Effect of Shellac Microcapsules Blended with Carbonyl Iron Powder and Carbon Nanotubes on the Self-Healing and Electromagnetic Wave Absorption Properties of Waterborne Coatings on Fiberboard Surface
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Experimental Materials and Instruments
2.2. Preparation of Shellac Microcapsules, CIP, CNT Blended Coatings (SCCBC)
2.2.1. Preparation of Shellac Microcapsules
2.2.2. Preparation of Shellac Microcapsules, CIP, CNT Blended Powders
2.2.3. Preparation of SCCBC on the Fiberboard
2.2.4. Preparation of SCCBC on the Glass Slide
2.3. Testing and Characterisation
2.3.1. Microscopic Characterization
2.3.2. Chemical Composition
2.3.3. Optical Properties
2.3.4. Mechanical Properties
2.3.5. Roughness
2.3.6. Liquid Resistance
2.3.7. Electromagnetic Wave Absorption Performance
3. Results and Discussion
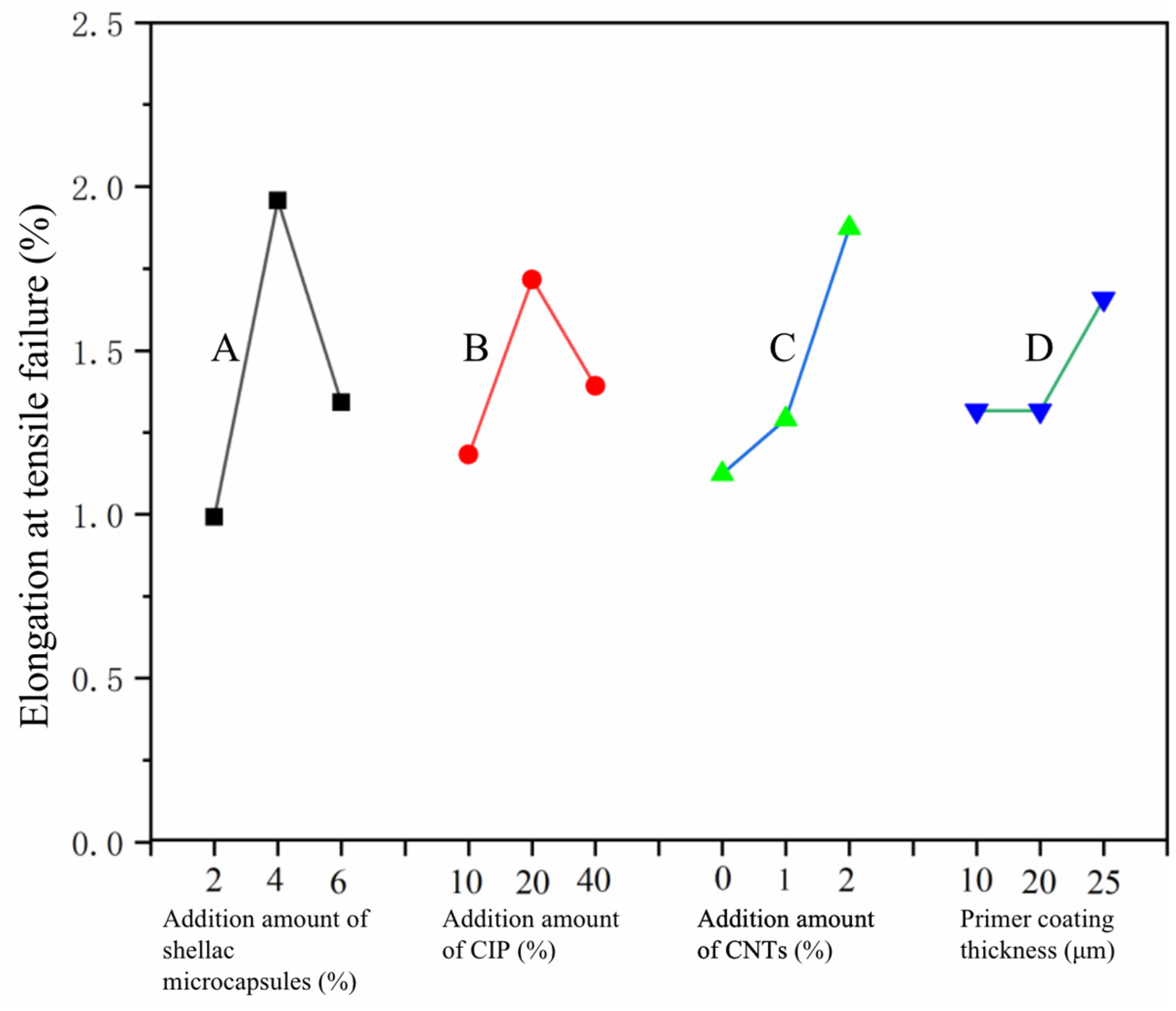
3.1. Analysis of Orthogonal Experimental Results
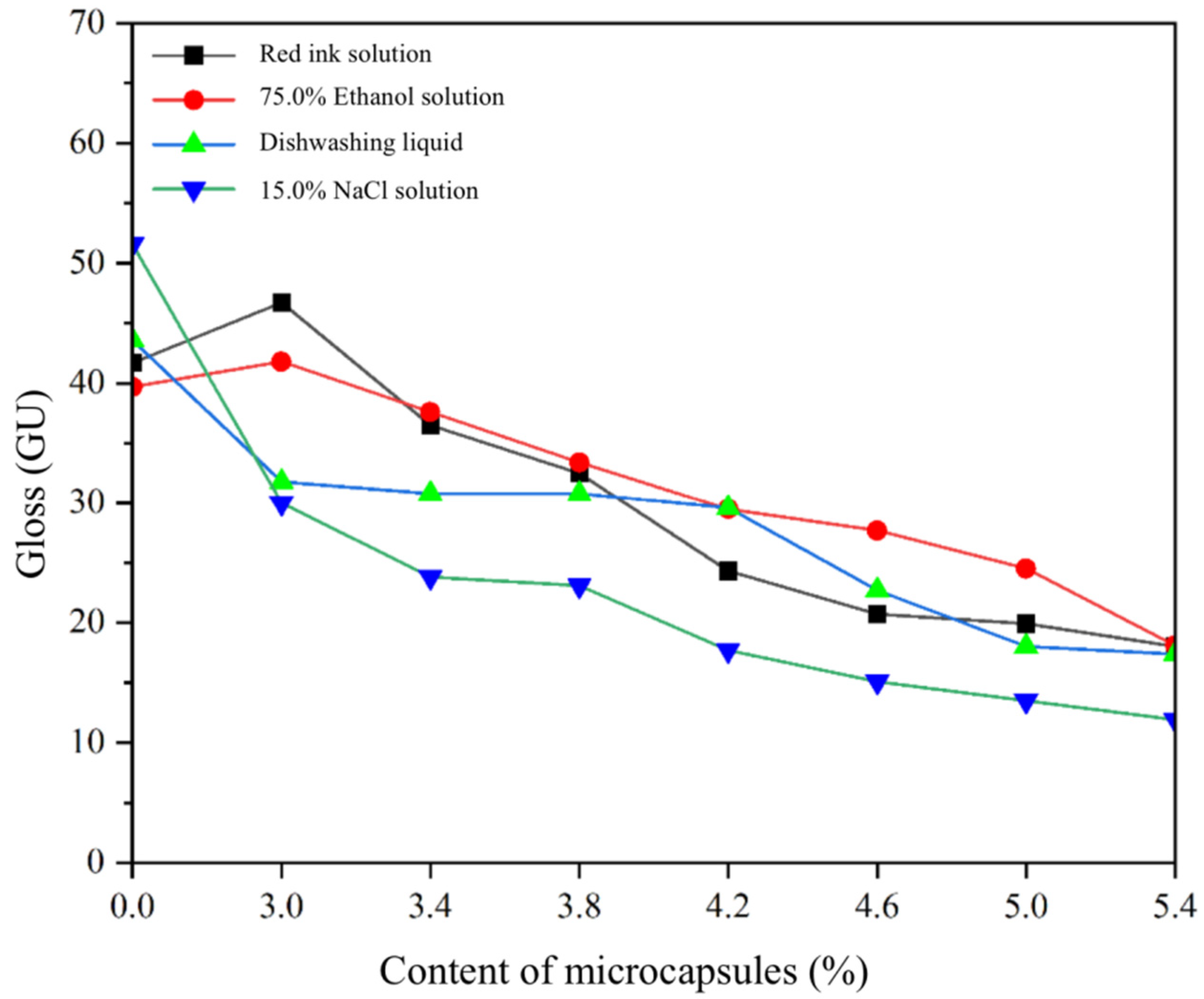
3.2. Analysis of Optical Properties of SCCBC
3.3. Analysis of Mechanical Properties and Roughness of SCCBC
3.4. Analysis of the Elongation at Tensile Failure and the Self-Healing Rate of SCCBC
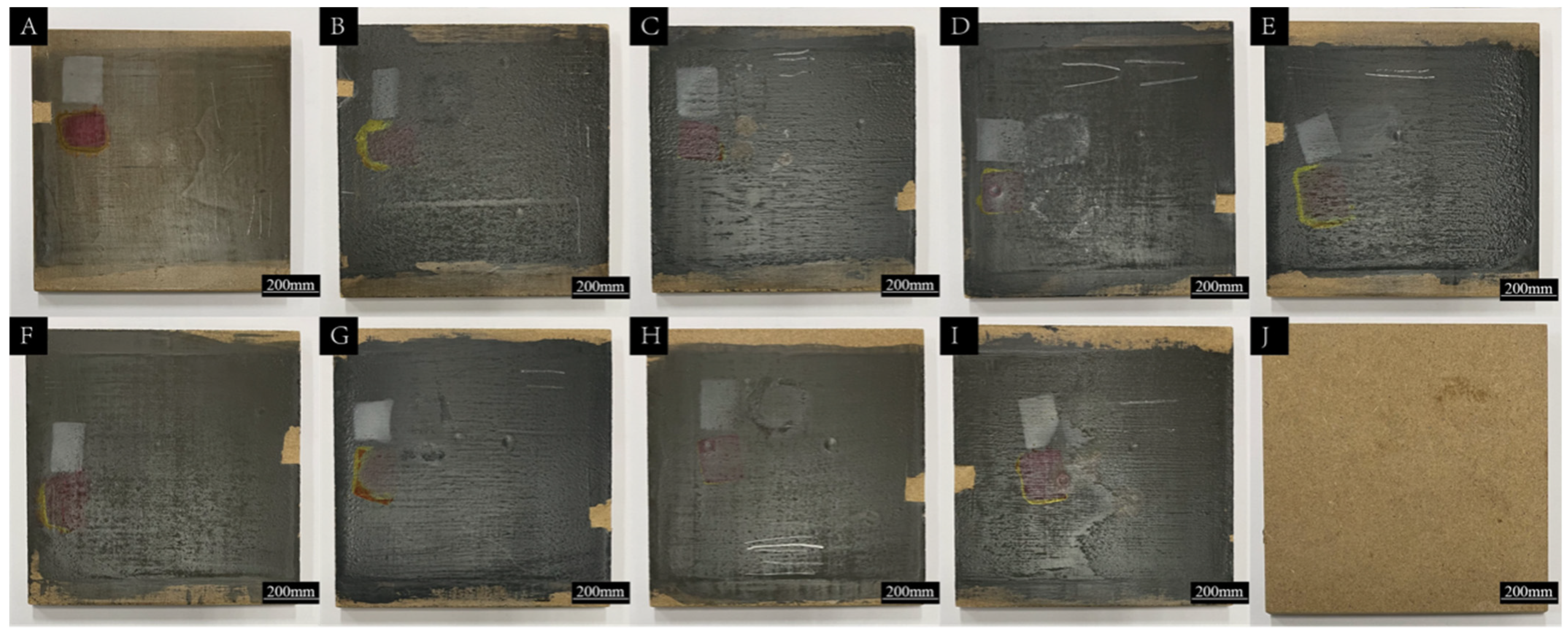
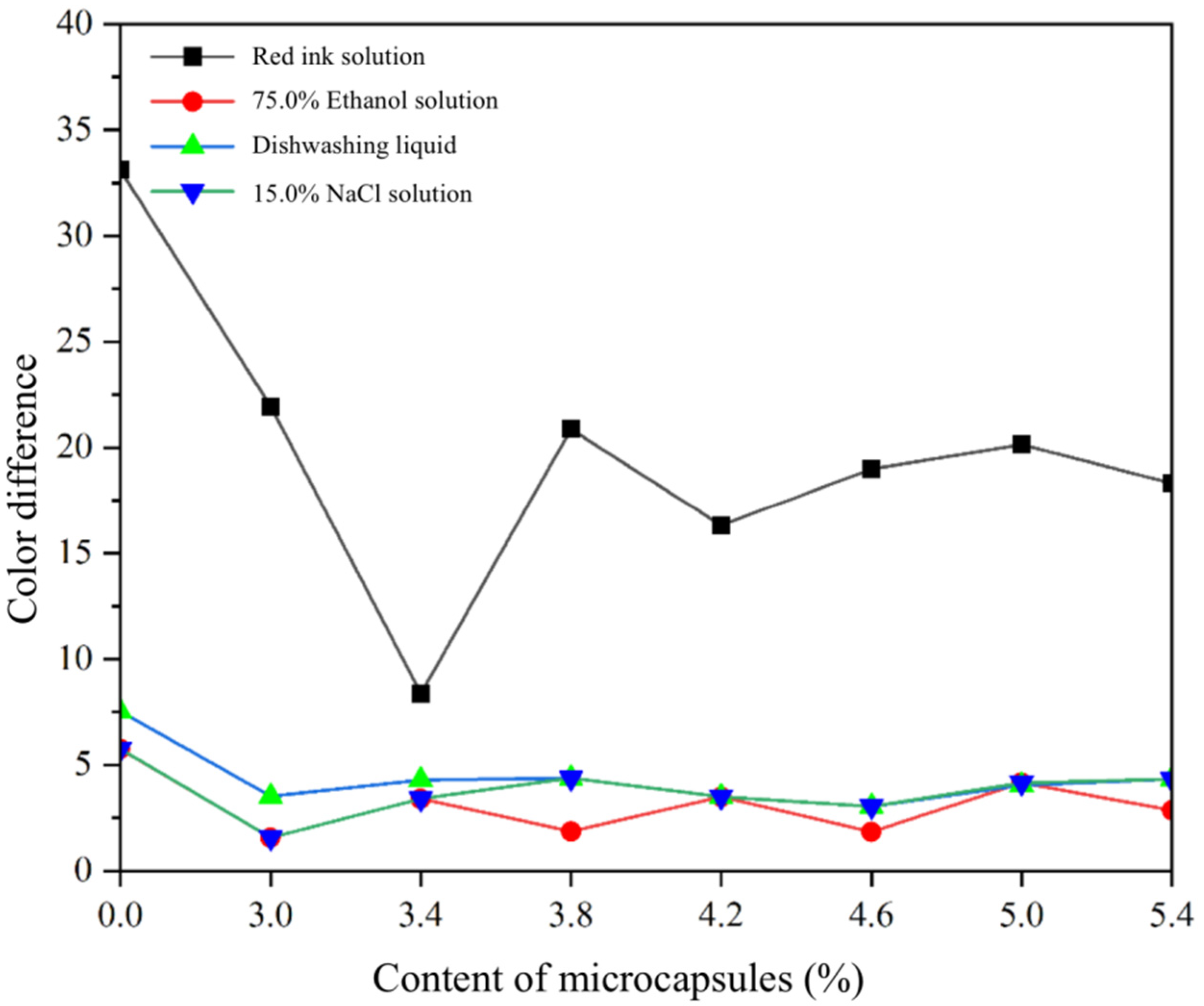
3.5. Analysis of Liquid Resistance of SCCBC
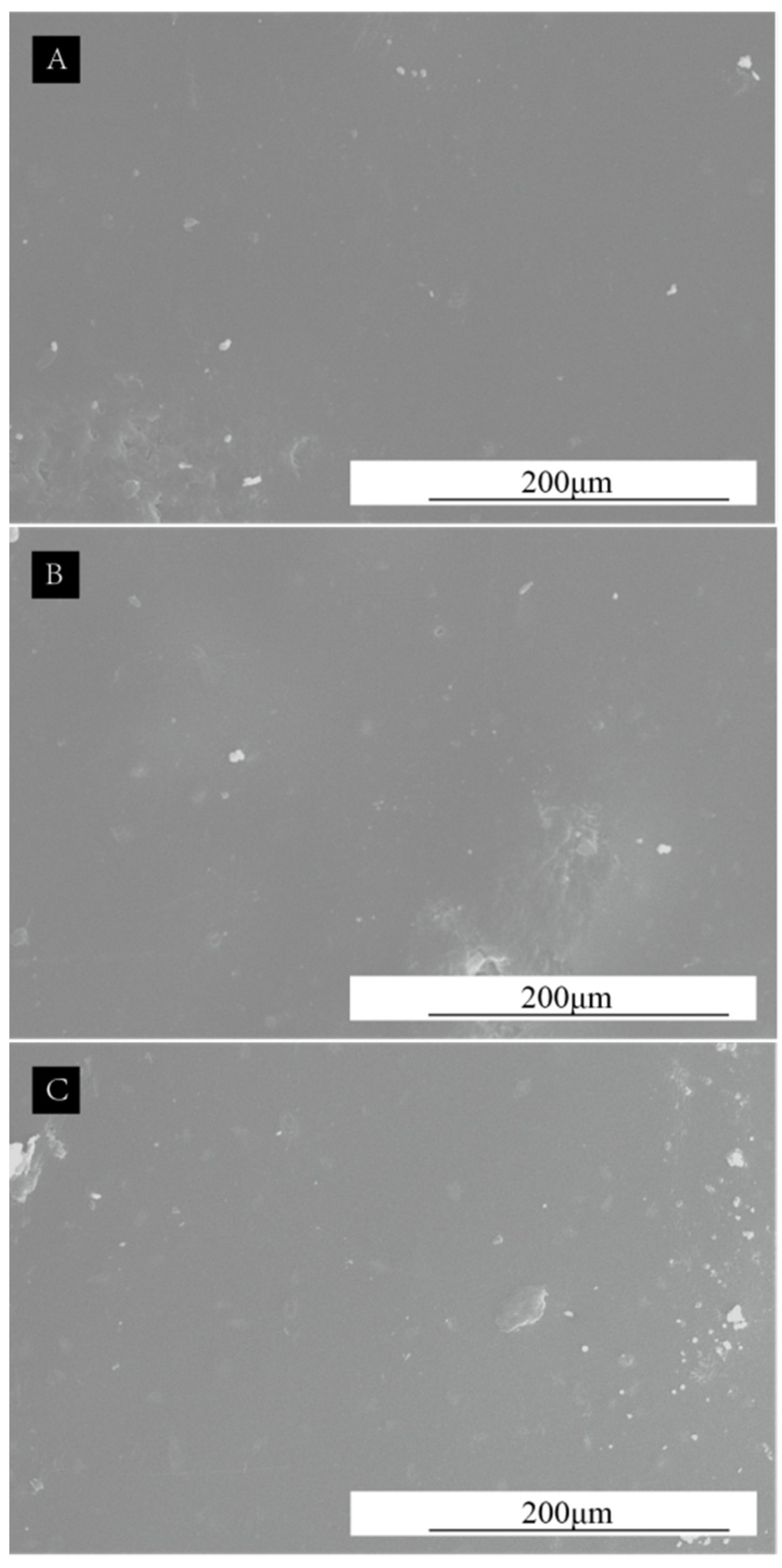
3.6. Analysis of Microscopic Characterization of SCCBC
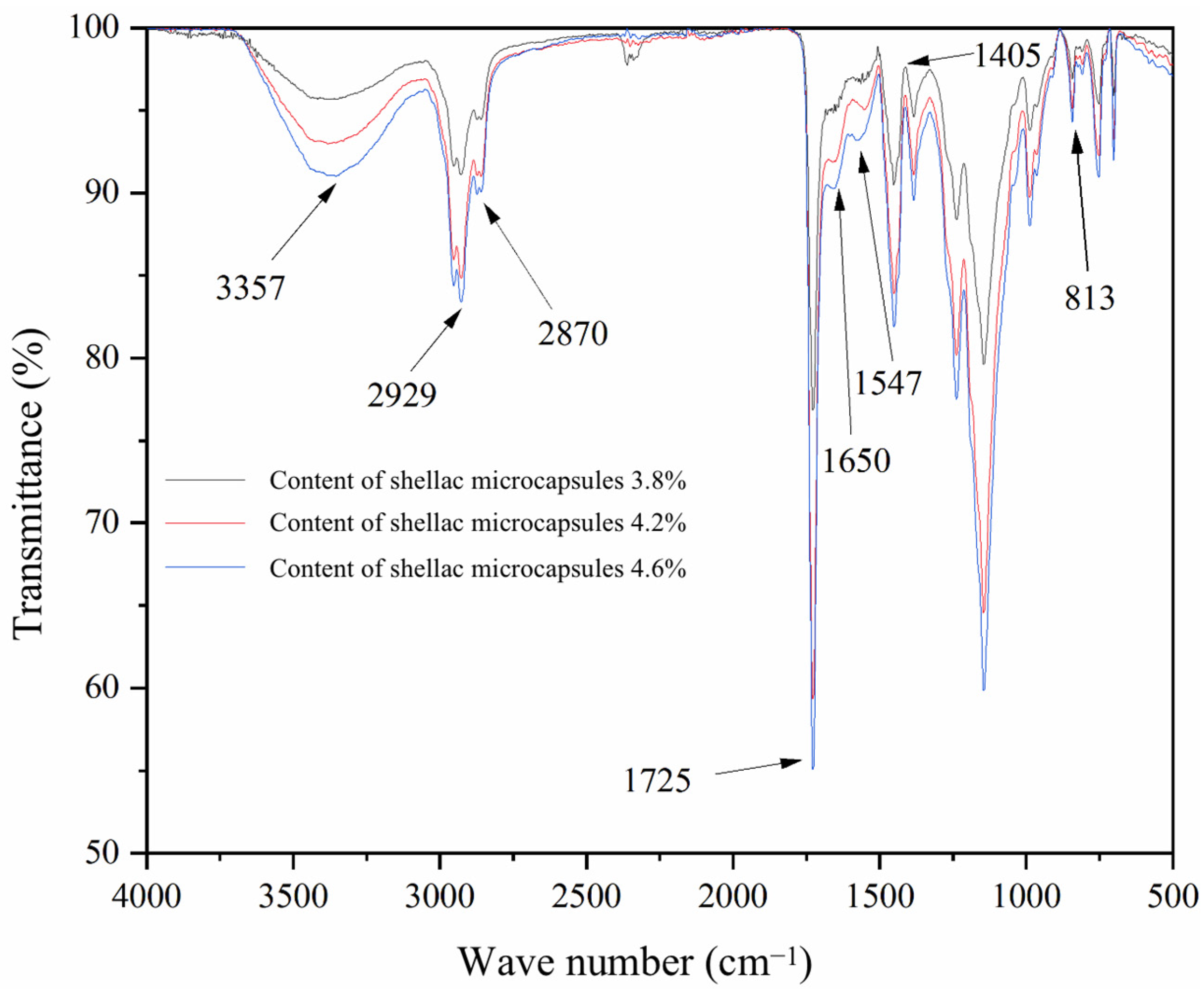
3.7. Infrared Analysis of SCCBC
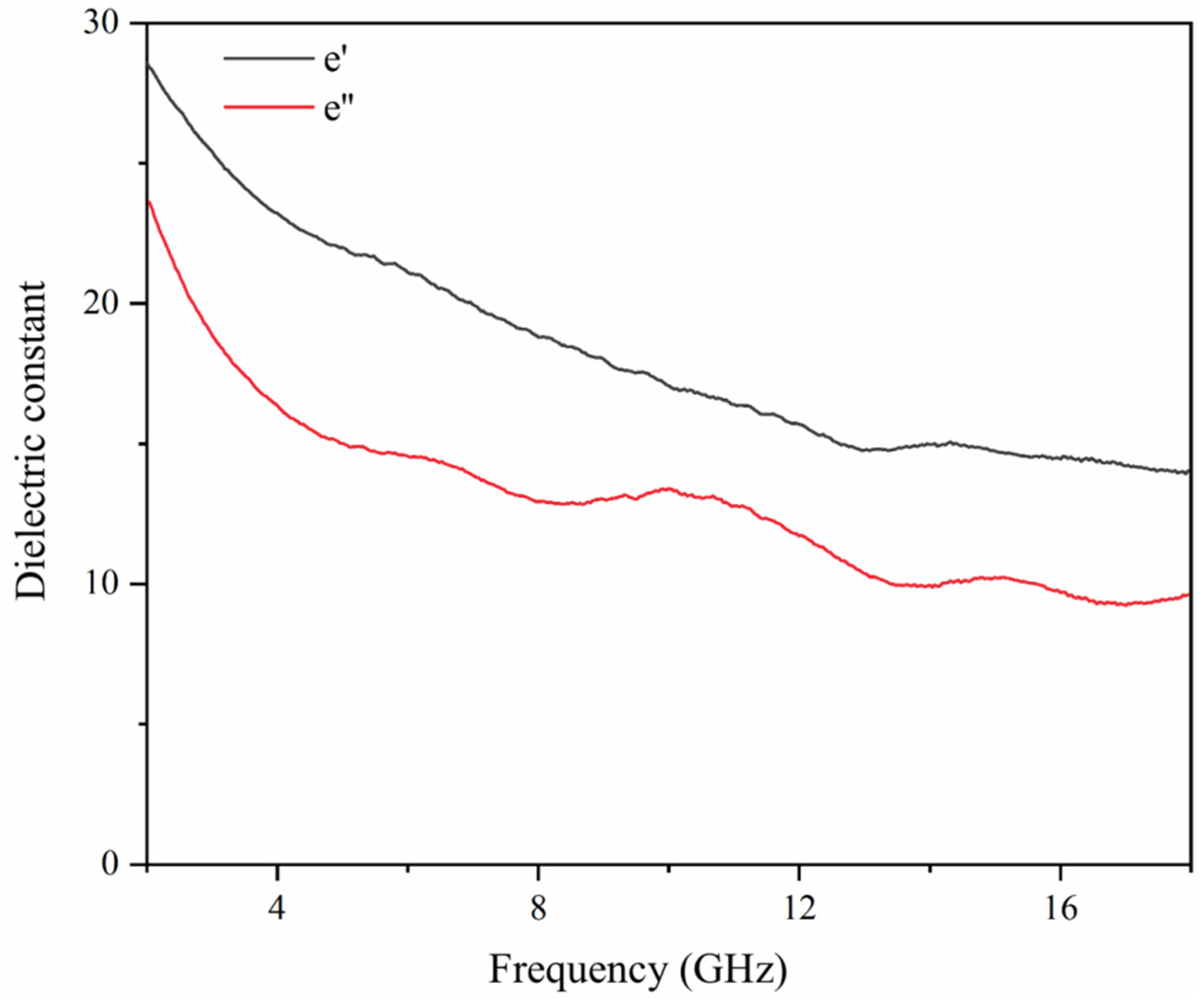
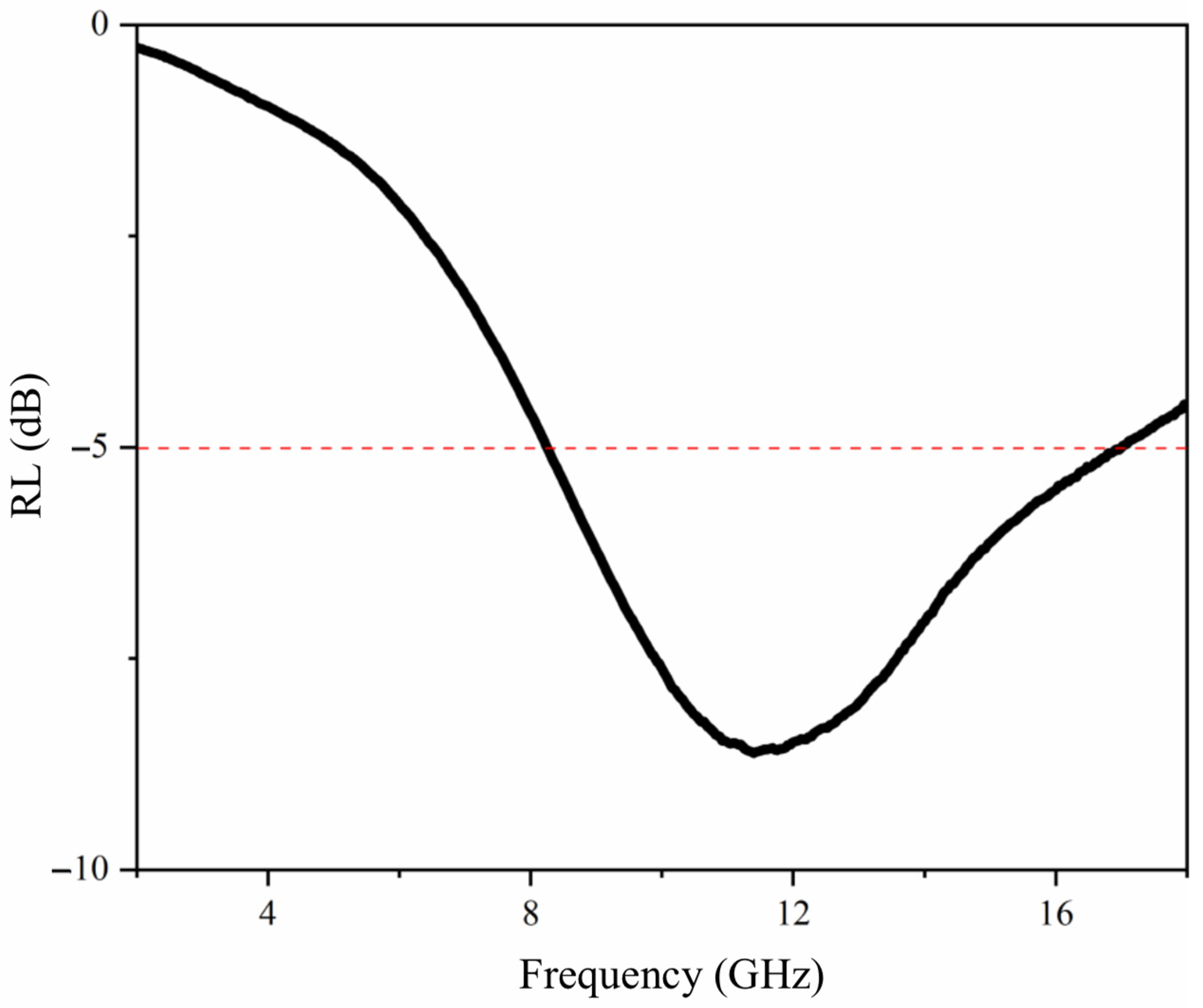
3.8. Analysis of the Electromagnetic Wave Absorption Performance of Shellac Microcapsules, CIP, CNT Blended Powders
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Z.N.; Niu, Y.T.; Chen, F.Y. Development and finishing technology of waterborne UV lacquer-coated wooden flooring. Bioresources 2021, 16, 1101–1114. [Google Scholar] [CrossRef]
- Guan, T.; Du, Z.K.; Chang, X.Y.; Zhao, D.L.; Yang, S.R.; Sun, N.; Ren, B.Y. A reactive hydrophobically modified ethoxylated urethane (HEUR) associative polymer bearing benzophenone terminal groups: Synthesis, thickening and photo-initiating reactivity. Polymers 2019, 178, 121552. [Google Scholar] [CrossRef]
