The Corrosion of Mn Coatings Electrodeposited from a Sulphate Bath with Te(VI) Additive and Influence of Phosphate Post-Treatment on Corrosion Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrodeposition
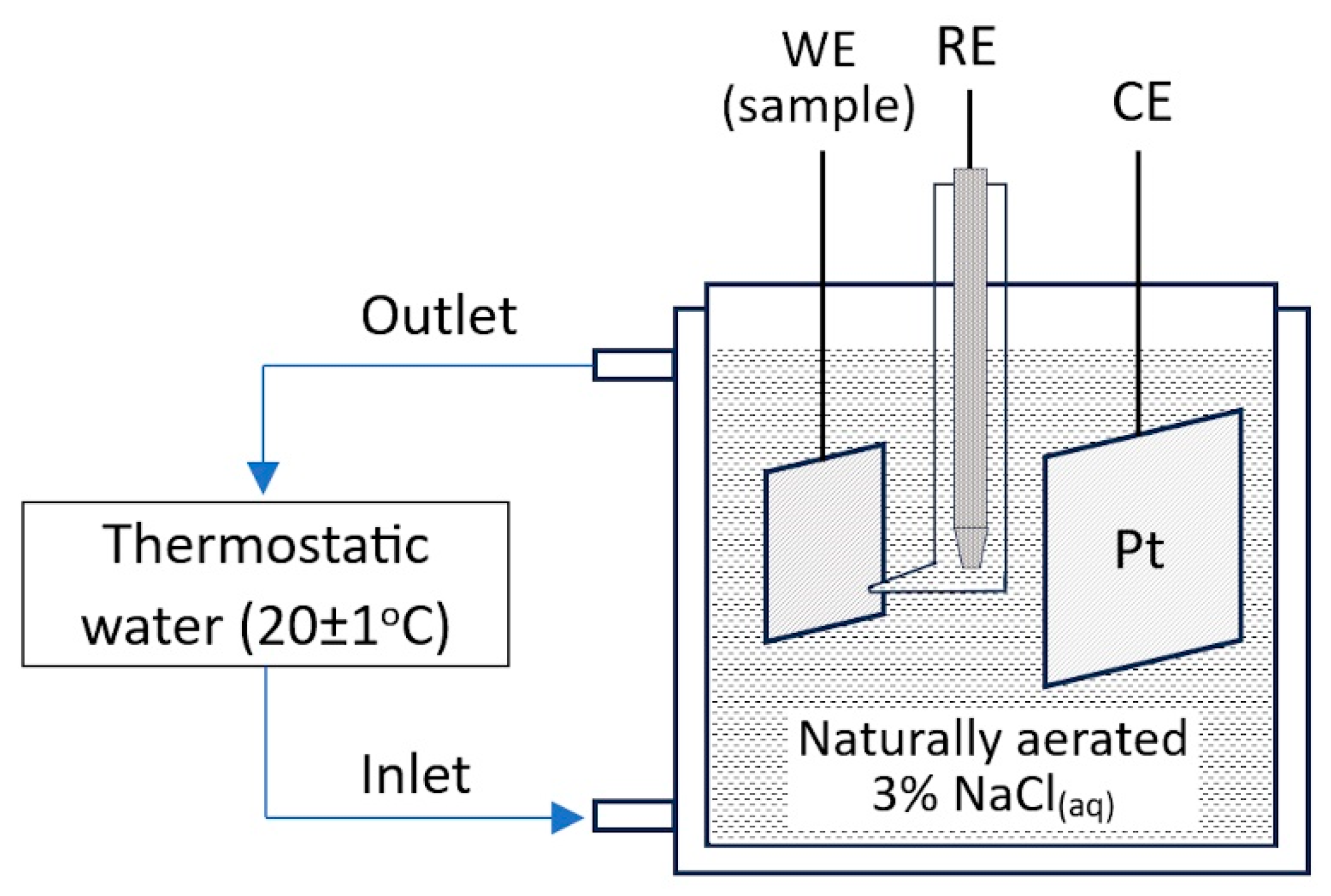
2.2. Electrochemical Tests
2.3. Salt Spray Test
2.4. Phosphate Treatment
