Mechanical and Barrier Properties Optimization of Carboxymethyl Chitosan-Gelatin-Based Edible Film Using Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.2.1. Preparation of CMCS-GL-Based Edible Coating Solutions
2.2.2. Method for Determination of Mechanical Properties and Barrier Properties of CMCS-GL-Based Edible Film
- Determination of film thickness
- 2.
- Determination of TS and EAB
- 3.
- Determination of WVP
- 4.
- Determination of OP
- 5.
- Determination of comprehensive scores for film mechanical and barrier performance
2.2.3. Single Factor Test for Performance Optimization of CMCS-GL-Based Edible Film
2.2.4. Response Surface Optimization Test of Performance of the CMCS-GL-Based Edible Film
2.3. Statistical Analysis
3. Results and Discussion
3.1. Univariate Test Results and Analysis
3.1.1. Effect of CMCS:GL (w:w) on Mechanical and Barrier Properties of Edible Film
3.1.2. The Effect of Glycerol Addition on the Mechanical and Barrier Properties of Edible Films
3.1.3. Effect of CaCl2 Addition on the Mechanical and Barrier Properties of Edible Films
3.1.4. Effect of Tween-20 Addition on the Mechanical and Barrier Properties of Edible Films
3.1.5. Effect of AA Addition on the Mechanical and Barrier Properties of Edible Films
3.2. Determination of the Comprehensive Scores of the Mechanical and Barrier Properties of the Edible Film
3.2.1. Results of Principal Component Analysis
3.2.2. Determination of Comprehensive Scores of Mechanical and Barrier Properties of Edible Membranes
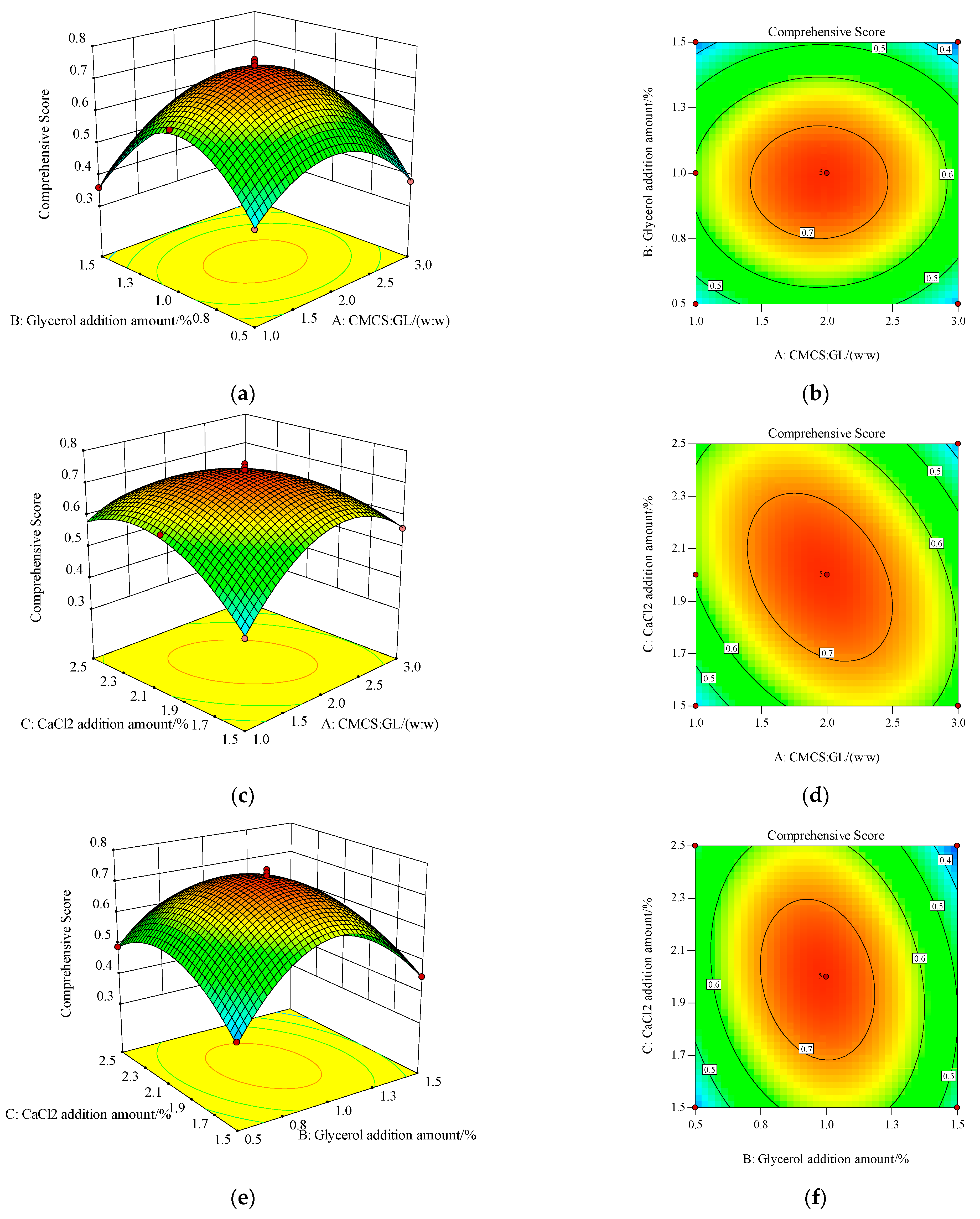
3.3. Response Surface Optimization Test Results and Analysis
3.4. Response Surface Optimization Test Graph Analysis
3.5. Determination and Verification of the Optimal Formulation of CMCS-GL-Based Edible Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mkandawire, M.; Aryee, A.N.A. Resurfacing and modernization of edible packaging material technology. Curr. Opin. Food Sci. 2018, 19, 104–112. [Google Scholar] [CrossRef]
- Nešić, A.; Cabrera-Barjas, G.; Dimitrijević-Branković, S.; Davidović, S.; Radovanović, N.; Delattre, C. Prospect of Polysaccharide-Based Materials as Advanced Food Packaging. Molecules 2020, 25, 135. [Google Scholar] [CrossRef] [PubMed]
