Abstract
Liquid phase deposition is a cost-effective and energy-efficient method for obtaining TiO2 films at low temperatures. This work demonstrates the use of a multifunctional template consisting of grafted gallic acid (GA) and hexamethylenediamine (HD) on a Ti substrate to regulate the deposition of TiO2 films. X-ray diffraction characterization shows that films deposited with the template have a more obvious anatase phase compared to direct deposition at 25 °C. The intensity of photoluminescence spectra also shows significant differences at 373.7 nm and 663.3 nm. Furthermore, electrochemical measurements indicate that TiO2 films adjusted by the template have excellent electrochemical conversion properties. This proposed method provides a new simple route for fabricating TiO2 films that are adjusted by GAHD templates, which may have good applications in the fields of semiconductor materials and biological materials.
1. Introduction
The technology for synthesizing inorganic oxide thin films using low-temperature liquid solutions has attracted increasing attention [1]. Unlike depositing thin films on substrates using chemical or mechanical methods under high-temperature conditions, which require higher output energy and more complex instruments [2,3], liquid phase deposition (LPD) preparation of oxide films does not require heat treatment or vacuum equipment, and a uniform thin film can be deposited on the substrate by immersing it in an appropriate reaction solution. The adhesion strength between the film and the substrate is more secure [4]. Liquid phase deposition has little demand for substrate morphology and can coat not only line-of-sight substrates but also non-planar ones [5,6]. Moreover, the equipment required for deposition is simple and easy to use. Finally, the solutions and reagents used in liquid phase deposition have a reduced environmental impact, as they do not rely on expensive organometallic precursors.
In the process of liquid phase deposition of TiO2 films, both solution parameters and functional groups on the substrate material play an important part in the deposition quality of the film. Therefore, effectively adjusting these two factors will to some extent affect the nucleation and growth of TiO2 particles, thereby obtaining high-quality films. Regarding the study of solution parameters, there are already mature processes, so more in-depth research is required in the area of template regulation on the substrate. The influence of substrates on the deposition of films from aqueous solutions has been systematically reported. In liquid phase deposition, surface modification of the substrate is essential to ensure the uniformity of thin film growth. Reasonable treatment methods may involve the formation of surface hydroxides, appropriate pre-deposition, or self-assembly of seed layers.
Taking SiO2 as an example, functionalization of the Si surface with -OH functional groups is the most effective coverage before immersion in the precursor solution, which can be obtained by appropriate etching of the silicon surface. Si-OH functional groups serve as seed layers and achieve film growth through a condensation reaction with silicic acid in the growth solution. In the method of growth in solution, another key factor affecting the growth is interfacial energy. When a low interfacial energy substrate is introduced into the growth solution compared to the substrate with homogeneous nucleation, heterogeneous growth is favorable. The mechanism of seed growth is defined by introducing low-interfacial-energy substrates into supersaturated solutions to promote heteroepitaxial growth. Lower interfacial energy is the result of surface modification and is also a natural attribute of materials. Equitable treatment methods may involve the formation of surface hydroxides, pre-deposition of suitable seed layers, or self-assembly. In the past, many research groups have contributed to studying the impact of functional groups on the substrate by using organic self-assembled monolayers (SAMs) or chemically modified substrate surfaces and have made great progress [7,8]. SAMs are strongly ordered, densely packed long-chain hydrocarbon molecules that are fixed to the solid substrate surface through strong covalent or ionic bonds. The functional groups at the SAM endpoints can anchor to the ceramic film surface and significantly affect its properties and deposition. Using this method, compact and uniform TiO2, SnO2, V2O5·nH2O, and other oxides have been successfully deposited [9,10,11]. Research has also found that SAMs promote the formation of oxide films and can facilitate solid heteronucleation because they decrease surface energy relative to the bare substrate surface. In cases where nucleation occurs in the bulk solution (away from the substrate), SAMs can increase the Van der Waals forces and electrostatic attraction between existing solid particles and SAMs, which is propitious for film formation [12]. Another method is to use organic polymer electrolytes to modify the substrate surface and study their effects on film deposition. This method has been utilized for the deposition of nanostructured polymer electrolyte/TiO2 (organic/inorganic) composite films containing alternating layers [13]. Researchers used polymer electrolytes such as poly (diallyldimethylammonium chloride), poly (acrylic acid), and polycyclic aromatic hydrocarbons to treat polystyrene latex particles [14,15,16], followed by LPD to prepare TiO2 microspheres. The polymer electrolytes contained rich functional groups such as amino, carboxyl, hydroxyl phenol, and sulfonic acid groups and were utilized to prepare single- or double-layer polymer electrolyte templates on the surface of polystyrene microspheres through layer-by-layer electrostatic adsorption [17,18]. Particle nucleation and growth were controlled during the liquid phase deposition process.
Researchers have concluded that functional groups such as -NH2, -OH, -COOH [19], etc., enhance the heterogeneous nucleation of solids on the self-assembled monolayer surface, respectively. However, there is little research on how multifunctional groups affect the deposition and growth of TiO2 at the same time. Gallic acid (GA), which contains the structures of o-phenols and benzoic acid, can also be adsorbed on metal surfaces and then undergo Michael addition reactions or Schiff base reactions through direct reactions between o-phenolic groups and amines and acid–base reactions between carboxyl groups and amines. Therefore, molecules with no less than two amine groups are introduced to react with GA to prepare copolymer films. Hexamethylenediamine (HD) with two amine groups and basicity is selected to perform a copolymerization reaction with GA. The introduction of GA and HD provides the GAHD film surface with three reactive groups: carboxyl, primary amine, and quinone. In this study, we selected the mixed solution of gallic acid and hexamethylenediamine as the template to create a grafted gallic acid and hexamethylenediamine (GAHD) film with multifunctional groups such as -NH2, -OH, -COOH, etc., simultaneously. These groups have hydrophilic properties [20], which is important for researching the organization and performance of TiO2 film that is adjusted by multifunctional group templates. In contrast, other surface modification methods, such as self-assembled monolayers (SAM), layered assembly, functionalized silane coating, and plasma polymer deposition, have limitations for specific material applications: these techniques require specific substrates and chemicals, special surface modifications, complex instrumentation, and multi-step implementation procedures. Therefore, the choice of these two substances for copolymerization reactions to obtain GAHD multifunctional templates is simple, efficient, and implementable [21].
Additionally, gallic acid is a biological molecule with properties such as oxidation resistance, anti-microbial, and anti-tumor [22,23], which is nontoxic to the human body. The use of liquid as a deposition medium allows the coating of non-planar substrates, expanding the range of substrates that can be covered, i.e., increasing the arbitrariness of substrate selection, being used for deposition on a variety of substrates such as glass, polymers, carbon fibers, and metals. Here, we chose pure titanium as a substrate considering its lighter mass, non-toxic and non-hazardous nature, higher compatibility, and the abundance of sources and material strength, thus ensuring a low toxicity of the substrate so that the samples prepared can be evaluated not only in the field of optoelectronics but also extended to the evaluation in the field of biomedicine. In the experiment, dopamine (DA) was used to pre-treat the Ti substrate to ensure strong adhesion of the GAHD film [24]. Polydopamine is prepared from the self-polymerization of dopamine. Through the o-phenolic structure obtained from mussel mucilage protein, dopamine can be adsorbed on the surface of various materials under alkaline conditions. At the same time, dopamine self-polymerizes with its specific amines via Michael addition reactions and Schiff base reactions. GA and HD form a thin film GAHD under alkaline conditions. Since molecules containing catechol or even benzoic acid can be fused by chemisorption onto metal particles, according to reports, the prepared templates were successfully immobilized on the substrate in the presence of polydopamine [25]. In this study, the TiO2 film on the Ti substrate was prepared by first immersing the Ti substrate in the DA solution and then using a dynamic water bath at 37 °C to make the GAHD film template. Finally, the TiO2 film was obtained using the LPD method. Material characterization and electrochemical analysis of the films were further performed, leading to interesting results.