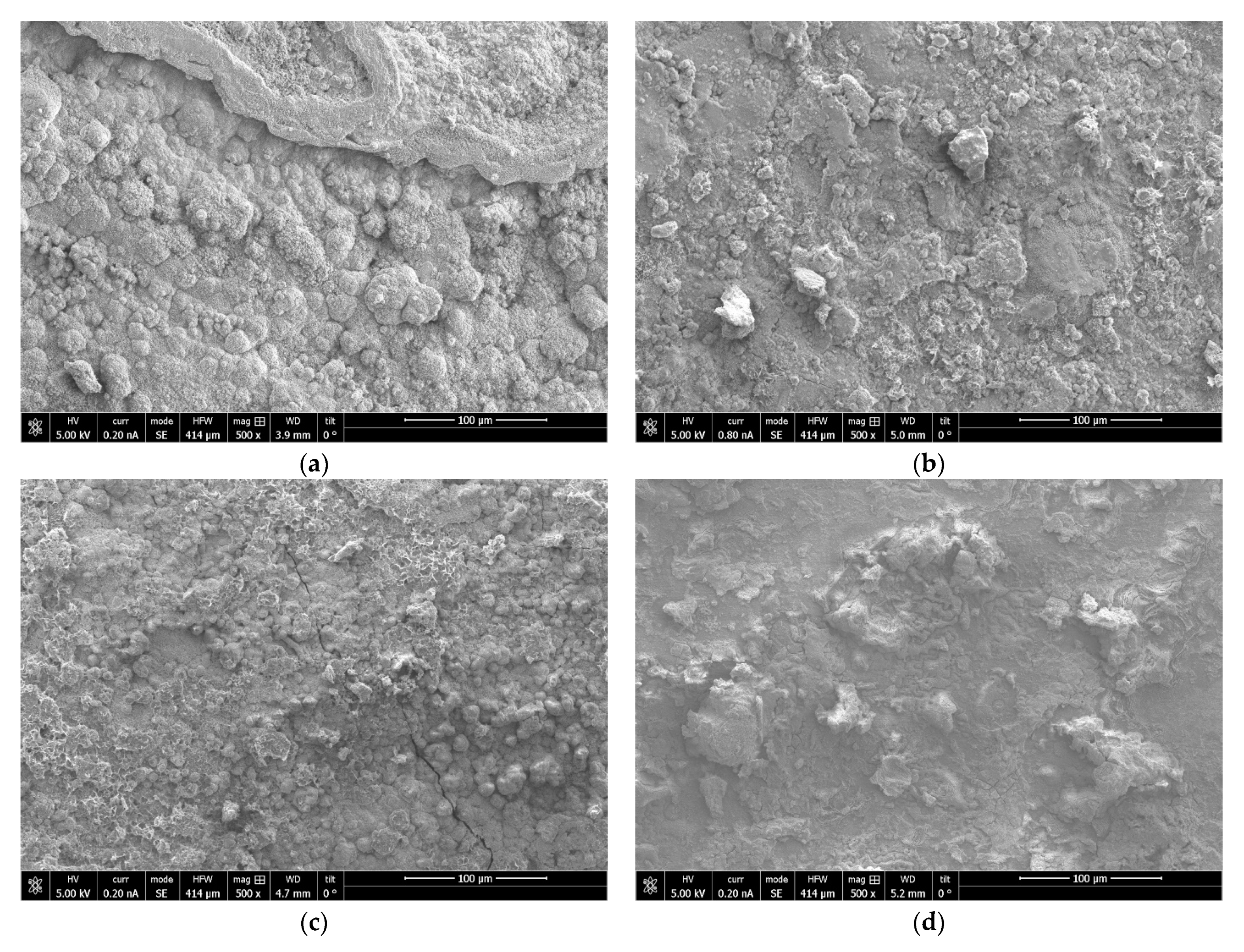
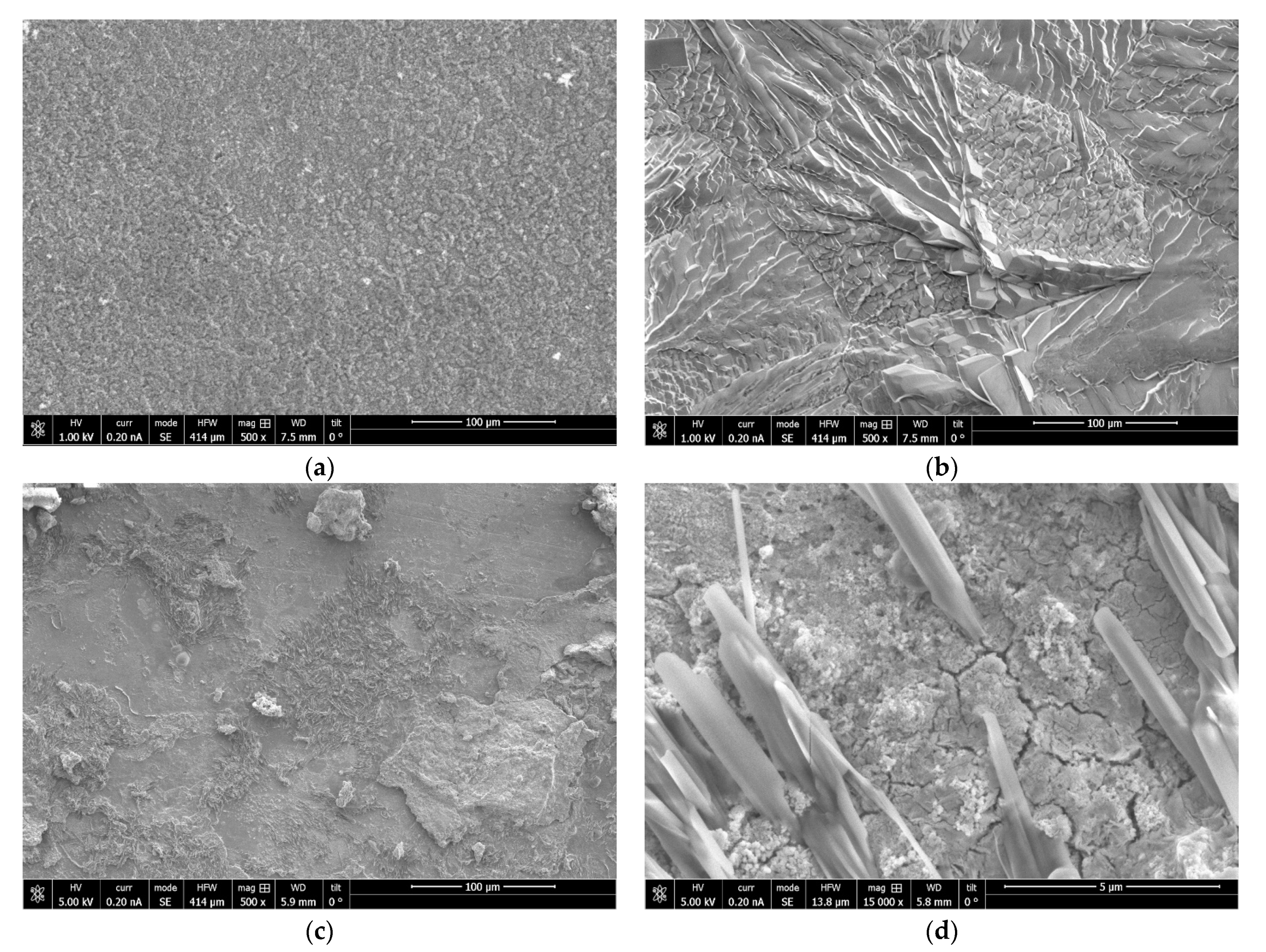
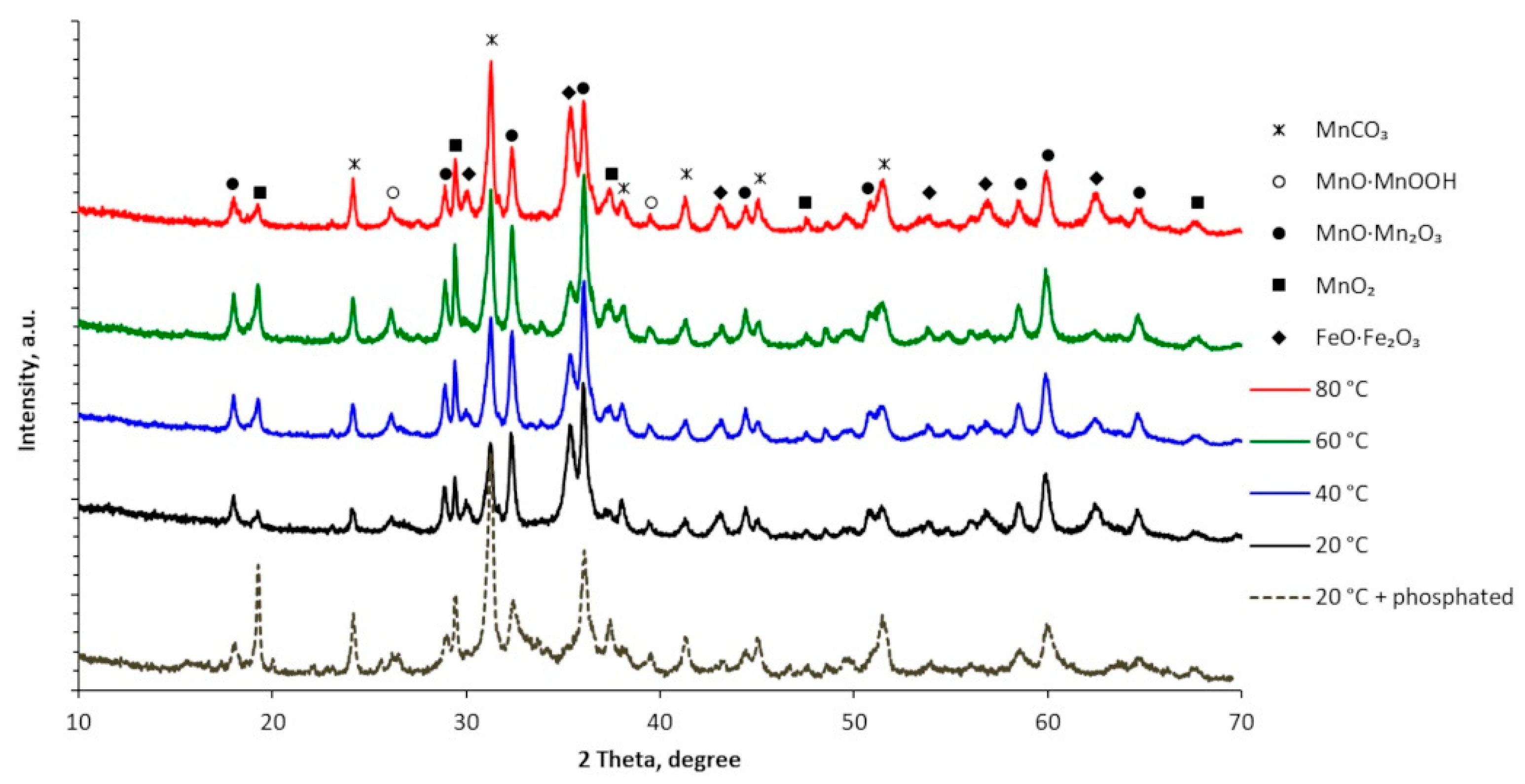
2.5. Structural, Elemental and Morphological Analysis
3. Results and Discussion
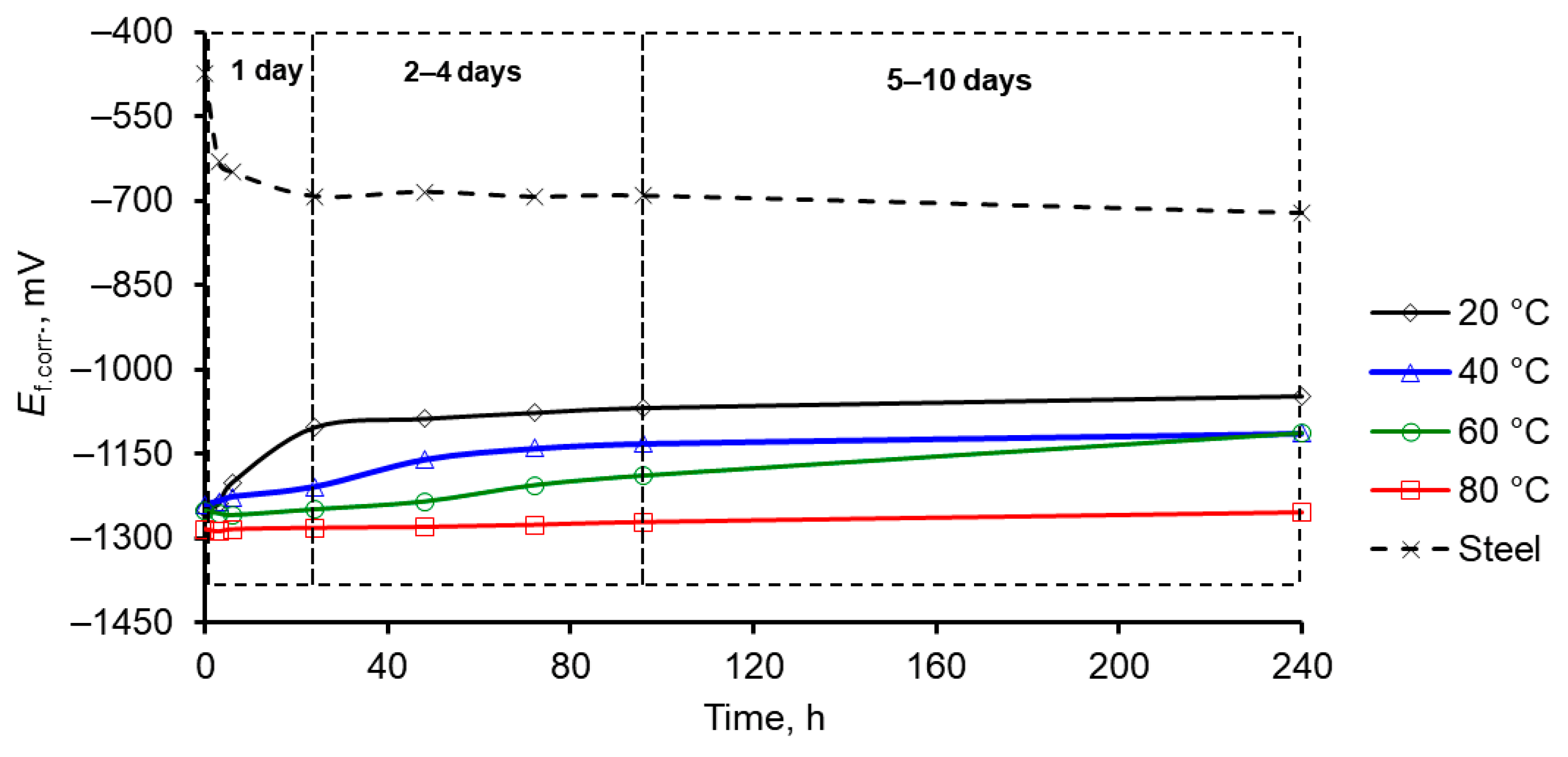
3.1. Corrosion of Mn Coatings in Naturally Aerated 3% NaCl Aqueous Solution
