Sodium Alginate–Montmorillonite Composite Film Coatings for Strawberry Preservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation Process
2.1.1. Preparation of Composite Films
2.1.2. Strawberry Preservation
2.2. Testing Method
2.2.1. Mechanical Properties
2.2.2. Permeability
2.2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.4. Weight Loss
2.2.5. MDA Content
2.2.6. Respiratory Intensity
2.2.7. Titratable Acid Content
2.2.8. Soluble Sugar Content
2.2.9. Total Phenol Content
2.2.10. Soluble Solid Content
2.2.11. Data Processing
3. Results
3.1. Film Properties
3.1.1. Mechanical Properties
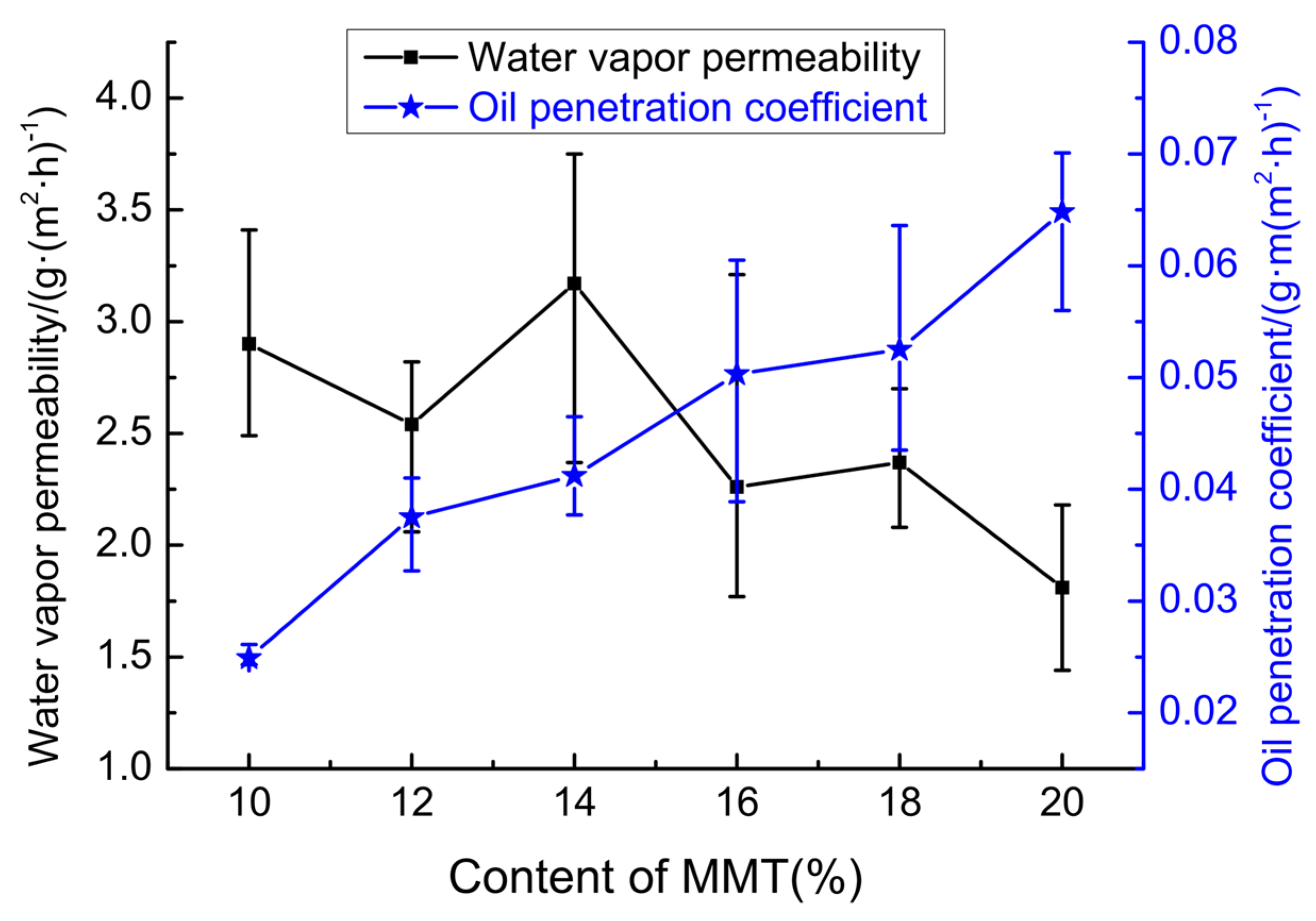
3.1.2. Permeabilities
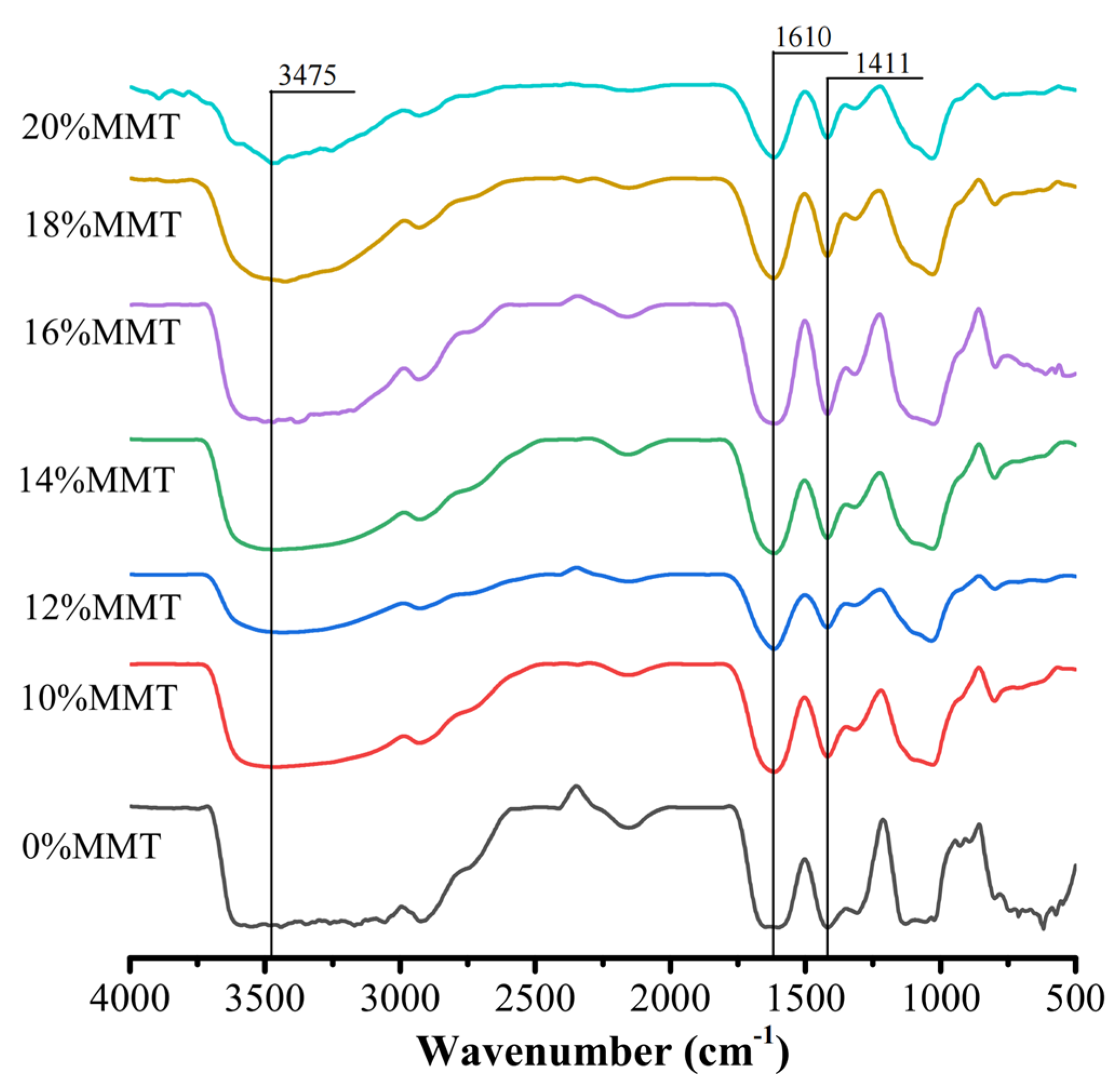
3.1.3. FTIR
3.2. Strawberry Preservation Analysis
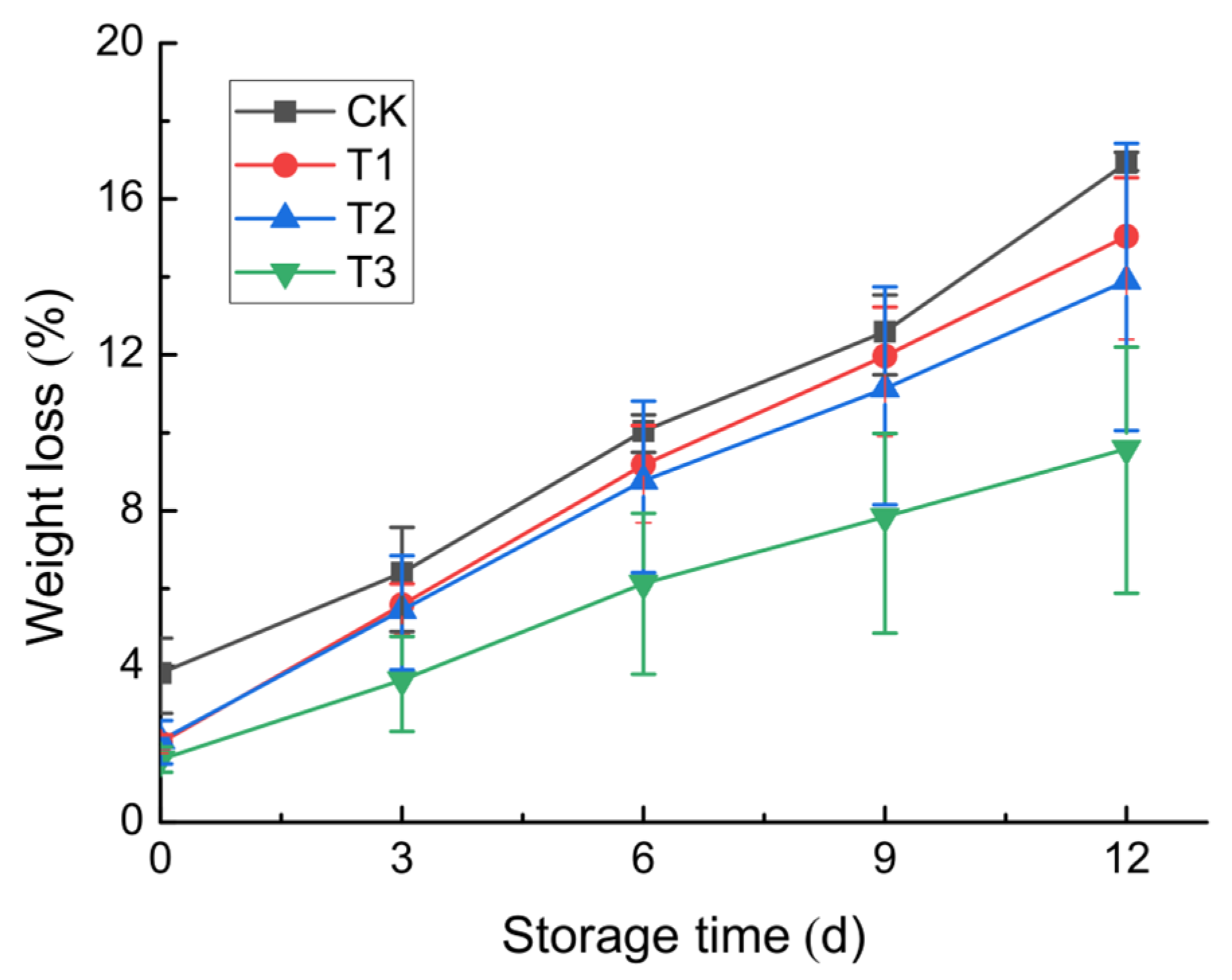
3.2.1. Weight Loss
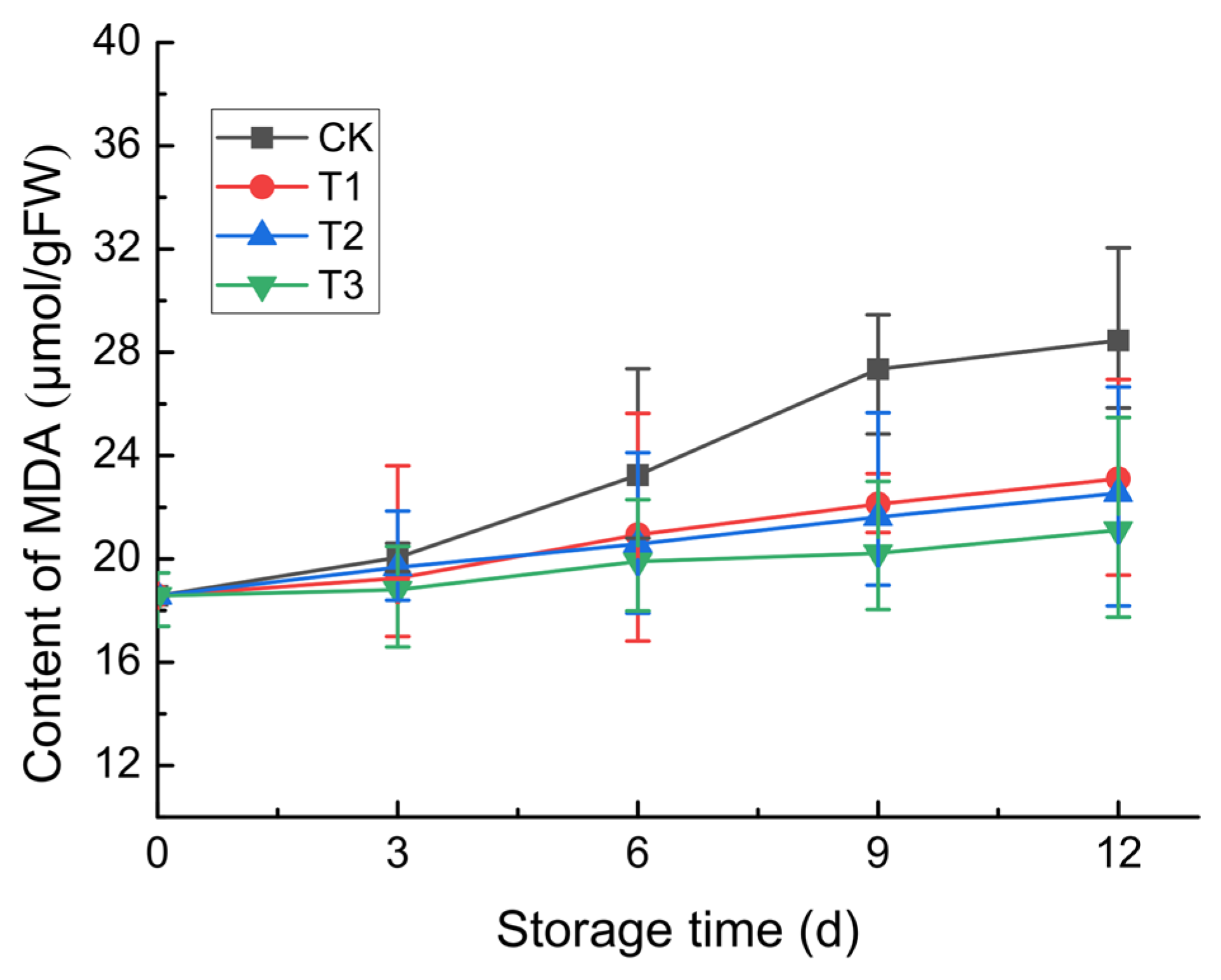
3.2.2. MDA Content
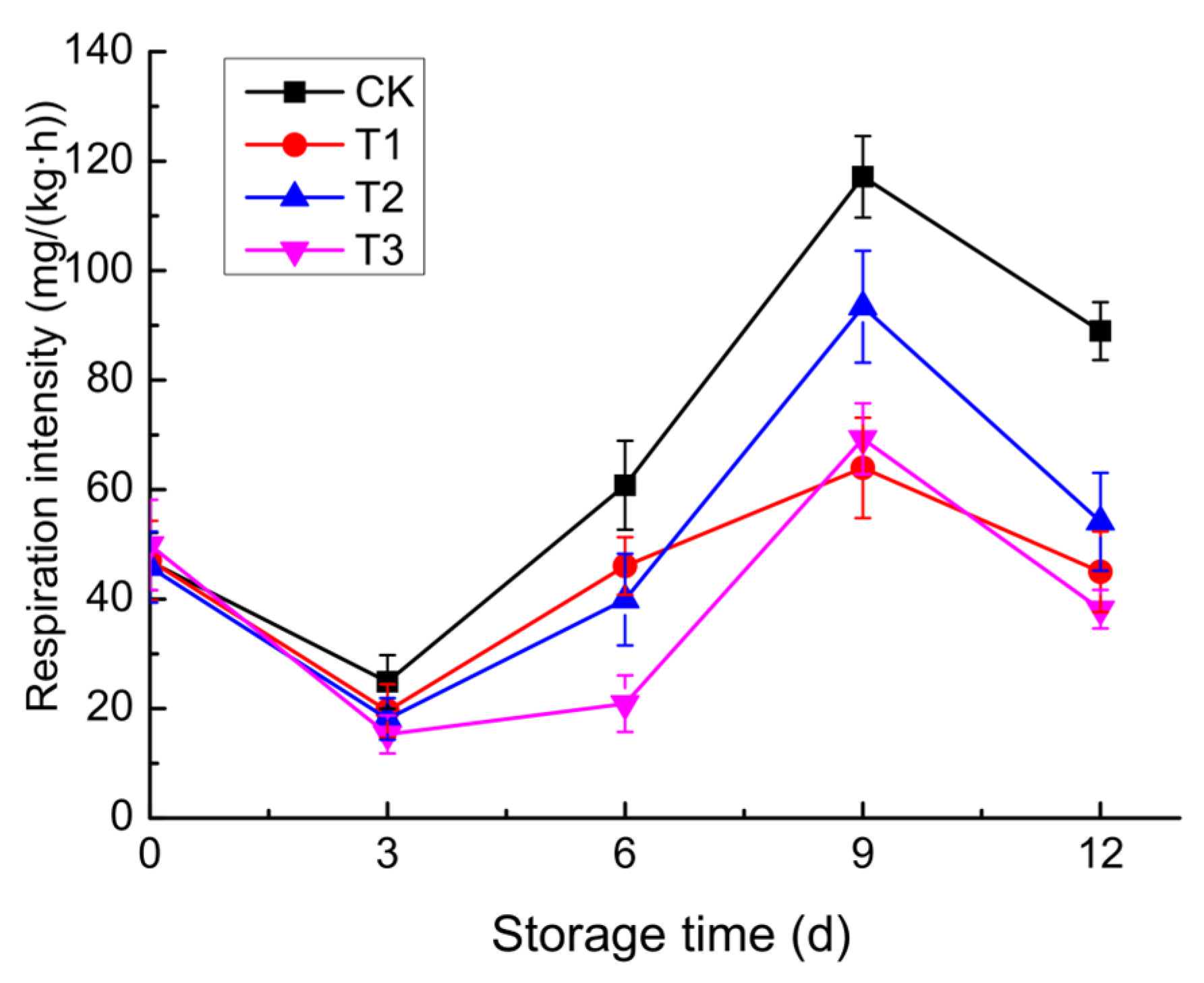
3.2.3. Respiratory Intensity
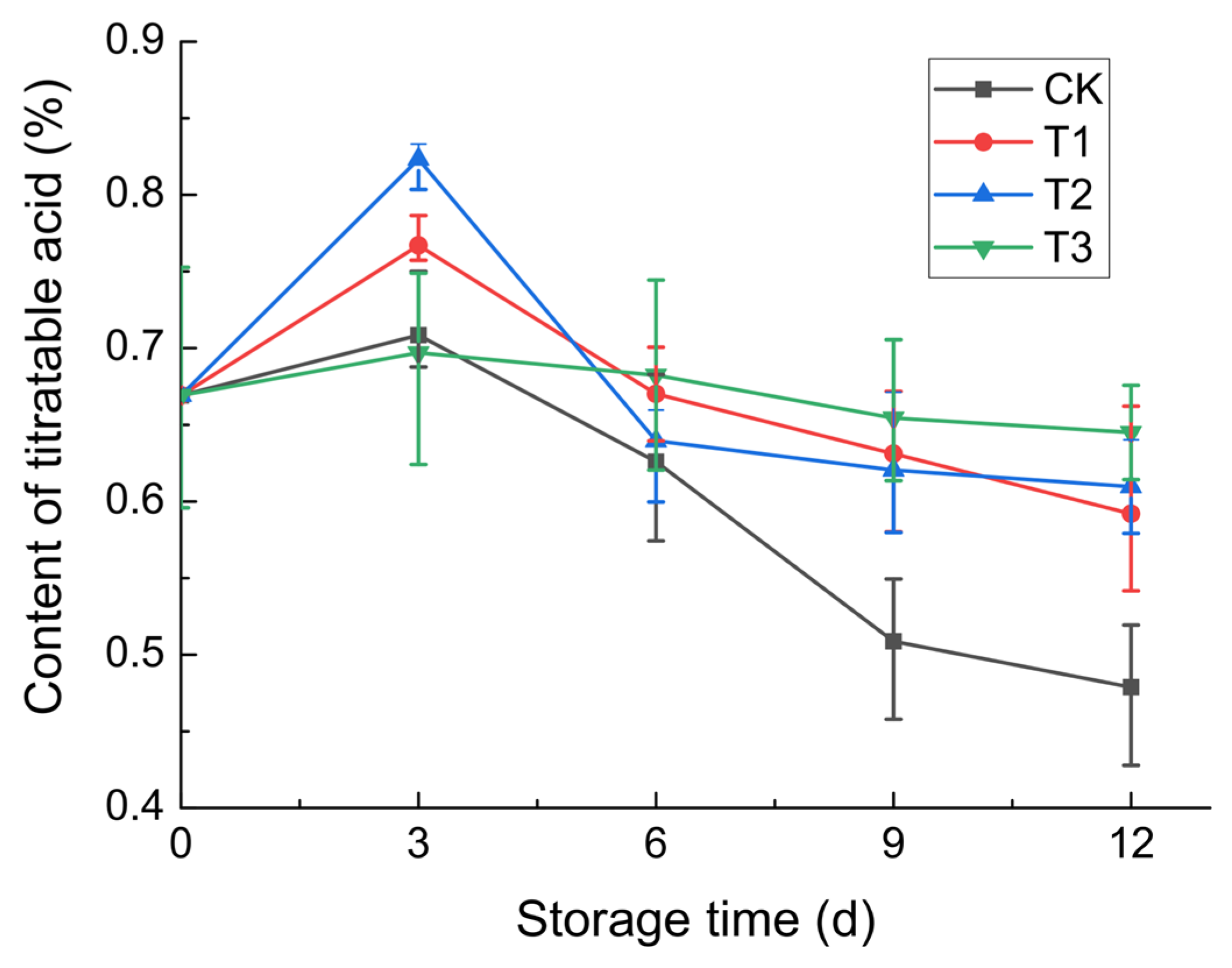
3.2.4. Titratable Acid Content
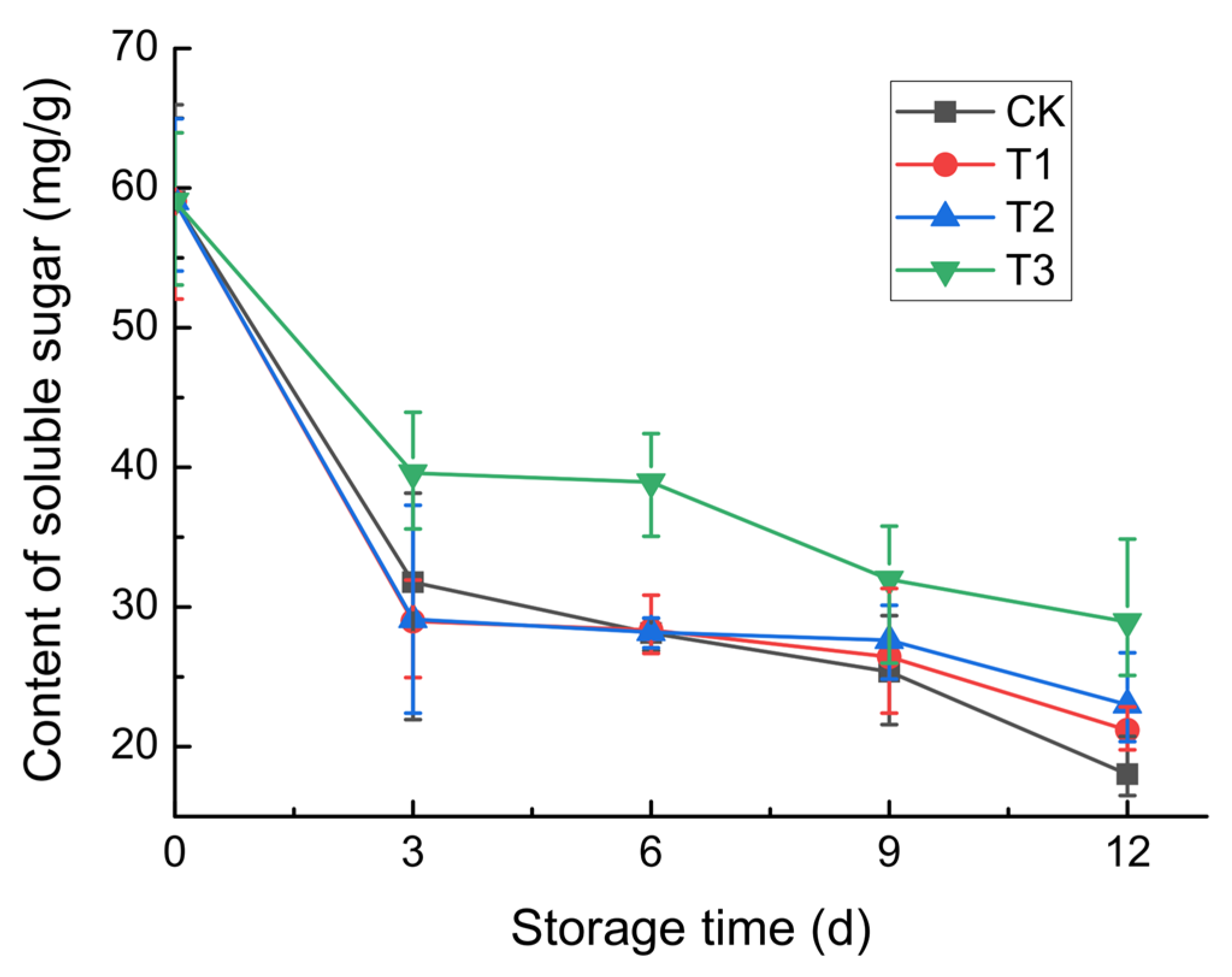
3.2.5. Soluble Sugar Content
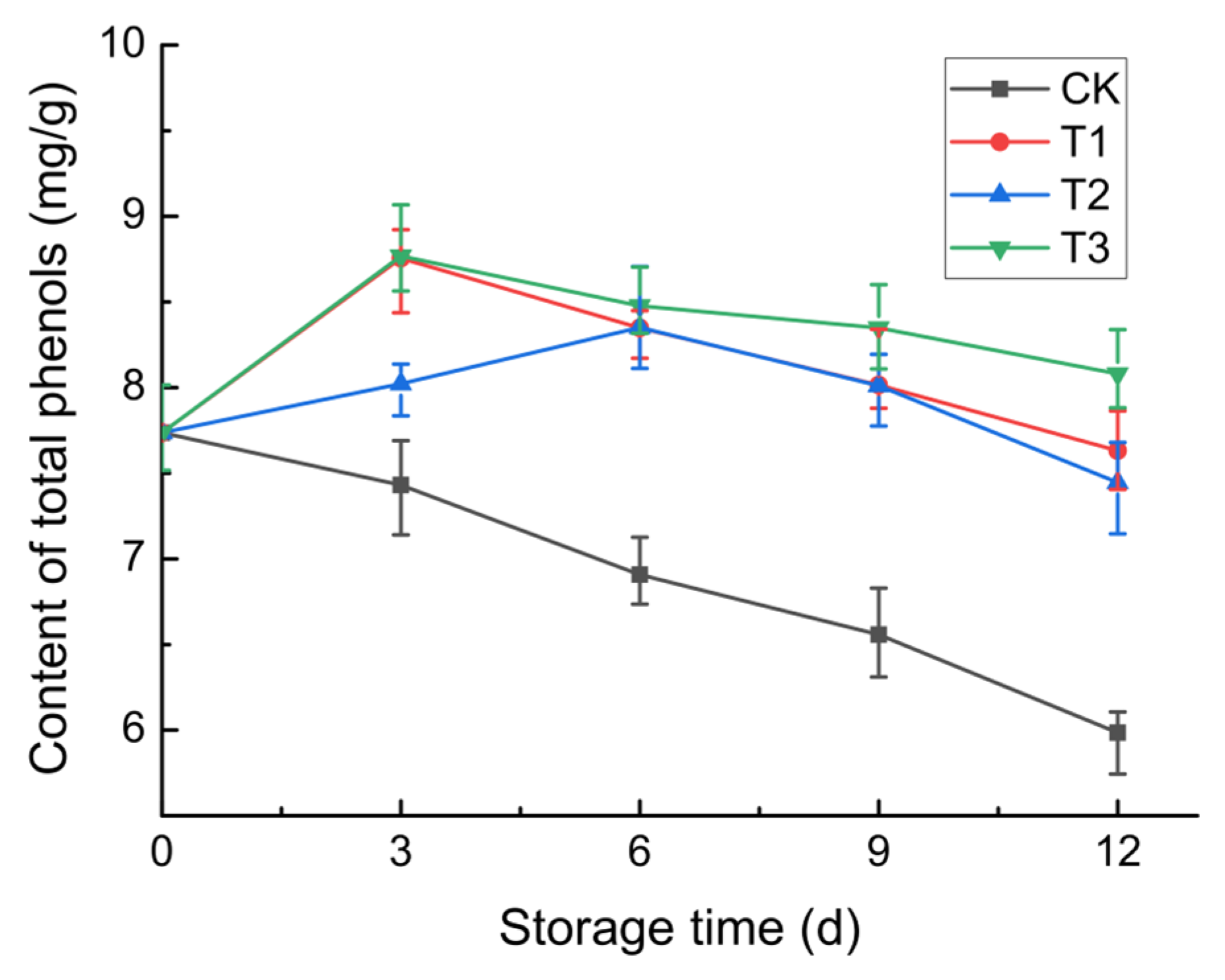
3.2.6. Total Phenol Content