2. Materials and Methods
2.1. Materials
In this experiment, double-distilled water was used for all experiments. Ammonium hexafluorotitanate (CP, 98%, M = 197.93) was purchased from Aladdin, and boric acid (M = 61.83) and hydrofluoric acid were obtained from ChangZheng regent. Dopamine, gallic acid, and hexamethylenediamine were purchased from Sigma-Aldrich (Burlington, MA, USA), and Tris (hydroxymethyl) aminomethane (Tris-base) was obtained from Kaizheng Biotechnology Co., Ltd. (Beijing, China). Commercially purchased pure titanium disks with dimensions of 10 mm and thickness of 2 mm were used as substrates.
2.2. Preparation of (GAHD) as a Template for TiO2 Film Deposition
Since Ti contains a natural oxide layer on its surface when placed in air, the experiments were carried out to eliminate this effect as much as possible by polishing it before each experiment. Firstly, mirror-polished Ti substrates were cleaned by ultrasonication in acetone (15 min × 3 times), followed by ultrasonication in absolute ethanol (15 min × 3 times), and finally, ultrasonication in distilled water (15 min × 3 times). This thorough cleaning ensured that the surfaces were well-prepared for the subsequent deposition step. Next, the cleaned titanium sheets were immersed in a fresh DA solution, prepared by dissolving dopamine hydrochloride (2 mg/mL) in Tris-base buffer (10 mM, pH = 8.5) at 20 °C for 12 h. The resulting polydopamine (PDA)-coated Ti substrate was then ultrasonically washed with double distilled water (10 min × 3 times). This process was replicated once, and then the PDA-coated Ti substrates were dried in a drying cabinet. Subsequently, in a 37 °C dynamic water bath, the samples were immersed in a mixture solution of GA and HD at a 1:2 mass ratio for 3 h to obtain the GAHD template. Afterward, the samples were ultrasonically cleaned with distilled water (5 min × 3 times) and allowed to dry naturally.
Finally, the TiO2 film was deposited onto the substrate. Ammonium hexafluorotitanate ([NH4]2TiF6) and boric acid (H3BO3) were separately dissolved in deionized water at 50 °C for 12 h. An appropriate amount of HF was added to the boric acid solution to control the pH, and an ammonium hexafluorotitanate solution was added. The substrate coated with the GAHD template was then immersed in the solution containing 0.02M [NH4]2TiF6 and 0.06 M H3BO3 at pH 2.8 and kept at 50 °C [26] to deposit TiO2 for 12, 24, and 36 h, respectively. At last, the obtained TiO2 film was ultrasonically washed with double-distilled water three times and then placed in a drying cabinet [5]. The approximate flow chart is shown in Figure 1.
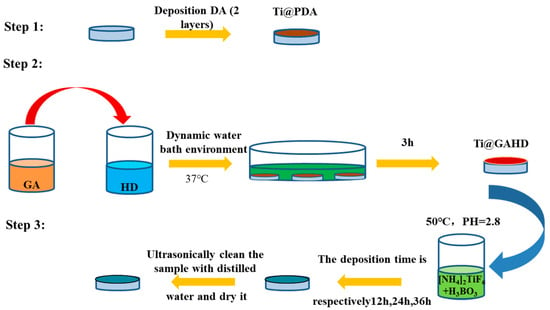
Figure 1.
Schematic diagram of the experimental procedure.
2.3. Characterization Methods
2.3.1. Optical Microscopy (OM), Scanning Electron Microscopy (SEM), and Atomic Force Microscopy (AFM)
The surface morphology was characterized by optical microscopy (MM6, Leitz Company, Oberkochen, Germany) and field-emission scanning electron microscopy (FESEM, JSM-7401F, JEOL, Tokyo, Japan). The surface morphology and roughness were analyzed by atomic force microscopy (AFM, SPI 3800, NSK, Inc., Tokyo, Japan) in tapping mode with a scanned range of 25 μm × 25 μm. Each sample was analyzed at more than three points to obtain the morphology.
2.3.2. Characterization of the GAHD Film
- Colorimetric staining with Acid Orange II and Fourier transform infrared spectroscopy (FTIR)
To determine the amine concentration of the GAHD film, a 5 μmol/mL solution of Acid Orange II (AOII, Sigma-Aldrich) in HCl (1 mM, pH = 3) was added to the surface of each sample in triplicate [8]. The samples were incubated for 12 h at 25 °C, after which the supernatant was removed, and the samples were washed three times for 15 min with HCl solution (1 mM, pH = 3). The samples were subsequently left to dry naturally, with both the surface and covers completely dry. Next, the samples were incubated in NaOH solution (0.01 M, pH = 12) at 25 °C for 15 min to elute the absorbed AOII from the surfaces of the samples. Finally, 150μL of the eluate from each sample were transferred to a 96-well plate, and the absorbance was monitored using a microplate reader (mQuant, Bio-tek instruments Inc., Winooski, VT, USA) at 485 nm. The NH2 concentration of each sample was identified using the standard curve of AOII solution (pH = 12).
The chemical bonding states of the PDA and GAHD template on the Ti substrate were identified using Fourier transform infrared spectroscopy (FTIR, Nicolet 5700, Thermo Electron Corporation, Waltham, MA, USA).
- 2.
- X-ray photoelectron spectroscopy (XPS) of Immobilized GAHD Film and obtained TiO2 Films
Surface chemical composition analysis was conducted using X-ray photoelectron spectroscopy (XPS, XSAM800, Kratos Ltd., Manchester, UK) with an Al Kα X-ray source at a power of excitation radiation (hν = 1253.6 eV). The X-ray source operated at 12 kV × 15 mA at a pressure of 2 × 10−7 Pa. The C1 peak (binding energy 284.7 eV) was used as a reference for charge correction.
- 3.
- X-ray diffractometer (XRD) of obtained TiO2 Films
The phase structure of TiO2 films on the GAHD template was identified using an X’pert X-ray diffractometer (TFXRD, Philip X’Pert) with a Cu Kα target (λ = 0.154 nm) and 2-theta ranged from 20–100° at a step size of 0.25°.
2.4. Evaluation Methods
- Contact Angle Testing
To obtain the polarity and surface energy of the TiO2 film, contact angle measurements were performed using static drops and a DSA100 (Krüss, Hamburg, Germany), depending on the manufacturer’s instructions at 25 °C and 60% relative humidity. Each sample was measured in five different fields on the sample surface to obtain statistically significant data. The results were analyzed, and the surface energy was calculated using the Owens and Wendt method with the data of the contact angle of water (polar solvent) and diiodomethane (nonpolar solvent).
- 2.
- Photoluminescence (PL)
Since the TiO2 film exhibits semiconductor properties, further information about the film’s surface can be obtained through photoluminescence spectra (PL) testing. The PL was detected using an iHR320 spectrometer (Jobin Yvon, Oberursel, Germany) with a helium-cadmium laser origin at room temperature. PL spectra were tested from samples deposited in the TiO2 film in the 320–700 nm range using laser wavelengths of 220 nm.
- 3.
- Electrochemical impedance spectroscopy (EIS) and Mott–Schottky plots
As the polar component of the sample surface has a relationship with its electrochemical performance, electrochemical measurements are necessary for further study of the film’s nature. Electrochemical impedance spectroscopy (EIS) and Mott–Schottky plots were conducted utilizing an electrochemical workstation (IM6, Zahner, Kronach, Germany). The workstation utilized a three-electrode setup with the specimen as the working electrode, a platinum sheet as the counter-electrode, and a saturated calomel electrode (SCE) as the reference electrode. The testing samples, which were encapsulated with silicone and exposed to the surface prepared in the experiment, were approximately 0.78 cm2. Additionally, the encapsulated sample was fixed onto copper for convenient experiment testing. Prior to testing, each sample was stabilized in phosphate-buffered saline (PBS, pH = 7.4) solutions used as electrolytes for 30 min to maintain a stable open-circuit potential at 37 ± 0.5 °C. EIS spectra were obtained by applying sinusoidal perturbations of 10 mV over the VOC at frequencies ranging from 10−3 to 105 Hz. EIS spectra were recorded as a Bode plot. In the Schottky testing, the potential was collected from −1 V to 1 V, and Mott–Schottky plots were recorded directly and analyzed using the Mott–Schottky equation as follows [27,28]:
where Csc is the measured space charge layer capacitance, Efb represents the flat band potential, ND is the density of donors in the space charge layer, ε is the relative dielectric constant of the titanium oxide film, ε0 is the permittivity of free space, K is the Boltzmann constant, and T is the absolute temperature. The space charge layer carrier concentration (Nsc, equivalent to donor density ND) and Efb of the samples were calculated by simulating the plot slope (using the Mott–Schottky equation). Electrochemical tests were repeated no fewer than two times for statistical purposes.