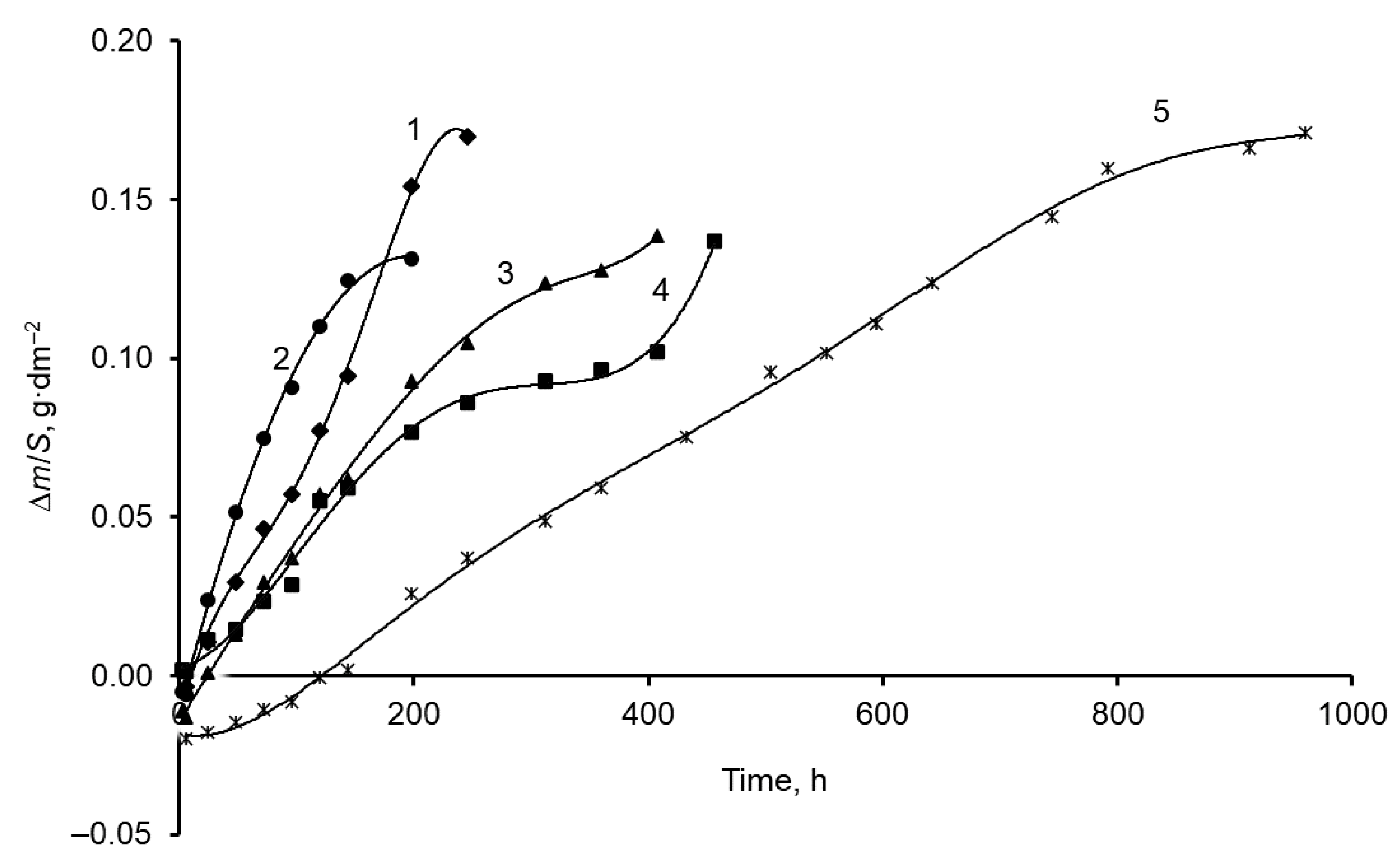
3.2. Corrosion of Mn Coatings in a Salt Spray Chamber
4. Conclusions
- The most resistant to corrosion were Mn coatings, electrodeposited from higher temperature (60–80 °C) MASB at 15 A·dm–2 cathodic current density and containing incorporated Te (about 1.1–1.4 wt. %). The increased corrosion resistance of Mn coatings, electrodeposited at higher temperatures, could be associated with the changes in their structure from the mixture of α-Mn and β-Mn phases to α-Mn phase and a two-fold decrease in crystallite sizes of Mn coatings [25], and all this ultimately leads to an overall improvement in the quality of Mn coatings.
