Optically Transparent TiO2 and ZnO Photocatalytic Thin Films via Salicylate-Based Sol Formulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. TiO2 and ZnO Sol Preparation and Thin Film Deposition
2.2.1. TiO2 and ZnO Sol Synthesis
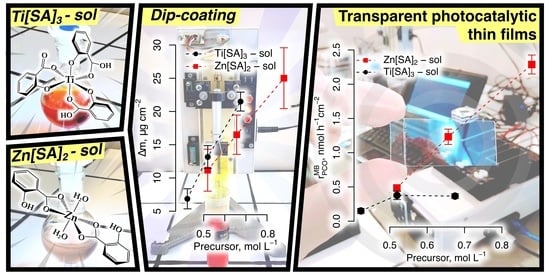
- TiO2 Sol: TTIP was added dropwise into the SA/IPA mixture. During this step, a light-yellow fluffy precipitate was observed, especially in the Ti:10 and Ti:15 mixtures, but it redissolved under magnetic stirring. The mixture was continuously stirred for 3 h at RT, resulting in a clear, intensively red-orange colored sol. The three sols are denoted as Ti:10, Ti:15, and Ti:20, with 1:3:10, 1:3:15, and 1:3:20 TTIP:SA:IPA molar ratios, respectively.
- ZnO Sol: The calculated amount of ZAD was added to the SA/IPA solution at RT and magnetic stirring. The mixture was then brought up to 60 °C under reflux and stirred for an additional 3 h until the ZAD was completely dissolved. Finally, a clear sol with a faint pink color was obtained. The three sols are denoted as Zn:10, Zn:15, and Zn:20, with 1:2:10, 1:2:15, and 1:2:20 ZAD:SA:IPA molar ratios, respectively.
2.2.2. Dip-Coating of TiO2 and ZnO Thin Films
2.3. Characterization Methods
2.3.1. Physico-Chemical Characteristics of the Sols
2.3.2. Instrumental Methods
2.4. Photocatalytic Activity Determination
- The MB saturation concentration () was estimated by fitting the non-dissociative Langmuir adsorption model (Equation (8)).where is the MB adsorption rate constant, and is the time coordinate. was then converted to molar saturation coverage per geometric area (nmol cm−2), as shown in Equation (9), where represents the reaction volume (15 mL), is the molecular weight of MB dye (319.85 g mol−1), and is the geometric area of the sample (3.9 cm2).The parameter is thus indicative of the differences in the specific surface area between the photocatalytic samples.
- The MB PCO reaction rate () and reaction rate constant () were determined from the decrease in during UV illumination. was obtained by linear regression, as shown in Equation (10), where represents the initial after the dark adsorption phase.was converted to molar MB PCO removal rate per geometric area (nmol h−1 cm−2) using a similar approach as that shown in Equation (9). Additionally, rate constants (h−1) were calculated using the pseudo-first-order kinetic model, commonly employed to describe MB PCO removal rates, as shown in Equation (11).
2.5. Computational Methods
2.5.1. Density Functional Theory (DFT) Computations
2.5.2. Data Treatment and Model Fitting
3. Results and Discussion
3.1. ATR-FTIR & DFT Investigation of the TiO2 and ZnO Salicylate Sols
DFT Modelling of Ti[SA] and Zn[SA] Complexes
3.2. Effect of Precursor Concentration on the Physicochemical Parameters of TiO2 and ZnO Deposition Sols and Resulting Thin Film Mass-Loading
3.3. Structure & Morphology of Salicylate-Based TiO2 and ZnO Thin Films
3.4. Optical Properties of the TiO2 and ZnO Thin Films
3.4.1. Transfer Matrix Method Modelling of TiO2 and ZnO Thin Film Transmittance Spectra
3.4.2. Results from Modelling of TiO2 and ZnO Thin Film Transmittance Spectra
3.5. Photocatalytic Activity of the TiO2 and ZnO Thin Films