3. Results
3.1. Material Characterization of Films
3.1.1. SEM and AFM
Figure 2 shows the surface morphology of all samples, according to the optical image in Figure 2a, indicating that the TiO2 films were all successfully deposited on the substrate. The microstructure surfaces of the TiO2 films are presented in Figure 2b. From the topography, we can point out that the surface of the Ti substrate undergoes significant changes after film deposition. The surface of pure titanium exhibits irregularities and uneven marks. However, upon fixing the GAHD template, the surface becomes notably smoother, effectively concealing the defective areas of the original titanium surface. After TiO2 film deposition, the surface is turned. Following 12 h of deposition, the obtained TiO2 film differs significantly from the nontemplate and GAHD template-adjusted deposition. Firstly, we found that the surface particle aggregation of the films, after template modulation, exhibits a more spherical morphology. Secondly, we observe cracks in some of the samples of TiO2 film. From the literature, we know that the film did not crack during cooling and rinsing with deionized water, only after complete drying. Capillary stresses arise when the solvent used for cleaning shrinks into the pores in the film. Natural drying in the initial stage does not completely evaporate the liquid in the pores, so the gradual disappearance of the meniscus of the cleaning agent in the pores of the film results in the cracking of the film. This can be avoided by cooling or washing the surface of the sample with a solvent and then drying it completely [8,29]. Based on the SEM results, it can be possible to conclude that both the GAHD and TiO2 films were successfully deposited on the substrate. Additionally, the presence of the multifunctional group template GAHD may indeed have an effect on the surface morphology of the resulting TiO2 films.
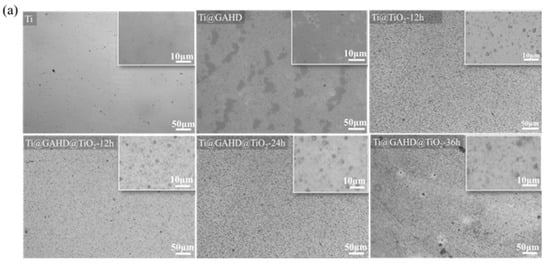
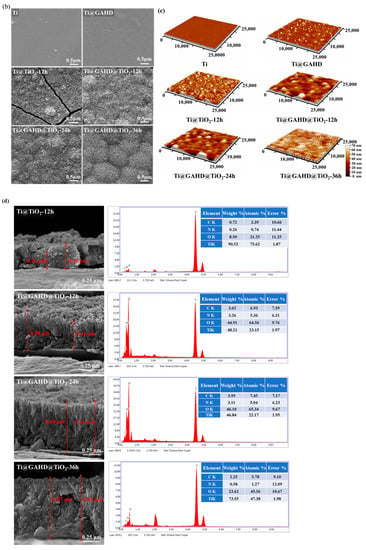
Figure 2.
OM, SEM, and AFM images and cross-sectional SEM and corresponding energy dispersive spectrometer (EDS) EDS spectra of samples: (a) OM images of Ti, GAHD template, and TiO2 films with different deposition methods and varying processing times; (b) SEM images of Ti, GAHD template, and TiO2 films with different deposition methods and varying processing times; (c) AFM images of Ti, GAHD template, and TiO2 films with different deposition methods and varying processing times; (d) cross-sectional SEM and corresponding energy dispersive spectrometer (EDS) EDS spectra with different deposition methods and varying processing times.
Figure 2c shows the AFM images of the substrate, GAHD template, and different TiO2 films. It is obvious that the roughness and thickness of the surface materials are larger, especially for the GAHD template, which exhibits a more uniform coverage on the substrate. After TiO2 deposition, the grains become almost spherical and uniformly distributed. The ripple morphology is observed due to the coalescence of grains, which is consistent with SEM.
The average particle size obtained in Table 1 is calculated by the AFM software based on the imported AFM images through direct particle identification. In comparison to pure titanium, when the surface is modified with a GAHD template, the roughness shows only a slight change, while the grain size experiences a significant increase. When compared to directly deposited TiO2 films, the films generated through template modulation exhibit slightly increased roughness but have smaller average grain sizes. This observation may be ascribed to the film deposition mechanism [30]. During the initial growth stage, the template-modulated film does not rapidly deposit directly on the substrate, resulting in larger agglomerations of TiO2 particles. Instead, it interacts with the functional groups on the template’s surface, forming a seed layer. With an extended deposition time of 24 h, the grain size of the film remains relatively unchanged while the roughness gradually increases. However, after 36 h of deposition, both the roughness and grain size undergo significant changes, consistent with the SEM results. Notably, the grains exhibit distinct island-like undulations. The observed undulating structure might have influenced the AFM test results, leading to a notable change in roughness values. The undulations could have impacted the AFM tip as it scanned the surface, resulting in the recorded increase in roughness. These findings regarding the roughness and grain size further support the impact of template modulation on the surface morphology of the TiO2 films.

Table 1.
All samples roughness and grain size of AFM.
Figure 2d illustrates cross-sectional views of various samples alongside their corresponding EDS analysis results. For statistical purposes regarding film thickness, we measured the thickest and thinnest segments of the film. Upon comparing TiO2 films generated through direct deposition and GAHD template modulation after 12 h, the following observations were made. Firstly, there was a notable disparity in film thickness. The TiO2 film thickness resulting from direct deposition measured approximately 0.26–0.39 μm, whereas the film thickness derived from GAHD template modulation was notably thicker at around 0.41–0.58 μm. Secondly, differences were observed in the uniformity of the film surface. The film subject to template modulation exhibited enhanced surface homogeneity. This can be attributed to the template’s rich assortment of functional groups such as -NH2, -COOH, -OH, etc. These groups facilitate heterogeneous nucleation during the initial stages, creating additional nucleation sites. Consequently, the seed layer becomes densely and uniformly structured. This uniformity significantly influences the later stages of film growth, resulting in closely packed particle spacing in the subsequent film. This foundational uniformity has a positive impact on the overall film growth process [7,8,19]. Lastly, variations in adhesive force between the film and substrate were noted. Films generated through template modulation displayed superior adhesion compared to those directly deposited. This improved adhesion is attributed to pre-treating the pure titanium substrate with dopamine prior to grafting the multifunctional template. The effective adhesion of dopamine contributes to the secure attachment of the template to the substrate. With deposition time extended to 24 h, film thickness exhibited a significant increase to approximately 0.69–0.74 μm. Further extension to 36 h led to a film thickness of 0.87–0.92 μm. This suggests that film thickness displays a gradual increase in tandem with prolonged deposition.
EDS analyses revealed that the generated TiO2 films comprised four surface elements: C, N, O, and Ti. The consistent presence of N across all samples may be attributed to residual water within the films. It is plausible that the inorganic salt ammonium fluorotitanate was retained during film generation [31,32]. Apart from these elements, no other components were detected within the TiO2 films. Consequently, it is assumed that these films are devoid of organic residues.
3.1.2. Colorimetric Staining with Acid Orange II and FTIR
In order to demonstrate that the GAHD films were successfully deposited on the substrate surface, amine groups were selectively labeled using Acid Orange II [20]. As seen in Figure 3a, the density of amine groups and optical density on the samples were measured. It is clear that the GAHD films have a maximum value of 4.1 ± 1.6 nmol/cm and corresponding optical density, which is higher than that of the other samples. This is primarily due to the contribution of HD in the template. Similarly, FT-IR spectra can also reflect the introduction of the GAHD template. The chemical structure of the PDA and GAHD template surface was analyzed using ATR-FTIR. As seen in Figure 3b, compared with the Ti@PDA surface, the Ti@GAHD surface spectra showed significant changes. Different peaks were observed at 1600 cm−1 (N–H bending and benzene skeleton C=C stretch vibration band), 1066 cm−1 (C-O vibration bands of phenolic hydroxyl groups) [22,23], and 630 cm−1–680 cm−1 (N-H out-of-plane bending vibration). These results are in agreement with previously reported findings and confirm that polydopamine was successfully fixed onto the GAHD template on the Ti substrate.
