Highly Efficient Photocathodic Protection Performance of ZIS@CNNs Composites under Visible Light
Abstract
:1. Introduction
2. Experimental
2.1. Materials
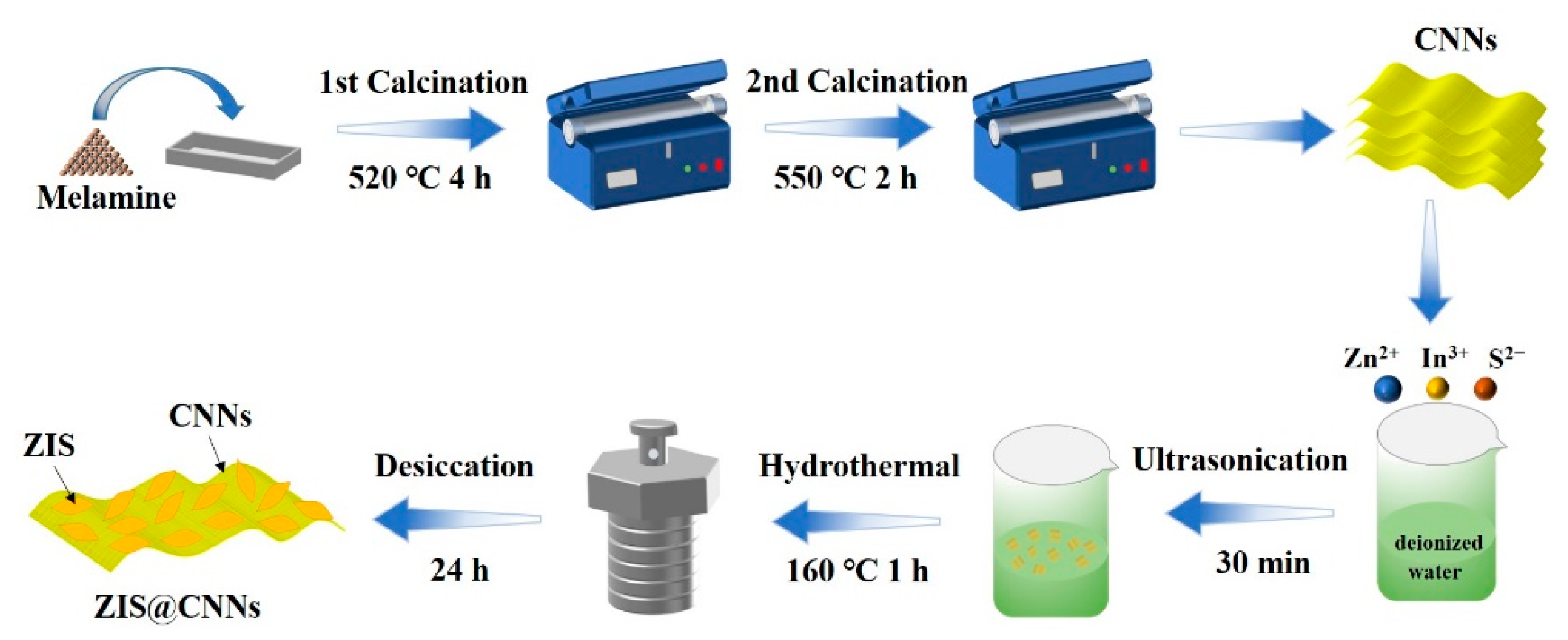
2.2. Preparation of CNNs
2.3. Fabrication of ZIS@CNNs Photoelectrodes
2.4. Characterization
2.5. Electrochemical Tests
3. Results and Discussion
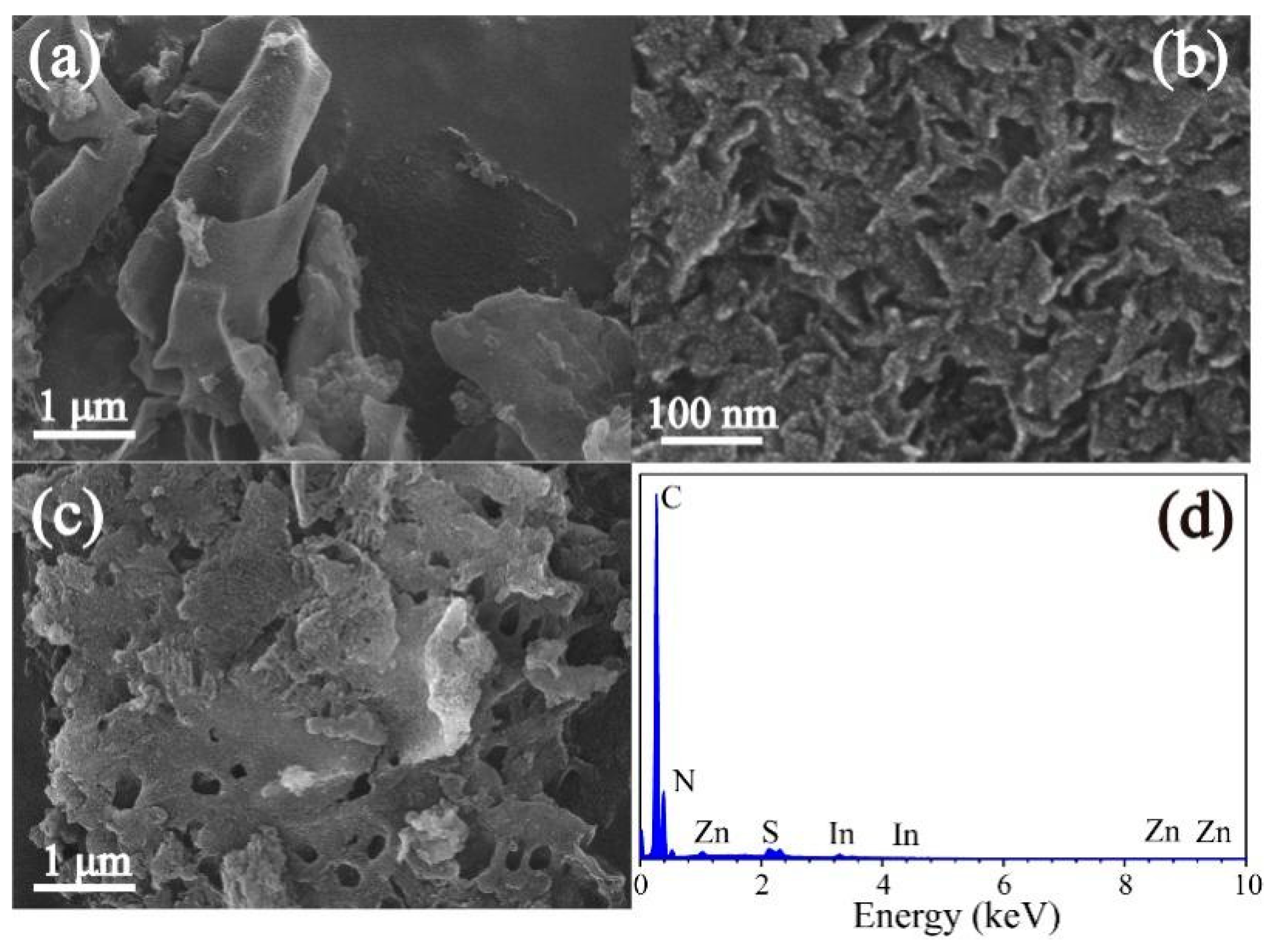
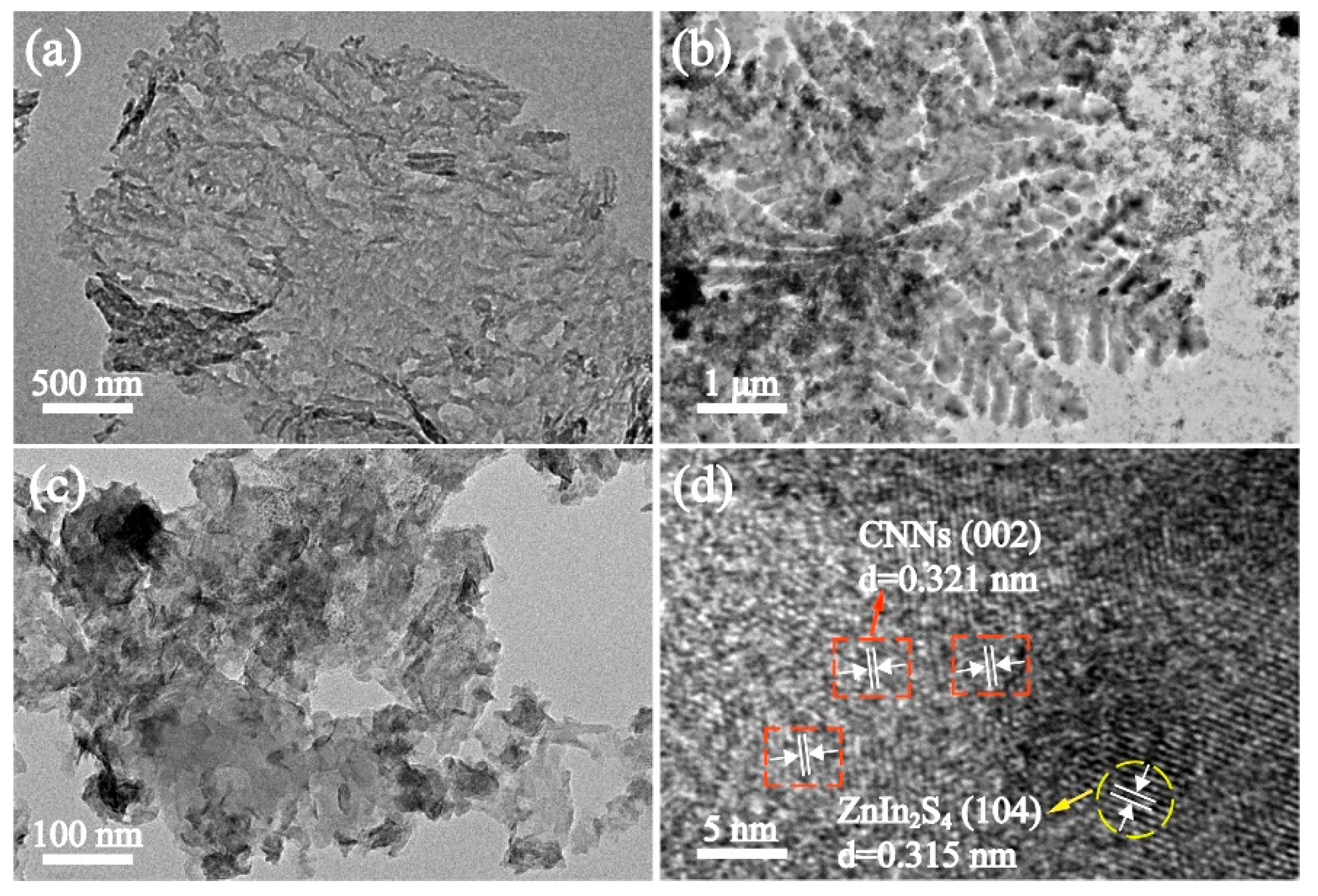
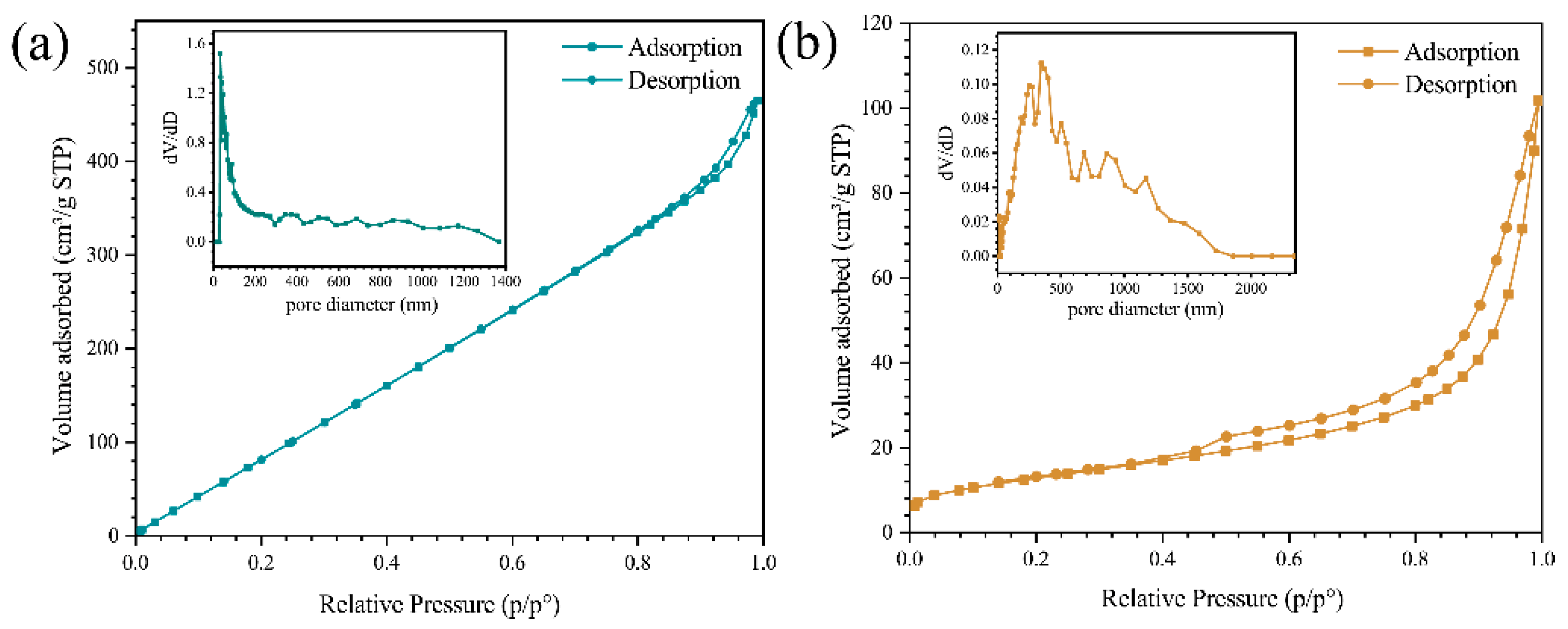
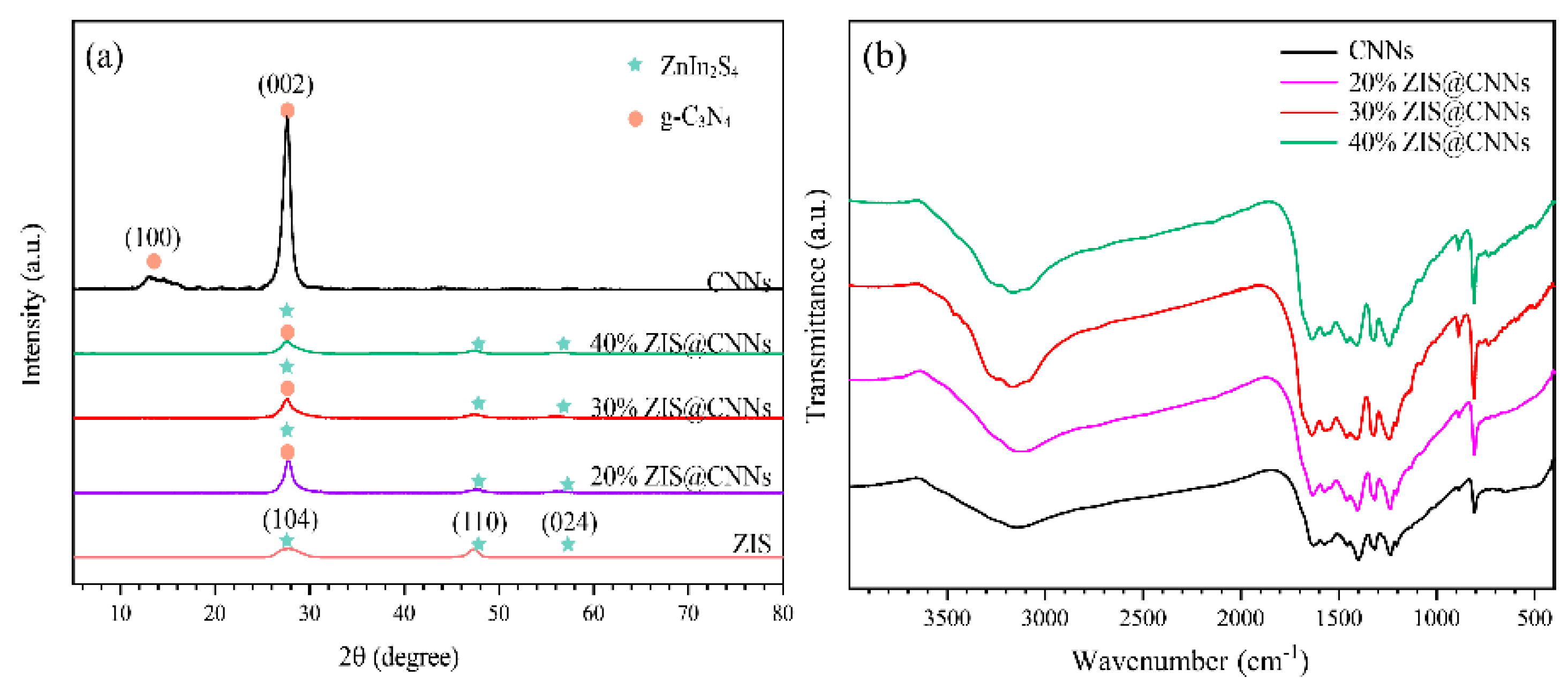