- The corrosion resistance of Mn coatings can be significantly increased by additionally coating them with a phosphate film. The corrosion resistance of phosphated Mn coatings, electrodeposited from room temperature (20 °C) MASB, increases by a factor of about five times and reaches 1000 h before corrosion of the steel substrate occurs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamaguchi, T.; Nagano, H.; Murai, R.; Sugimori, H.; Sekiguchi, C.; Sumi, I. Development of Mn recovery process from waste dry cell batteries. J. Mater. Cycles Waste Manag. 2018, 20, 1909–1917. [Google Scholar] [CrossRef]
- Sun, Y.; Tian, X.K.; He, B.B.; Yang, C.; Pi, Z.; Wang, Y.; Zhang, S. Studies of the reduction mechanism of selenium dioxide and its impact on the microstructure of manganese electrodeposit. Electrochim. Acta 2011, 56, 8305–8310. [Google Scholar] [CrossRef]
- Lu, J.; Dreisinger, D.; Glück, T. Manganese electrodeposition—A literature review. Hydrometallurgy 2014, 141, 105–116. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, Z.; Zhang, X.; Yang, L.; Chang, J.; Tao, C. Electrochemical oscillation on anode regulated by sodium oleate in electrolytic metal manganese. J. Electroanal. Chem. 2019, 845, 13–21. [Google Scholar] [CrossRef]
- Wei, Q.F.; Ren, X.L.; Du, J.; Wei, S.J.; Hu, S.R. Study of the electrodeposition ditions of metallic manganese in an electrolytic membrane reactor. Miner. Eng. 2010, 23, 578–586. [Google Scholar] [CrossRef]
- Fernández-Barcia, M.; Hoffmann, V.; Oswald, S.; Giebeler, L.; Wolff, U.; Uhlemann, M.; Gebert, A. Electrodeposition of manganese layers from sustainable sulfate based electrolytes. Surf. Coat. Technol. 2018, 334, 261–268. [Google Scholar] [CrossRef]
- Caoa, X.; Dreisinger, D.B.; Lua, J.; Belangerb, F. Electrorefining of high purity manganese. Hydrometallurgy 2017, 171, 412–421. [Google Scholar] [CrossRef]
- Diaz-Arista, P.; Trejo, G. Electrodeposition and characterization of manganese coatings obtained from acidic chloride bath containing ammonium thiocyanate as an additive. Surf. Coat. Technol. 2006, 201, 3359–3367. [Google Scholar] [CrossRef]
- Padhy, S.K.; Patnaik, P.; Thipathy, B.; Bhattacharya, I. Microstructural aspects of manganese metal during its electrodeposition from sulphate solutions in the presence of quaternary amines. Mater. Sci. Eng. 2015, 193, 83–90. [Google Scholar] [CrossRef]
- Gonsalves, M.; Pletcher, P. A study of the electrodeposition of manganese from aqueous chloride electrolytes. J. Electroanal. Chem. 1990, 285, 185–193. [Google Scholar] [CrossRef]
- Mulin, E.V.; Kistosturov, N.I.; Tarasenkova, V.P.; Solovjov, V.A. Influence of catholite circulation and ammonium chloride on manganese current efficiency during electrolysis. Russ. J. Electrochem. 1984, 20, 1429–1434. [Google Scholar]
- Yang, F.; Jiang, L.; Yu, X.; Lai, Y.; Li, J. The effects of SeO2 additive on Mn electrodeposition on Al substrate in MnSO4-(NH4)2SO4-H2O solution. Hydrometallurgy 2020, 192, 105285. [Google Scholar] [CrossRef]
- Xu, F.; Dan, Z.; Zhao, W.; Han, G.; Sun, Z.; Xiao, K.; Jiang, L.; Duan, N. Electrochemical analysis of manganese electrodeposition and hydrogen evolution from pure aqueous sulfate electrolytes with addition of SeO2. J. Electroanal. Chem. 2015, 741, 149–156. [Google Scholar] [CrossRef]
- Rojas-Montes, J.C.; Pérez-Garibay, R.; Uribe-Salas, A.; Bello-Teodoro, S. Selenium reaction mechanism in manganese electrodeposition process. J. Electroanal. Chem. 2017, 803, 65–71. [Google Scholar] [CrossRef]
- P´erez-Garibay, R.; Rojas-Montes, J.; Bello-Teodoro, S.; Flores-Álvarez, J.M. Effect of SeO2 on the morphology of electrodeposited manganese. J. Appl. Electrochem. 2020, 50, 1291–1299. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, Z.; Tao, C.; Li, C.; Chang, J. Production of electrolytic manganese metal using a new hyperchaotic circuit system. J. Mater. Res. Technol. 2022, 18, 4804–4815. [Google Scholar] [CrossRef]
- Thakur, A.; Sharma, S.; Ganjoo, R.; Assad, H.; Kumar, A. Anti-Corrosive Potential of the Sustainable Corrosion Inhibitors Based on Biomass Waste: A Review on Preceding and Perspective Research. J. Phys. Conf. Ser. 2022, 2267, 012079. [Google Scholar] [CrossRef]