4. Conclusions and Outlook
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37. [Google Scholar] [CrossRef]
- Armaković, J.; Savanović, M.M.; Armaković, S. Titanium dioxide as the most used photocatalyst for water purification: An overview. Catalysts 2022, 13, 26. [Google Scholar] [CrossRef]
- Muscetta, M.; Russo, D. Photocatalytic Applications in Wastewater and Air Treatment: A Patent Review (2010–2020). Catalysts 2021, 11, 834. [Google Scholar] [CrossRef]
- Wetchakun, K.; Wetchakun, N.; Sakulsermsuk, S. An overview of solar/visible light-driven heterogeneous photocatalysis for water purification: TiO2- and ZnO-based photocatalysts used in suspension photoreactors. J. Ind. Eng. Chem. 2019, 71, 19–49. [Google Scholar] [CrossRef]
- Ahmad, K.; Ghatak, H.R.; Ahuja, S.M. A review on photocatalytic remediation of environmental pollutants and H2 production through water splitting: A sustainable approach. Environ. Technol. Innov. 2020, 19, 100893. [Google Scholar] [CrossRef]
- Wang, J.; Guo, R.; Bi, Z.; Chen, X.; Hu, X.; Pan, W. A review on TiO2−x-based materials for photocatalytic CO2 reduction. Nanoscale 2022, 14, 11512–11528. [Google Scholar] [CrossRef]
- Hamid, S.B.A.; Teh, S.J.; Lai, C.W. Photocatalytic Water Oxidation on ZnO: A Review. Catalysts 2017, 7, 93. [Google Scholar] [CrossRef]
- Castro-Muñoz, R. The Role of New Inorganic Materials in Composite Membranes for Water Disinfection. Membranes 2020, 10, 101. [Google Scholar] [CrossRef]
- Arun, J.; Nachiappan, S.; Rangarajan, G.; Alagappan, R.P.; Gopinath, K.P.; Lichtfouse, E. Synthesis and application of titanium dioxide photocatalysis for energy, decontamination and viral disinfection: A review. Environ. Chem. Lett. 2023, 21, 339–362. [Google Scholar] [CrossRef]
- Olea, M.; Bueno, J.; Pérez, A. Nanometric and surface properties of semiconductors correlated to photocatalysis and photoelectrocatalysis applied to organic pollutants—A review. J. Environ. Chem. Eng. 2021, 9, 106480. [Google Scholar] [CrossRef]
- Navidpour, A.H.; Hosseinzadeh, A.; Zhou, J.L.; Huang, Z. Progress in the application of surface engineering methods in immobilizing TiO2 and ZnO coatings for environmental photocatalysis. Catal. Rev. Sci. Eng. 2021, 65, 822–873. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Yang, B.; Ma, Z.; Wang, Q.; Yang, J. Synthesis and Photoelectrocatalytic Applications of TiO2/ZnO/Diatomite Composites. Catalysts 2022, 12, 268. [Google Scholar] [CrossRef]
- Mani, J.; Sakeek, H.; Habouti, S.; Dietze, M.; Es-Souni, M. Macro–meso-porous TiO2, ZnO and ZnO–TiO2-composite thick films. Properties and application to photocatalysis. Catal. Sci. Technol. 2012, 2, 379–385. [Google Scholar] [CrossRef]
- Wiatrowski, A.; Wojcieszak, D.; Mazur, M.; Kaczmarek, D.; Domaradzki, J.; Kalisz, M.; Kijaszek, W.; Pokora, P.; Mańkowska, E.; Lubanska, A.; et al. Photocatalytic Coatings Based on TiOx for Application on Flexible Glass for Photovoltaic Panels. J. Mater. Eng. Perform. 2022, 31, 6998–7008. [Google Scholar] [CrossRef]
- Zhao, W.; Lu, H. Self-Cleaning Performance of Super-Hydrophilic Coatings for Dust Deposition Reduction on Solar Photovoltaic Cells. Coatings 2021, 11, 1059. [Google Scholar] [CrossRef]