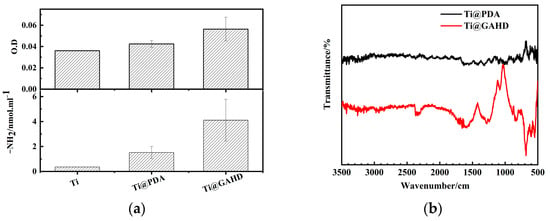
Figure 3.
Amino content test results and FTIR spectrum of samples. (a) Amino content test results for bare Ti, PDA-treated Ti (Ti@PDA), and Ti modified with GA and HD (Ti@GAHD); (b) FTIR spectrum comparison of PDA-treated Ti (Ti@PDA) and Ti modified with GA and HD (Ti@GAHD).
3.1.3. XPS
To obtain detailed information about the chemical composition of each sample, we performed high-resolution XPS investigations of N 1s, O 1s, and Ti 2p. Figure 4a,b depicts the N 1s and O 1s core-level spectra of Ti@PDA and Ti@GAHD, respectively, while Figure 4c,d shows the O 1s and Ti 2p core-level spectra of Ti@GAHD@TiO2-12 h, Ti@@GAHD@TiO2-24 h, and Ti@GAHD@TiO2-36 h.
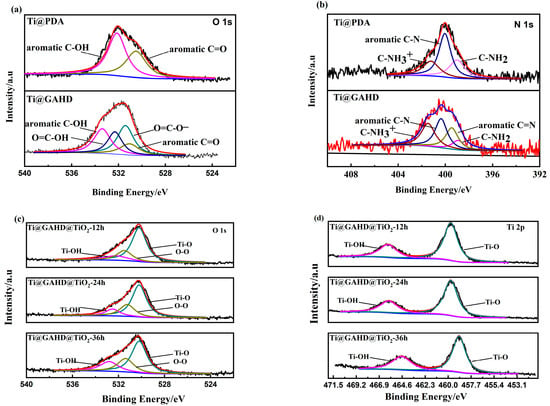
Figure 4.
High-resolution XPS of samples. (a) High-resolution XPS analysis of Ti@PDA and Ti @GAHD for C 1s regions; (b) XPS Ti@PDA and Ti@GAHD for N 1s regions; (c) high-resolution XPS analysis of template-adjusted TiO2 films with varying processing times for O 1s; (d) high-resolution XPS analysis of template-adjusted TiO2 films with varying processing times for Ti 2p.
The curve in Figure 4a,b states that the peak positions of O and N have significant overlap, which may indicate differences in the chemical structures of the different films. In the PDA XPS spectrum, the O 1s peak is determined by two components: 531.1 eV assigned to aromatic C=O (quinone) and 533.6 eV assigned to aromatic C-OH (phenolic hydroxyl group) [7]. By contrast, after the deposition of the GAHD film, two new peaks appear in the spectrum at 531.7 eV and 532.7 eV, which are allocated to O=C-O- and O=C-OH, respectively. These peaks could be allocated to the O element of carboxy groups in the gallic acid molecules. The N1s peak in the PDA spectrum is resolved into three components: 399 eV assigned to C-NH2, 400.2 eV due to aromatic C-N, and 401.3 eV assigned to C-NH3+. Similarly, after the deposition of the GAHD film, a new peak emerges at 399.5 eV due to aromatic C=N, which is contributed by a Schiff-base reaction between GA and HD. These results suggest that the GAHD film was successfully deposited on the substrate.
Figure 4c,d shows the XPS spectra of Ti 2p and O 1s, respectively. From Figure 4c, it can be seen that the O 1s region is composed of three peaks. Depositing films for different lengths of time resulted in peaks appearing at 530.2 eV (Ti-O), 532.3 eV (Ti-OH), and 531.9 eV (O-O) on the surface of the thin film. During the growth stage of the thin film in the later stages of deposition, chelation occurs between TiO2 and phenolic hydroxyl groups, resulting in the production of Ti-OH on the surface, which is consistent with the previously discussed mechanism of film formation; O-O (531.4 eV) ascribed to the adventitious O2 from the ambient atmosphere can be found commonly. At 530.2 eV, the oxygen element of TiO2 is present, and the other peak at Eb = 532.3 eV is related to H2O and Ti-OH on the surface. These results indicate that the TiO2 film was deposited on the template of the GAHD film. From Figure 4d, a main doublet composed of two symmetric peaks, Eb (Ti 2p3/2) = 458.9 eV and Eb (Ti 2p1/2) = 464.5 eV, is assigned to Ti4+ in the Ti 2p spectrum. The high proportion of Ti 2p1/2 indicates that the TiO2 film is composed of the stoichiometric anatase phase of titania, which is consistent with the results reported in the literature for liquid phase deposition [27,33]. With an increase in the deposition time, the peaks have a tendency to move in the lower binding energy direction. This could be because there is a rise in the number of oxygen vacancies on the film surface, causing a shift of the Ti 2p peak towards a lower binding energy due to changes in the chemical environment of the Ti4+ ions. Although the number of Ti3+ ions on the TiO2 surface may be too low to be detected by XPS, the presence of Ti3+ can increase the electron density, thus leading to a weakening of the binding energy, resulting in a shift towards lower binding energy directions [34,35,36,37].
3.1.4. XRD
Figure 5 depicts the typical XRD pattern obtained from the prepared films. The TiO2 thin film, prepared using the liquid phase deposition method, shows distinct peak positions at 25° and 37.8°, which are observed beyond the peaks originating from the Ti substrate (35.1°, 38.4°, 40.1°), indicating the presence of the anatase phase [38]. Combined with data from Table 2, the (101) peak maintains a slow growth, but it is useful to note that when the TiO2 film is directly deposited on the substrate for 12 h, it exhibits a higher peak intensity of about 709 at 37.8° compared to the film generated under template modulation about 550. This difference can be ascribed to a few factors. On the one hand, it is easy to achieve a continuous film on the untreated template, as there are no additional functional groups present. On the other hand, after template modulation, the introduction of multifunctional groups increases the density of nucleation sites on the substrate. The TiO2 seed layer formed during template modulation allows subsequent TiO2 particles to grow in a specific direction. Consequently, during the initial stage of deposition, TiO2 directly deposited on the substrate without a template undergoes rapid growth [39,40,41]. We can see from Table 2 that with an extension of the deposition time from 24 h to 36 h, we observed noteworthy changes in the X-ray diffraction patterns. The (101) peak intensity increased from 542 to 688, and the (004) peak intensity from 935 to 1190. However, the ratio of peak intensities remained relatively stable between the two crystal planes, fluctuating between 0.580 and 0.578. Similarly, the peak integral area ratio ranged from 0.523 to 0.532. These observations suggest that, as the deposition time is prolonged, the alterations in the preferred orientation growth of the grains are not significant. Furthermore, for the Ti@TiO2-12 h sample, an additional peak position at 40.9° was observed. After consulting the relevant XRD standard cards for Ti and TiO2, no corresponding peaks were found; therefore, this peak might be attributed to an impurity peak.
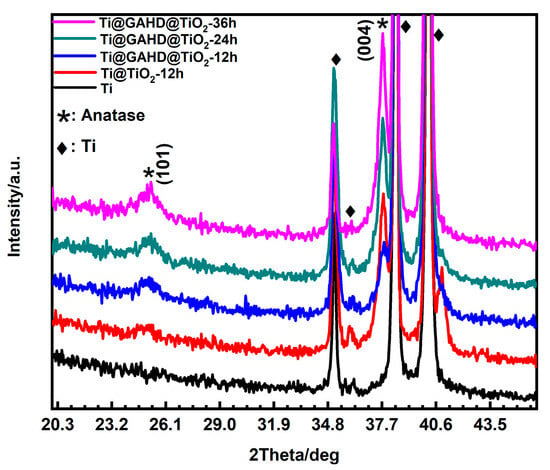
Figure 5.