- Padhy, S.K.; Patnaik, P.; Tripathy, B.C.; Ghosh, M.K.; Bhattacharya, I.N. Electrodeposition of manganese metal from sulphate solutions in the presence of sodium octyl sulphate. Hydrometallurgy 2016, 165, 73–80. [Google Scholar] [CrossRef]
- Padhy, S.K.; Tripathy, B.C.; Alfantazi, A. Effect of Sas additives on electrodeposition manganese metal (Emm) from sulfate solutions in the presence of sodium metabisulphite. Hydrometallurgy 2018, 177, 227–236. [Google Scholar] [CrossRef]
- Xue, J.R.; Wang, S.; Zhong, H.; Li, C.X.; Wu, F.F. Influence of sodium oleate on manganese electrodeposition in sulfate solution. Hydrometallurgy 2016, 160 (Suppl. C), 115–122. [Google Scholar] [CrossRef]
- Fan, X.; Xi, S.Y.; Sun, D.G.; Liu, Z.H.; Du, J.; Tao, C.Y. Mn–Se interactions at the cathode interface during the electrolytic-manganese process. Hydrometallurgy 2012, 127–128, 24–29. [Google Scholar] [CrossRef]
- Etxebarria, N.; Arana, G.; Antolin, R.; Borge, G.; Posata, T.; Raposa, J.C. Selenium in electrolytic manganese as a reference material for the quality control of aluminium melts. Accred. Qual. Assur. 2007, 12, 575–580. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Siu, A.C.; Leung, K.T. Anomalous electrodeposition of metallic Mn nanostructured films on H-terminated Si(100) at anodic potential. Chem. Mater. 2007, 19, 6414–6420. [Google Scholar] [CrossRef]
- Sharma, R.K.; Rastogi, A.C.; Singh, G. Electrochemical growth and characterization of Manganese telluride thin films. Mater. Chem. Phys. 2004, 84, 46–51. [Google Scholar] [CrossRef]
- Griskonis, E.; Sulcius, A.; Zmuidzinaviciene, N. Influence of temperature on the properties of Mn coatings electrodeposited from the electrolyte containing Te(VI) additive. J. Appl. Electrochem. 2014, 44, 1117–1125. [Google Scholar] [CrossRef]
- Galvanauskaite, N.; Sulcius, A.; Griskonis, E.; Diaz-Arista, P. Influence of Te(VI) additive on manganese electrodeposition at room temperature and coating properties. Trans. IMF 2011, 89, 325–332. [Google Scholar] [CrossRef]
- Kondratas, D.Z.; Stulpinas, B.B.; Petroshevichyute, O.S. Corrosion properties of selenium-containing manganese galvanic deposits alloyed with iron subgroup metals. Scientific works of universities Lit. SSR. Chem. Chem. Technol. 1976, 18, 164–170. [Google Scholar]
- Li, W.P.; Zuo, X.L.; Liang, J.H.; He, J.H.; Zhang, S.T. Effect of acetate on electrodeposition of manganese from chloride electrolyte with SeO2 additives. Adv. Mater. Res. 2014, 937, 193–199. [Google Scholar] [CrossRef]
- Galvan-Reyes, C.; Fuentes-Aceituno, J.C.; Salinas-Rodríguez, A. The role of alkalizing agent on the manganese phosphating of a high strength steel part 1: The individual effect of NaOH and NH4OH. Surf. Coat. Technol. 2016, 291, 179–188. [Google Scholar] [CrossRef]
- Hosseini, M.R.; Sarabi, A.A.; Mohammadloo, E.E.; Sarayloo, M. The performance improvement of Zr conversion coating through Mn incorporation: With and without organic coating. Surf. Coat. Technol. 2016, 258, 437–446. [Google Scholar] [CrossRef]
- Duszczyk, J.; Siuzdak, K.; Klimczuk, T.; Strychalska-Nowak, J.; Zaleska-Medynska, A. Manganese Phosphatizing Coatings: The Effects of Preparation Conditions on Surface Properties. Materials 2018, 11, 2585. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Macías, G.; Fuentes-Aceituno, J.C.; Rodríguez, A.S.; Rodríguez-Varela, F.J. Understanding the Nature of the Manganese Hot Dip Phosphatizing Process of Steel. J. Mex. Chem. Soc. 2013, 57, 328–336. [Google Scholar] [CrossRef]
- Yingsamphancharoen, T.; Srisuwan, N.; Rodchanarowan, A. The electrochemical investigation of the corrosion rates of welde pipe ASTM A106 Grade B. Metals 2016, 6, 207. [Google Scholar] [CrossRef]
- Available online: https://matweb.com/search/datasheet.aspx?matguid=67e1b53cedc944b19ae5a9d46cef3096&ckck=1 (accessed on 12 June 2023).
- EN ISO 9227:2022; Corrosion Tests in Artificial Atmospheres. Salt Spray Tests. ISO: Geneva, Switzerland, 2022; p. 18.