3.2.7. Soluble Solid Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruvinov, E.; Cohen, S. Alginate biomaterial for the treatment of myocardial infarction: Progress, translational strategies, and clinical outlook From ocean algae to patient bedside. Adv. Drug Deliver. Rev. 2016, 96, 54–76. [Google Scholar] [CrossRef]
- Hu, C.H.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y.P. Ions-induced gelation of alginate: Mechanisms and applications. Int. J. Biol. Macromol. 2021, 177, 578–588. [Google Scholar] [CrossRef]
- Zeng, Y.B.; Wang, Y.; Tang, J.H.; Zhang, H.T.; Dai, J.W.; Li, S.Q.; Yan, J.; Qin, W.; Liu, Y.W. Preparation of sodium alginate/konjac glucomannan active films containing lycopene microcapsules and the effects of these films on sweet cherry preservation. Int. J. Biol. Macromol. 2022, 215, 67–78. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Zhao, W.X.; Lin, Z.H.; Tang, Z.F.; Lin, B.F. Carboxymethyl chitosan/sodium alginate hydrogel films with good biocompatibility and reproducibility by in situ ultra-fast crosslinking for efficient preservation of strawberry. Carbohyd. Polym. 2023, 316, 121073. [Google Scholar] [CrossRef]
- Duong, N.T.C.; Uthairatanakij, A.; Laohakunjit, N.; Jitareerat, P.; Kaisangsri, N. Cross-linked alginate edible coatings incorporated with hexyl acetate: Film characteristics and its application on fresh-cut rose apple. Food Biosci. 2023, 52, 102410. [Google Scholar] [CrossRef]
- Montone, A.M.I.; Malvano, F.; Taiano, R.; Capparelli, R.; Capuano, F.; Albanese, D. Alginate coating charged by hydroxyapatite complexes with lactoferrin and quercetin enhances the pork meat shelf life. Foods 2023, 12, 553. [Google Scholar] [CrossRef]
- Nie, X.B.; Wang, L.H.; Wang, Q.; Lei, J.L.; Hong, W.S.; Huang, B.S.; Zhang, C.F. Effect of a sodium alginate coating infused with tea polyphenols on the quality of fresh Japanese sea bass (Lateolabrax japonicas) fillets. J. Food Sci. 2018, 83, 1695–1700. [Google Scholar] [CrossRef]
- Yan, P.L.; Lan, W.Q.; Xie, J. Modification on sodium alginate for food preservation: A review. Trends Food Sci. Tech. 2024, 143, 104217. [Google Scholar] [CrossRef]
- Nair, M.S.; Tomar, M.; Punia, S.; Kukula-Koch, W.; Kumar, M. Enhancing the functionality of chitosan- and alginate-based active edible coatings/films for the preservation of fruits and vegetables: A review. Int. J. Biol. Macromol. 2020, 164, 304–320. [Google Scholar] [CrossRef]
