1. Introduction
Coating technology is an economical and effective surface treatment technology, which is widely used to prevent the corrosion and destruction of metal constructions in a marine environment [
1,
2]. A cathodic protection coating is one of many protective coatings [
3,
4]. The protection mechanism of cathodic protection coating is reflected in multiple ways. On the one hand, cathodic protection coatings act as a sacrificial anode to guard the substrate by making use of the potential difference between it and the substrate. On the other hand, the coating is also an excellent physical shielding coating, involving the shielding of the coating itself and the blocking of the corrosion products forming a film via the degradation of the coating [
5].
Zn-based coatings are a promising cathodic protective coating that can prevent the corrosion of carbon steel due to their lower self-corrosion potential (around −1.1 V
SCE~−1.3 V
SCE) compared to that of low-carbon steel (−0.72 V
SCE) [
6,
7,
8,
9,
10,
11]. For instance, the self-corrosion potential for a hot-dipped Zn-Al coating is −1.05 V
SCE [
6] and that for cold-sprayed Zn-xNi is around −0.98 V
SCE~−1.01 V
SCE [
7]. Among the Zn-based composite coatings, the Zn-Al coating has certain advantages. For example, the potential difference between Zn and Al is tiny, meaning that the galvanic corrosion tendency in the coating is not obvious. Moreover, the corrosion products of Zn-Al are mainly insoluble layered double hydroxides (LDHs), which could delay the penetration of the corrosive medium [
10]. Therefore, Zn-Al coatings often act as an anode to protect low-carbon steel.
Many researchers have focused on the fabrication and anti-corrosion performance of Zn and Zn-Al coatings as a protective coating for steel in marine environments [
9,
10,
11,
12,
13,
14]. Tachiban et al. [
9] studied the corrosion resistance of hot-dipped Zn and Zn-Al coatings in a coastal area. Xue et al. [
11] investigated the microstructure and corrosion resistance behavior of a Zn-Al coating co-deposited on low-carbon steel via pack cementation. How the Al content affects the corrosion behavior of arc-sprayed Zn-Al coatings was further studied in the work of Zhu et al. [
12]. The results indicated that the Zn-Al coating has a higher corrosion resistance to seawater than the pure Zn coating, and Zn-30Al exhibited the highest corrosion resistance. The experiments of Kim et al. [
13] made clear that Zn-Al coatings improve the corrosion resistance of the base material in marine environments by forming ZnO and Zn
5(OH)
8Cl
2·H
2O corrosion products. However, the pure Zn-30Al coating represented a limited cathodic protection efficiency in a harsh corrosive environment [
15].
Usually, the potential difference between the coating and the substrate is increased to improve the efficiency of the cathodic protection of the coating. Liu [
16] and Zhu et al. [
17] added active elements Mg and Cu to a Zn-Al coating, respectively. Their experiments show that the obtained coatings have a lower electrode potential and express an enhanced cathodic protection efficiency. As a rule, a lower electrode potential means a higher active dissolution rate, which greatly reduces the service life of the coating. Hence, the key point when improving the comprehensive protective performance of Zn-Al coatings is to attain a balance between sacrificial behavior and dissolution resistance. Huang [
15] increased the cathodic protection without causing too fast a coating dissolution by matching the ratio of Zn and Al in a Zn-Al coating. Arrighi [
4] added Ce(III) as a potential corrosion inhibitor of sacrificial Zn-Fe coatings that were electrodeposited on steel. Liu [
16] added an active Mg element to Al-Zn-Si, which decreased the electrode potential and enhanced the cathodic protection efficiency of an Al-Zn-Si alloy. Moreover, an appropriate amount of magnesium in the alloy can promote the formation of a MgZn phase, which could inhibit the corrosion of the alloy. This means that the appropriate alloying element Mg has a balancing effect on the service life and cathodic protection property of Al-Zn-Si-Mg alloys. Is there an adequate additive that could enhance both the service life and cathodic protection property of Zn-Al coatings?
Reduced graphene oxide (rGO; in this work, rGO is denoted as
G) is a form of graphene. It is a special corrosion protection material with a large surface area and strong conductivity and a promising and versatile additive [
18]. The anti-sepsis characteristics of graphene are related to its distribution state and the properties of the composite materials. When a complete graphene coating is distributed on the substrate surface [
19], or a graphene composite is made with organic and inorganic coatings [
20], graphene mainly plays a shielding role, using its large surface area to extend the propagation path of corrosive media. If graphene is composited with metals, the graphene in these composites may promote the dissolution of metal by accelerating electron transfer when in a corrosive environment, by which passive metals could form a passive film [
21] and active metal would rapidly be degraded [
22,
23]. Based on the abovementioned points, it can be speculated that
G is a suitable additive, which can enhance both the service life and cathodic protection properties of a Zn-Al coating, and the key is to distribute
G evenly in the coating.
We deposited a Zn-
G/Al composite coating on low-carbon steel using LPCS [
24,
25]. Using
G-coated Al powders prepared by chemical reduction as a feedstock,
G was successfully embedded in the interface of Zn and Al. According to experiments [
24],
G lowered the potential of the Zn-
G/Al coating and thus enhanced its potential difference with the substrate. In this way, the boosting potential difference facilitates the cathode polarization behavior and induces the formation of corrosion products when in a harsh environment. In this work, Zn-
G/Al composite coatings were immersed in NaCl solution, and the medium-term immersion behavior was observed to study how the embedded
G affects the medium-term protection performance of Zn-
G/Al coatings.
