEZC2 | 30.65 | 0.56 | 19.47 | 46.08 | 3.24 | - |
| SEZC3 | 31.33 | 0.32 | 19.52 | 47.73 | 0.94 | 0.16 |
| Sample | Roughness (μm) | Porosity (%) | Thickness (µm) |
|---|---|---|---|
| SE | 1.45 | 11.45 | 2.7 |
| SEZ | 1.91 | 10.75 | 4.1 |
| SEZC1 | 1.94 | 8.29 | 6.2 |
| SEZC2 | 1.95 | 7.34 | 7.3 |
| SEZC3 | 1.97 | 7.15 | 7.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mojsilović, K.; Tadić, N.; Stojanović, S.; Damjanović-Vasilić, L.; Vasilić, R. Facile Preparation of Composite Coatings with Incorporated 13X Zeolite and CeO2. Coatings 2024, 14, 1516. https://doi.org/10.3390/coatings14121516
Mojsilović K, Tadić N, Stojanović S, Damjanović-Vasilić L, Vasilić R. Facile Preparation of Composite Coatings with Incorporated 13X Zeolite and CeO2. Coatings. 2024; 14(12):1516. https://doi.org/10.3390/coatings14121516
Chicago/Turabian StyleMojsilović, Kristina, Nenad Tadić, Srna Stojanović, Ljiljana Damjanović-Vasilić, and Rastko Vasilić. 2024. "Facile Preparation of Composite Coatings with Incorporated 13X Zeolite and CeO2" Coatings 14, no. 12: 1516. https://doi.org/10.3390/coatings14121516
APA StyleMojsilović, K., Tadić, N., Stojanović, S., Damjanović-Vasilić, L., & Vasilić, R. (2024). Facile Preparation of Composite Coatings with Incorporated 13X Zeolite and CeO2. Coatings, 14(12), 1516. https://doi.org/10.3390/coatings14121516








