Enhancing Magnesium Phosphate Cement Paste for Efficient Fluoride Adsorption
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Preparation
2.3. Analysis Method
2.4. Adsorption Study
3. Results and Discussion
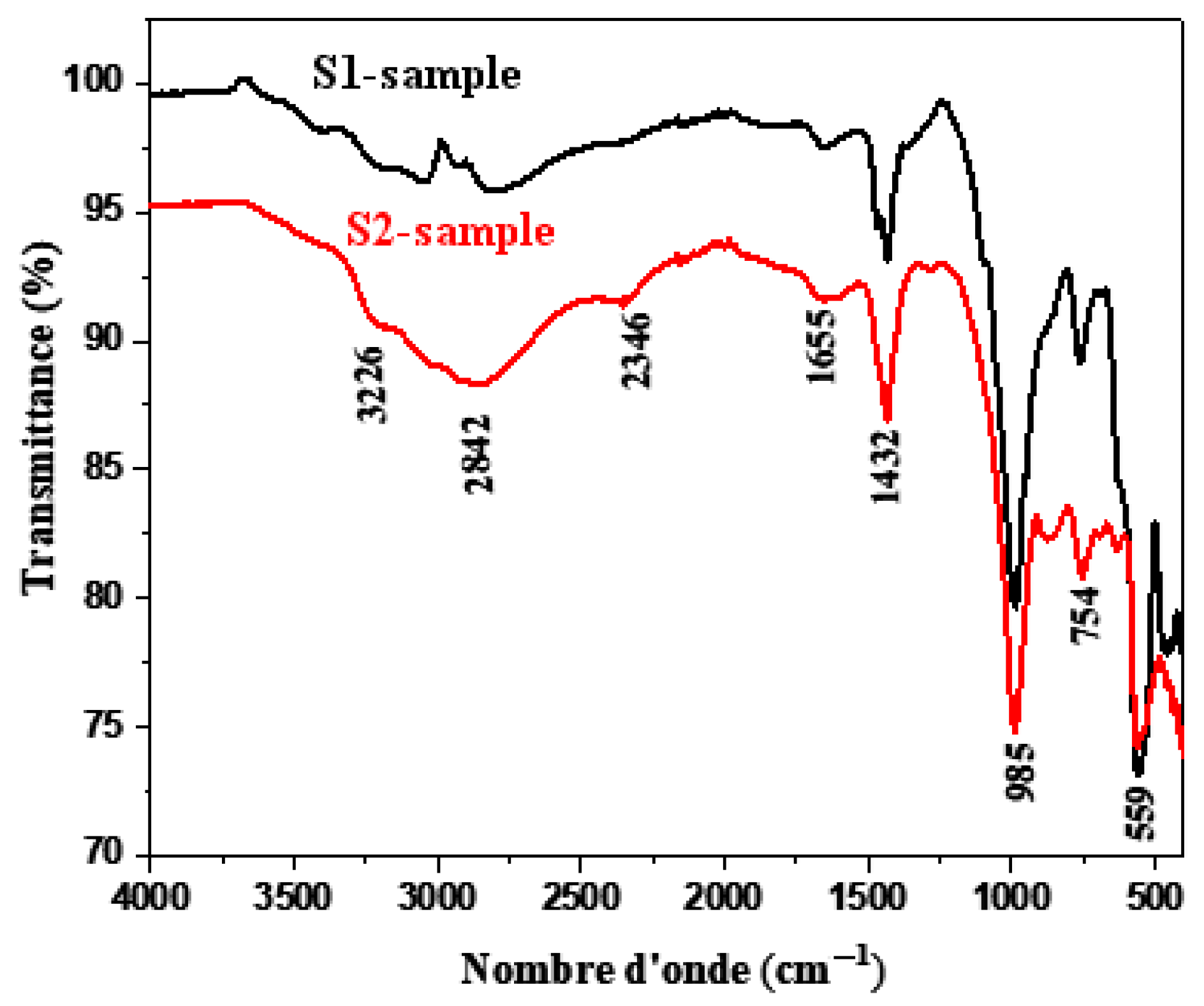
3.1. Composites Analyses
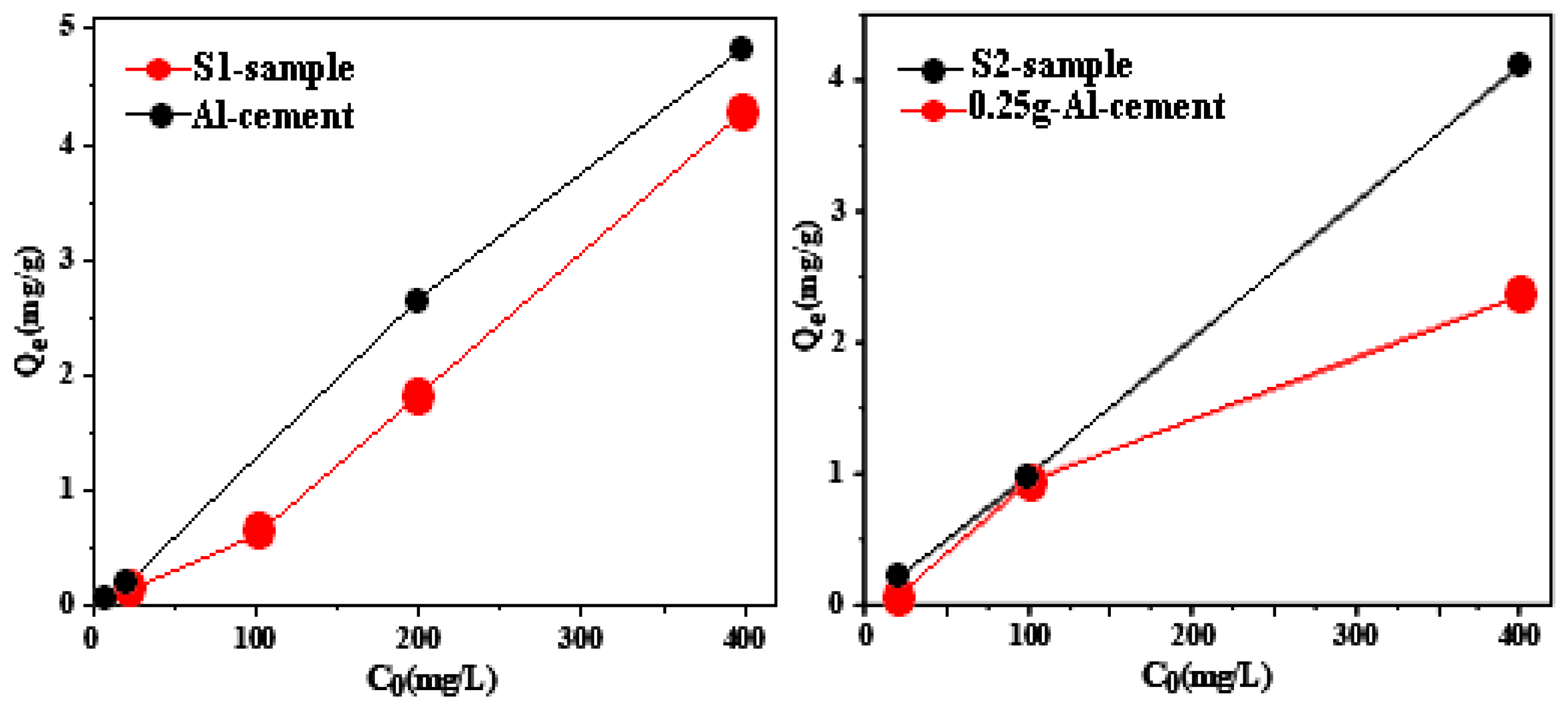
3.2. Adsorption Study
3.3. Comparison with Other Used Adsorbents
4. Conclusions
- The addition of iron oxide, aluminum, and alumina significantly enhances the strength of the cement matrix, improving its resistance to water and dry conditions even after several days of direct contact. Notably, the addition of alumina has the additional benefit of reducing the exothermicity of the reaction, improving the cement’s setting time.
- The addition of aluminum or alumina to the cement matrix enables an improvement in the specific surface area of the material.
- The highest adsorption capacity for fluoride observed was 4.84 mg/g, achieved with the material synthesized using 1.5 g of aluminum.
- Incorporating iron oxide into a matrix containing 1.5 g of aluminum slightly decreases the adsorbed quantity, shifting from 4.84 mg/g to 4.29 mg/g.
- An alumina addition markedly improves adsorption, especially for the composite initially containing only 0.25 g of aluminum, increasing from 2.35 mg/g to 4.10 mg/g with the addition of a mere 0.5 g of alumina.