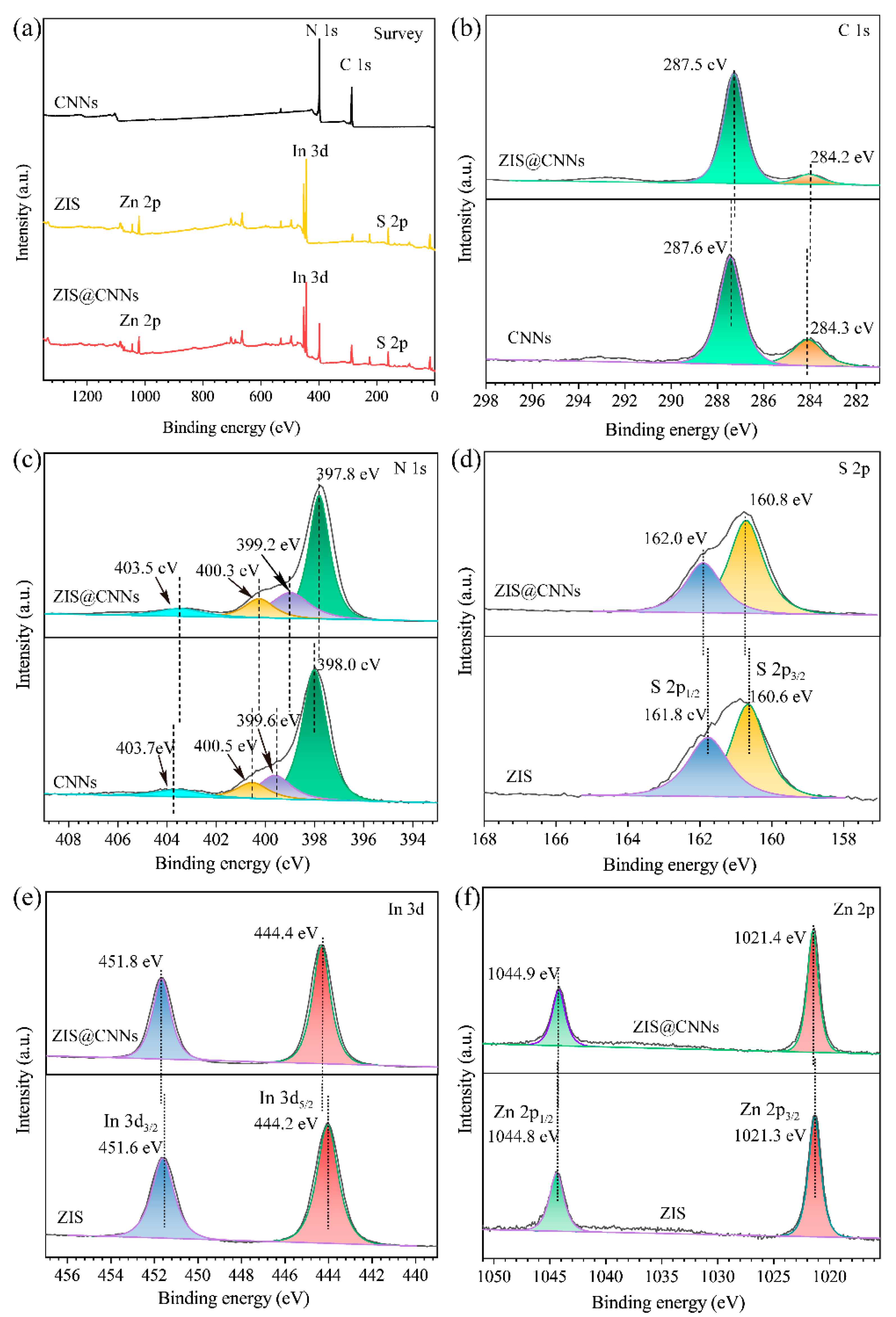
3.1. Characterization of ZIS@CNNs Composites
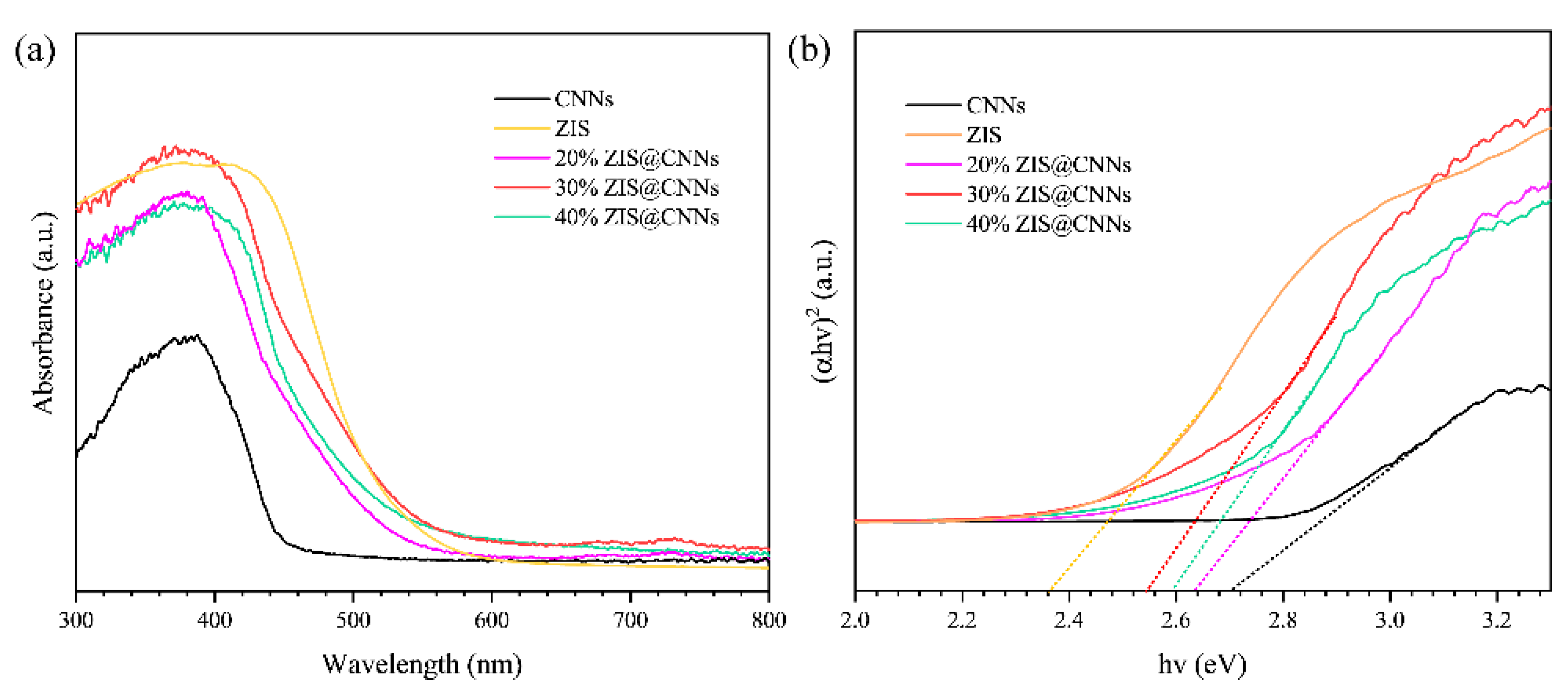
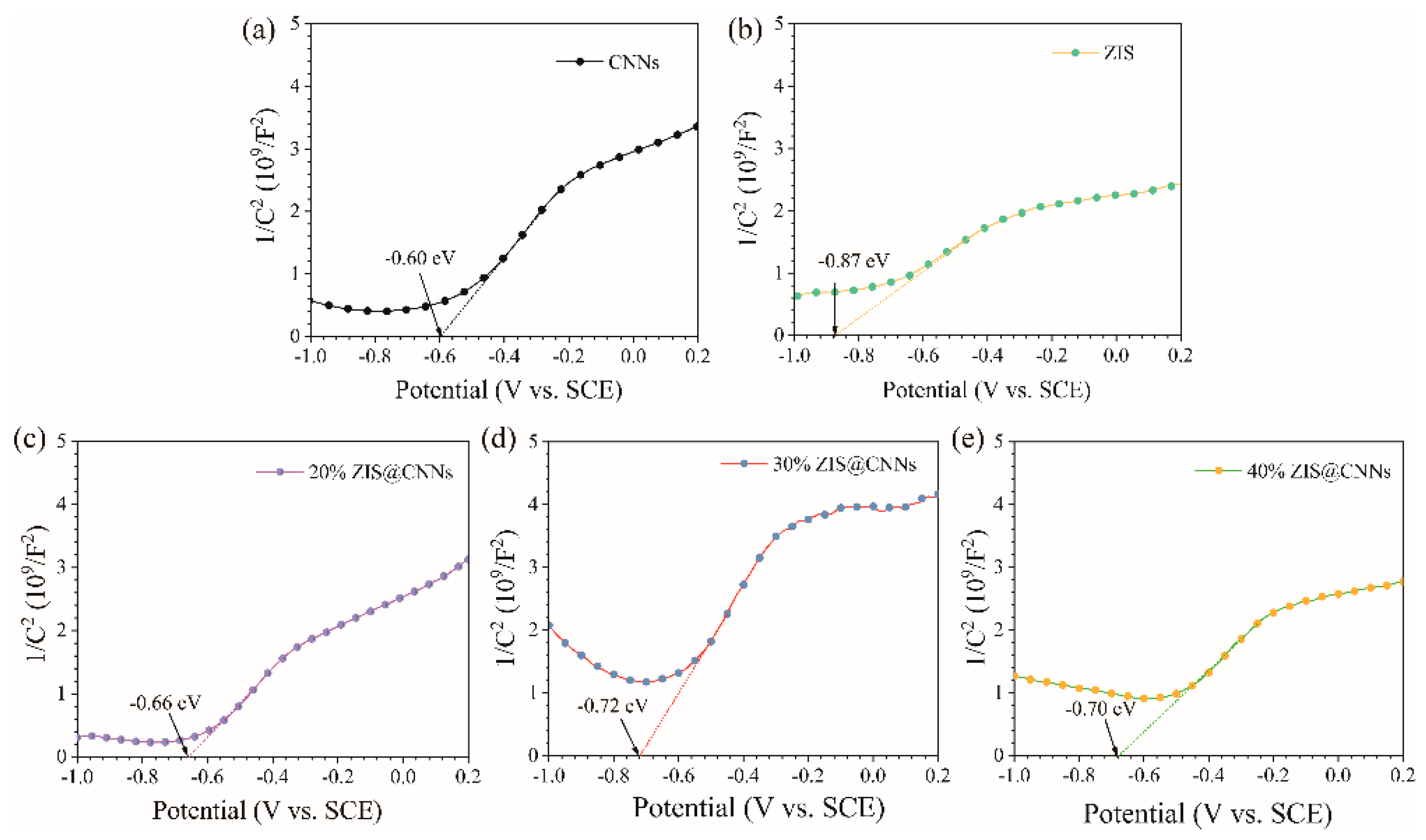
3.2. Light Absorption Characteristics
3.3. PCP Effects
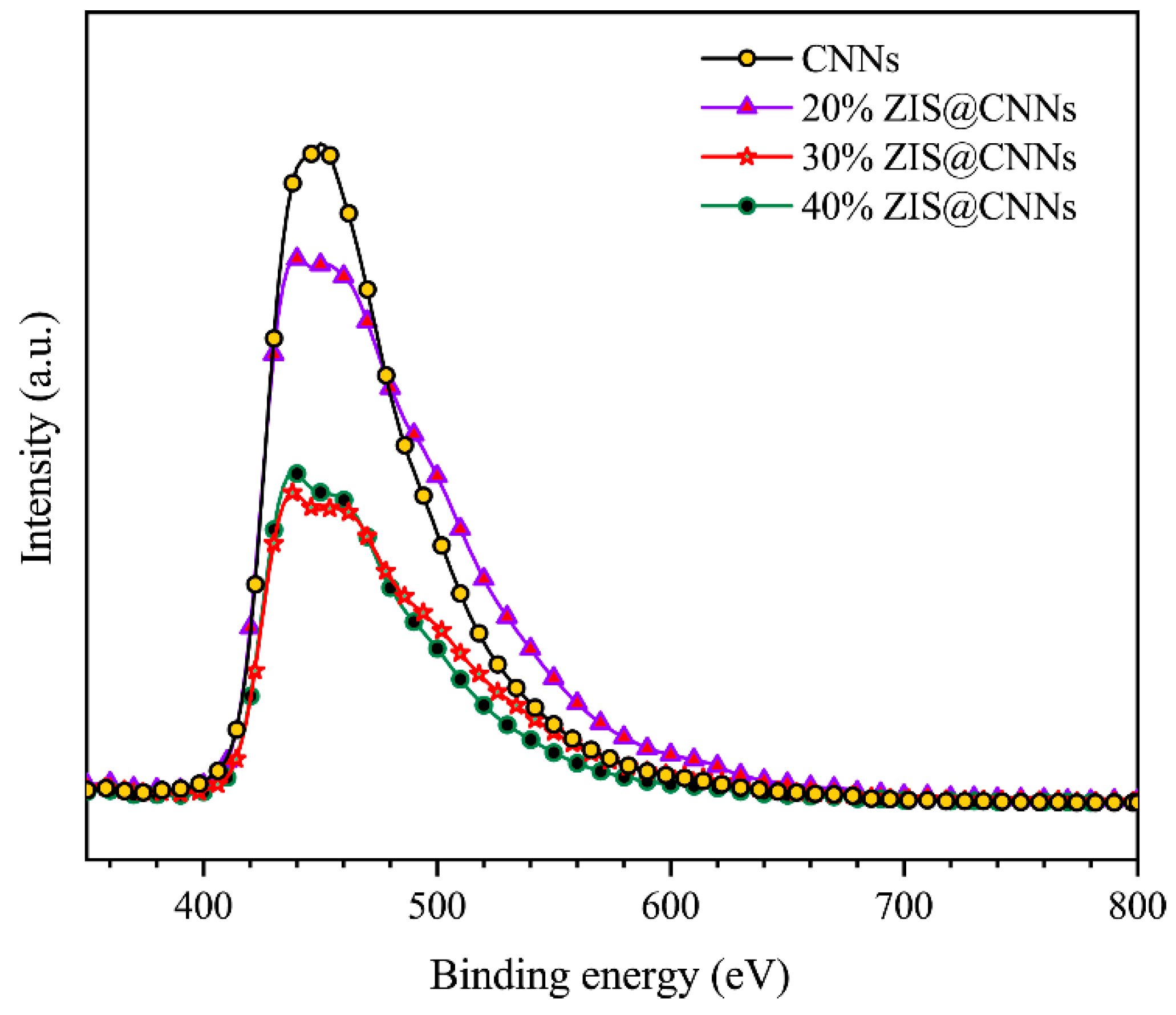
3.4. Mechanism Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, H.; Zhang, Y.; Wang, S.; Li, L.; Wang, W.; Sun, Q. In-situ generation of Bi2S3 to construct WO3/BiVO4/Bi2S3 heterojunction for photocathodic protection of 