3. Results and Discussion
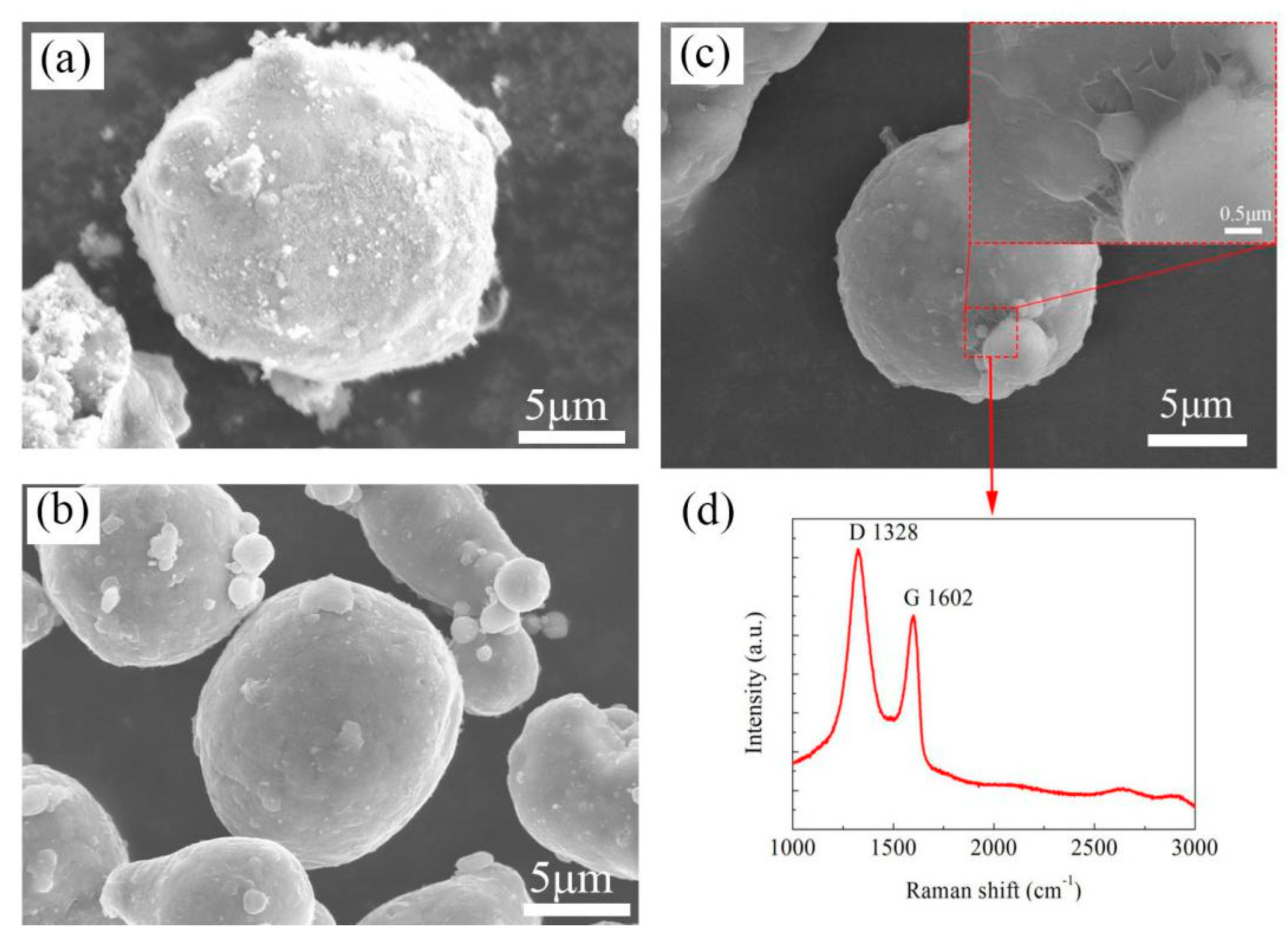
The SEM morphologies of Zn powders, Al powders and 0.2 wt.%
G/Al are presented in
Figure 1. The size for Zn and Al is around 15–20 μm; some wrinkles appeared on the 0.2 wt.%
G/Al powders’ surface, as shown in
Figure 1c. A Raman test was conducted to measure
G; D (1310 cm
−1) and G (1595 cm
−1) peaks were observed on the Raman spectrum acquired from the surface of 0.2 wt.%
G/Al powder (
Figure 1d). As depicted, the D/G intensity ratio of G (1.13) was higher than that of GO (0.84), which confirmed that GO was reduced and coated on the Al powder surface.