- Feng, B.; Wang, D.; Li, Y.H.; Qian, J.P.; Yu, C.L.; Wang, M.S.; Luo, D.N.; Wei, S.Y. Mechanical properties of a soy protein isolate-grafted-acrylate (SGA) copolymer used for wood coatings. Polymers 2020, 12, 1137. [Google Scholar] [CrossRef]
- Luo, Y.R.; Xu, W. Optimization of Panel Furniture Plates Rework Based on Intelligent Manufacturing. Bioresources 2023, 18, 5198–5208. [Google Scholar] [CrossRef]
- Zhou, C.M.; Gu, W.H.; Luo, X.; Kaner, J. Building a 4E interview-grounded theory model: A case study of demand factors for customized furniture. PLoS ONE 2023, 18, 0282956. [Google Scholar] [CrossRef]
- Guo, S.W.; Wang, D.; Shi, J.Y.; Li, X.; Feng, B.; Meng, L.L.; Cai, Z.Y.; Qiu, H.K.; Wei, S.Y. Study on waterborne acrylate coatings modified with biomass silicon. Prog. Org. Coat. 2020, 135, 601–607. [Google Scholar] [CrossRef]
- Hao, W.F.; Hao, H.; Kanwal, H.; Jiang, S.P. Evaluation of Self-Healing Efficiency of Microcapsule-Based Self-Healing Cementitious Composites Based on Acoustic Emission. J. Renew. Mater. 2023, 11, 1687–1697. [Google Scholar] [CrossRef]
- Wang, H.P.; Rong, M.Z.; Zhang, M.Q. Self-Healing Polymers and Polymer-Based Composites Containing Microcapsules. Prog. Chem. 2010, 22, 2397–2407. [Google Scholar]
- Liu, Q.Q.; Gao, D.; Xu, W. Effect of Polyurethane Non-Transparent Coating Process on Coating Performance Applied on Modified Poplar. Coatings 2022, 12, 39. [Google Scholar] [CrossRef]
- Jiang, S.P.; Lin, Z.Y.; Tang, C.; Hao, W.F. Preparation and Mechanical Properties of Microcapsule-Based Self-Healing Cementitious Composites. Materials 2021, 14, 4866. [Google Scholar] [CrossRef]
- Ma, Y.X.; Liu, J.T.; Zhang, Y.R.; Ge, Y.; Wu, R.; Song, X.H.; Zhang, P.; Wu, J. Mechanical behavior and self-healing mechanism of polyurea-based double-walled microcapsule/epoxy composite films. Prog. Org. Coat. 2021, 157, 106283. [Google Scholar]
- Qin, Y.Z.; Yan, X.X. Effect of the Addition of Shellac Self-Healing and Discoloration Microcapsules on the Performance of Coatings Applied on Ebiara Solid Board. Coatings 2022, 12, 1627. [Google Scholar]