- The Langmuir isotherm aptly describes the adsorption process, indicating monolayer adsorption, where adsorbate molecules form a single layer on the adsorbent surface. This model assumes a homogeneous surface with a fixed number of identical adsorption sites and does not account for interactions between adsorbate molecules. Additionally, the calculated separation factor, RL, further confirms the favorable nature of the adsorption process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schweitzer, L.; Noblet, J. Chapter 3.6—Water Contamination and Pollution. Green Chem. 2018, 261–290. [Google Scholar] [CrossRef]
- Ben Mbarek, W.; Pineda, E.; Escoda, L.; Suñol, J.J.; Khitouni, M. High-efficiency decolorization of azo dye Reactive Black 5 by Ca-Al particles. J. Environ. Chem. Eng. 2017, 5, 6107–6113. [Google Scholar] [CrossRef]
- Hafshejani, L.D.; Tangsir, S.E.; Daneshvar, M.; Maljanen, A.; Lähde, J.; Jokiniemi, M.; Naushad, M.; Bhatnagar, A. Optimization of fluoride removal from aqueous solution by Al2O3 nanoparticles. J. Mol. Liq. 2017, 238, 254–262. [Google Scholar] [CrossRef]
- Borgohain, X.; Boruah, A.; Sarma, G.K.; Rashid, M.H. Rapid and extremely high adsorption performance of porous MgO nanostructures for fluoride removal from water. J. Mol. Liq. 2020, 305, 112799. [Google Scholar] [CrossRef]
- Karkar, S.; Debnath, S.; De, P.; Parashar, K.; Pillay, K.; Sashikumar, P.; Ghosh, U.C. Preparation, characterization and evaluation of fluoride adsorption efficiency from water of iron-aluminium oxide-graphene oxide composite material. Chem. Eng. J. 2016, 306, 269–279. [Google Scholar] [CrossRef]