- Petkoska, A.T.; Daniloski, D.; D’Cunha, N.M.; Naumovski, N.; Broach, A.T. Edible packaging: Sustainable solutions and novel trends in food packaging. Food Res. Int. 2021, 140, 109981. [Google Scholar] [CrossRef]
- Amin, U.; Khan, M.U.; Majeed, Y.; Rebezov, M.; Khayrullin, M.; Bobkova, E.; Shariati, M.A.; Chung, I.M.; Thiruvengadam, M. Potentials of polysaccharides, lipids and proteins in biodegradable food packaging applications. Int. J. Biol. Macromol. 2021, 183, 2184–2198. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, B.; Qadri, O.S.; Srivastava, A.K. Recent developments in shelf-life extension of fresh-cut fruits and vegetables by application of different edible coatings: A review. LWT Food Sci. Technol. 2018, 89, 198–209. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current advancements in chitosan-based film production for food technology; A review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef]
- Zhang, C.; Gong, H.; Liu, Y. Effects of postharvest coating using chitosan combined with natamycin on physicochemical and microbial properties of sweet cherry during cold storage. Int. J. Biol. Macromol. 2022, 214, 1–9. [Google Scholar] [CrossRef]
- Tokatlı, K.; Demirdöven, A. Effects of chitosan edible film coatings on the physicochemical and microbiological qualities of sweet cherry (Prunus avium L.). Sci. Hortic. 2020, 259, 108656. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, H.; Huang, Q.; Xu, J.; Duan, M.; Yu, S.; Zhi, Z.; Pang, J.; Wu, C. Carboxymethyl chitosan incorporated with gliadin/phlorotannin nanoparticles enables the formation of new active packaging films. Int. J. Biol. Macromol. 2022, 203, 40–48. [Google Scholar] [CrossRef]
- Bai, R.; Zhang, X.; Yong, H.; Wang, X.; Liu, Y.; Liu, J. Development and characterization of antioxidant active packaging and intelligent Al3+-sensing films based on carboxymethyl chitosan and quercetin. Int. J. Biol. Macromol. 2019, 126, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Dayarian, S.; Zamani, A.; Moheb, A.; Masoomi, M. Physico-Mechanical Properties of Films of Chitosan, Carboxymethyl Chitosan, and Their Blends. J. Polym. Environ. 2014, 22, 409–416. [Google Scholar] [CrossRef]