- Singh, K.; Rani, N. Study of role of phosphate coating properties electrophoretic paint performance by EIS and simulated corrosion tests. Trans. IMF 2014, 92, 153–160. [Google Scholar] [CrossRef]
- Crozier, B.M.; Liu, Q.; Ivey, D. Electrodeposition of an iron–cobalt phase isostructural to α-Mn. ECS Trans. 2009, 16, 141–154. [Google Scholar] [CrossRef]
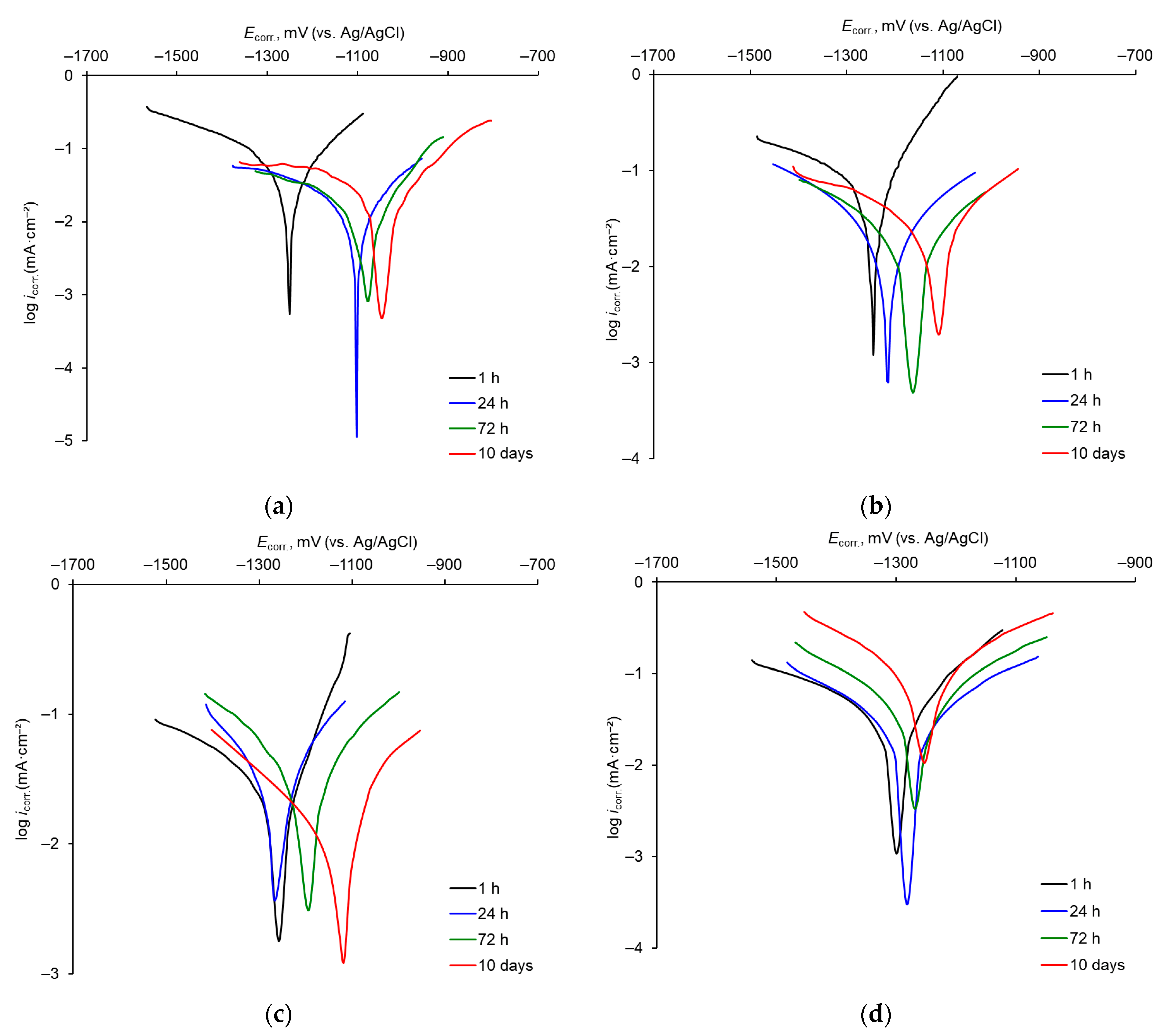
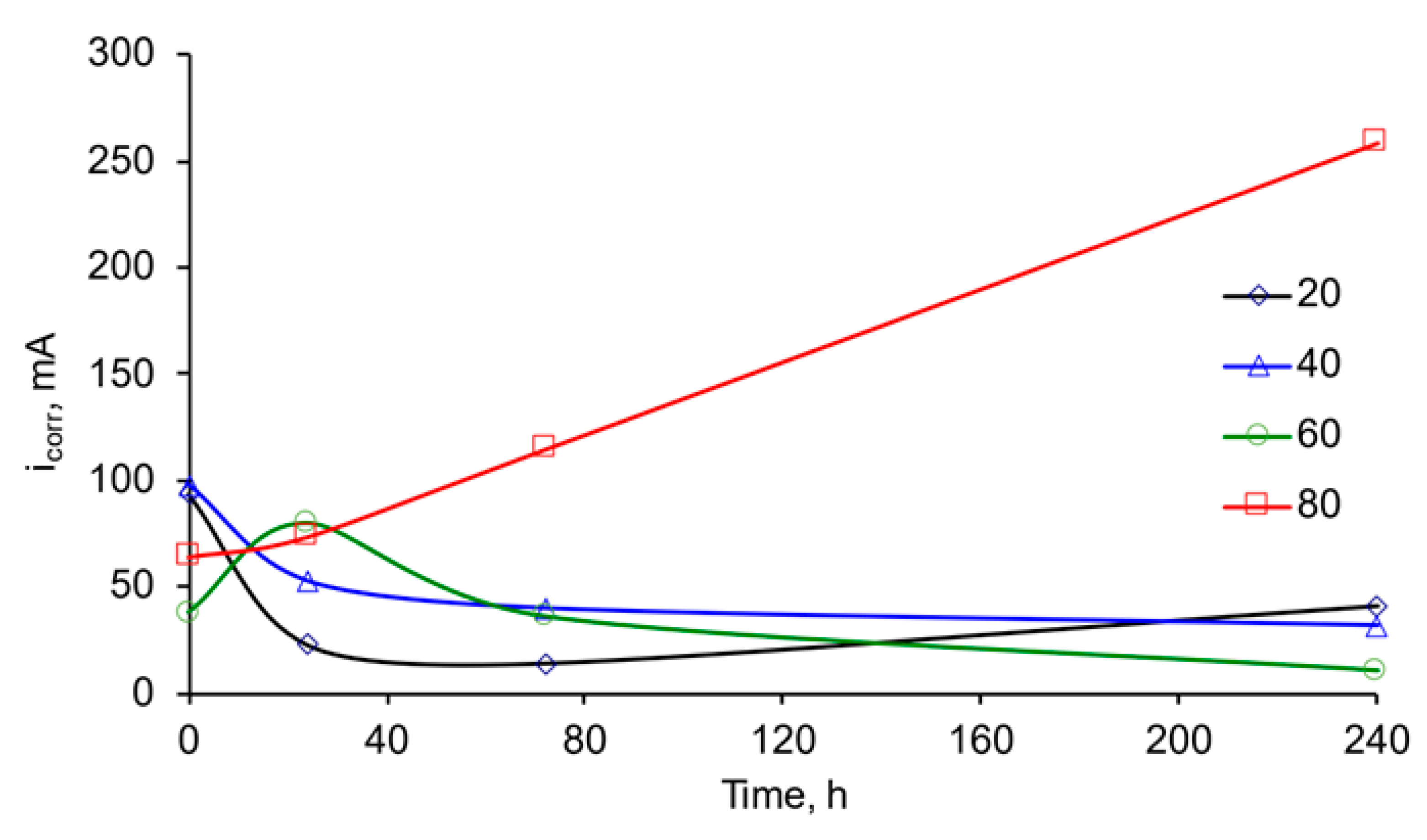
| Temperature of MASB, °C | Corrosion Time, h | Ecorr., V (vs. Ag/AgCl) | icorr., μA·cm–2 | −βc, mV·dec–1 | βa, mV·dec–1 |
|---|---|---|---|---|---|
| 20 | 1 | −1251 | 93.4 | 66.7 | 37.5 |
| 24 | −1104 | 22.6 | 96.2 | 37.1 | |
| 72 | −1082 | 14.1 | 60.6 | 23.6 | |
| 240 | −1048 | 41.1 | 233.2 | 44.1 | |
| 40 | 1 | −1249 | 97.7 | 99.8 | 24.8 |
| 24 | −1215 | 52.4 | 81.8 | 70.4 | |
| 72 | −1169 | 39.6 | 96.5 | 72.9 | |
| 240 | −1114 | 31.5 | 122.8 | 38.8 | |
| 60 | 1 | −1257 | 37.9 | 68.4 | 18.4 |
| 24 | −1267 | 79.8 | 126.1 | 84.8 | |
| 72 | −1193 | 35.9 | 125.0 | 96.8 | |
| 240 | −1117 | 10.4 | 54.1 | 20.1 | |
| 80 | 1 | −1288 | 64.2 | 94.2 | 34.4 |
| 24 | −1282 | 73.6 | 89.9 | 73.8 | |
| 72 | −1269 | 114.8 | 87.4 | 70.1 | |
| 240 | −1253 | 258.8 | 83.1 | 84.5 |
| Temperature of MASB, °C | The Appearance of: | |||||
|---|---|---|---|---|---|---|
| Individual Dark Brown or Black Dots | Bluish Spots | Dark Brown Spots | White Spots | Orange Spots | Corrosion of Steel Substrate | |
| 20 | 6 h | 6 h | 72 h | 66 h | 72 h | 240 h |
| 40 | 3 h | 6 h | 36 h | 126 h | 84 h | 192 h |
| 60 | 12 h | 24 h | 42 h | 48 h | 108 h | after 408 h, corroded coating separates from the steel substrate |