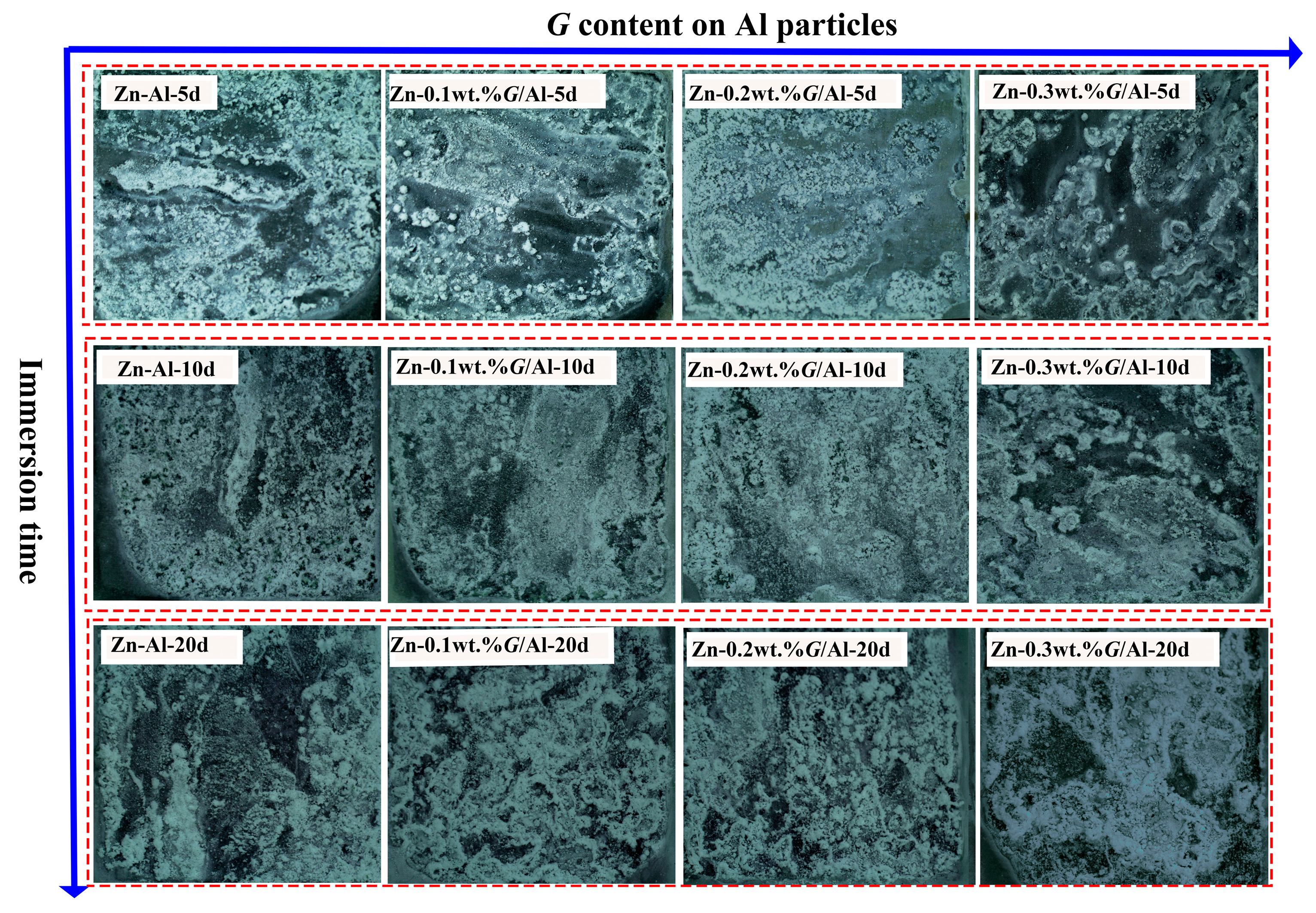
Figure 2 shows the macroscopic corrosion appearance of the Zn-x wt.%
G/Al coating after immersion in 3.5 wt.% NaCl solution for 5 d, 10 d and 20 d. As presented, the white corrosion products gradually covered the coating surface during the immersion time. During the early and middle immersion time (5 d and 10 d), the area of the coating surface covered by corrosion products differed. The cover area is arranged in the order of Zn-0.2 wt.%
G/Al, Zn-0.1 wt.%
G/Al, Zn-0.3 wt.%
G/Al, Zn-Al, from large to small. The results indicate that the presence of
G in the coating could accelerate the dissolution of the coating when in a corrosive solution, and the Zn-0.2 wt.%
G/Al coating experienced the fastest corrosion rate at the initial immersion time. With prolonged immersion, the corrosion products on Zn-x wt.%
G/Al coatings become more compact and evenly distributed.
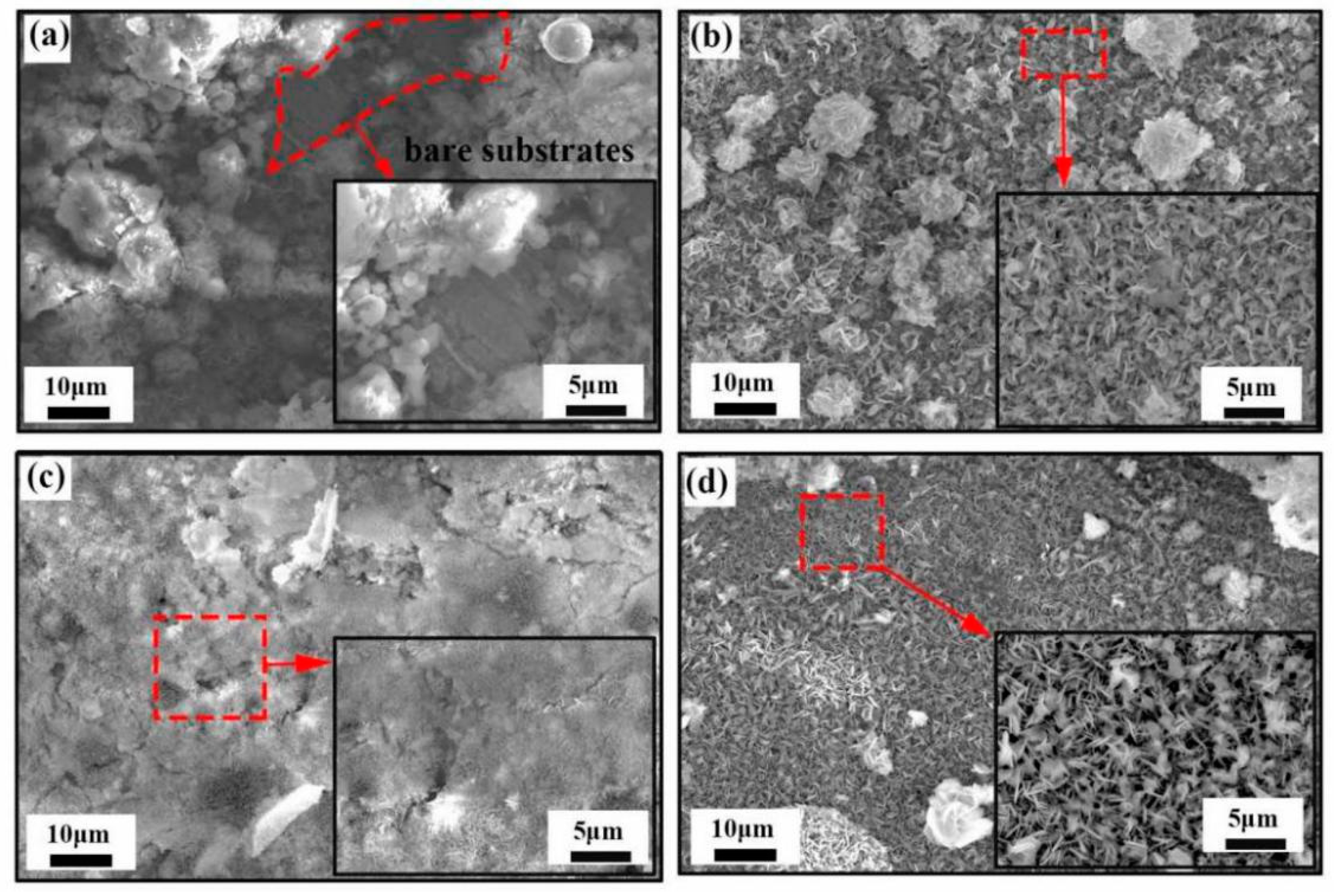
Figure 3 shows the SEM morphology of corrosion products generated on Zn-x wt.%
G/Al coatings’ surface after immersion in 3.5 wt.% NaCl for 10 d. As demonstrated in
Figure 3a, some loose corrosion products accumulated on the Zn-Al coating surface, and part of the bare coating substrate was still exposed after 10 d of immersion. The Zn-0.1 wt.%