- Fan, S.; Wang, D.; Wen, X.; Li, X.; Fang, F.; Richel, A.; Xiao, N.; Fauconnier, M.-L.; Hou, C.; Zhang, D. Incorporation of cinnamon essential oil-loaded Pickering emulsion for improving antimicrobial properties and control release of chitosan/gelatin films. Food Hydrocoll. 2023, 138, 108438. [Google Scholar] [CrossRef]
- Yadav, S.; Mehrotra, G.; Bhartiya, P.; Singh, A.; Dutta, P. Preparation, physicochemical and biological evaluation of quercetin based chitosan-gelatin film for food packaging. Carbohydr. Polym. 2020, 227, 115348. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Nguyen, D.H.; Nguyen, H.V. Combination effects of calcium chloride and nano-chitosan on the postharvest quality of strawberry (Fragaria x ananassa Duch.). Postharvest Biol. Technol. 2020, 162, 111103. [Google Scholar] [CrossRef]
- Kou, X.-H.; Guo, W.-L.; Guo, R.-Z.; Li, X.-Y.; Xue, Z.-H. Effects of Chitosan, Calcium Chloride, and Pullulan Coating Treatments on Antioxidant Activity in Pear cv. “Huang guan” During Storage. Food Bioprocess Technol. 2014, 7, 671–681. [Google Scholar] [CrossRef]
- Liu, K.; Yuan, C.; Chen, Y.; Li, H.; Liu, J. Combined effects of ascorbic acid and chitosan on the quality maintenance and shelf life of plums. Sci. Hortic. 2014, 176, 45–53. [Google Scholar] [CrossRef]
- Aleryani-Raqeeb, A.; Mahmud, T.M.; Omar, S.S.; Za, A.M. Effects of Calcium Infiltration and Chitosan Coating on Storage Life and Quality Characteristics During Storage of Papaya (Carica papaya L.). Int. J. Agric. Res. 2008, 3, 296–306. [Google Scholar] [CrossRef][Green Version]
- Tian, Z.; Guo, Y.; Yang, X.; Guo, K.; Ji, J.; Hao, S. Nano Calcium-Deficient Hydroxyapatite/O-carboxymethyl Chitosan-CaCl2 Microspheres Loaded with Rhein for Bone Defect Repair. J. Bionic Eng. 2022, 19, 1087–1099. [Google Scholar] [CrossRef]
- Mohamood, N.F.A.-Z.T.; Halim, A.H.A.; Zainuddin, N. Carboxymethyl Cellulose Hydrogel from Biomass Waste of Oil Palm Empty Fruit Bunch Using Calcium Chloride as Crosslinking Agent. Polymers 2021, 13, 4056. [Google Scholar] [CrossRef]