304 SS. J. Electroanal. Chem. 2022, 907, 116033. [Google Scholar] [CrossRef]
- Pu, J.; Wang, X.; Liu, J.; Ren, M.; Nan, Y.; Liu, M.; Xu, H.; Yang, L.; Huang, Y.; Hou, B. PDA decorated spaced TiO2 nanotube array photoanode material for photocathodic protection of 304 stainless steels. J. Electroanal. Chem. 2022, 914, 116319. [Google Scholar] [CrossRef]
- Wang, X.; Xu, H.; Nan, Y.; Sun, X.; Duan, J.; Huang, Y.; Hou, B. Research progress of TiO2 photocathodic protection to metals in marine environment. J. Oceanol. Limnol. 2020, 38, 1018–1044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, H.; Wang, Y.; Cui, X.; Wen, Z.; Liu, Y.; Fan, L.; Feng, J. Facile fabrication of SnO2 modified TiO2 nanorods film for efficient photocathodic protection of 304 stainless steel under simulated solar light. Corros. Sci. 2020, 176, 108927. [Google Scholar] [CrossRef]
- Qiu, J.; Wei, S.; Pan, J.; Liang, Z.; Ma, J.; Liang, Q.; Li, C. g-C3N4-loaded carbon nanofiber for efficient photocatalytic hydrogen production. Integr. Ferroelectr. 