- Panžić, I.; Juraić, K.; Krstulović, N.; Šantić, A.; Belić, D.; Blažeka, D.; Plodinec, M.; Mandić, V.; Macan, J.; Hammud, A.; et al. ZnO@TiO2 Core Shell Nanorod Arrays with Tailored Structural, Electrical, and Optical Properties for Photovoltaic Application. Molecules 2019, 24, 3965. [Google Scholar] [CrossRef]
- Hanková, A.; Kuzminova, A.; Kylián, O. Nanostructured Semi-Transparent TiO2 Nanoparticle Coatings Produced by Magnetron-Based Gas Aggregation Source. Coatings 2023, 13, 51. [Google Scholar] [CrossRef]
- Ehrmann, N.; Reineke-Koch, R. Ellipsometric studies on ZnO:Al thin films: Refinement of dispersion theories. Thin Solid Films 2010, 519, 1475–1485. [Google Scholar] [CrossRef]
- Vajargah, P.H.; Abdizadeh, H.; Ebrahimifard, R.; Golobostanfard, M. Sol–gel derived ZnO thin films: Effect of amino-additives. Appl. Surf. Sci. 2013, 285, 732–743. [Google Scholar] [CrossRef]
- Lu, B.-J.; Lin, K.-T.; Kuo, Y.-M.; Tsai, C.-H. Preparation of High-Transparency, Superhydrophilic Visible Photo-Induced Photocatalytic Film via a Rapid Plasma-Modification Process. Coatings 2021, 11, 784. [Google Scholar] [CrossRef]
- Brinker, C.J.; Frye, G.C.; Hurd, A.J.; Ashley, C.S. Fundamentals of sol-gel dip coating. Thin Solid Films 1991, 201, 97–108. [Google Scholar] [CrossRef]
- Ohya, Y.; Saiki, H.; Tanaka, T.; Takahashi, Y. Microstructure of TiO2 and ZnO Films Fabricated by the Sol-Gel Method. J. Am. Ceram. Soc. 1996, 79, 825–830. [Google Scholar] [CrossRef]
- Toyoda, M.; Watanabe, J.; Matsumiya, T. Evolution of structure of the precursor during sol-gel processing of ZnO and low temperature formation of thin films. J. Sol-Gel Sci. Technol. 1999, 16, 93–99. [Google Scholar] [CrossRef]
- Amakali, T.; Daniel, L.S.; Uahengo, V.; Dzade, N.Y.; de Leeuw, N.H. Structural and Optical Properties of ZnO Thin Films Prepared by Molecular Precursor and Sol–Gel Methods. Crystals 2020, 10, 132. [Google Scholar] [CrossRef]
- Vilà, A.; Gómez-Núñez, A.; Alcobé, X.; Palacios, S.; Puig Walz, T.; López, C. Influence of the Nature of Aminoalcohol on ZnO Films Formed by Sol-Gel Methods. Nanomaterials 2023, 13, 1057. [Google Scholar] [CrossRef]
- Znaidi, L. Sol–gel-deposited ZnO thin films: A review. Mater. Sci. Eng. B 2010, 174, 18–30. [Google Scholar] [CrossRef]
- Rodríguez-Gattorno, G.; Santiago-Jacinto, P.; Rendon-Vázquez, L.; Németh, J.; Dékány, I.; Díaz, D. Novel Synthesis Pathway of ZnO Nanoparticles from the Spontaneous Hydrolysis of Zinc Carboxylate Salts. J. Phys. Chem. B 2003, 107, 12597–12604. [Google Scholar] [CrossRef]
- Spiridonova, J.; Katerski, A.; Danilson, M.; Krichevskaya, M.; Krunks, M.; Oja Acik, I. Effect of the Titanium Isopropoxide:Acetylacetone Molar Ratio on the Photocatalytic Activity of TiO2 Thin Films. Molecules 2019, 24, 4326. [Google Scholar] [CrossRef]
- Verma, A.; Samanta, S.B.; Bakhshi, A.K.; Agnihotry, S.A. Effect of stabilizer on structural, optical and electrochemical properties of sol–gel derived spin coated TiO2 films. Sol. Energy Mater. Sol. Cells 2005, 88, 47–64. [Google Scholar] [CrossRef]
- Sato, M.; Hara, H.; Nishide, T.; Sawada, Y. A water-resistant precursor in a wet process for TiO2 thin film formation. J. Mater. Chem. 1996, 6, 1767–1770. [Google Scholar] [CrossRef]