XRD patterns of bare Ti, TiO2 film (Ti@TiO2-12 h), and TiO2 film modified with GAHD template (Ti@GAHD@TiO2-12 h, Ti@GAHD@TiO2-24 h, Ti@GAHD@TiO2-36 h).

Table 2.
The integral area of the peak, the peak intensity, and the integral area and peak intensity ratio.
3.2. Performance Testing
3.2.1. Contact Angle
The water contact angles (WCA) and surface energy of the different samples are provided in Figure 6. From Figure 6, the contact angle changes after the deposition of the template and TiO2 film can be seen. Compared to the bare substrate, Ti@GAHD WCA decreased significantly, suggesting that the surface of the template is hydrophilic. Furthermore, with the same deposition time, when GAHD is invoked as a template, the WCA decreases even further. This can be attributed to the hydrophilic functional groups in the template, such as -NH2, -COOH, -OH, and so on, which can improve the heterogeneous nucleation of solids on the template surface, resulting in a decrease in the surface energy of the template relative to that of a bare substrate. Table 3 shows the water and diiodomethane contact angles, surface energy, and polar dispersion data of samples.
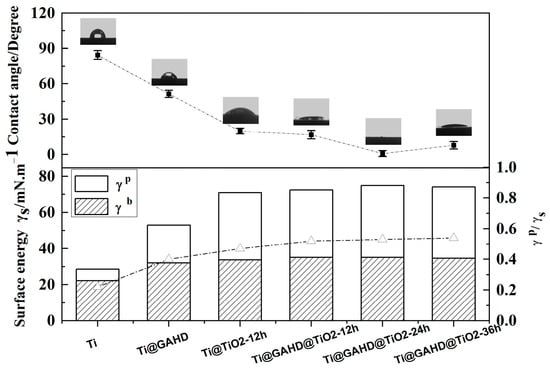
Figure 6.
Water contact angle images and the ratio of polar components to surface energy of bare Ti, GAHD template (Ti@GAHD), TiO2 film (Ti@TiO2-12 h), and TiO2 film modified with GAHD template (Ti@GAHD@TiO2-12 h, Ti@GAHD@TiO2-24 h, Ti@GAHD@TiO2-36 h).

Table 3.
Water contact angles and the ratio of polar to dispersive components of surface energy for bare Ti, GAHD template (Ti @GAHD), TiO2 film (Ti@TiO2-12 h), and GAHD template adjusted TiO2 films (Ti@GAHD@TiO2-12 h, Ti@GAHD@TiO2-24 h, Ti@GAHD@TiO2-36 h).
Specifically, the original Ti substrate presented a WCA of 84 ± 3.7°. The WCA had a significant decrease after fixing the GAHD template on the substrate, which is about 51 ± 3.1°. When TiO2 was directly deposited on the Ti substrate for 12 h, it was about 20 ± 2.4°. However, using the GAHD film as a template, the WCA varied, which was reduced by approximately 3°. This may be ascribed to the more -OH groups on the surface of the TiO2 film, which is consistent with the mechanism that phenols react with TiO2 in the film-forming process by LPD.
As the deposition time of TiO2 increases, the WCA has an obvious decrease, especially at the deposition time of 24 h, where the surface of the sample exhibited a super-hydrophilicity phenomenon, which corresponds to the surface energy and polar components of the sample [42]. The higher the ratio of γp/(γd + γp) the materials have, the more hydrophilic the surface is [43]. Using the GAHD film as a template, at the deposition time of 24 h, the surface energy and polar components reach a maximum. The obtained film is so hydrophilic that it may be due to three reasons: firstly, TiO2 itself has relatively good hydrophilicity; secondly, with the extension of the TiO2 film deposition time, the surface of the samples produces a relatively large number of Ti-OH groups; thirdly, this may be related to grain size and surface roughness [44,45]. We know that the surface hydrophilicity of the material is an essential factor influencing biocompatibility, so the obtained materials may be favorable for improving biocompatibility.
3.2.2. Photoluminescence Spectra
Figure 7 displays the photocurrent spectra of different samples under 220 nm wavelength. As TiO2 is a semiconductor material, discussing its energy levels is necessary to study its electrical properties. Comparing it with the Ti substrate, after the deposition of TiO2, two new peaks appeared at about 373.7 nm and 663.3 nm, corresponding to the band gap of 3.32 eV and 1.87 eV, respectively. The bandgap energy of anatase is 3.2 eV and corresponds to the excitation wavelength of 387 nm, so the peak of all samples has a blue shift at 373.7 nm [46]. This is proof of the TiO2 absorption peak and the transition of electrons in the band gap. The strength of the differences is determined by the number of the carrier concentration. Depositing the same amount of time, the peak of Ti@GAHD@TiO2-12 h is stronger than Ti@TiO2-12 h due to the former’s superior film thickness and density over the latter. When the two samples are exposed to the constant excitation light, more electrons of the former will be inspired, and therefore, they have greater chances of electron-hole composite, showing a strong photoluminescence peak. As the deposition time increases, the carrier concentration increases, and the corresponding photoluminescence peak gradually enhances.
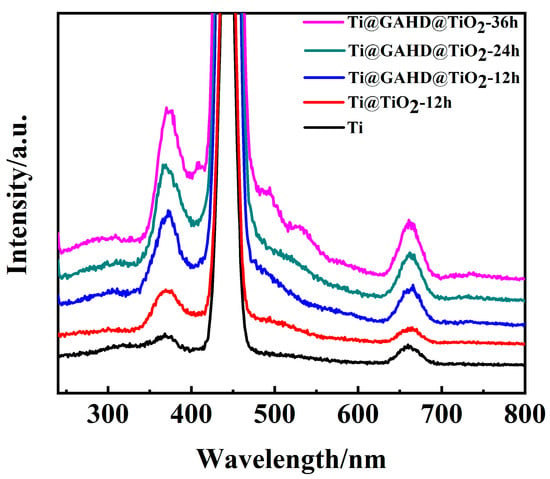
Figure 7.
Photoluminescence spectra of bare Ti, TiO2 film (Ti@TiO2-12 h), and template-adjusted TiO2 films (Ti@GAHD@TiO2-12 h, Ti@GAHD@TiO2-24 h, Ti@GAHD@TiO2-36 h).
There is just an apparent difference in the strength of the peak position at 663.3 nm. The intensity of Ti@GAHD@TiO2-12 h is considerably higher than that of Ti@TiO2-12 h. We are aware that in the peak of 663.3 nm, an emission peak from the bound exciton luminescence appears. The reason for this phenomenon is the small size of nanoparticles of TiO2 particles or the surface of a large number of oxygen vacancies and defects. At the same time, the larger the oxygen vacancy and defect content, the more efficiently the photoelectrons and holes can be separated, so the higher the probability of exciton occurrence and the stronger the PL signal. Therefore, we can conclude that differences in the intensity of the peaks occur owing to GAHD template adjustments, resulting in more oxygen vacancies and defects in the TiO2 film [47,48]. Additionally, the emitted light intensity of all template-modulated TiO2 films is significantly stronger compared to that of directly deposited TiO2 films, and it further increases with the deposition time. This enhancement can be attributed, in part, to the improved crystalline quality of titanium dioxide and the increased thickness of the TiO2 films. When TiO2 film is excited by light, electronics in the film absorb energy and transition to the valence band. In the excited state, the electronic spontaneously transitions to the low-energy state and implements luminescence through emitted photons. Therefore, prolonging the reaction time, the PL emission intensity of the film increases significantly at 36 h, exhibiting the largest photocurrent in UV light. Oxygen vacancies and surface states can strongly affect photocatalytic reactions. Therefore, the TiO2 obtained, adjusted by the GAHD template, can be implemented in the field of photocatalysis.