- Özdemir, K.S.; Gökmen, V. Effect of Chitosan-Ascorbic Acid Coatings on the Refrigerated Storage Stability of Fresh-Cut Apples. Coatings 2019, 9, 503. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Cui, Q.-L.; Wang, Y.; Shi, F.; Liu, Y.-P.; Liu, J.-L.; Nie, G.-W. Effect of carboxymethyl chitosan-gelatin-based edible coatings on the quality and antioxidant properties of sweet cherry during postharvest storage. Sci. Hortic. 2021, 289, 110462. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Cui, Q.-L.; Wang, Y.; Shi, F.; Fan, H.; Zhang, Y.-Q.; Lai, S.-T.; Li, Z.-H.; Li, L.; Sun, Y.-K. Effect of Edible Carboxymethyl Chitosan-Gelatin Based Coating on the Quality and Nutritional Properties of Different Sweet Cherry Cultivars during Postharvest Storage. Coatings 2021, 11, 396. [Google Scholar] [CrossRef]
- Martínez-Camacho, A.; Cortez-Rocha, M.; Ezquerra-Brauer, J.; Graciano-Verdugo, A.; Rodriguez-Félix, F.; Castillo-Ortega, M.; Yépiz-Gómez, M.; Plascencia-Jatomea, M. Chitosan composite films: Thermal, structural, mechanical and antifungal properties. Carbohydr. Polym. 2010, 82, 305–315. [Google Scholar] [CrossRef]
- Haghighi, H.; De Leo, R.; Bedin, E.; Pfeifer, F.; Siesler, H.W.; Pulvirenti, A. Comparative analysis of blend and bilayer films based on chitosan and gelatin enriched with LAE (lauroyl arginate ethyl) with antimicrobial activity for food packaging applications. Food Packag. Shelf Life 2019, 19, 31–39. [Google Scholar] [CrossRef]
- Fan, H.Y.; Duquette, D.; Dumont, M.-J.; Simpson, B.K. Salmon skin gelatin-corn zein composite films produced via crosslinking with glutaraldehyde: Optimization using response surface methodology and characterization. Int. J. Biol. Macromol. 2018, 120, 263–273. [Google Scholar] [CrossRef]
- Ziani, K.; Oses, J.; Coma, V.; Maté, J.I. Effect of the presence of glycerol and Tween 20 on the chemical and physical properties of films based on chitosan with different degree of deacetylation. LWT Food Sci. Technol. 2008, 41, 2159–2165. [Google Scholar] [CrossRef]