- Gharsallah, S.; Alsawi, A.; Hammami, B.; Khitouni, M.; Charnay, C.; Chemingui, M. Synthesis and Characterization of New Composite Materials Based on Magnesium Phosphate Cement for Fluoride Retention. Materials 2023, 16, 718. [Google Scholar] [CrossRef] [PubMed]
- Mondal, P.; Purkait, M.K. Preparation and characterization of novel green synthesized iron aluminum nanocomposite and studying its efficiency in fluoride removal. Chemosphere 2019, 235, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Gasparotto, J.M.; Roth, D.; Perilli, D.O.; Franco, D.S.P.; Carissimi, E.; Foletto, E.L.; Jahn, S.L.; Dotto, G.L. A novel Fe-Al-La trioxide composite: Synthesis, characterization, and application for fluoride ions removal from the water supply. J. Environ. Chem. Eng. 2021, 9, 106350. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, M.; Li, Y.; Chen, Z.; Yang, H.; Wang, X. Advanced porous materials and emerging technologies for radionuclides removal from Fukushima radioactive water. Eco-Environ. Health 2023, 2, 252–256. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, G.; Hou, D.; Wang, Z. Fluoride adsorption properties of three modified forms of activated alumina in drinking water. J. Water Health 2014, 12, 715–721. [Google Scholar] [CrossRef]
- Gharsallah, S.; Mallah, A.; Alsawi, A.; Hammami, B.; Khitouni, M.; Charnay, C.; Chemingui, M. Study of Modified Magnesium Phosphate Cement for Fluoride Removal. Materials 2023, 16, 5749. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, G.; Hou, D.; Wang, Z. Nanoscale insight on the initial hydration mechanism of magnesium phosphate cement. Constr. Build. Mater. 2021, 276, 122213. [Google Scholar] [CrossRef]