G/Al and Zn-0.3 wt.%
G/Al coatings’ surfaces were covered by a mass of nanoplatelets with a size of 2–3 μm and the nanoplatelets were vertically cross-linked, as presented in
Figure 3b,d. Corrosion products on the Zn-0.2 wt.%
G/Al coating were the most compact, and nanosheets for the products were very tiny, with a size of 2–3 μm, as shown in
Figure 3c.
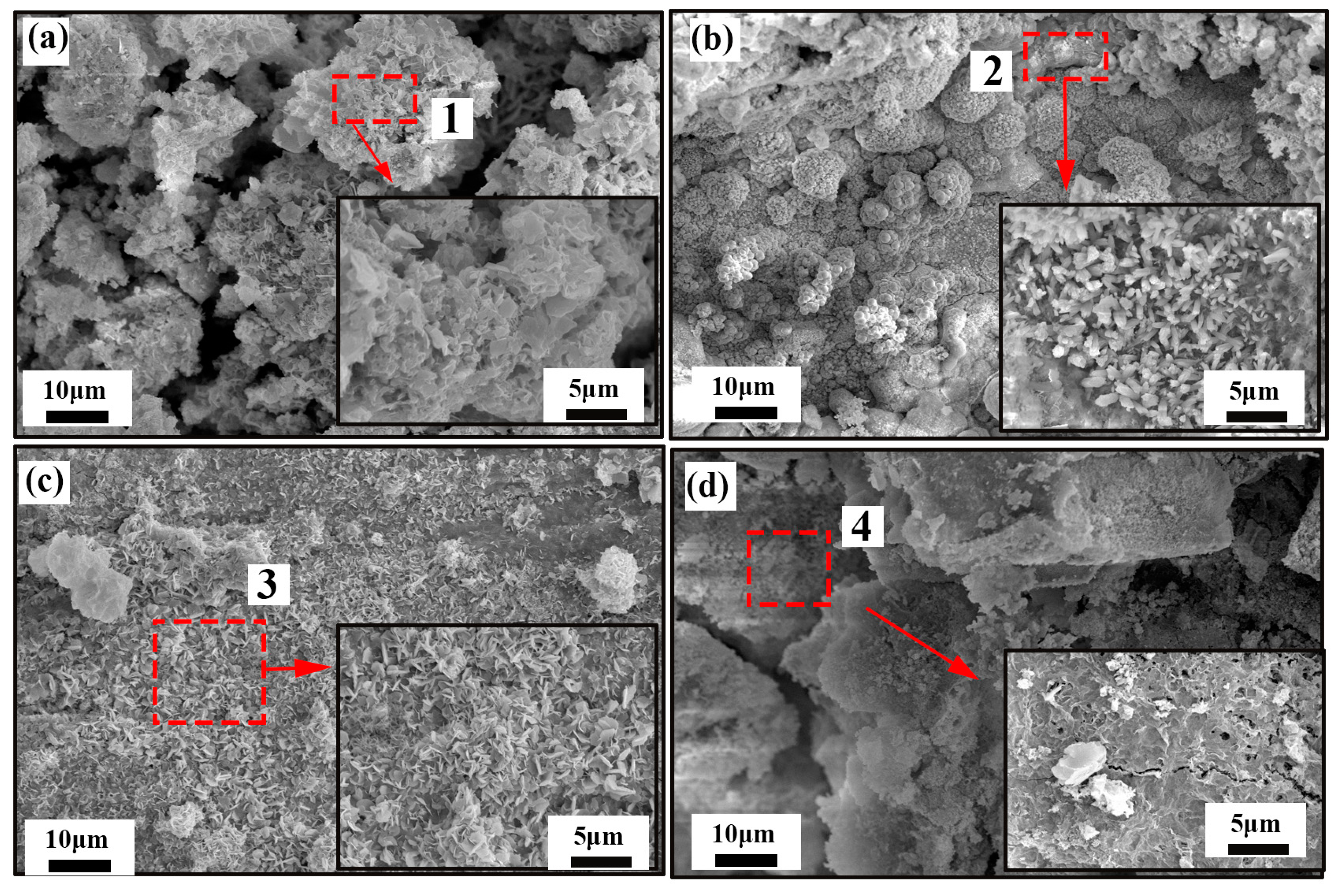
Figure 4 shows the SEM morphology of corrosion products generated on Zn-x wt.%
G/Al coatings’ surface after immersion in 3.5 wt.% NaCl solution for 20 d. After 20 d immersion, clusters of corrosion products accumulated loosely on the Zn-Al coating surface (
Figure 4a), with apparent gaps between the cluster layers. Rod-shaped nanoparticles clustered and densely covered the Zn-0.1 wt.%
G/Al coating surface to form a film of corrosion products, as shown in
Figure 4b. For the Zn-0.2 wt.%
G/Al coating after 20 d immersion, as presented in
Figure 4c, the corrosion product layer is compact and composed of nanosheets. Flake-like corrosion products were formed on the Zn-0.3 wt.%
G/Al coating surface, and some cracks and holes occurred on the corrosion product layer’s surface.