- Greathouse, J.A.; Cygan, R.T. Molecular dynamics simulation of uranyl(vi) adsorption equilibria onto an external montmorillonite surface. Phys. Chem. Chem. Phys. 2005, 7, 3580–3586. [Google Scholar] [CrossRef]
- Cao, S.; Liu, C.; Zhou, L.; Zhang, H.C.; Zhao, Y.X.; Liu, Z.A. Bioapplication of cyclodextrin-containing montmorillonite. J. Mater. Chem. B 2021, 9, 9241–9261. [Google Scholar] [CrossRef]
- Yang, L.L.; Zhang, Y.W.; Ao, T.; Jiang, G.C.; Liu, H.Q.; Huang, L.S.; Song, W.Y.; Wang, X.X.; Wang, A.J. Bead and necklace-like silica-based hybrid materials for controlling montmorillonite dispersion filtration. Colloid. Surface. A 2023, 668, 131457. [Google Scholar] [CrossRef]
- Wu, L.; He, X.; Zhao, Y.F.; Huang, K.L.; Tong, Z.F.; Liao, B.; Pang, H. Montmorillonite-based materials for electrochemical energy storage. Green Chem. 2024, 26, 678–704. [Google Scholar] [CrossRef]
- Bhat, A.H.; Rangreez, T.A.; Inamuddin; Chisti, H.T.N. Wastewater Treatment and Biomedical Applications of Montmorillonite Based Nanocomposites: A Review. Curr. Anal. Chem. 2022, 18, 269–287. [Google Scholar] [CrossRef]