| 80 | 12 h | 42 h | 42 h | 42 h | 78 h | after 456 h, only at the corners of the samples |
| 20+ phosphated | 24 h | 312 h | 192 h | 264 h | - | 960 h |
| Temperature of MASB, °C | Element, at. % |
|---|---|
| 20 | O—25.58 |
| Na—1.13 | |
| Si—0.09 | |
| S—0.02 | |
| Cl—0.91 | |
| Mn—49.16 | |
| Fe—22.75 | |
| Te—0.35 | |
| 20 + phosphated | O—30.8 |
| Na—0.00 | |
| Si—0.15 | |
| S—0.10 | |
| Cl—0.76 | |
| Mn—63.33 | |
| Fe—2.04 | |
| P—3.03 | |
| Te—0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žmuidzinavičienė, N.; Griškonis, E.; Šulčius, A. The Corrosion of Mn Coatings Electrodeposited from a Sulphate Bath with Te(VI) Additive and Influence of Phosphate Post-Treatment on Corrosion Resistance. Coatings 2023, 13, 1617. https://doi.org/10.3390/coatings13091617
Žmuidzinavičienė N, Griškonis E, Šulčius A. The Corrosion of Mn Coatings Electrodeposited from a Sulphate Bath with Te(VI) Additive and Influence of Phosphate Post-Treatment on Corrosion Resistance. Coatings. 2023; 13(9):1617. https://doi.org/10.3390/coatings13091617
Chicago/Turabian StyleŽmuidzinavičienė, Nerita, Egidijus Griškonis, and Algirdas Šulčius. 2023. "The Corrosion of Mn Coatings Electrodeposited from a Sulphate Bath with Te(VI) Additive and Influence of Phosphate Post-Treatment on Corrosion Resistance" Coatings 13, no. 9: 1617. https://doi.org/10.3390/coatings13091617
APA StyleŽmuidzinavičienė, N., Griškonis, E., & Šulčius, A. (2023). The Corrosion of Mn Coatings Electrodeposited from a Sulphate Bath with Te(VI) Additive and Influence of Phosphate Post-Treatment on Corrosion Resistance. Coatings, 13(9), 1617. https://doi.org/10.3390/coatings13091617