3.2.3. EIS Bode Spectra and Schottky
Figure 8 illustrates the electrochemistry performance of the TiO2 film deposited on a multifunctional group template. Electrochemical impedance spectroscopy (EIS) measurements were carried out using a small perturbation over the open circuit potential. Figure 8a shows the EIS Bode spectra of samples in PBS. The top of the impedance data reveals that the impedance values of Ti@GAHD@TiO2-12 h are approximately 1E3 in the high-frequency region, while the impedance values of Ti@TiO2-12 h are roughly 1E2. This indicates that Ti@GAHD@TiO2-12 h has better corrosion resistance than Ti@TiO2-12 h [49]. The characteristic frequency of TiO2 deposited on GAHD film, shown in the Bode phase plots, increases two times, particularly in the high frequency, where a new peak CPE-1 appears at around 103 Hz. This peak is distinct from CPE-1’ at around 10Hz, as compared to the deposited TiO2 without a template. It can be inferred that the response at high frequency is related to the space charge layer capacitance Csc and resistance of Ti–O film Rf, while the response at low frequency at around 10−1 Hz can be associated with the double layer capacitance Cdl and interfacial reaction resistance Rct [50]. Thus, the GAHD template played a decisive role in adjusting the deposition of the TiO2 film.
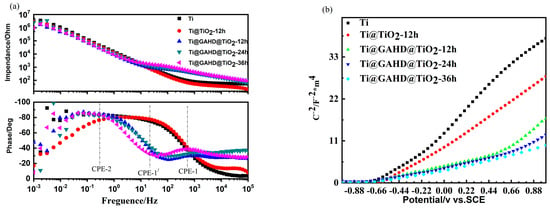
Figure 8.
Electrochemistry impedance spectroscopy (EIS) and Schottky of samples. (a) Electrochemistry impedance spectroscopy (EIS) of bare Ti, TiO2 film (Ti@TiO2-12 h), and template-adjusted TiO2 film (Ti@GAHD@TiO2-12 h, Ti@GAHD@TiO2-24 h, Ti@GAHD@TiO2-36 h); (b) Schottky of bare Ti, TiO2 film (Ti@TiO2-12 h), and template-adjusted TiO2 film (Ti@GAHD@TiO2-12 h, Ti@ GAHD@TiO2-24 h, Ti@GAHD@TiO2-36 h).
To gain a better understanding of the differences in the TiO2 film with and without the template deposition, impedance techniques and Mott-Schottky (MS) measurements were performed. Figure 8b shows the sigmoidal plot shape that is consistent with that of typical n-type semiconductors. The slope of the Mott–Schottky plot provides a rough estimate of the donor density in the TiO2 film [51] and the flat band potential obtained by extrapolation of the Mott–Schottky plot. Table 4 displays the data of Efb and NSC of samples in detail. Compared with the Ti substrate, the rate of the curve had an observable reduction, and the flat band potential decreased from −0.57 V/SCE to −0.79 V/SCE, which shifted to a more negative potential consistently after being adjusted by the GAHD template. A more negative potential of Efb relates to a higher surface Fermi energy level position, indicating that the samples were more prone to electronic overflow. Additionally, the rate of the latter curve is gentler, which suggests a higher donor density [52]. This may be explained by the use of the GAHD film as a template, causing a difference in the deposited TiO2 [53,54]. Table 4 presents the data of Nsc and Efb of all samples analyzed by the Mott–Schottky equation.

Table 4.
Donor density data of bare Ti, TiO2 film (Ti@TiO2-12 h), and template-adjusted TiO2 films (Ti@GAHD@TiO2-12 h, Ti@GAHD@TiO2-24 h, Ti@GAHD@TiO2-36 h).
4. Discussion
The mechanism of the formation of the TiO2 film deposited on a multifunctional group template involves two steps: heterogeneous nucleation and homogeneous growth. Using the LPD method, the mix solution of (NH4)2TiF6 and H3BO3 contains many cation and anion ions, such as F−, BF4−, Ti(OH)62−, H+, and others [4,5]. TiO2 particles are obtained from the hydrolysis of (NH4)2TiF6 and H3BO3, and crystals and films are produced according to the following hydrolysis reactions:
Template selection is critical in controlling the deposition of thin films. In this experiment, a template with better hydrophilicity than a Ti bare substrate was supposed to promote liquid-phase deposition of the film. In solution, various ions such as F−, BO33−, BF4−, TiF62−, and H+ exist, providing the TiO2 particles with different positive and negative charges. The substrate surface properties affect the deposition results during the initial stage of particle deposition. Therefore, the selection of functional groups on the template surface is also essential. As noted in the literature, functional groups such as -NH2, -COOH, and -OH can promote the deposition of the film, whether as a single functional group or as a template formed by alternate deposition of polyelectrolytes. Therefore, it is necessary to choose a substance that simultaneously possesses these three functional groups to avoid the incompleteness of single functional group regulation and the complexity of the template formation process by layer-by-layer polyelectrolyte deposition. Finally, the product obtained by reacting gallic acid with hexamethylenediamine was selected as a multifunctional group template. Furthermore, the product has the conditions of containing these three functional groups simultaneously, and it presents hydrophilic characteristics. The experimental process is easy and feasible.
Since there are various positive and negative ions present in the solution that adsorb on the surface of the initial TiO2 particles, the TiO2 particles become charged. The template we prepared contains -NH2, -COOH, and -OH groups, which cause TiO2 particles to be attracted to the template due to the attractive electrostatic interaction and form a TiO2 film. This multifunctionality, combined with the hydrophilicity of the template, reduces the surface energy compared to the bare substrate. As a result, in the deposition process, the GAHD template promotes the deposition of TiO2 particles, enhancing the heterogeneous nucleation of TiO2 on the GAHD template. Then, the completion of heterogeneous nucleation by the group of -OH chelates with TiO2 particles [7,8,19], and this is performed through the following reactions:
As a consequence, the template can provide more nucleation sites for the deposition of TiO2 particles. This may be a major reason why the film deposited by the template improves the density and uniformity. Next, the layer of nucleation TiO2 particles on the substrate acts as a seed layer that promotes the uniform condensation of particles, leading to growth. After ultrasonication of the final TiO2 film for 20 min, it was found that the film had a strong force with the substrate through the template-adjusted deposition method compared to the direct deposition method. Therefore, deposition from templates adjusting mechanism can improve the binding force of the TiO2 film and template. As seen in Figure 9, it represents a schematic diagram illustrating the regulation of TiO2 film growth using the GAHD template.
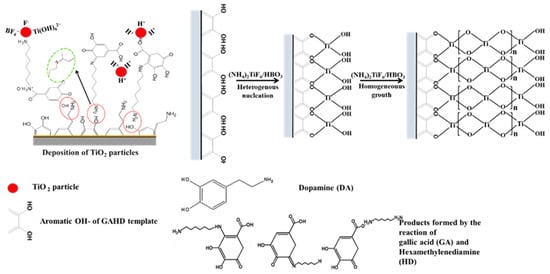
Figure 9.
GAHD template regulates the growth mechanism of TiO2 thin films.
5. Conclusions
A TiO2 composite film was successfully synthesized using the GAHD film as a template on the DA pre-treated substrate by the LPD method. The multifunctional groups had an effect on the structure and electrochemical properties of TiO2 films. Corroborative results from SEM, AFM, XRD, and XPS revealed the composition and porous anatase crystalline structure of the films. The contact angle indicated that the TiO2 film had super-hydrophilicity properties and higher surface energies, which may be favorable for biocompatibility. From the electrochemical measurement results, it was observed that the TiO2 film had better luminous and corrosion resistance performance than the traditional method, which is deposited directly on the substrate. Hence, we conclude that the TiO2 film would be a potential material for biology and photocatalysts.
Author Contributions
Conceptualization, H.L. and G.W.; methodology, H.L.; validation, H.L.; formal analysis, S.C.; investigation, C.L.; resources, G.W.; data curation, X.Z.; writing—original draft preparation, H.L.; writing—review and editing, G.W.; visualization, H.L.; supervision, X.Z.; project administration, G.W.; funding acquisition, G.W. All authors have read and agreed to the published version of the manuscript.
Funding
National Key Research and Development Program of China (2016YFE1102500); Sichuan Province Science and Technology Support Program (2020YFH0077); Science and Technology of Guangxi for Base and Talent Project (AD22035222).
Data Availability Statement
Data underlying the results presented in this paper are available from the author upon request.