- Al Kausor, M.; Sen Gupta, S.; Bhattacharyya, K.G.; Chakrabortty, D. Montmorillonite and modified montmorillonite as adsorbents for removal of water soluble organic dyes: A review on current status of the art. Inorg. Chem. Commun. 2022, 143, 109686. [Google Scholar] [CrossRef]
- Jayrajsinh, S.; Shankar, G.; Agrawal, Y.K.; Bakre, L. Montmorillonite nanoclay as a multifaceted drug-delivery carrier: A review. J. Drug. Deliv. Sci. Tec. 2017, 39, 200–209. [Google Scholar] [CrossRef]
- Ungureanu, C.; Tihan, G.; Zgârian, R.; Pandelea, G. Bio-coatings for preservation of fresh fruits and vegetables. Coatings 2023, 13, 1420. [Google Scholar] [CrossRef]
- Yu, D.W.; Regenstein, J.M.; Xia, W.S. Bio-based edible coatings for the preservation of fishery products: A Review. Crit. Rev. Food Sci. 2019, 59, 2481–2493. [Google Scholar] [CrossRef]
- Qian, Y.; Huang, Z.; Zhou, G.; Chen, C.; Sang, Y.; Yu, Z.; Jiang, L.; Mei, Y.; Wei, Y. Preparation and properties of organically modified Na-montmorillonite. Materials 2023, 16, 3184. [Google Scholar] [CrossRef]