- Luo, Z.Y.; Xu, W.; Wu, S.S. Performances of Green Velvet Material (PLON) Used in Upholstered Furniture. Bioresources 2023, 18, 5108–5119. [Google Scholar] [CrossRef]
- Han, Y.; Yan, X.X.; Tao, Y. Effect of Transparent, Purple, and Yellow Shellac Microcapsules on the Optical Properties and Self-Healing Performance of Waterborne Coatings. Coatings 2022, 12, 1056. [Google Scholar] [CrossRef]
- Qu, Z.W.; Wang, Y.; Yang, P.A.; Zheng, W.; Li, N.; Bai, J.Y.; Zhang, Y.W.; Li, K.L.; Wang, D.S.; Liu, Z.H.; et al. Enhanced Electromagnetic Wave Absorption Properties of Ultrathin MnO2 Nanosheet-Decorated Spherical Flower-Shaped Carbonyl Iron Powder. Molecules 2022, 27, 135. [Google Scholar]
- He, Z.F.; Fang, Y.; Wang, X.J.; Pang, H. Microwave absorption properties of PANI/CIP/Fe3O4 composites. Synth. Met. 2011, 161, 420–425. [Google Scholar]
- Yin, C.L.; Fan, J.M.; Bai, L.Y.; Ding, F.; Yuan, F.L. Microwave absorption and antioxidation properties of flaky carbonyl iron passivated with carbon dioxide. J. Magn. Magn. Mater. 2013, 340, 65–69. [Google Scholar]
- Qiao, Y.J.; Yao, Z.D.; Li, Q.W.; Ji, Y.; Li, Z.N.; Zheng, T.; Zhang, X.H.; Wang, X.D. Preparation and microwave absorption of CIP/EP hollow spheres lattice composites. Compos. Part A Appl. Sci. Manuf. 2021, 150, 106626. [Google Scholar]
- Chen, Q.L.; Li, L.Y.; Wang, Z.L.; Ge, Y.C.; Zhou, C.S.; Yi, J.H. Synthesis and enhanced microwave absorption performance of CIP@ SiO2@Mn0.6Zn0.4Fe2O4 ferrite composites. J. Alloys Compd. 2019, 779, 720–727. [Google Scholar] [CrossRef]
- Zhou, J.J.; Wang, X.Y.; Ge, K.Y.; Yang, Z.Y.; Li, H.Q.; Guo, C.F.; Wang, J.Y.; Shan, Q.; Xia, L. Core-shell structured nanocomposites formed by silicon coated carbon nanotubes with anti-oxidation and electromagnetic wave absorption. J. Colloid. Interf. Sci. 2021, 607, 881–889. [Google Scholar]
- Ding, X.W.; Lyu, L.F.; Wang, F.L.; Yang, Y.F.; Qiao, J.; Xu, D.M.; Zhang, X.; Kong, L.X.; Liu, J.R. Novel ternary Co3O4/CeO2/CNTs composites for high-performance broadband electromagnetic wave absorption. J. Alloys Compd. 2021, 864, 158141. [Google Scholar]