- Ribeiro, D.V.; Morelli, M.R. Influence of the addition of grinding dust to a magnesium phosphate cement matrix. Constr. Build. Mater. 2009, 23, 3094–3102. [Google Scholar] [CrossRef]
- Le Rouzic, M.; Chaussadent, T.; Stefan, L.; Saillio, M. On the influence of Mg/P ratio on the properties and durability of magnesium potassium phosphate cement pastes. Cem. Concr. Res. 2017, 96, 27–41. [Google Scholar] [CrossRef]
- Tansel, B.; Lunn, G.; Monje, O. Struvite formation and decomposition characteristics for ammonia and phosphorus recovery: A review of magnesium-ammonia-phosphate interactions. Chemosphere 2018, 194, 504–514. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, X.; Ma, R.; Sun, S.; Fang, L.; Lin, J.; Luo, J. Solid waste-based magnesium phosphate cements: Preparation, performance and solidification/stabilization mechanism. Constr. Build. Mater. 2021, 297, 123761. [Google Scholar] [CrossRef]
- Lu, X.; Chen, B. Experimental study of magnesium phosphate cements modified by metakaolin. Constr. Build. Mater. 2016, 123, 719–726. [Google Scholar] [CrossRef]
- Bouaoun, I.; Hammi, H.; M’nif, A. Box-Behnken design optimization of magnesium potassium phosphate cement properties using sodium chloride as retarder. J. Tunis. Chem. Soc. 2016, 18, 152–159. [Google Scholar]
- Limousin, G.; Gaudet, J.P.; Charlet, L.; Szenknect, S.; Barthès, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Ding, Z.; Dong, B.; Xing, F.; Han, N.; Li, Z. Cementing mechanism of potassium phosphate based magnesium phosphate cement. Ceram. Int. 2012, 38, 6281–6288. [Google Scholar] [CrossRef]