- Wei, Y.X.; Huang, Z.E.; Yu, Z.L.; Han, C.; Yang, C.R. Preparation and properties of fractionated soybean protein isolate films. Materials 2021, 14, 5436. [Google Scholar] [CrossRef]
- Mirzaei-Mohkam, A.; Garavand, F.; Dehnad, D.; Keramat, J.; Nasirpour, A. Physical, mechanical, thermal and structural characteristics of nanoencapsulated vitamin E loaded carboxymethyl cellulose films. Prog. Org. Coat. 2020, 138, 105383. [Google Scholar] [CrossRef]
- Yu, Z.L.; Wei, Y.X.; Fu, C.C.; Sablani, S.S.; Huang, Z.E.; Han, C.; Li, D.N.; Sun, Z.J.; Qin, H.Z. Antimicrobial activity of gamma-poly (glutamic acid), a preservative coating for cherries. Colloid. Surface. B 2023, 225, 113272. [Google Scholar] [CrossRef]
- Zhang, L.M.; Liu, Z.L.; Sun, Y.; Wang, X.M.; Li, L. Combined antioxidant and sensory effects of active chitosan/zein film containing a-tocopherol on Agaricus bisporus. Food Packag. Shelf 2020, 24, 100470. [Google Scholar] [CrossRef]
- Xu, B.; Wu, S.J. Preservation of mango fruit quality using fucoidan coatings. LWT Food Sci. Technol. 2021, 143, 111150. [Google Scholar] [CrossRef]
- GB/T 12456-2008; Determination of Total Acid in Food. Standards Press of China: Beijing, China, 2008.