Figure 5 is EDX results acquired from regions 1, 2, 3 and 4 shown in
Figure 4. After 20 d immersion, the corrosion products are mainly composed of elements Zn, Al, Cl, C and O. The element Cl came from corrosive media, and C is derived from CO
2 that dissolved in solution. As shown in EDX results, it can be seen that O content in corrosion products formed on the Zn-0.2 wt.%
G/Al surface is highest, which may be related to the formation of ZnAl
2CO
3(OH)
16·4H
2O.
Figure 6 shows the XRD results for corrosion products on the Zn-x wt.%
G/Al coating after 10 d and 20 d immersion in NaCl solution. After 10 d immersion, an outstanding peak clearly appeared, centered around 11.3°, 43° and associated with hydrozincite [
22]. Peaks caused by Zn
4CO
3(OH)
6·H
2O were present at 37°, 54° and 72° in the XRD pattern [
20]. The peak of zinc oxide (ZnO) at 39.5° [
26] was relatively higher than the peaks of simonkolleite (Zn
5(OH)
8Cl
2) at 11.3°, ZnAl
2CO
3(OH)
16·4H
2O around 11.3° and 43° and Zn
4CO
3(OH)
6·H
2O at 54° and 72° [
10]. There were some changes in the intensity of the diffraction peak of the corrosion products when immersion time was prolonged to 20 d, as shown in
Figure 6. The diffraction peak intensity of ZnO at 39.5° decreased, and the peak intensity of Zn
4CO
3(OH)
6·H
2O at 54°, 72° and ZnAl
2CO
3(OH)
16·4H
2O at 43° was strengthened. Some mini peaks around 33.5°, ascribed to Zn
5(OH)
8Cl
2 and Zn
4CO
3(OH)
6·H
2O, successively appeared, which may be due to the transformation of ZnO. ZnO is unstable, which means that it readily reacts with water and gradually turns into simonkolleite with low dissolving properties in water. After immersion in NaCl solution, simonkolleite could provide protection for the coating. For Zn-0.2 wt.%
G/Al coating, the diffraction peak intensity of ZnAl
2CO
3(OH)
16·4H
2O at 43° strengthened remarkably, which may be related to the translation of Al.
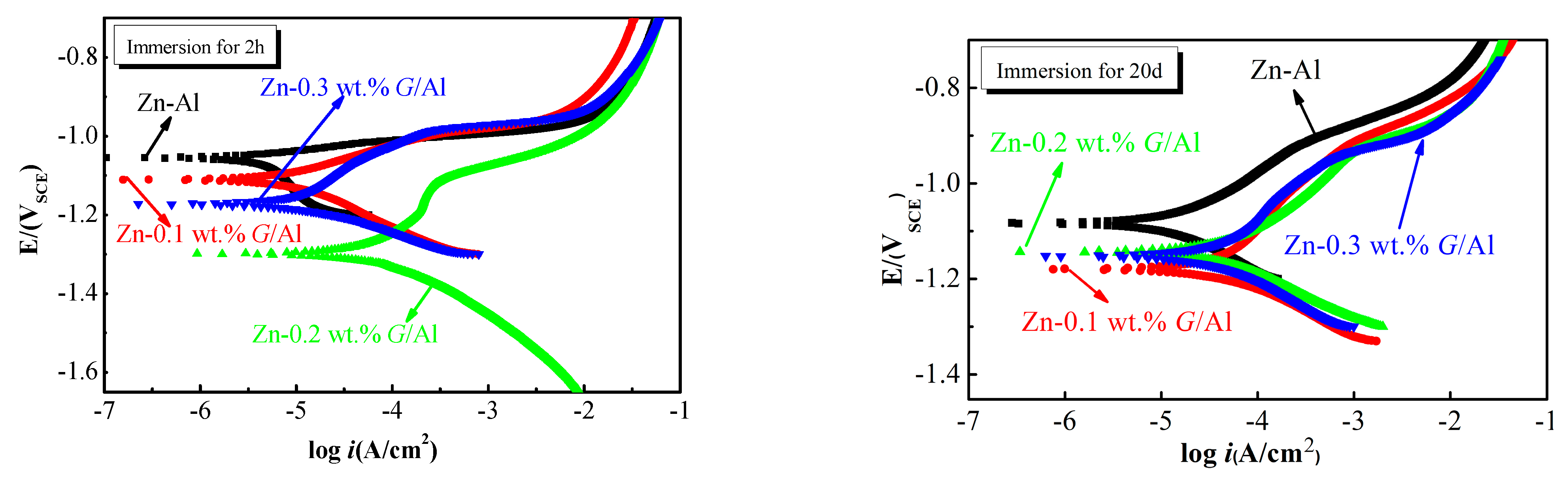
Figure 7 presents the potentiodynamic polarization curves (PPCs) of Zn-x wt.%
G/Al coatings after immersion for 2 h and 20 d. The specific
Ecorr and
icorr values fitted from the PPC are displayed in
Table 1. For the Zn-Al coating, the
Ecorr value of the Zn-Al coating after immersion for 2 h is −1.055 V
SCE; the value for the coating after 20 d immersion decreased to −1.083 V
SCE. After 20 d immersion, the
icorr value for the Zn-Al coating is 15.8 μA/cm
2, which is two and a half times higher than the value of the Zn-Al coating after immersion for 2 h (6.508 μA/cm
2). The
Ecorr values for Zn-0.1 wt.%
G/Al, Zn-0.2 wt.%
G/Al and Zn-0.3 wt.%
G/Al coatings are −1.180 V
SCE, −1.143 V
SCE and −1.152 V
SCE after 20 d immersion, which are elevated compared with the coating values acquired at 2 h immersion time. The variation trend of the
icorr value for the Zn-
G/Al coating is the opposite. The
icorr value for Zn-0.1 wt.%
G/Al and Zn-0.3 wt.%
G/Al coatings increased, but that for the Zn-0.2 wt.%
G/Al coating decreased.
In PPCs, the Ecorr value indicates the corrosion tendency of the coating and a higher Ecorr value means a lower corrosion tendency; the icorr value represents the corrosion rate of the coating and the value of icorr is inversely proportional to its anti-corrosion properties. For the steel with a protective coating, the higher the potential difference between the coating and steel matrix, the stronger the coating’s propensity for cathodic protection. A higher corrosion current density indicated a better cathodic protection efficiency. Compared with the Ecorr value for steel (−0.72 VSCE), Zn-G/Al coating values were all below -1VSCE, which indicates that all Zn-G/Al coatings are cathodic protection coatings. Increasing with immersion time, the Ecorr values for Zn-0.1 wt.