- Nishide, T.; Sato, M.; Hara, H. Crystal structure and optical property of TiO2 gels and films prepared from Ti-edta complexes as titania precursors. J. Mater. Sci. 2000, 35, 465–469. [Google Scholar] [CrossRef]
- Gigant, K.; Rammal, A.; Henry, M. Synthesis and Molecular Structures of Some New Titanium(IV) Aryloxides. J. Am. Chem. Soc. 2001, 123, 11632–11637. [Google Scholar] [CrossRef] [PubMed]
- Gusev, A.; Baluda, Y.; Braga, E.; Kryukova, M.; Kiskin, M.; Chuyan, E.; Ravaeva, M.; Cheretaev, I.; Linert, W. Mn(II), Co(II), Ni(II) and Zn salicylates: Synthesis, structure and biological properties studies. Inorg. Chim. Acta 2021, 528, 120606. [Google Scholar] [CrossRef]
- Lee, B.P.; Narkar, A.; Wilharm, R. Effect of metal ion type on the movement of hydrogel actuator based on catechol-metal ion coordination chemistry. Sens. Actuators B Chem. 2016, 227, 248–254. [Google Scholar] [CrossRef]
- Kuznetsova, S.; Mongush, E.; Lisitsa, K. Zinc oxide films obtained by sol-gel method from film-forming solutions. J. Phys. Conf. Ser. 2019, 1145, 012020. [Google Scholar] [CrossRef]
- Stefanov, B.I.; Milusheva, V.S.; Kolev, H.G.; Tzaneva, B.R. Photocatalytic activation of TiO2-functionalized anodic aluminium oxide for electroless copper deposition. Catal. Sci. Technol. 2022, 12, 7027–7037. [Google Scholar] [CrossRef]
- Pang, F.; Seng, C.; Teng, T.; Ibrahim, M.H. Densities and viscosities of aqueous solutions of 1-propanol and 2-propanol at temperatures from 293.15 K to 333.15 K. J. Mol. Liq. 2007, 136, 71–78. [Google Scholar] [CrossRef]
- Hoke, B.C., Jr.; Chen, J.C. Binary aqueous-organic surface tension temperature dependence. J. Chem. Eng. Data 1991, 36, 322–326. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Stefanov, B.I. Photocatalytic reactor for in situ determination of supported catalysts activity in liquid-phase based on 3D-printed components and Arduino. In Proceedings of the 2020 XXIX International Scientific Conference Electronics (ET), Sozopol, Bulgaria, 16–18 September 2020. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Barca, G.M.; Bertoni, C.; Carrington, L.; Datta, D.; De Silva, N.; Deustua, J.E.; Fedorov, D.G.; Gour, J.R.; Gunina, A.O.; Guidez, E.; et al. Recent developments in the general atomic and molecular electronic structure system. J. Chem. Phys. 2020, 152, 154102. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colic-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.; Seeger, R.; Pople, J. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Hay, J.P.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Hay, J.P.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Pritchard, B.P.; Altaravwy, D.; Didier, B.; Gibson, T.D.; Windus, T.L. A New Basis Set Exchange: An Open, Up-to-date Resource for the Molecular Sciences Community. J. Chem. Inf. Model 2019, 59, 4814–4820. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 4 September 2023).
- Nash, J.C. {optimr}: A Replacement and Extension of the ‘Optim’ Function. 2016. Available online: https://CRAN.R-project.org/package=optimr (accessed on 4 September 2023).
- Borchers, H. Pracma: Practical Numerical Math Functions. R Package Version 2.4.2. 2022. Available online: https://CRAN.R-project.org/package=pracma (accessed on 4 September 2023).