- Rocha, G.O.; Farias, M.G.; de Carvalho, C.W.P.; Ascheri, J.L.R.; Galdeano, M.C. Filmes compostos biodegradáveis a base de amido de mandioca e proteína de soja. Polim. Cienc. Tecnol. 2014, 24, 587–595. [Google Scholar] [CrossRef]
- Xu, J.; Wei, R.; Jia, Z.; Song, R. Characteristics and bioactive functions of chitosan/gelatin-based film incorporated with ε-polylysine and astaxanthin extracts derived from by-products of shrimp (Litopenaeus vannamei). Food Hydrocoll. 2020, 100, 105436. [Google Scholar] [CrossRef]
- Zugravu, M.V.; Smith, R.A.; Reves, B.T.; Jennings, J.A.; Cooper, J.O.; Haggard, W.O.; Bumgardner, J.D. Physical properties and in vitro evaluation of collagen–chitosan–calcium phosphate microparticle-based scaffolds for bone tissue regeneration. J. Biomater. Appl. 2012, 28, 566–579. [Google Scholar] [CrossRef]
- Maringgal, B.; Hashim, N.; Tawakkal, I.S.M.A.; Mohamed, M.T.M. Recent advance in edible coating and its effect on fresh/fresh-cut fruits quality. Trends Food Sci. Technol. 2020, 96, 253–267. [Google Scholar] [CrossRef]
- Davidović, S.; Miljković, M.; Tomić, M.; Gordić, M.; Nešić, A.; Dimitrijević, S. Response surface methodology for optimisation of edible coatings based on dextran from Leuconostoc mesenteroides T3. Carbohydr. Polym. 2018, 184, 207–213. [Google Scholar] [CrossRef] [PubMed]
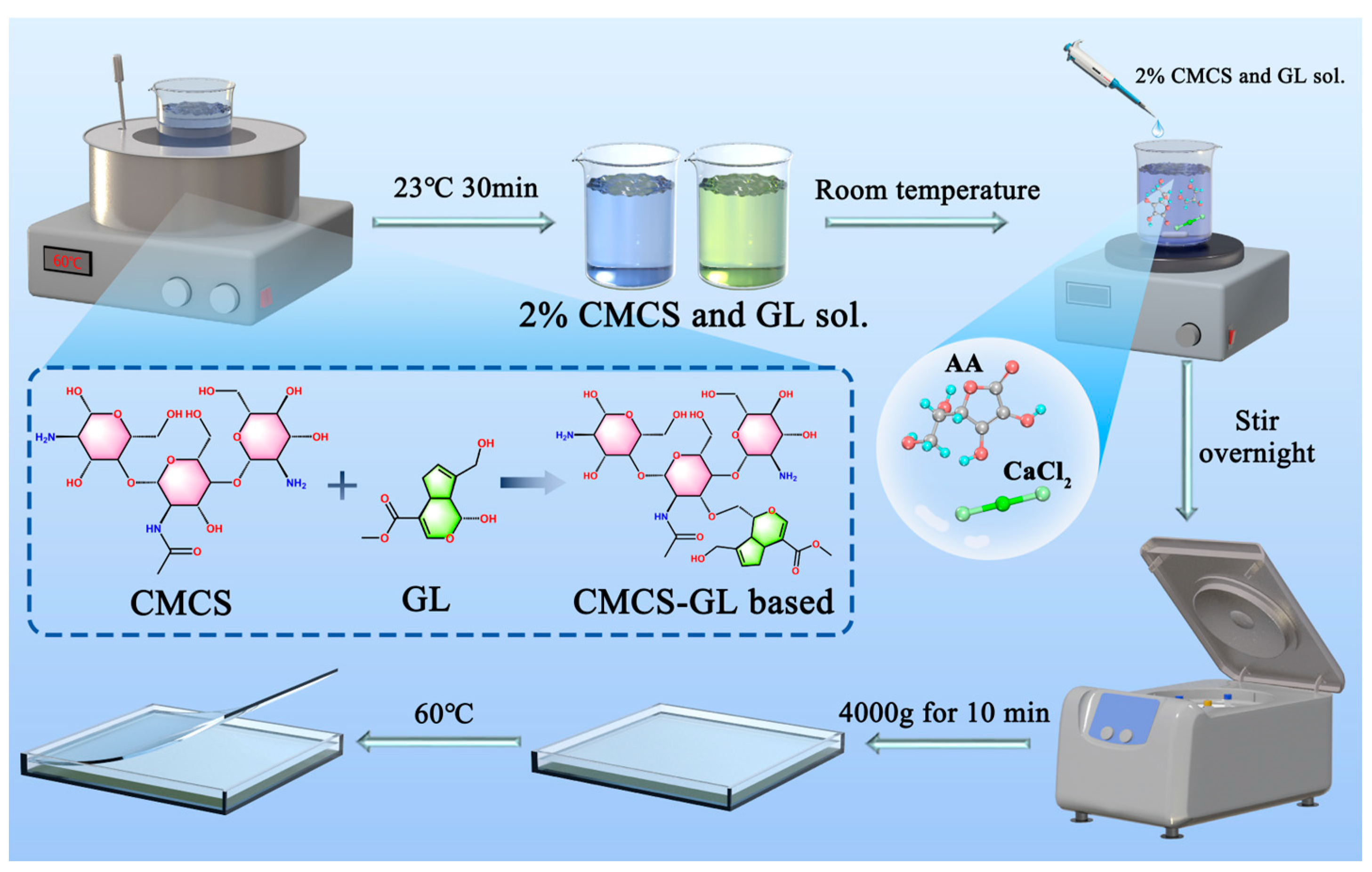
| Levels | The Quality Ratio between CMCS and GL | Glycerol Addition Amount/% | CaCl2 Addition Amount/% | Tween-20 Addition Amount/% | AA Addition Amount/% |
|---|---|---|---|---|---|
| 1 | 6:0 | 0 | 1 | 0 | 0 |
| 2 | 4:2 | 0.5 | 1.5 | 0.1 | 1 |
| 3 | 3:3 | 1 | 2 | 0.2 | 2 |
| 4 | 2:4 | 1.5 | 2.5 | 0.3 | 3 |
| 5 | 0:6 | 2 | 3 | 0.4 | 4 |
| The Quality Ratio between CMCS and GL | Glycerol Addition Amount/% | CaCl2 Addition Amount/% |
|---|---|---|
| 1:1 (1) | 0.5 | 1.5 |
| 2:1 (2) | 1 | 2 |
| 3:1 (3) | 1.5 | 2.5 |
| Factors | AA Addition Amount/% | Thickness/mm | TS/MPa | EAB/% | WVP/ 10−12 g·cm/ (cm2·s·Pa) | OP/ 10−11 cm3·cm/ (m2·s·Pa) |
|---|---|---|---|---|---|---|
| CMCS:GL (w:w) | 6:0 | 0.052 ± 0.006 a | 17.61 ± 0.57 a | 41.74 ± 1.77 a | 1.85 ± 0.12 a | 6.51 ± 0.19 a |
| 4:2 | 0.053 ± 0.005 a | 20.09 ± 0.71 b | 79.84 ± 2.54 b | 1.53 ± 0.08 b | 5.37 ± 0.16 b | |