%G/Al, Zn-0.2 wt.%G/Al and Zn-0.3 wt.%G/Al coatings at 20 d increased by 0.5%, 12% and 1.7%, respectively, in comparison with the value at the initial immersion time but were still lower than that of steel (−0.72 VSCE). Therefore, the coating could protect steel through its sacrificial anode action during full immersion.
Cathodic protection is the method of sacrificing the anode to avoid the corrosion of the cathode. The sacrificial ability of the anode increases with the increase in dissolution rate of the coating layer, while the large dissolution rate of the coating layer decreases service life for the coating [
8]. The key point is the balance between sacrificial behavior and dissolution resistance. In the work of Jonathan Elvins et al. [
27], the effect of magnesium additions on cut edge corrosion resistance of zinc aluminum alloy galvanized steel was studied. They measured individual anodic activity against anode number for the cut edge by SVET. Results indicated that the addition of Mg resulted in an increase in zinc loss, an increase in active anode numbers and an increase in the number of long-lived anodes. Ding et al. [
23] prepared graphene/zinc-containing coatings on steel, and electrochemical test results indicated that 0.3%graphene-70%Zn coating has the highest current corrosion identity, which presented that adding graphene prolonged the duration and increased the values of the stable cathodic protection currents of the coating. Hence, a higher cathodic protection efficiency calls for a higher corrosion current density during the process of cathodic protection. As shown in
Table 1, the
icorr value for the Zn-
G/Al coating is higher than that of Zn-Al, which means that adding G to the coating could enhance the activity and strengthen the cathodic protection effect of the Zn-Al composite coating during the full-immersion process.
During the anodic protection of metals, the anodic protection coating is also required to have a low corrosion rate to extend its service life. However, the higher the anodic protection efficiency of the coating, the higher the corrosion rate. Therefore, if the coating can generate a protective corrosion product layer to reduce the degradation rate during the anodic protection process, then this contradiction may be alleviated. It is surprising that the icorr value for Zn-0.2 wt.%G/Al went down after 20 d immersion compared with the value at the initial immersion time, which demonstrated that the corrosion products generated on the Zn-0.2 wt.%G/Al coating could delay the degradation and prolong the service life of the coating. Therefore, we conclude that inserting G into the coating endowed the coating with an enduring activity and equipped it with a persistent cathodic protection ability during full immersion. When the G content in powders of the Al surface is 0.2 wt.%, the obtained composite coating surface generated a protective corrosion product film after 20 d immersion, which could retard the degradation of coating.
Compared with casted Zn-Al alloy [
28] or hot-dipped Zn-Al composite coating (E
corr is around −1.0) [
16], Zn-
G/Al coating has an excellent cathodic protection efficiency. However, the
icorr value for Zn-
G/Al coating is still higher than that of traditional cathodic protection coating.