- Li, H.B.; Wu, W.H.; Hao, X.X.; Wang, S.; You, M.Y.; Han, X.Z.; Zhao, Q.; Xing, B.S. Removal of ciprofloxacin from aqueous solutions by ionic surfactant-modified carbon nanotubes. Environ. Pollut. 2018, 243, 206–217. [Google Scholar]
- Deng, R.; Gao, X.; Hou, J.; Lin, D.H. Multi-omics analyses reveal molecular mechanisms for the antagonistic toxicity of carbon nanotubes and ciprofloxacin to Escherichia coli. Sci. Total Environ. 2020, 726, 138288. [Google Scholar] [PubMed]
- Tao, Y.; Yan, X.X. Influence of HLB Value of Emulsifier on the Properties of Microcapsules and Self-Healing Properties of Waterborne Coatings. Polymers 2022, 14, 1304. [Google Scholar] [CrossRef]
- Li, W.B.; Yan, X.X. Effects of Shellac Self-Repairing and Carbonyl Iron Powder Microcapsules on the Properties of Dulux Waterborne Coatings on Wood. Polymers 2023, 15, 2016. [Google Scholar]
- Huang, Y.Y.; Wu, J. Preparation and Characterization of Graphene Oxide/Polyaniline/Carbonyl Iron Nanocomposites. Materials 2022, 15, 484. [Google Scholar]
- Zhou, J.C.; Xu, W. Toward interface optimization of transparent wood with wood color and texture by silane coupling agent. J. Mater. Sci. 2022, 57, 5825–5838. [Google Scholar] [CrossRef]
- Hu, W.G.; Yu, R.Z. Mechanical and acoustic characteristics of four wood species subjected to bending load. Maderas-Cienc. Tecnol. 2023, 25, 39. [Google Scholar]
- Sun, H.M.; Shen, X.D.; Cui, S.; Xu, N. Preparation and absorption properties in near infrared wavelength of carbon nanotubes/acrylate coatings. Chin. J. Chem. Eng. 2007, 20, 784–788. [Google Scholar]
- Li, R.R.; He, C.J.; Wang, X.D. Evaluation and modeling of processability of laser removal technique for bamboo outer layer. JOM 2021, 73, 2423–2430. [Google Scholar]
- Li, R.R.; He, C.J.; Wang, X.D. Effects of processing parameters on mass loss and coating properties of poplar plywood during CO2 laser modification. Eur. J. Wood Wood Prod. 2022, 80, 899–906. [Google Scholar] [CrossRef]