- Khamar, D.; Zeglinski, J.; Mealey, D.; Rasmuson, Å.C. Investigating the role of solvent–solute interaction in crystal nucleation of salicylic acid from organic solvents. J. Am. Chem. Soc. 2014, 136, 11664–11673. [Google Scholar] [CrossRef]
- Humbert, B.; Alnot, M.; Quilès, F. Infrared and Raman spectroscopical studies of salicylic and salicylate derivatives in aqueous solution. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1998, 54, 465–476. [Google Scholar] [CrossRef]
- Alvarez-Ros, M.C.; Sánchez-Cortés, S.; García-Ramos, J.V. Vibrational study of the salicylate interaction with metallic ions and surfaces. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2000, 56, 2471–2477. [Google Scholar] [CrossRef]
- Koksharova, T.V.; Kurando, S.V.; Stoyanova, I.V. Coordination compounds of 3d-metals salicylates with thiosemicarbazide. Russ. J. Gen. Chem. 2012, 82, 1481–1484. [Google Scholar] [CrossRef]
- Rodríguez, I.; Fernández-Vega, L.; Maser-Figueroa, A.N.; Sang, B.; González-Pagán, P.; Tinoco, A.D. Exploring Titanium(IV) Complexes as Potential Antimicrobial Compounds. Antibiotics 2022, 11, 158. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hu, J.; Liu, W.; Zhu, F.; Wang, G.; Tung, C.; Wang, Y. Binding Modes of Salicylic Acids to Titanium-Oxide Molecular Surfaces. Chem. Eur. J. 2020, 26, 2666–2674. [Google Scholar] [CrossRef] [PubMed]
- Klug, H.P.; Alexander, L.E.; Sumner, G.G. The crystal structure of zinc salicylate dihydrate. Acta Cryst. 1958, 11, 41–46. [Google Scholar] [CrossRef]
- Landau, L.; Levich, B. Dragging of a Liquid by a Moving Plate. In Dynamics of Curved Fronts; Pelcé, P., Ed.; Academic Press: Cambridge, MA, USA, 1988; pp. 141–153. [Google Scholar] [CrossRef]
- Kuznetsov, A.; Xiong, M. Effect of evaporation on thin film deposition in dip coating process. Int. Commun. Heat Mass Transf. 2002, 29, 35–44. [Google Scholar] [CrossRef]
- Han, J.; Qiu, W.; Gao, W. Potential dissolution and photo-dissolution of ZnO thin films. J. Hazard. Mater. 2010, 178, 115–122. [Google Scholar] [CrossRef]
- Knittl, Z. Optics of Thin Films (An Optical Multilayer Theory); Wiley: London, UK, 1976. [Google Scholar]
- Katsidis, C.C.; Siapkas, D.I. General transfer-matrix method for optical multilayer systems with coherent, partially coherent, and incoherent interference. Appl. Opt. 2002, 41, 3978–3987. [Google Scholar] [CrossRef]
- González-Leal, J.; Prieto-Alcón, R.; Angel, J.; Minkov, D.A.; Márquez, E. Influence of substrate absorption on the optical and geometrical characterization of thin dielectric films. Appl. Opt. 2002, 41, 7300–7308. [Google Scholar] [CrossRef]
- Makhlouka, Y.; Sanaâ, F.; Gharbia, M. Ordinary and Extraordinary Complex Refractive Indices Extraction of a Mylar Film by Transmission Spectrophotometry. Polymers 2022, 14, 1805. [Google Scholar] [CrossRef]
- Blanco, E.; González-Leal, J.; Ramírez-del Solar, M. Photocatalytic TiO2 sol-gel thin films: Optical and morphological characterization. Sol. Energy 2015, 122, 11–23. [Google Scholar] [CrossRef]
- Jellison, G.E., Jr.; Modine, F.A. Parameterization of the optical functions of amorphous materials in the interband region. Appl. Phys. Lett. 1996, 69, 371–373, Erratum in Appl. Phys. Lett. 1996, 69, 2137. [Google Scholar] [CrossRef]