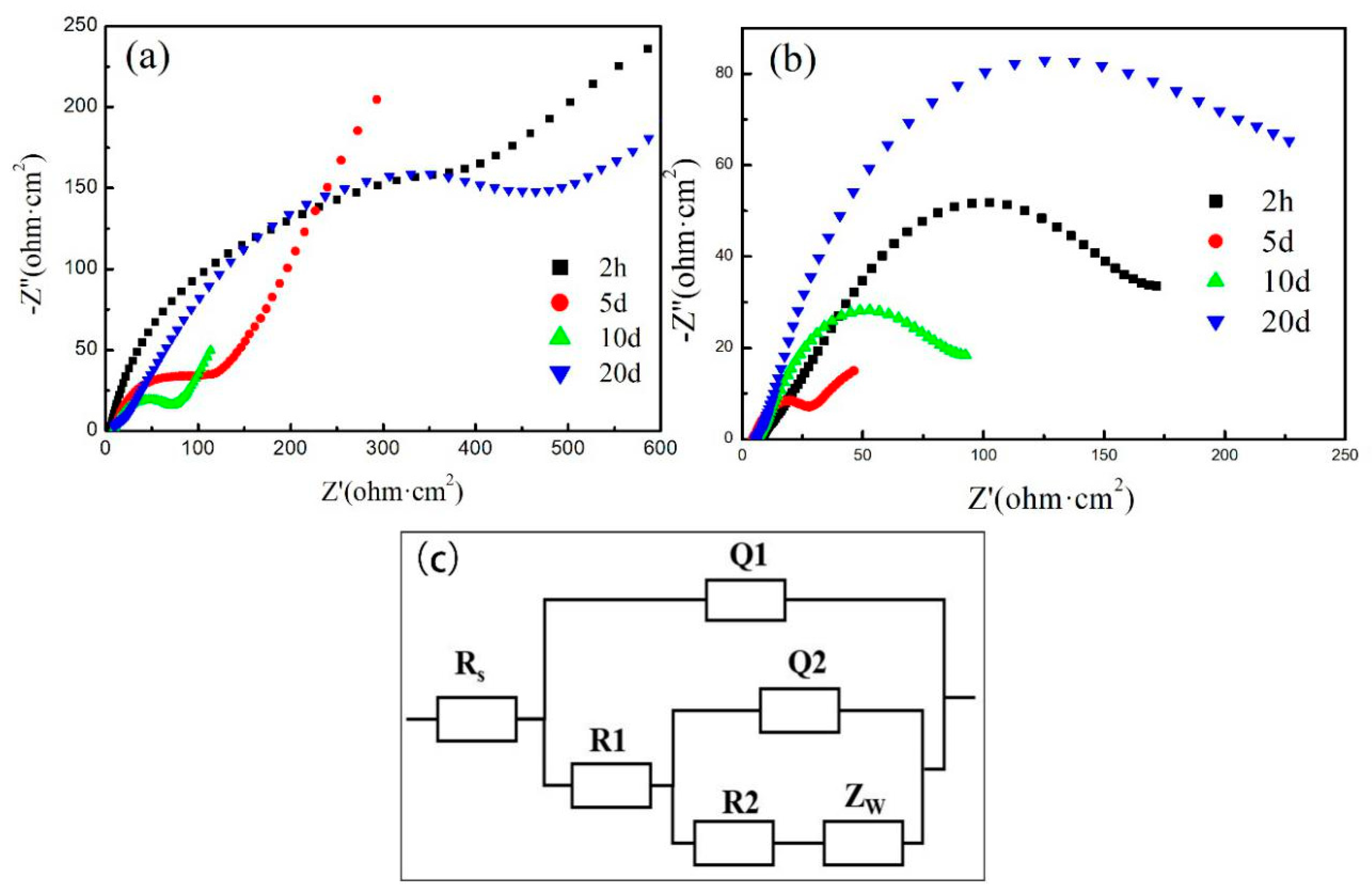
To illustrate the equilibrium effect, the impedance change for Zn-Al and Zn-0.2 wt.%
G/Al coatings during the full immersion is presented in
Figure 8. Actually, Zn-
G/Al coating during immersion experienced two processes: firstly, the generation of corrosion products; secondly, the compaction of corrosion products. The densification of corrosion products is related to activity of the coating. For the Zn-Al coating, the electrochemical impedance spectrum (EIS) after 5–10 d immersion was fitted by the equivalent circuit
Rs(
Q1(
R1Q2(
R2Zw))) [
29]. Rs, corresponding to a high-frequency region, represents the resistance of the electrolyte;
Q1 and
R1 are linked to the capacitance and resistance of the corrosion products stacked on the coating surface, respectively. The reactions of the coating are described by the charge transfer resistance
R2 and the double-layer capacitance
Q2 which correspond to the low-frequency region. As demonstrated in EIS curves, a 45° near-line appears in the low-frequency region, which is caused by the diffusion of the porous corrosion product layer, for which a corresponding semi-infinite-length Warburg element
ZW is added in series with
R2. The EIS after 20d immersion was fitted by the equivalent circuit
Rs(
Q1(
R1(
R2Q2))) [
30]; there was no apparent diffusion phenomenon due to the corrosion product layer has basically formed. For the Zn-0.2 wt.%G/Al coating, before 5 d immersion, EIS curves were fitted by an equivalent circuit of
Rs(
Q1(
R1Q2(
R2Zw))). After 10 d immersion, corrosion products were generated and stacked on the Zn-0.2 wt.%G/Al coating surface to form a layer. Therefore, the equivalent circuit
Rs(
Q1(
R1(
R2Q2)) can be used to fit the EIS curve of the coating after 10 d immersion.