- Sista, K.S.; Dwarapudi, S.; Kumar, D.; Sinha, G.R.; Moon, A.P. Carbonyl iron powders as absorption material for microwave interference shielding: A review. J. Alloys Compd. 2020, 853, 157251. [Google Scholar] [CrossRef]
- Chang, Y.J.; Yan, X.X. Preparation and Self-Repairing Properties of MF-Coated Shellac Water-Based Microcapsules. Coatings 2022, 10, 778. [Google Scholar] [CrossRef]
- Ataei, S.; Khorasani, S.N.; Torkaman, R.; Neisiany, R.E.; Koochaki, M.S. Self-healing performance of an epoxy coating containing microencapsulated alkyd resin based on coconut oil. Prog. Org. Coat. 2018, 120, 160–166. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Niu, Y.G. Influence of phenolic resin surface treatment agent on interfacial adhesion of carbon fibre reinforced epoxy resin composites. Adv. Compos. Lett. 2007, 16, 237–241. [Google Scholar] [CrossRef]
- Tezel, O.; Cigil, A.B.; Kahraman, M.V. Design and development of self-healing coating based on thiol-epoxy reactions. React. Funct. Polym. 2019, 142, 69–76. [Google Scholar] [CrossRef]
- Liu, M.; Xu, G.L.; Wang, J.A.; Tu, X.W.; Liu, X.Y.; Wu, Z.H.; Lv, J.F.; Xu, W. Effects of Shellac Treatment on Wood Hygroscopicity, Dimensional Stability and Thermostability. Coatings 2020, 10, 881. [Google Scholar] [CrossRef]
- Ni, X.; Luo, J.; Liu, R.; Liu, X.Y. A novel flexible UV-cured carbon nanotube composite film for humidity sensing. Sens. Actuat. B-Chem. 2019, 297, 126785. [Google Scholar] [CrossRef]
- Zhou, M.; Gu, W.H.; Wang, G.H.; Zheng, J.; Pei, C.C.; Fan, F.Y.; Ji, G.B. Sustainable wood-based composites for microwave absorption and electromagnetic interference shielding. J. Mater. Chem. A 2020, 8, 24267–24283. [Google Scholar] [CrossRef]


| Level | Addition Amount of Shellac Microcapsules (%) | Addition Amount of CIP (%) | Addition Amount of CNTs (%) | Primer Coating Thickness (μm) |
|---|---|---|---|---|
| 1 | 2.0 | 10.0 | 0 | 10 |
| 2 | 4.0 | 20.0 | 1.0 | 20 |
| 3 | 6.0 | 40.0 | 2.0 | 25 |
| Sample (#) | Addition Amount of Shellac Microcapsules (%) | Addition Amount of CIP (%) | Addition Amount of CNTs (%) | Primer Coating Thickness (μm) |
|---|---|---|---|---|
| 1 | 2.0 | 10.0 | 0 | 10 |