- Tanguy, C. Optical dispersion by Wannier excitons. Phys. Rev. Lett. 1995, 75, 4090. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Martínez, E.; Navarrete-Astorga, E.; Ramos-Barrado, J.; Gabás, M. Evolution of Al: ZnO optical response as a function of doping level. Appl. Surf. Sci. 2017, 421, 680–686. [Google Scholar] [CrossRef]
- Tanguy, C. Refractive index of direct bandgap semiconductors near the absorption threshold: Influence of excitonic effects. IEEE J. Quantum Electron. 1996, 32, 1746–1751. [Google Scholar] [CrossRef]
- Bouzourâa, M.; En Naciri, A.; Moadhen, A.; Rinnert, H.; Guendouz, M.; Battie, Y.; Chaillou, A.; Zaïbi, M.; Oueslati, M. Effects of silicon porosity on physical properties of ZnO films. Mater. Chem. Phys. 2016, 175, 233–240. [Google Scholar] [CrossRef]
- Brinkley, D.; Engel, T. Active site density and reactivity for the photocatalytic dehydrogenation of 2-propanol on TiO2 (110). Surf. Sci. 1998, 415, L1001–L1006. [Google Scholar] [CrossRef]
- Krýsa, J.; Novotná, P.; Kment, Š.; Mills, A. Effect of glass substrate and deposition technique on the properties of sol gel TiO2 thin films. J. Photochem. Photobiol. A 2011, 222, 81–86. [Google Scholar] [CrossRef]
- Elangovan, S.; Chandramohan, V.; Sivakumar, N.; Senthil, T. Synthesis and characterization of sodium doped ZnO nanocrystals and its application to photocatalysis. Superlattices Microstruct. 2015, 85, 901–907. [Google Scholar] [CrossRef]
- Mills, A.; Wang, J.; Ollis, D.F. Kinetics of Liquid Phase Semiconductor Photoassisted Reactions: Supporting Observations for a Pseudo-Steady-State Model. J. Phys. Chem. B 2006, 110, 14386–14390. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Silva, Y.d.J.; Toledano-Ayala, M.; Gallardo-Hernández, S.; Godínez, L.A.; Méndez-López, A. Investigation of TiO2 Deposit on SiO2 Films: Synthesis, Characterization, and Efficiency for the Photocatalytic Discoloration of Methylene Blue in Aqueous Solution. Nanomaterials 2023, 13, 1403. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, Z.S.; Shaban, M.; Ahmed, I.A. Photocatalytic Degradation of Methyl Orange and Methylene Blue Dyes by Engineering the Surface Nano-Textures of TiO2 Thin Films Deposited at Different Temperatures via MOCVD. Molecules 2023, 28, 1160. [Google Scholar] [CrossRef] [PubMed]
- Abboudi, A.; Iaiche, S.; Djelloul, A.; Chala, A.; Kezzoula, F.; Bensouici, F.; Bououdina, M.; Humayun, M. Effect of fluoric acid concentration on the structural, optical, and photocatalytic properties of TiO2 thin films. Inorg. Chem. Commun. 2023, 155, 111073. [Google Scholar] [CrossRef]
- Seifi, A.; Salari, D.; Khataee, A.; Çoşut, B.; Arslan, L.Ç.; Niaei, A. Enhanced photocatalytic activity of highly transparent superhydrophilic doped TiO2 thin films for improving the self-cleaning property of solar panel covers. Ceram. Int. 2023, 49, 1678–1689. [Google Scholar] [CrossRef]
- Widyastuti, E.; Chiu, C.T.; Hsu, J.L.; Lee, Y.C. Photocatalytic antimicrobial and photostability studies of TiO2/ZnO thin films. Arab. J. Chem. 2023, 16, 105010. [Google Scholar] [CrossRef]