- Sotiriadis, K.; Mácová, P.; Mazur, A.S.; Tolstoy, P.M.; Viani, A. A solid state NMR and in-situ infrared spectroscopy study on the setting reaction of magnesium sodium phosphate cement. J. Non-Cryst. Solids 2018, 498, 49–59. [Google Scholar] [CrossRef]
- Spirovski, F.; Kuzmanovski, I.; Lutz, H.D.; Engelen, B. Infrared and Raman spectra of magnesium ammonium phosphate hexahydrate (struvite) and its isomorphous analogs. I. Spectra of protiated and partially deuterated magnesium potassium phosphate hexahydrate. Mol. Struct. 2004, 689, 110. [Google Scholar] [CrossRef]
- Chu, Y.; Khan, M.A.; Zhu, S.; Xia, M.; Lei, W.; Wang, F.; Xu, Y. Microstructural modification of organo-montmorillonite with Gemini surfactant containing four ammonium cations: Molecular dynamics (MD) simulations and adsorption capacity for copper ions. J. Chem. Technol. Biotechnol. 2019, 94, 3585–3594. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Zhu, S.; Khan, M.A.; Wang, F.; Bano, Z.; Xia, M. Exploration of adsorption mechanism of 2-phosphonobutane-1,2,4-tricarboxylic acid onto kaolinite and montmorillonite via batch experiment and theoretical studies. J. Hazard. Mater. 2021, 403, 123810. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, M.; Jain, N.; Maiti, A. A comparative adsorption kinetic modeling of fluoride adsorption by nanoparticles and its polymeric nanocomposite. J. Environ. Chem. Eng. 2021, 9, 105595. [Google Scholar] [CrossRef]