| 3:3 | 0.053 ± 0.006 a | 16.03 ± 0.38 c | 70.95 ± 2.14 c | 1.38 ± 0.07 b,c | 5.19 ± 0.15 b | |
| 2:4 | 0.050 ± 0.005 a | 13.08 ± 0.29 d | 64.15 ± 2.50 d | 1.29 ± 0.08 c | 5.23 ± 0.10 b | |
| 0:6 | 0.053 ± 0.006 a | 8.40 ± 0.38 e | 62.15 ± 1.96 d | 1.23 ± 0.13 c | 4.52 ± 0.40 c | |
| Glycerol addition amount/% | 0 | 0.055 ± 0.004 a | 20.17 ± 0.63 a | 45.06 ± 1.99 a | 1.75 ± 0.09 a | 6.46 ± 0.09 a |
| 0.5 | 0.053 ± 0.003 a | 18.10 ± 0.79 b | 52.51 ± 1.38 b | 1.54 ± 0.07 b | 5.66 ± 0.11 b | |
| 1 | 0.057 ± 0.004 a | 16.18 ± 0.43 c | 70.91 ± 1.97 c | 1.41 ± 0.03 c | 5.18 ± 0.13 b | |
| 1.5 | 0.055 ± 0.003 a | 14.36 ± 0.79 d | 74.64 ± 1.94 d | 1.58 ± 0.05 b | 5.84 ± 0.11 b | |
| 2 | 0.054 ± 0.003 a | 13.06 ± 0.67 e | 76.20 ± 1.81 d | 1.81 ± 0.08 a | 6.38 ± 0.15 a | |
| CaCl2 addition amount/% | 1 | 0.055 ± 0.004 a | 13.37 ± 0.58 a,b | 78.66 ± 1.93 a | 1.88 ± 0.07 a,d | 6.61 ± 0.24 a |
| 1.5 | 0.052 ± 0.005 a | 14.84 ± 0.80 b | 75.68 ± 1.69 a | 1.61 ± 0.06 b | 5.66 ± 0.19 b | |
| 2 | 0.055 ± 0.003 a | 16.22 ± 0.52 c | 71.92 ± 1.83 b | 1.45 ± 0.08 c | 5.20 ± 0.15 c | |
| 2.5 | 0.053 ± 0.004 a | 14.14 ± 0.73 b | 57.37 ± 1.46 c | 1.75 ± 0.08 a | 5.82 ± 0.25 b,d | |
| 3 | 0.054 ± 0.003 a | 12.45 ± 0.66 a | 46.82 ± 1.96 d | 1.96 ± 0.09 d | 6.19 ± 0.21 d | |
| Tween-20 addition amount/% | 0 | 0.053 ± 0.004 a | 15.83 ± 0.54 a | 66.23 ± 1.50 a | 1.45 ± 0.03 a | 5.36 ± 0.20 a |
| 0.1 | 0.054 ± 0.003 a | 15.75 ± 0.50 a | 65.61 ± 1.70 a | 1.47 ± 0.06 a | 5.25 ± 0.25 a | |
| 0.2 | 0.055 ± 0.003 a | 15.77 ± 0.93 a | 66.01 ± 1.61 a | 1.49 ± 0.08 a | 5.33 ± 0.20 a | |
| 0.3 | 0.054 ± 0.003 a | 15.92 ± 0.21 a | 65.64 ± 1.46 a | 1.46 ± 0.06 a | 5.38 ± 0.21 a | |
| 0.4 | 0.055 ± 0.002 a | 16.06 ± 0.67 a | 65.20 ± 1.84 a | 1.42 ± 0.04 a | 5.35 ± 0.12 a | |
| AA addition amount/% | 0 | 0.056 ± 0.004 a | 15.41 ± 0.31 a,b | 64.42 ± 1.04 a | 1.44 ± 0.04 a | 5.34 ± 0.23 a |
| 1 | 0.055 ± 0.004 a | 15.55 ± 0.28 b | 64.47 ± 0.62 a | 1.42 ± 0.05 a | 5.50 ± 0.08 a | |
| 2 | 0.053 ± 0.003 a | 15.50 ± 0.33 b | 64.99 ± 1.17 a | 1.45 ± 0.05 a | 5.44 ± 0.10 a | |
| 3 | 0.056 ± 0.005 a | 14.92 ± 0.21 a,b | 64.30 ± 1.08 a | 1.47 ± 0.05 a | 5.46 ± 0.12 a | |