| 2 | 2.0 | 20.0 | 1.0 | 20 |
| 3 | 2.0 | 40.0 | 2.0 | 25 |
| 4 | 4.0 | 10.0 | 1.0 | 25 |
| 5 | 4.0 | 20.0 | 2.0 | 10 |
| 6 | 4.0 | 40.0 | 0.0 | 20 |
| 7 | 6.0 | 10.0 | 2.0 | 20 |
| 8 | 6.0 | 20.0 | 0.0 | 25 |
| 9 | 6.0 | 40.0 | 1.0 | 10 |
| Sample (#) | Shellac Microcapsules (g) | CIP (g) | CNTs (g) | Deionized Water (g) | Sodium Dodecyl Benzene Sulfonate (g) | Primer (g) |
|---|---|---|---|---|---|---|
| 1 | 0.08 | 0.40 | 0 | 23.50 | 0.02 | 3.52 |
| 2 | 0.08 | 0.80 | 0.04 | 45.04 | 0.04 | 3.08 |
| 3 | 0.08 | 1.60 | 0.08 | 86.16 | 0.08 | 2.24 |
| 4 | 0.16 | 0.40 | 0.04 | 29.37 | 0.03 | 3.40 |
| 5 | 0.16 | 0.80 | 0.08 | 50.91 | 0.05 | 2.96 |
| 6 | 0.16 | 1.60 | 0 | 86.16 | 0.08 | 2.24 |
| 7 | 0.24 | 0.40 | 0.08 | 35.25 | 0.03 | 3.28 |
| 8 | 0.24 | 0.80 | 0 | 50.91 | 0.05 | 2.96 |
| 9 | 0.24 | 1.60 | 0.04 | 92.03 | 0.09 | 2.12 |
| Content of Shellac Microcapsules (%) | Shellac Microcapsules (g) | CIP (g) | CNTs (g) | Water (g) | Sodium Dodecyl Benzene Sulfonate (g) | Primer (g) |
|---|---|---|---|---|---|---|
| 3.0 | 0.12 | 0.80 | 0.08 | 48.95 | 0.05 | 3.00 |
| 3.4 | 0.13 | 0.80 | 0.08 | 49.44 | 0.05 | 2.99 |
| 3.8 | 0.15 | 0.80 | 0.08 | 50.42 | 0.05 | 2.97 |
| 4.2 | 0.17 | 0.80 | 0.08 | 51.44 | 0.05 | 2.95 |
| 4.6 | 0.18 | 0.80 | 0.08 | 52.95 | 0.05 | 2.94 |
| 5.0 | 0.20 | 0.80 | 0.08 | 53.95 | 0.05 | 2.92 |
| 5.4 | 0.22 | 0.80 | 0.08 | 54.94 | 0.06 | 2.90 |
| Liquid Resistance Level | Standard |
|---|---|
| 1 | No modification is observed in the coating. |
| 2 | A slight change in color becomes visible as the light source moves nearer. |
| 3 | There are slight marks that can be observed. |
| 4 | The imprints are quite severe, but the overall structure and appearance of the coating remains unchanged. |
| 5 | Severe imprinting, changes in material structure, such as blistering, cracking. |
| SCCBC | Shellac Microcapsules (%) | CIP (%) | CNTs (%) | Primer Coating Thickness (μm) | Elongation at Tensile Failure (%) |
|---|---|---|---|---|---|
| 1 # | 2.0 | 10.0 | 0 | 10 | 0.3 |
| 2 # | 2.0 | 20.0 | 1.0 | 20 | 1.0 |
| 3 # | 2.0 | 40.0 | 2.0 | 25 | 1.6 |
| 4 # | 4.0 | 10.0 | 1.0 | 25 | 1.8 |