- Shyamal, D.S.; Ghosh, P.K. Efficiency of Portland Pozzolana Cement as an adsorbent in removing excess fluoride from groundwater. Groundw. Sustain. Dev. 2019, 9, 100248. [Google Scholar] [CrossRef]
- Fan, X.; Parker, D.J.; Smith, M.D. Adsorption kinetics of fluoride on low-cost materials. Water Res. 2003, 37, 4929–4937. [Google Scholar] [CrossRef]
- Mondal, P.; George, S. Removal of Fluoride from Drinking Water Using Novel Adsorbent Magnesia-Hydroxyapatite. Water. Air. Soil Pollut. 2015, 226, 241. [Google Scholar] [CrossRef]
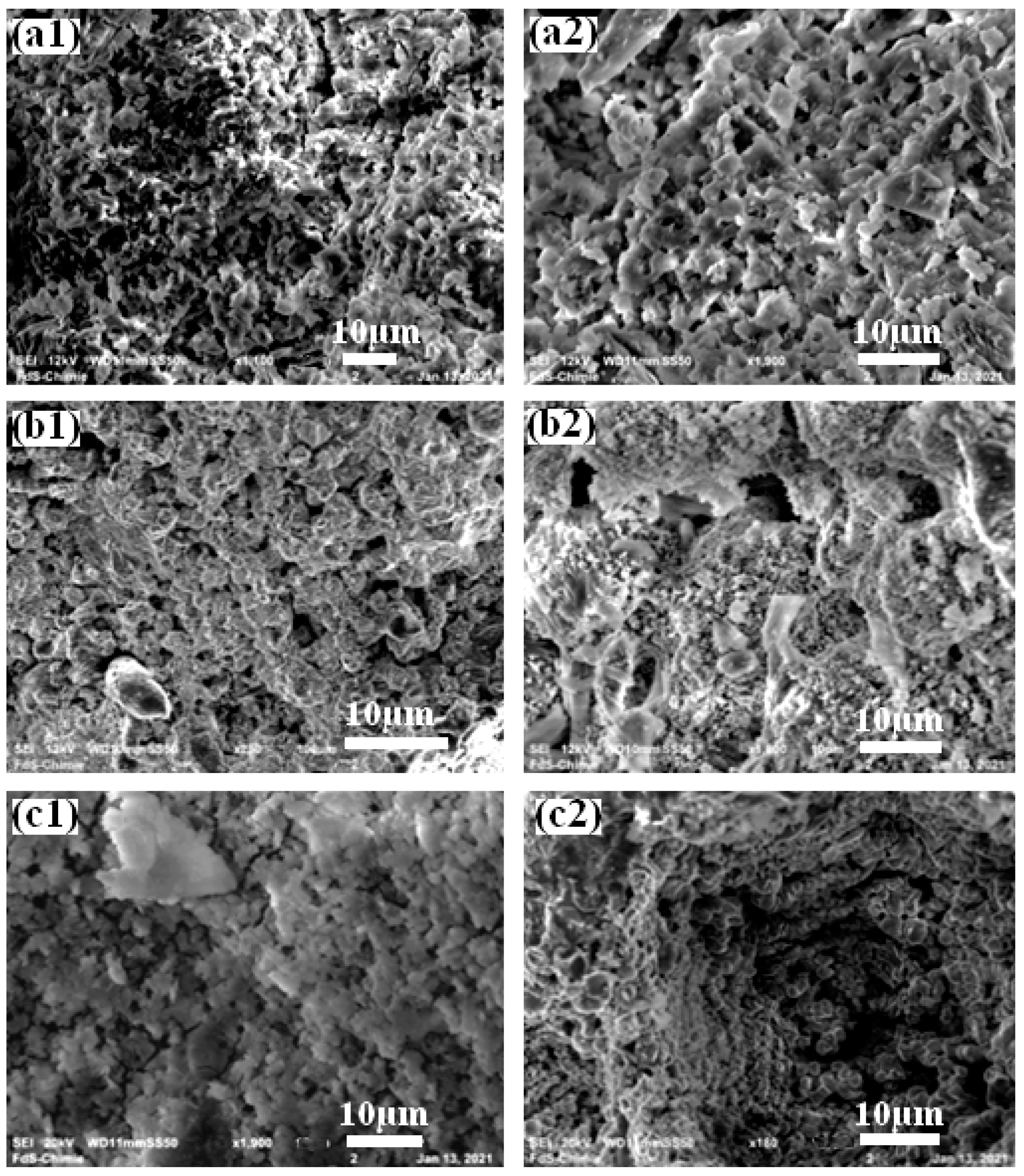

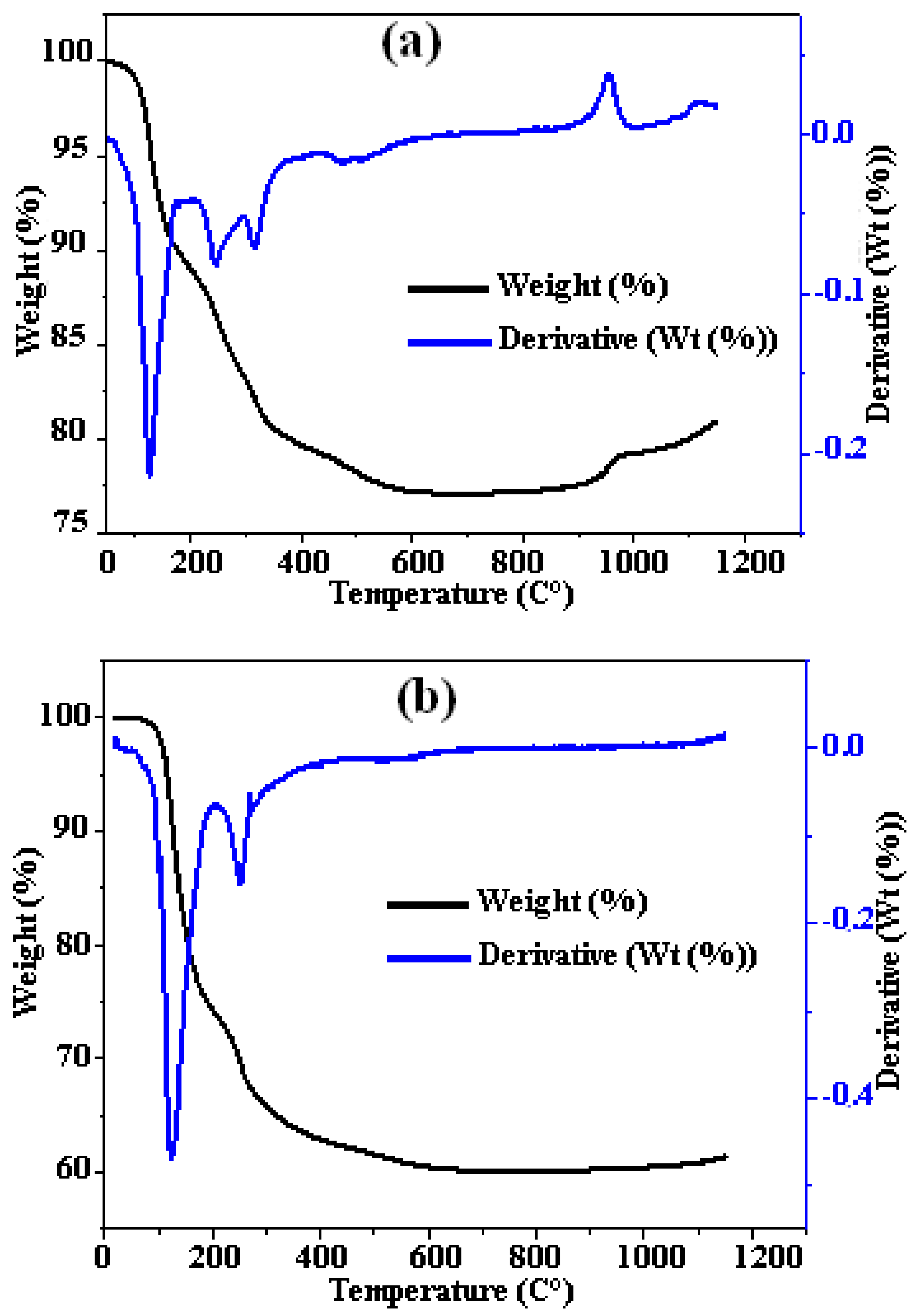
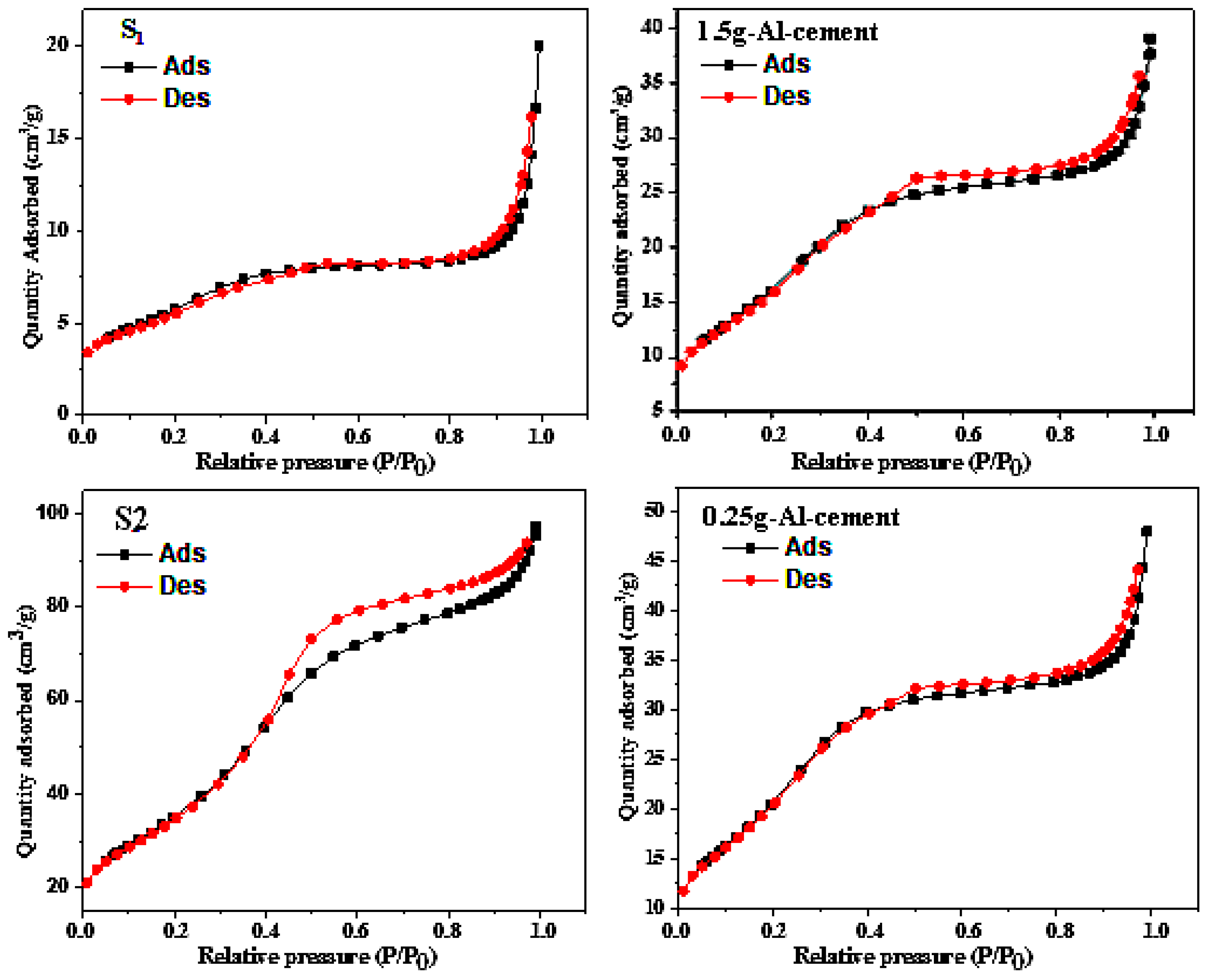
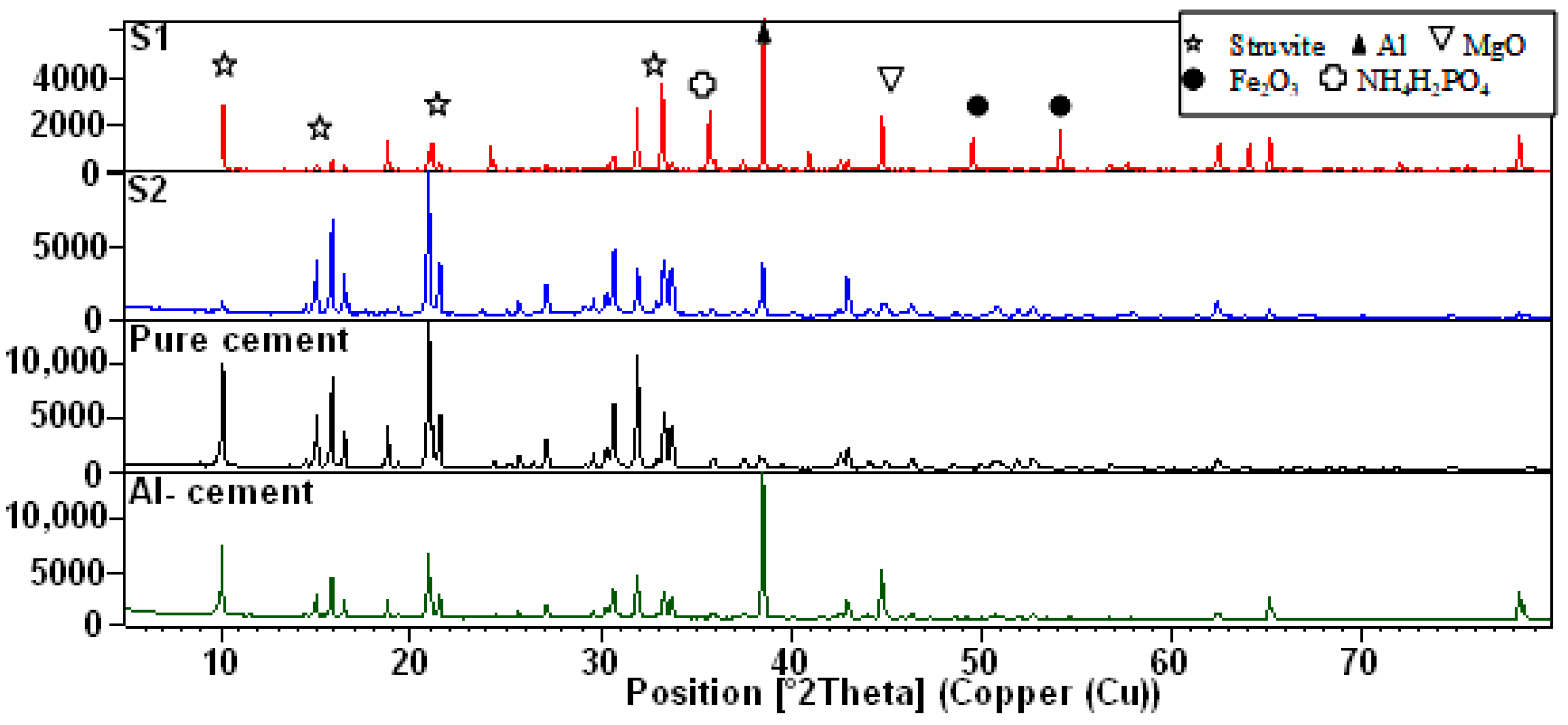

| MgO (g) | NH4H2PO4 (g) | Borax (g) | Al (g) | Al2O3 (g) | Fe2O3(g) | H2O (mL) | |
|---|---|---|---|---|---|---|---|
| Al-cement | 1.0680 | 2.8580 | 0.3115 | 1.5320 | - | - | 2 |
| S1 | 1.0320 | 2.8628 | 0.3330 | 1.5640 | - | 1.5320 | 2 |
| 0.25 g Al-cement | 1.0790 | 2.8800 | 0.3310 | 0.2560 | - | - | 2 |
| S2 | 1.0390 | 2.8580 | 0.3250 | 0.2500 | 0.5470 | - | 2 |
| BET Surface | Pore Volume | Pore Size | |
|---|---|---|---|
| 1.5 g-Al-cement | 64.0342 ± 3.7 m2/g | 0.060498 cm3/g | 37.7913 Å |
| S1 | 21.8228 ± 1.2 m2/g | 0.030965 cm3/g | 56.7565 Å |
| 0.25 g-Al-cement | 83.524 ± 3.3 m2/g | 0.074313 cm3/g | 35.5886 Å |
| S2 | 134.673 ± 2.3 m2/g | 0.15054 cm3/g | 44.715 Å |
| Isotherm | Equation | S1 | S2 |
|---|---|---|---|
| Langmuir | b = 0.205 L/g q0 = 4.87 mg/g Rl = 0.806 Kap = −0.0010 R2 = 0.998 | b = 0.023 L/g q0 = 43.47 mg/g Rl = 0.993 Kap = 0.0016 R2 = 0.988 | |
| Freundlich | 1/n = 1.197 Kf = 0.0038 R2 = 0.999 | 1/n = 0.968 Kf = 0.0131 R2 = 0.918 | |