| 4 | 0.057 ± 0.006 a | 14.73 ± 0.66 a | 64.38 ± 1.20 a | 1.49 ± 0.07 a | 5.47 ± 0.06 a |
| Types | Number | TS/MPa | EAB/% | WVP/ 10−12 g·cm/(cm2·s·Pa) | OP 10−11 cm3·cm/(m2·s·Pa) |
|---|---|---|---|---|---|
| Raw test data | 1 | 17.61 ± 0.57 | 41.74 ± 1.77 | 1.85 ± 0.12 | 6.51 ± 0.19 |
| 2 | 20.09 ± 0.71 | 79.84 ± 2.54 | 1.53 ± 0.08 | 5.37 ± 0.16 | |
| 3 | 18.10 ± 0.79 | 52.51 ± 1.38 | 1.54 ± 0.07 | 5.66 ± 0.11 | |
| 4 | 16.18 ± 0.43 | 70.91 ± 1.97 | 1.41 ± 0.03 | 5.18 ± 0.13 | |
| 5 | 16.22 ± 0.52 | 71.92 ± 1.83 | 1.45 ± 0.08 | 5.20 ± 0.15 | |
| 6 | 14.14 ± 0.73 | 57.37 ± 1.46 | 1.75 ± 0.08 | 5.82 ± 0.25 | |
| Standardized Test data | 1 | 0.55 ± 0.08 | 0.04 ± 0.04 | 0.20 ± 0.19 | 0.11 ± 0.11 |
| 2 | 0.90 ± 0.24 | 0.93 ± 0.49 | 0.73 ± 0.19 | 0.79 ± 0.45 | |
| 3 | 0.82 ± 0.24 | 0.63 ± 0.47 | 0.51 ± 0.29 | 0.64 ± 0.36 | |
| 4 | 0.65 ± 0.10 | 0.51 ± 0.06 | 0.80 ± 0.14 | 0.67 ± 0. 09 | |
| 5 | 0.73 ± 0.21 | 0.72 ± 0.40 | 0.78 ± 0. 06 | 0.71 ± 0.09 | |
| 6 | 0.59 ± 0.15 | 0.45 ± 0.42 | 0.77 ± 0.03 | 0.69 ± 0.03 |
| Component | Eigenvalues | Variance Contribution Rate/% | Cumulative Variance Contribution Rate/% |
|---|---|---|---|
| Z1 | 2.619 | 65.475 | 65.475 |
| Z2 | 1.008 | 25.188 | 90.663 |
| Z3 | 0.280 | 6.993 | 97.656 |
| Z4 | 0.094 | 2.344 | 100.000 |
| Component | TS (X1) | EAB (X2) | WVP (X3) | OP (X4) |
|---|---|---|---|---|
| Z1 | 0.922 | 0.186 | 0.914 | 0.948 |
| Z2 | 0.044 | 0.981 | −0.030 | −0.206 |
| Component | TS | EAB | WVP | OP |
|---|---|---|---|---|
| Y1 | 0.570 | 0.115 | 0.565 | 0.948 |
| Y2 | 0.044 | 0.997 | −0.030 | −0.205 |
| H | 0.423 | 0.115 | 0.565 | 0.586 |
| W | 0.251 | 0.068 | 0.334 | 0.347 |
| Number | CMCS:GL (w:w, X1) | Glycerol Addition Amount/% (X2) | CaCl2 Addition Amount/% (X3) | Comprehensive Score Y |
|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 0.76 |
| 2 | −1 | 0 | 0 | 0.62 |
| 3 | 0 | 1 | −1 | 0.45 |
| 4 | 0 | −1 | −1 | 0.40 |
| 5 | 0 | −1 | 1 | 0.49 |
| 6 | 0 | 0 | 0 | 0.75 |
| 7 | 1 | −1 | 0 | 0.38 |
| 8 | 0 | 0 | 0 | 0.74 |
| 9 | 1 | 1 | 0 | 0.33 |
| 10 | −1 | −1 | 0 | 0.42 |
| 11 | 0 | 0 | 0 | 0.71 |
| 12 | −1 | 0 | −1 | 0.41 |
| 13 | −1 | 1 | 0 | 0.36 |