Acknowledgments
We would like to thank the Analytical and Testing Center of Southwest Jiaotong University for the characterization analysis and discussion.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhong, W.-L.; Li, C.; Liu, X.-M.; Bai, X.-K.; Zhang, G.-S.; Lei, C.-X. Liquid phase deposition of flower-like TiO2 microspheres decorated by ZIF-8 nanoparticles with enhanced photocatalytic activity. Microporous Mesoporous Mater. 2020, 306, 110401. [Google Scholar] [CrossRef]
- Phuinthiang, P.; Trinh, D.T.T.; Channei, D.; Ratananikom, K.; Sirilak, S.; Khanitchaidecha, W.; Nakaruk, A. Novel strategy for the development of antibacterial TiO2 thin film onto polymer substrate at room temperature. Nanomaterials 2021, 11, 1493. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, C. TiO2 coated ZnO nanorods by mist chemical vapor deposition for application as photoanodes for dye-sensitized solar cells. Nanomaterials 2019, 9, 1339. [Google Scholar] [CrossRef]
- Huang, J.-J.; Ou, S.-L.; Hsu, C.-F.; Shen, X.-Q. The effect of boric acid concentration on the TiO2 compact layer by liquid-phase deposition for dye-sensitized solar cell. Appl. Surf. Sci. 2019, 477, 7–14. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, X.; Chen, Y.; Zhang, W.; Qian, J.; Soliman, H.; Qu, A.; Liu, Q.; Pu, S.; Huang, N.; et al. Ultraviolet irradiation assisted liquid phase deposited titanium dioxide (TiO2)-incorporated into phytic acid coating on magnesium for slowing-down biodegradation and improving osteo-compatibility. Mat. Sci. Eng. C-Mater. 2020, 108, 110487. [Google Scholar] [CrossRef]
- Dahlan, D.; Saad, S.K.M.; Berli, A.U.; Bajili, A.; Umar, A.A. Synthesis of two-dimensional nanowall of Cu-Doped TiO2 and its application as photoanode in DSSCs. Phys. E Low Dimens. Syst. Nanostruct. 2017, 91, 185–189. [Google Scholar] [CrossRef]
- McDonald, B.T.; Cui, T. Superhydrophilic surface modification of copper surfaces by Layer-by-Layer self-assembly and Liquid Phase Deposition of TiO2 thin film. J. Colloid Interface Sci. 2011, 354, 1–6. [Google Scholar] [CrossRef]
- Attar, A.S.; Hassani, Z. Fabrication and growth mechanism of single-crystalline rutile TiO2 nanowires by liquid-phase deposition process in a porous alumina template. J. Mater. Sci. Technol. 2015, 31, 828–833. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Rodiansyah, A.; Sawant, J.; Park, K.C. Patterning of Silicon Substrate with Self-Assembled Monolayers Using Vertically Aligned Carbon Nanotube Electron Sources. Nanomaterials 2022, 12, 4420. [Google Scholar] [CrossRef]
- HLee, A.; Ma, Y.; Zhou, F.; Hong, S.; Lee, H. Material-independent surface chemistry beyond polydopamine coating. Acc. Chem. Res. 2019, 52, 704–713. [Google Scholar]
- Pandey, P.; Pandey, A.; Shukla, N.K. Surface modification by self-assembled monolayer and carbon nanotubes. Emerg. Mater. Res. 2017, 6, 15–20. [Google Scholar] [CrossRef]
- Yumnam, N.; Wagner, V. Controlled growth of ZnO nanorods via self-assembled monolayer. J. Appl. Electrochem. 2018, 48, 85–94. [Google Scholar] [CrossRef]
- Dianat, S.; Hatefi-Mehrjardi, A.; Mahmoodzadeh, K. Electrochemical behavior of inorganic–organic hybrid polyoxometalate ((Cys)3[PW12O40]) nanostructure self-assembled monolayer on polycrystalline gold electrode surfaces. New J. Chem. 2019, 43, 1388–1397. [Google Scholar] [CrossRef]
- Liu, S.; Shan, H.; Xia, S.; Yan, J.; Yu, J.; Ding, B. Polymer template synthesis of flexible SiO2 nanofibers to upgrade composite electrolytes. ACS Appl. Mater. Interfaces 2020, 12, 31439–31447. [Google Scholar] [CrossRef] [PubMed]
- Mashentseva, A.; Kozlovskiy, A.; Zdorovets, M. Electrochemical template synthesis of copper nanotubes from nitrate and sulfate electrolytes. Russ. J. Gen. Chem. 2019, 89, 988–993. [Google Scholar] [CrossRef]
- Park, M.S.; Kim, D.J.; Cho, H.H.; Kim, J.H. Solid polymer electrolyte dye-sensitized solar cells with organized mesoporous TiO2 interfacial layer templated by poly (vinyl alcohol)–poly (methyl methacrylate) comb copolymer. Solid State Ion. 2017, 300, 195–204. [Google Scholar]
- Duan, G.-W.; Xie, L.; Zhao, C.; Wang, M.; Ge, X.-W. Influence of the Surface Electrical Property of Polymer Template Microspheres on the Morphology of Hollow Silica Microspheres. Acta Polym. Sin. 2017, 5, 785–792. [Google Scholar]
- Park, S.S.; Ha, C.S. Hollow mesoporous functional hybrid materials: Fascinating platforms for advanced applications. Adv. Funct. Mater. 2018, 28, 1703814. [Google Scholar] [CrossRef]
- Lei, C.; Zhou, H.; Feng, Z.; Zhu, Y.; Du, R. Liquid phase deposition (LPD) of TiO2 thin films as photoanodes for cathodic protection of stainless steel. J. Alloys Compd. 2012, 513, 552–558. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Yang, Z.; Zhou, S.; Luo, R.; Maitz, M.F.; Zhao, Y.; Wang, J.; Xiong, K.; Huang, N. A simple one-step modification of various materials for introducing effective multi-functional groups. Colloids Surf. B Biointerfaces 2014, 113, 125–133. [Google Scholar] [CrossRef]
- Qiao, H.; Xiao, H.; Huang, Y.; Yuan, C.; Zhang, X.; Bu, X.; Wang, Z.; Han, S.; Zhang, L.; Su, Z.; et al. SiO2 loading into polydopamine-functionalized TiO2 nanotubes for biomedical applications. Surf. Coat. Technol. 2019, 364, 170–179. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.; Luo, R.; Maitz, M.F.; Jing, F.; Sun, H.; Huang, N. The covalent immobilization of heparin to pulsed-plasma polymeric allylamine films on 316L stainless steel and the resulting effects on hemocompatibility. Biomaterials 2010, 31, 2072–2083. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yang, P.; Qin, W.; Maitz, M.F.; Zhou, S.; Huang, N. The effect of coimmobilizing heparin and fibronectin on titanium on hemocompatibility and endothelialization. Biomaterials 2011, 32, 4691–4703. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Y.; Sheng, M.; Cui, S.; Wang, Z.; Zhang, X.; Yang, C.; Yu, Z.; Zhang, Y.; Tian, S.; et al. Enhanced Adhesion of Carbon Nanotubes by Dopamine Modification. Langmuir 2019, 35, 4527–4533. [Google Scholar] [CrossRef]
- Wu, K.; Wang, Y.; Zhitomirsky, I. Electrophoretic deposition of TiO2 and composite TiO2–MnO2 films using benzoic acid and phenolic molecules as charging additives. J. Colloid Interface Sci. 2010, 352, 371–378. [Google Scholar] [CrossRef]
- Pizem, H.; Sukenik, C.N.; Sampathkumaran, U.; McIlwain, A.K.; De Guire, M.R. Effects of substrate surface functionality on solution-deposited titania films. Chem. Mater. 2002, 14, 2476–2485. [Google Scholar] [CrossRef]
- Lee, K.; Lee, S.; Cho, H.; Jeong, S.; Kim, W.D.; Lee, S.; Lee, D.C. Cu+-incorporated TiO2 overlayer on Cu2O nanowire photocathodes for enhanced photoelectrochemical conversion of CO2 to methanol. J. Energy Chem. 2018, 27, 264–270. [Google Scholar] [CrossRef]