| Temkin | A = 0.040 B = 1.281 R2 = 0.740 | A = 0.185 B =0.705 R2 = 0.658 | |
| Dubinin–Radushkevich | qd = 0.951 B = −135.2 R2 = 0.750 | qd = 1.799 B = 101.7 R2 = 0.729 |
| Adsorbent | Removal Capacity (mg/g) | pH | Refs. |
|---|---|---|---|
| Portland Pozzolana Cement | 0.25 | 6–11.8 | [27] |
| Calcite | 0.39 | 6 | [28] |
| Mg-HAP | 1.40 | 9–10 | [29] |
| Alumina cement | 1.61 | 8–10 | [10] |
| 0.25 g Al-cement | 2.35 | 8–9 | Present study |
| 1.5 g Al-cement | 4.84 | 8–9 | Present study |
| Composite S1 cement | 4.29 | 8–9 | Present study |
| Composite S2 cement | 4.10 | 8–9 | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gharsallah, S.; Alsawi, A.; Alsulami, A.H.; Charnay, C.; Chemingui, M. Enhancing Magnesium Phosphate Cement Paste for Efficient Fluoride Adsorption. Coatings 2024, 14, 9. https://doi.org/10.3390/coatings14010009
Gharsallah S, Alsawi A, Alsulami AH, Charnay C, Chemingui M. Enhancing Magnesium Phosphate Cement Paste for Efficient Fluoride Adsorption. Coatings. 2024; 14(1):9. https://doi.org/10.3390/coatings14010009
Chicago/Turabian StyleGharsallah, Sana, Abdulrahman Alsawi, Abdulelah H. Alsulami, Clarence Charnay, and Mahmoud Chemingui. 2024. "Enhancing Magnesium Phosphate Cement Paste for Efficient Fluoride Adsorption" Coatings 14, no. 1: 9. https://doi.org/10.3390/coatings14010009
APA StyleGharsallah, S., Alsawi, A., Alsulami, A. H., Charnay, C., & Chemingui, M. (2024). Enhancing Magnesium Phosphate Cement Paste for Efficient Fluoride Adsorption. Coatings, 14(1), 9. https://doi.org/10.3390/coatings14010009