| 14 | 0 | 0 | 0 | 0.73 |
| 15 | 1 | 0 | −1 | 0.56 |
| 16 | 1 | 0 | −1 | 0.37 |
| 17 | 0 | 1 | 0 | 0.31 |
| Source of Variance | Sum of Squares | DF | Mean Square | F Value | p Value | Significance |
|---|---|---|---|---|---|---|
| Model | 0.44 | 9 | 0.049 | 188.15 | <0.0001 | ** |
| X1 | 2.1 × 10−3 | 1 | 2.1 × 10−3 | 8.10 | 0.0248 | * |
| X2 | 7.2 × 10−3 | 1 | 7.2 × 10−3 | 27.78 | 0.0012 | ** |
| X3 | 4.22 × 10−3 | 1 | 4.22 × 10−3 | 1.63 | 0.2427 | |
| X1X2 | 2.5 × 10−3 | 1 | 2.5 × 10−3 | 0.096 | 0.7652 | |
| X1X3 | 0.018 | 1 | 0.018 | 68.01 | <0.0001 | ** |
| X2X3 | 0.013 | 1 | 0.013 | 51.02 | 0.0002 | ** |
| 0.085 | 1 | 0.085 | 325.98 | <0.0001 | ** | |
| 0.18 | 1 | 0.18 | 690.45 | <0.0001 | ** | |
| 0.040 | 1 | 0.040 | 155.97 | <0.0001 | ** | |
| Residual | 1.815 × 10−3 | 7 | 2.592 × 10−3 | |||
| Lack of fit | 3.345 × 10−3 | 3 | 1.115 × 10−3 | 0.30 | 0.8240 | no |
| Pure Error | 1.48 × 10−3 | 4 | 3.7 × 10−3 | |||
| Cor total | 0.44 | 16 | ||||
| R2 | 0.9959 | |||||
| 0.9906 | ||||||
| CV/% | 3.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-L.; Cui, Q.-L.; Wang, Y.; Liu, J.-L.; Zhang, Y.-Q. Mechanical and Barrier Properties Optimization of Carboxymethyl Chitosan-Gelatin-Based Edible Film Using Response Surface Methodology. Coatings 2023, 13, 1529. https://doi.org/10.3390/coatings13091529
Zhang Y-L, Cui Q-L, Wang Y, Liu J-L, Zhang Y-Q. Mechanical and Barrier Properties Optimization of Carboxymethyl Chitosan-Gelatin-Based Edible Film Using Response Surface Methodology. Coatings. 2023; 13(9):1529. https://doi.org/10.3390/coatings13091529
Chicago/Turabian StyleZhang, Yu-Lei, Qing-Liang Cui, Yu Wang, Jin-Long Liu, and Yan-Qing Zhang. 2023. "Mechanical and Barrier Properties Optimization of Carboxymethyl Chitosan-Gelatin-Based Edible Film Using Response Surface Methodology" Coatings 13, no. 9: 1529. https://doi.org/10.3390/coatings13091529
APA StyleZhang, Y.-L., Cui, Q.-L., Wang, Y., Liu, J.-L., & Zhang, Y.-Q. (2023). Mechanical and Barrier Properties Optimization of Carboxymethyl Chitosan-Gelatin-Based Edible Film Using Response Surface Methodology. Coatings, 13(9), 1529. https://doi.org/10.3390/coatings13091529