| 5 # | 4.0 | 20.0 | 2.0 | 10 | 2.6 |
| 6 # | 4.0 | 40.0 | 0 | 20 | 1.5 |
| 7 # | 6.0 | 10.0 | 2.0 | 20 | 1.4 |
| 8 # | 6.0 | 20.0 | 0 | 25 | 1.6 |
| 9 # | 6.0 | 40.0 | 1.0 | 10 | 1.0 |
| mean value 1 | 0.992 | 1.183 | 1.125 | 1.317 | |
| mean value 2 | 1.958 | 1.717 | 1.292 | 1.317 | |
| mean value 3 | 1.342 | 1.392 | 1.875 | 1.658 | |
| Range | 0.966 | 0.534 | 0.750 | 0.341 | |
| DEVSQ | 1.536 | 0.496 | 0.916 | 0.269 | |
| degrees of freedom | 2 | 2 | 2 | 2 | |
| F ratio | 1.910 | 0.617 | 1.139 | 0.334 | |
| F critical value | 4.460 | 4.460 | 4.460 | 4.460 | |
| significance |
| Content of Shellac Microcapsules (%) | L1 | a1 | b1 | L2 | a2 | b2 | ΔL | Δa | Δb | ΔE |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 49.60 | 9.12 | 25.32 | 49.65 | 9.19 | 25.6 | −0.05 | −0.07 | −0.28 | 0.29 |
| 3.0 | 25.31 | 0.95 | 1.83 | 24.87 | 1.52 | 1.64 | 0.44 | −0.57 | 0.19 | 0.74 |
| 3.4 | 24.07 | 1.01 | 1.79 | 23.98 | 0.65 | 0.96 | 0.09 | 0.36 | 0.83 | 0.91 |
| 3.8 | 22.30 | −0.29 | 0.45 | 23.07 | 0.58 | 0.79 | −0.77 | −0.87 | −0.34 | 1.21 |
| 4.2 | 21.35 | 0.66 | 0.62 | 20.23 | 0.31 | 0.15 | 1.12 | 0.35 | 0.47 | 1.26 |
| 4.6 | 19.33 | 0.38 | 0.13 | 20.87 | 0.96 | 0.78 | −1.54 | −0.58 | −0.65 | 1.77 |
| 5.0 | 20.58 | −0.16 | 0.84 | 18.87 | 0.46 | 0.43 | 1.71 | −0.62 | 0.41 | 1.85 |
| 5.4 | 18.09 | 0.75 | 0.35 | 19.83 | −0.26 | 0.88 | −1.74 | 1.01 | −0.53 | 2.08 |
| Content of Shellac Microcapsules (%) | 20° Glossiness Value (GU) | 60° Glossiness Value (GU) | 85° Glossiness Value (GU) |
|---|---|---|---|
| 0 | 20.5 | 50.1 | 52.4 |
| 3.0 | 19.4 | 46.4 | 49.1 |
| 3.4 | 18.3 | 44.3 | 48.6 |
| 3.8 | 17.3 | 44.4 | 49.8 |
| 4.2 | 13.9 | 37.8 | 45.3 |
| 4.6 | 6.4 | 23.2 | 29.1 |
| 5.0 | 4.9 | 20.2 | 25.1 |
| 5.4 | 4.3 | 18.6 | 23.6 |
| Content of Shellac Microcapsules (%) | Adhesion (Degree) | Hardness | Impact Resistance (kg∙cm) | Roughness (μm) |
|---|---|---|---|---|
| 0 | 1 | H | 6 | 0.489 |
| 3.0 | 1 | H | 7 | 2.151 |
| 3.4 | 1 | H | 9 | 2.365 |
| 3.8 | 2 | 2H | 11 | 2.564 |
| 4.2 | 2 | 2H | 15 | 2.593 |
| 4.6 | 3 | 3H | 13 | 2.826 |
| 5.0 | 4 | 3H | 12 | 3.024 |