- Deepthi, V.; Vidhya, B.; Sebastian, A. Photocatalytic degradation of model pollutants using ZnO/Ag3PO4 heterostructure thin films under visible and solar irradiation. Opt. Mater. 2023, 138, 113646. [Google Scholar] [CrossRef]
- Daher, E.A.; Riachi, B.; Chamoun, J.; Laberty-Robert, C.; Hamd, W. New approach for designing wrinkled and porous ZnO thin films for photocatalytic applications. Colloids Surf. A 2023, 658, 130628. [Google Scholar] [CrossRef]













| Vibrational Modes * | SA | H2Ti[SA]3 | Zn[SA]2·2H2O | |||
|---|---|---|---|---|---|---|
| DFT, cm−1 | Observed, cm−1 | DFT, cm−1 | Observed, cm−1 | DFT, cm−1 | Observed, cm−1 | |
| ν(C=O) | 1731 | 1670 | 1657 | 1670 | - | - |
| νas(COO−) + ν(C=C) | 1623 1577 | 1610 1588 | 1616, 1608 1595 | 1604 1583 | 1627 1584 1540 | 1630 1599 1569 |
| ν(C=C) | 1483 1470 | 1487 1465 | 1466 1451, 1438 | 1487 1460 | 1482 1460 | 1487 1464 |
| νs(COO−) | 1380 | 1379 | 1327 | 1338 | 1397, 1371 | 1397, 1376 |
| δ(OH) + ν(COO−) | 1324, 1312 | 1301 | 1404 1296 | 1400 1310 | - | - |
| ν(Ph-OH) | 1240 | 1245 | 1269, 1242 | 1245, 1224 | 1247 | 1248 |
| δ(Ph-OH) | 1236 | 1219 | - | - | 1227 | 1223 |
| δ(CH) | 1181 1155 1100 | 1159 1126 1107 | 1182 1150 1123, 1090 | 1159 1126 1107 | 1162 1143 | 1159 1126 1107 |
| δ(C=C) | 1023 842 631 | 1032 850 657 | 1034 845 - | 1032 850 - | 1029 815 - | 1032 - - |
| δ(C=C), δ(CH) + δ(COO−) out of plane | 753 695 | 757 701 | 770 712 | 757 701 | 754 708 | 757 704 |
| ν(Me-O) | - | - | 883 680 | 887 671 | 875 674 | 866 671 |
| Composition | * g cm−3 | * mN m−2 | * mPa s−1 | µg cm−2 | µg cm−2 | µg cm−2 | µg cm−2 |
|---|---|---|---|---|---|---|---|
| Ti:10 | 0.913 ± 0.005 | 26.80 ± 1.03 | 8.38 ± 0.01 | 17.31 | 21.49 ± 1.42 | 33.63 ± 1.70 | 55.12 ± 2.16 |
| Ti:15 | 0.878 ± 0.004 | 24.26 ± 0.73 | 6.07 ± 0.03 | 11.32 | 13.16 ± 1.70 | 13.64 ± 1.54 | 26.80 ± 2.38 |
| Ti:20 | 0.856 ± 0.001 | 22.19 ± 0.54 | 4.77 ± 0.01 | 8.15 | 6.86 ± 1.51 | 10.80 ± 3.10 | 17.66 ± 3.32 |
| Zn:10 | 0.960 ± 0.001 | 25.62 ± 0.53 | 6.83 ± 0.01 | 18.21 | 25.00 ± 4.58 | 18.70 ± 2.80 | 43.71 ± 4.42 |
| Zn:15 | 0.912 ± 0.001 | 24.34 ± 0.14 | 4.46 ± 0.01 | 10.59 | 16.50 ± 2.96 | 6.89 ± 1.55 | 23.40 ± 2.30 |
| Zn:20 | 0.876 ± 0.001 | 23.09 ± 0.81 | 3.53 ± 0.01 | 7.37 | 11.17 ± 3.15 | 8.49 ± 3.14 | 19.65 ± 1.40 |
| TiO2 Sample | (eV) | Tauc—Lorentz model fitting parameters | (nm) | *2 | *3 (%) | |||||||
| (eV) | (eV) | (eV) | (eV) | |||||||||
| Ti:10 | 3.34 | 1.88 | 3.39 | 111.25 | 4.36 | 1.203 | 0.49 | 205 | 2.02 | 21 | ||
| Ti:15 | 3.36 | 1.77 | 3.44 | 111.49 | 4.53 | 1.161 | 0.50 | 137 | 1.99 | 22 | ||
| Ti:20 | 3.43 | 2.28 | 3.47 | 114.26 | 4.35 | 0.897 | 0.51 | 86 | 1.91 | 27 | ||
| ZnO Sample | (eV) | Tanguy model fitting parameters | χ2 | d (nm) | *2 | *3 (%) | ||||||
| (eV) | (eV3/2) | (meV) | (meV) | (eV2) | (eV) | |||||||
| Zn:10 | 3.30 | 1 *1 | 3.45 | 8.22 | 60 *1 | 48 | 26 *1 | 7.1 *1 | 0.88 | 72 | 1.57 | 34 |
| Zn:15 | 3.29 | 3.46 | 7.68 | 60 | 0.57 | 52 | 1.55 | 36 | ||||
| Zn:20 | 3.28 | 3.45 | 5.69 | 68 | 0.44 | 35 | 1.48 | 43 | ||||
| Sample | nmol cm−2 | IUV mW cm−2 | nmol h−1 cm−2 | h−1 |
|---|---|---|---|---|
| P25 | 1.447 ± 0.078 | 0.87 ± 0.09 | 1.733 ± 0.096 | 0.153 ± 0.009 |
| 2.08 ± 0.31 | 2.495 ± 0.201 | 0.228 ± 0.020 | ||
| 4.85 ± 0.33 | 2.878 ± 0.206 | 0.266 ± 0.021 | ||