The variation in the EIS radius for the coating during mid-term immersion showed that the Zn-x wt.%
G/Al coating experienced three phases: the first is the shielding of the coating, the second is the erosion of the coating, which provides cathodic protection, and the third is the barrier of the formed erosion product layer. For the Zn-Al coating, the cathodic protection stage occurred in the first 10 d of immersion. The arc radius increased with immersion time due to the corrosion product layer formed on the Zn-Al coating. Although the service life of the coating can be extended due to the shielding protection of the corrosion product layer, the cathodic protection efficiency is still low, as shown in
Table 1 (
Ecorr value of Zn-Al at 20d immersion time is −1.083 V
SCE, which is higher than that of Zn-x wt.%
G/Al coating). For Zn-0.2 wt.%
G/Al, the cathodic protection stage occurred in the first 5 d of immersion, and the
R1 value (arc radius value) dramatically decreased. After 5 d immersion, the arc radius value of the EIS curve gradually increased. The increased
R1 value shows that the formed corrosion product layer has a better shielding protection effect. With a further increase in immersion time (after 20 d), the shielding protection effects of the corrosion product layer significantly increased beyond the shielding protection of the Zn-0.2 wt.%
G/Al coating at the initial immersion time, as shown by the largest arc radius value of the EIS curve. The excellent protection of the corrosion products on the Zn-0.2 wt.%
G/Al coating surface could be the result of the shielding effect of
G in the corrosion products. The Zn-0.2 wt.%
G/Al coating has a superior cathodic protection efficiency after 20 d immersion (with a lower
Ecorr value of −1.152 V
SCE), which can be ascribed to the bridge connection with the conductivity of
G.
4. Mechanism
According to the results of PPS and EIS, we deduced that the Zn-0.2 wt.%
G/Al coating possesses a continuous cathodic protection ability compared with the other three coatings after 20 d immersion, and the corrosion product on Zn-0.2 wt.%
G/Al coating could delay the dissolution of the coating, which could prolong the service life of the coating during the full-immersion process. The special protective properties of the Zn-0.2 wt.%
G/Al coating produced during medium-term immersion can be attributed to the equilibrium effect of
G. Actually, graphene is a double-edged sword when applied in the corrosion protection of a coating [
22]. When
G completely coats metal surfaces or is used as an additive in organic or inorganic coatings,
G prolongs the propagation path of the corrosive medium through its large specific surface area; thus, the use of
G in the coating mainly plays a role in shielding protection [
31,
32]. However, the conductivity of
G is inclined to expedite the metal degradation when
G metal matrix composites are used [
18]. Due to the potential difference between Zn, Al and
G, the reaction process of the Zn-
G/Al coating in an aggressive medium involves galvanic reactions.
Figure 9 shows the microbattery reaction process of the two coatings soaked in an aggressive NaCl medium.
For the Zn-Al coating (
Figure 9a), Al with a low self-corrosion potential is preferentially dissolved and promotes the release of OH
−, by which the 3.5 wt.% NaCl solution gradually becomes alkaline and promotes the dissolution of Zn. The dissolved zinc and aluminum first formed oxides that were easily hydrolyzed. The oxides hydrolyzed to hydroxides Zn(OH)
42− and Al(OH)
4−, which continuously promote the dissolution of the coating. When the hydroxide content in solution is high enough, hydroxides coordinate to form stable bimetallic hydroxides and deposit on the coating surface. In the Zn-
G/Al coating (
Figure 9b), the addition of conductive
G transforms Zn and Al into anode, so that Zn and Al dissolve simultaneously and promote the formation of corrosion products, as demonstrated in
Figure 2. During the corrosion product layer formation, the solvent-less
G in the Zn-Al coating was exposed by the dissolution of Zn and Al and entered into the corrosion product layer, accompanied by the stacking of corrosion products. According to the XRD results exhibited in
Figure 4, corrosion products mainly consist of LDH. LDH is an inorganic compound with an impotent electron conductivity, which would weaken the cathodic protection property of the Zn-Al coating.
G in the corrosion product layer acts as both a barrier and means of electron conduction. Firstly, the barrier property of
G prolongs the permeation path of the erosion media, which enhanced the shielding ability of the corrosion product film. Secondly,
G in the corrosion product layer bridged the coating and the corrosive medium and facilitated electron propagation, which provided the coating with continuous cathodic protection.
Among the four coatings, we deduced that the Zn-0.2 wt.%G/Al coating possesses a continuous cathodic protection ability, and the corrosion product film generated on Zn-0.2 wt.%G/Al coating after immersion in the medium has the best shielding ability. This phenomenon is related to the distribution and concentration of G in the coating. In our previous work, we proved that the deposition effect of Al powders is strongly associated with the G concentration when coated on Al. During cold spraying, properly increasing the difference in hardness between the sprayed particles can enhance the particle deposition efficiency; however, an exorbitant particle hardness will cause particles to violently rebound. G possesses an excellent mechanical strength; metal materials combined with G have a significantly enhanced hardness. In addition, a mechanically bonded heterogeneous interface is formed between G and Zn particles during cold spraying; therefore, with an increase in G concentration on the Al surface, the interface energy between G and Zn will increase and the G/Al in the coating will be distributed more evenly. In the Zn-Al coating, poorly dispersed Al agglomerates are found. When the G-coated Al is applied, the distribution of Al becomes uniform; moreover, G/Al in the Zn-0.2 wt.%G/Al composite coating is significantly decreased and uniformly scattered. When the concentration of G coated on Al is increased to 0.3 wt%, the main component of the coating is Zn, due to the rebound of 0.3 wt.%G/Al. Therefore, among the four coatings, the Zn-0.2 wt.%G/Al coating is equipped with a continuous cathodic protection ability and the corrosion products on the Zn-G/Al coating surface have better shielding properties than the initial Zn-G/Al coating due to the shielding of G, which could delay the dissolution of that coating when dipped in aggressive solution.





.jpg)