| 5.4 | 4 | 4H | 9 | 3.125 |
| Content of Microcapsules (%) | Elongation at Tensile Failure (%) | Self-Healing Rate (%) | ||
|---|---|---|---|---|
| Original Sample | After Scratching | After Repairing | ||
| 0 | 5.3 | 3.8 | 3.1 | - |
| 3.0 | 5.8 | 4.2 | 4.4 | 12.5 |
| 3.4 | 6.3 | 4.3 | 4.7 | 20.0 |
| 3.8 | 6.7 | 4.8 | 5.2 | 21.1 |
| 4.2 | 8.2 | 5.1 | 5.8 | 24.1 |
| 4.6 | 7.8 | 4.8 | 5.3 | 16.7 |
| 5.0 | 7.3 | 4.7 | 5.1 | 15.4 |
| 5.4 | 6.9 | 4.3 | 4.6 | 11.5 |
| Content of Shellac Microcapsules (%) | Liquid Resistance (Level) | |||
|---|---|---|---|---|
| 75.0% Ethanol Solution | Red Ink Solution | Dishwashing Liquid | 15.0% NaCl Solution | |
| 0 | 1 | 4 | 1 | 1 |
| 3.0 | 1 | 4 | 1 | 1 |
| 3.4 | 1 | 4 | 1 | 1 |
| 3.8 | 1 | 4 | 1 | 1 |
| 4.2 | 1 | 5 | 1 | 1 |
| 4.6 | 1 | 5 | 1 | 1 |
| 5.0 | 1 | 5 | 1 | 1 |
| 5.4 | 1 | 5 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Li, W.; Yan, X. Effect of Shellac Microcapsules Blended with Carbonyl Iron Powder and Carbon Nanotubes on the Self-Healing and Electromagnetic Wave Absorption Properties of Waterborne Coatings on Fiberboard Surface. Coatings 2023, 13, 1572. https://doi.org/10.3390/coatings13091572
Xia Y, Li W, Yan X. Effect of Shellac Microcapsules Blended with Carbonyl Iron Powder and Carbon Nanotubes on the Self-Healing and Electromagnetic Wave Absorption Properties of Waterborne Coatings on Fiberboard Surface. Coatings. 2023; 13(9):1572. https://doi.org/10.3390/coatings13091572
Chicago/Turabian StyleXia, Yongxin, Wenbo Li, and Xiaoxing Yan. 2023. "Effect of Shellac Microcapsules Blended with Carbonyl Iron Powder and Carbon Nanotubes on the Self-Healing and Electromagnetic Wave Absorption Properties of Waterborne Coatings on Fiberboard Surface" Coatings 13, no. 9: 1572. https://doi.org/10.3390/coatings13091572
APA StyleXia, Y., Li, W., & Yan, X. (2023). Effect of Shellac Microcapsules Blended with Carbonyl Iron Powder and Carbon Nanotubes on the Self-Healing and Electromagnetic Wave Absorption Properties of Waterborne Coatings on Fiberboard Surface. Coatings, 13(9), 1572. https://doi.org/10.3390/coatings13091572


.jpg)