- Hao, C.; Wang, W.; Zhang, R.; Zou, B.; Shi, H. Enhanced photoelectrochemical water splitting with TiO2@Ag2O nanowire arrays via p-n heterojunction formation. Sol. Energy Mater. Sol. Cells 2018, 174, 132–139. [Google Scholar] [CrossRef]
- Ma, B.; Goh, G.K.; Ma, J.; White, T.J. Growth kinetics and cracking of liquid-phase-deposited anatase films. J. Electrochem. Soc. 2007, 154, D557. [Google Scholar] [CrossRef]
- Herbig, B.; Löbmann, P. TiO2 photocatalysts deposited on fiber substrates by liquid phase deposition. J. Photoch. Photobio. A 2004, 163, 359–365. [Google Scholar] [CrossRef]
- Mu, W.; Shi, S. Fabrication and Characterization of TiO2-Organic Thin Film from an Aqueous Solution. Polym. Polym. Compos. 2014, 22, 699–704. [Google Scholar] [CrossRef]
- Fuchs, T.M.; Hoffmann, R.C.; Niesen, T.P.; Tew, H.; Bill, J.; Aldinger, F. Deposition of titania thin films from aqueous solution by a continuous flow technique. J. Mater. Chem. 2002, 12, 1597–1601. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, C.; Zhu, L.; Zhang, J. Photoelectrochemical activity of liquid phase deposited TiO2 film for degradation of benzotriazole. J. Hazard. Mater. 2010, 175, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Guan, Z.; Zhang, M.; He, C.; Li, Q.; Yang, J. Adjusting the ratio of bulk single-electron-trapped oxygen vacancies/surface oxygen vacancies in TiO2 for efficient photocatalytic hydrogen evolution. Catal. Sci. Technol. 2018, 8, 2809–2817. [Google Scholar] [CrossRef]
- Matharu, J.; Cabailh, G.; Thornton, G. Synthesis of TiO2(110) ultra-thin films on W(100) and their reactions with H2O. Surf. Sci. 2013, 616, 198–205. [Google Scholar] [CrossRef]
- Shin, J.Y.; Min, W.J.; Chang, H.S.; Kim, K.J. Thickness Measurement of Ultra-thin TiO2 Films by Mutual Calibration Method. Appl. Sci. Converg. Technol. 2020, 29, 50–54. [Google Scholar] [CrossRef]
- Singh, S.A.; Madras, G. Sonochemical synthesis of Pt, Ru doped TiO2 for methane reforming. Appl. Catal. A-Gen. 2016, 518, 102–114. [Google Scholar] [CrossRef]
- Yu, J.C.; Yu, J.; Ho, W.; Zhang, J. Effects of F-doping on the photocatalytic activity and microstructures of nanocrystalline TiO2 powders. Chem. Mater. 2002, 14, 3808–3816. [Google Scholar] [CrossRef]
- Jiang, Y.; Shi, K.; Tang, H.; Wang, Y. Enhanced wettability and wear resistance on TiO2/PDA thin films prepared by sol-gel dip coating. Surf. Coat. Technol. 2019, 375, 334–340. [Google Scholar] [CrossRef]
- Krumdieck, S.; Gorthy, R.; Gardecka, A.J.; Lee, D.; Miya, S.S.; Talwar, S.D.; Polson, M.I.; Bishop, C. Characterization of photocatalytic, wetting and optical properties of TiO2 thin films and demonstration of uniform coating on a 3-D surface in the mass transport controlled regime. Surf. Coat. Technol. 2017, 326, 402–410. [Google Scholar] [CrossRef]
- Mesilov, V.; Galakhov, V.; Gubkin, A.; Sherstobitova, E.; Zakharova, G.; Uimin, M.; Yermakov, A.Y.; Kvashnina, K.; Smirnov, D. X-ray diffraction and X-ray spectroscopy studies of cobalt-doped anatase TiO2: Co nanopowders. J. Phys. Chem. C 2017, 121, 24235–24244. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, S.; Chang, J.; Huan, Z.; Li, H. TiO2 Nanotubes Enhance Vascularization and Osteogenic Differentiation Through Stimulating Interactions Between Bone Marrow Stromal Cells and Endothelial Cells. J. Biomed. Nanotechnol. 2018, 14, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Li, P.; Xiang, X.; Zhou, J.; Huang, N. Responsive surface charge transfer doping effect of reductive bio-molecules (glucose, fucoidan, and heparin) contacting on semiconducting titanium oxide films. J. Mater. Sci. 2013, 48, 4109–4116. [Google Scholar] [CrossRef]
- Peng, C.; Izawa, T.; Zhu, L.; Kuroda, K.; Okido, M. Tailoring Surface Hydrophilicity Property for Biomedical 316L and 304 Stainless Steels: A Special Perspective on Studying Osteoconductivity and Biocompatibility. ACS Appl. Mater. Interfaces 2019, 11, 45489–45497. [Google Scholar] [CrossRef]
- Li, J.-H.; Yan, B.-F.; Shao, X.-S.; Wang, S.-S.; Tian, H.-Y.; Zhang, Q.-Q. Influence of Ag/TiO2 nanoparticle on the surface hydrophilicity and visible-light response activity of polyvinylidene fluoride membrane. Appl. Surf. Sci. 2015, 324, 82–89. [Google Scholar] [CrossRef]
- Komaraiah, D.; Radha, E.; James, J.; Kalarikkal, N.; Sivakumar, J.; Reddy, M.V.R.; Sayanna, R. Effect of particle size and dopant concentration on the Raman and the photoluminescence spectra of TiO2:Eu3+ nanophosphor thin films. J. Lumin. 2019, 211, 320–333. [Google Scholar] [CrossRef]
- Ma, S.; Reish, M.E.; Zhang, Z.; Harrison, I.; Yates, J.T. Anatase-Selective Photoluminescence Spectroscopy of P25 TiO2 Nanoparticles: Different Effects of Oxygen Adsorption on the Band Bending of Anatase. J. Phys. Chem. C 2017, 121, 1263–1271. [Google Scholar] [CrossRef]
- Hosseini, M.; Haghighatzadeh, A.; Mazinani, B. Enhanced third-order optical susceptibility in heterogeneous wurtzite ZnO/anatase TiO2 core/shell nanostructures via controlled TiO2 shell thickness. Opt. Mater. 2019, 92, 1–10. [Google Scholar] [CrossRef]
- Nabavi, H.F.; Aliofkhazraei, M. Morphology, composition and electrochemical properties of bioactive-TiO2/HA on CP-Ti and Ti6Al4V substrates fabricated by alkali treatment of hybrid plasma electrolytic oxidation process (estimation of porosity from EIS results). Surf. Coat. Technol. 2019, 375, 266–291. [Google Scholar] [CrossRef]
- Babaei, M.; Dehghanian, C.; Vanaki, M. Effect of additive on electrochemical corrosion properties of plasma electrolytic oxidation coatings formed on CP Ti under different processing frequency. Appl. Surf. Sci. 2015, 357, 712–720. [Google Scholar] [CrossRef]
- Sellers, M.C.; Seebauer, E.G. Measurement method for carrier concentration in TiO2 via the Mott–Schottky approach. Thin Solid Films 2011, 519, 2103–2110. [Google Scholar] [CrossRef]
- Çomaklı, O.; Yazıcı, M.; Yetim, T.; Yetim, A.F.; Çelik, A. The effect of calcination temperatures on structural and electrochemical properties of TiO2 film deposited on commercial pure titanium. Surf. Coat. Technol. 2016, 285, 298–303. [Google Scholar] [CrossRef]
- Wang, A.; Wu, S.; Dong, J.; Wang, R.; Wang, J.; Zhang, J.; Zhong, S.; Bai, S. Interfacial facet engineering on the Schottky barrier between plasmonic Au and TiO2 in boosting the photocatalytic CO2 reduction under ultraviolet and visible light irradiation. Chem. Eng. J. 2021, 404, 127145. [Google Scholar] [CrossRef]
- Boutelala, A.; Bourfa, F.; Mahtali, M. Effect of light on electrical and photoelectrical characteristics of Al/TiO2/p-Si Schottky diode. J. Mater. Sci. Mater. Electron. 2020, 31, 11379–11389. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).