- Zhu, D.; Guo, R.; Li, W.X.; Song, J.Y.; Cheng, F.S. Improved postharvest preservation effects of pholiota nameko mushroom by sodium alginate-based edible composite coating. Food Bioprocess Technol. 2019, 12, 587–598. [Google Scholar] [CrossRef]
- Lagnika, C.; Zhang, M.; Mothibe, K.J. Effects of ultrasound and high pressure argon on physico-chemical properties of white mushrooms (Agaricus bisporus) during postharvest storage. Postharvest Bio. Technol. 2013, 82, 87–94. [Google Scholar] [CrossRef]
- Cissé, M.; Polidori, J.; Montet, D.; Loiseau, G.; Ducamp-Collin, M.N. Preservation of mango quality by using functional chitosan-lactoperoxidase systems coatings. Postharvest Bio. Technol. 2015, 101, 10–14. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Yin, H.; Zhou, X.F.; Wang, D.F.; Zhong, Y.; Xia, Q.; Deng, Y.; Zhao, Y.Y. Antimicrobial, antioxidant and physical properties of chitosan film containing Akebia trifoliata (Thunb.) Koidz. peel extract/montmorillonite and its application. Food Chem. 2021, 361, 130111. [Google Scholar] [CrossRef]
- Das, D.; Panesar, P.S.; Saini, C.S. Effect of montmorillonite (MMT) on the properties of soybean meal protein isolate-based nanocomposite film loaded with debittered kinnow peel powder. Food Res. Int. 2024, 185, 114292. [Google Scholar] [CrossRef]
- Keshavarz, M.; Alizadeh, P. On the role of alginate coating on the mechanical and biological properties of 58S bioactive glass scaffolds. Int. J. Biol. Macromol. 2021, 167, 947–961. [Google Scholar] [CrossRef]
- Comaposada, J.; Gou, P.; Marcos, B.; Arnau, J. Physical properties of sodium alginate solutions and edible wet calcium alginate coatings. LWT Food Sci. Technol. 2015, 64, 212–219. [Google Scholar] [CrossRef]
- Sothornvit, R.; Hong, S.I.; An, D.J.; Rhim, J.W. Effect of clay content on the physical and antimicrobial properties of whey protein isolate/organo-clay composite films. LWT-Food Sci. Technol. 2010, 43, 279–284. [Google Scholar] [CrossRef]
- Olivo, P.M.; Scapim, M.R.D.; Miazaki, J.; Madrona, G.S.; Maia, L.F.; Rodrigues, B.M.; Pozza, M.S.D. Sodium alginate with turmeric coating for ripened cheeses. J. Food Sci. Technol. Mys. 2020, 57, 2364–2369. [Google Scholar] [CrossRef]
- Li, B.; Li, J.; Huang, S.W.; Qin, S.H.; Liu, N. Design of novel polylactide composite films with improved gas barrier and mechanical properties using epoxy chain extender-grafted organic montmorillonite. J. Polym. Sci. 2023, 61, 1572–1583. [Google Scholar] [CrossRef]
- Tayeb, A.H.; Tajvidi, M. Sustainable barrier system via self-assembly of colloidal montmorillonite and cross-linking resins on nanocellulose interfaces. ACS Appl. Mater. Interfaces 2019, 11, 1604–1615. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.J.; Zhao, J.C.; Guo, Y.; Zhu, P.; Wang, D.Y. Bio-based barium alginate film: Preparation, flame retardancy and thermal degradation behavior. Carbohyd. Polym. 2016, 139, 106–114. [Google Scholar] [CrossRef]
- Karimi, A.; Daud, W. Comparison the properties of PVA/Na+-MMT nanocomposite hydrogels prepared by physical and physicochemical crosslinking. Polym. Composite. 2016, 37, 897–906. [Google Scholar] [CrossRef]
- Ying, T.T.; Wu, P.J.; Gao, L.L.; Wang, C.C.; Zhang, T.H.; Liu, S.S.; Huang, R.A. Isolation and characterization of a new strain of Bacillus amyloliquefaciens and its effect on strawberry preservation. LWT Food Sci. Technol. 2022, 165, 113712. [Google Scholar] [CrossRef]
- Peretto, G.; Du, W.X.; Avena-Bustillos, R.J.; Berrios, J.D.; Sambo, P.; McHugh, T.H. Electrostatic and conventional spraying of alginate-based edible coating with natural antimicrobials for preserving fresh strawberry quality. Food Bioprocess Technol. 2017, 10, 165–174. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Bioch. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Yu, L.; Hu, Y.W.; Zhu, Z.C.; Zhuang, C.J.; Zhao, Y.Y.; Zhong, Y. he preservation performance of chitosan coating with different molecular weight on strawberry using electrostatic spraying technique. Int. J. Biol. Macromol. 2020, 151, 278–285. [Google Scholar] [CrossRef]
- Zhang, X.H.; Xu, J.; Wang, M.Z.; Gan, C.Y.; Ren, Y.; Zhao, X.Q.; Yuan, Z.H. Riboflavin application regulates sugar and energy metabolism in strawberries during cold storage. LWT Food Sci. Technol. 2023, 187, 115301. [Google Scholar] [CrossRef]
- Miele, N.A.; Volpe, S.; Torrieri, E.; Cavella, S. Improving physical properties of sodium caseinate based coating with the optimal formulation: Effect on strawberries’ respiration and transpiration rates. J. Food Eng. 2022, 331, 111123. [Google Scholar] [CrossRef]