| Ti:10 | 2.426 ± 0.213 | 0.88 ± 0.09 | 0.366 ± 0.035 | 0.031 ± 0.003 |
| 2.01 ± 0.26 | 0.324 ± 0.055 | 0.027 ± 0.005 | ||
| 4.13 ± 0.27 | 0.768 ± 0.318 | 0.068 ± 0.029 | ||
| Ti:15 | 1.756 ± 0.127 | 0.88 ± 0.09 | 0.377 ± 0.061 | 0.032 ± 0.005 |
| 2.04 ± 0.28 | 0.347 ± 0.057 | 0.029 ± 0.005 | ||
| 4.42 ± 0.29 | 0.475 ± 0.067 | 0.040 ± 0.006 | ||
| Ti:20 | 1.441 ± 0.132 | 0.88 ± 0.09 | 0.149 ± 0.037 | 0.012 ± 0.003 |
| 2.09 ± 0.29 | 0.228 ± 0.035 | 0.019 ± 0.003 | ||
| 4.30 ± 0.24 | 0.404 ± 0.071 | 0.034 ± 0.006 | ||
| Zn:10 | 1.159 ± 0.103 | 0.87 ± 0.09 | 2.288 ± 0.138 | 0.206 ± 0.014 |
| 2.01 ± 0.26 | 1.931 ± 0.204 | 0.173 ± 0.019 | ||
| 4.46 ± 0.36 | 1.691 ± 0.176 | 0.150 ± 0.017 | ||
| Zn:15 | 1.169 ± 0.128 | 0.88 ± 0.09 | 1.232 ± 0.112 | 0.107 ± 0.010 |
| 1.95 ± 0.25 | 1.040 ± 0.164 | 0.090 ± 0.015 | ||
| 4.31 ± 0.36 | 0.750 ± 0.077 | 0.064 ± 0.007 | ||
| Zn:20 | 1.007 ± 0.070 | 0.87 ± 0.09 | 0.485 ± 0.043 | 0.041 ± 0.004 |
| 2.11 ± 0.28 | 0.508 ± 0.041 | 0.043 ± 0.004 | ||
| 4.23 ± 0.25 | 0.468 ± 0.063 | 0.040 ± 0.005 |
| Film (Thickness)/Substrate | Deposition Method | Reaction Conditions | h−1 | Ref. |
|---|---|---|---|---|
| TiO2 (198 nm)/Glass | Sol-gel dip-coating TTIP:SA:IPA | 15 mL, 1 mg L−1 MB 3 W UV LED (365 nm) | 0.068 | This work |
| ZnO (90 nm)/Glass | Sol-gel dip-coating ZAD:SA:IPA | 0.206 | ||
| TiO2 (262 nm)/Glass | Sol-gel dip-coating TTIP:HCl:IPA | 3 mL, 5.4 mg L−1 MB 15 W UV lamp (254 nm) | 0.406 | [78] |
| TiO2 (171 nm)/SiO2/Glass | 0.397 | |||
| TiO2 (219 nm)/SiO2/Glass | 0.498 | |||
| TiO2 (262 nm)/SiO2/Glass | 0.798 | |||
| TiO2 (203 nm)/Quartz | Metal-Organic CVD TTIP precursor | 10 mg L−1 MB Natural sunlight | 0.462 * | [79] |
| TiO2 (192 nm)/Glass | Sol-gel dip-coating Ti(oEt)4:EtOH:HNO3:H2O | 35 mL, 2.5×10−5 mol L−1 MB 15 W UV lamp (254 nm) | 0.882 * | [80] |
| TiO2 (199 nm)/Glass | Sol-gel dip-coating TTIP:AcAc:EtOH:HNO3:SDS | 50 mL, 5 mg L−1 MB 11 W UV lamp | 0.255 * | [81] |
| TiO2 (132 nm)/Glass | Magnetron Sputtering | 10 mL, 10 mg L−1 MB 8 W UV tube | 0.145 * | [82] |
| ZnO (122 nm)/Glass | 0.412 * | |||
| ZnO (324 nm)/Glass | Spin-coating + hydrothermal ZAD:MEA:EtOH | 100 mL, 20 mg L−1 MB 300 W Tungsten-Halogen | 0.360 * | [83] |
| ZnO (47 nm)/Glass | Sol-gel dip-coating ZAD:MEA:IPA | 45 mL, 10−5 mol L−1 MB 500 lx UV lamp (365 nm) | 0.246 * | [84] |
| ZnO (130 nm)/Glass | 0.276 * | |||
| ZnO (250 nm)/Glass | 0.294 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanov, B.I. Optically Transparent TiO2 and ZnO Photocatalytic Thin Films via Salicylate-Based Sol Formulations. Coatings 2023, 13, 1568. https://doi.org/10.3390/coatings13091568
Stefanov BI. Optically Transparent TiO2 and ZnO Photocatalytic Thin Films via Salicylate-Based Sol Formulations. Coatings. 2023; 13(9):1568. https://doi.org/10.3390/coatings13091568
Chicago/Turabian StyleStefanov, Bozhidar I. 2023. "Optically Transparent TiO2 and ZnO Photocatalytic Thin Films via Salicylate-Based Sol Formulations" Coatings 13, no. 9: 1568. https://doi.org/10.3390/coatings13091568
APA StyleStefanov, B. I. (2023). Optically Transparent TiO2 and ZnO Photocatalytic Thin Films via Salicylate-Based Sol Formulations. Coatings, 13(9), 1568. https://doi.org/10.3390/coatings13091568