- Cortez-Vega, W.R.; Pizato, S.; de Souza, J.T.A.; Prentice, C. Using edible coatings from Whitemouth croaker (Micropogonias furnieri) protein isolate and organo-clay nanocomposite for improve the conservation properties of fresh-cut ‘Formosa’ papaya. Innov. Food Sci. Emerg. 2014, 22, 197–202. [Google Scholar] [CrossRef]
- Deng, P.P.; Zhang, Y.X.; Deng, Q.X.; Sun, Y.F.; Li, Y.W.; Wang, Z.H.; Jiang, F.T. Multifunctional sodium alginate-based self-healing edible cross-linked coating for banana preservation. Food Hydrocolloid. 2024, 151, 109753. [Google Scholar] [CrossRef]
- Gautam, A.; Gill, P.P.S.; Singh, N.P.; Jawandha, S.K.; Arora, R.; Singh, A. Composite coating of xanthan gum with sodium nitroprusside alleviates the quality deterioration in strawberry fruit. Food Hydrocolloid. 2024, 155, 110208. [Google Scholar] [CrossRef]
- Hajji, S.; Younes, I.; Affes, S.; Boufi, S.; Nasri, M. Optimization of the formulation of chitosan edible coatings supplemented with carotenoproteins and their use for extending strawberries postharvest life. Food Hydrocolloid. 2018, 83, 375–392. [Google Scholar] [CrossRef]
- Chiabrando, V.; Giacalone, G. Quality evaluation of blueberries coated with chitosan and sodium alginate during postharvest storage. Int. Food Res. J. 2017, 24, 1553–1561. [Google Scholar]
- Wu, X.Y.; Han, W.; Yang, Z.Q.; Zhang, Y.X.; Zheng, Y.J. The difference in temperature between day and night affects the strawberry soluble sugar content by influencing the photosynthesis, respiration and sucrose phosphatase synthase. Hortic. Sci. 2021, 48, 174–182. [Google Scholar] [CrossRef]
- Omer, T.A.; Majeed, S.N.; Farkha, T.K.J.; Abdullah, S.N. Determination of total phenol, antioxidant and antimicrobial activity of beetroot and strawberry in sulaimani city-kurdistan region, Iraq. Egypt. J. Chem. 2022, 65, 583–593. [Google Scholar] [CrossRef]
- Rastegar, S.; Khankahdani, H.H.; Rahimzadeh, M. Effectiveness of alginate coating on antioxidant enzymes and biochemical changes during storage of mango fruit. J. Food Biochem. 2019, 43, e12990. [Google Scholar] [CrossRef]
- Robles-Flores, G.D.; Abud-Archila, M.; Ventura-Canseco, L.M.C.; Meza-Gordillo, R.; Grajales-Lagunes, A.; Ruiz-Cabrera, M.A.; Gutiérrez-Miceli, F.A. Development and evaluation of a film and edible coating obtained from the Cajanus cajan seed applied to fresh strawberry fruit. Food Bioprocess Technol. 2018, 11, 2172–2181. [Google Scholar] [CrossRef]
- Falcó, I.; Flores-Meraz, P.L.; Randazzo, W.; Sánchez, G.; López-Rubio, A.; Fabra, M.J. Antiviral activity of alginate-oleic acid based coatings incorporating green tea extract on strawberries and raspberries. Food Hydrocolloid. 2019, 87, 611–618. [Google Scholar] [CrossRef]
- Antunes, G.V.; Sodré, G.M.; Radünz, M.; Rosa, R.S.D.; Rodrigues, A.; Gularte, M.A.; Gandra, T.K.V.; Gandra, E.A. Bioactive coating of sodium alginate and agar added with essential oils of thyme (Thymus vulgaris L.) and sweet orange (Citrus aurantium var. dulcis) with antimicrobial properties applied over strawberries. Rev. Chil. Nutr. 2023, 50, 281–290. [Google Scholar] [CrossRef]
- Alharaty, G.; Ramaswamy, H.S. The effect of sodium alginate-calcium chloride coating on the quality parameters and shelf life of strawberry cut fruits. J. Compos. Sci. 2020, 4, 123. [Google Scholar] [CrossRef]
- Emamifar, A.; Bavaisi, S. Nanocomposite coating based on sodium alginate and nano-ZnO for extending the storage life of fresh strawberries (Fragaria x ananassa Duch.). J. Food Meas. Charact. 2020, 14, 1012–1024. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Yu, Z.; Chen, Y.; Han, C.; Wei, Y.; Yang, F.; Qian, Y.; Wang, Y. Sodium Alginate–Montmorillonite Composite Film Coatings for Strawberry Preservation. Coatings 2024, 14, 1331. https://doi.org/10.3390/coatings14101331
Yan X, Yu Z, Chen Y, Han C, Wei Y, Yang F, Qian Y, Wang Y. Sodium Alginate–Montmorillonite Composite Film Coatings for Strawberry Preservation. Coatings. 2024; 14(10):1331. https://doi.org/10.3390/coatings14101331
Chicago/Turabian StyleYan, Xiaoping, Zuolong Yu, Yao Chen, Chao Han, Yunxiao Wei, Fan Yang, Yan Qian, and Yong Wang. 2024. "Sodium Alginate–Montmorillonite Composite Film Coatings for Strawberry Preservation" Coatings 14, no. 10: 1331. https://doi.org/10.3390/coatings14101331
APA StyleYan, X., Yu, Z., Chen, Y., Han, C., Wei, Y., Yang, F., Qian, Y., & Wang, Y. (2024). Sodium Alginate–Montmorillonite Composite Film Coatings for Strawberry Preservation. Coatings, 14(10), 1331. https://doi.org/10.3390/coatings14101331