2023, 231, 70–77. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, H.; Li, C.; Bai, J. 1D CeO2/g-C3N4 type II heterojunction for visible-light-driven photocatalytic hydrogen evolution. Inorg. Chem. Commun. 2022, 144, 109838. [Google Scholar] [CrossRef]
- Ren, T.; Dang, Y.; Xiao, Y.; Hu, Q.; Deng, D.; Chen, J.; He, P. Depositing Ag nanoparticles on g-C3N4 by facile silver mirror reaction for enhanced photocatalytic hydrogen production. Inorg. Chem. Commun. 2021, 123, 108367. [Google Scholar] [CrossRef]
- Li, X.; Liu, T.; Tian, F.; Tao, X.; Wu, Z.S. Enhanced carrier transport and visible light response in CA-β-CD/g-C3N4/Ag2O 2D/0D heterostructures functionalized with cyclodextrin for effective organic degradation. Korean J. Chem. Eng. 2022, 39, 2972–2982. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, X.; Zhao, J.; Xu, L.; Yan, J. Flower-like shaped Bi12TiO20/g-C3N4 heterojunction for effective elimination of organic pollutants: Preparation, characterization, and mechanism study. Appl. Organomet. Chem. 2020, 34, e5702. [Google Scholar] [CrossRef]
- Mao, N.; Jiao, Y.; Duan, X. g-C3N4/ZnO heterojunction as Fenton-like catalyst for degradation of organic pollution. Mater. Res. Bull. 2022, 151, 111818. [Google Scholar] [CrossRef]
- Zhu, X.; Deng, H.; Cheng, G. Facile construction of g-C3N4-W18O49 heterojunction with improved charge transfer for solar-driven CO2 photoreduction. Inorg. Chem. Commun. 2021, 132, 108814. [Google Scholar] [CrossRef]
- Huang, M.; Chen, C.; Wang, T.; Sui, Q.; Zhang, K.; Li, B. Cadmium-sulfide/gold/graphitic-carbon-nitride sandwich heterojunction photocatalyst with regulated electron transfer for boosting carbon-dioxide reduction to hydrocarbon. J. Colloid Interface Sci. 2022, 613, 575–586. [Google Scholar] [CrossRef]
- Zhang, R.; Cao, Y.; Doronkin, D.; Ma, M.; Dong, F.; Zhou, Y. Single-atom dispersed Zn-N3 active sites bridging the interlayer of g-C3N4 to tune NO oxidation pathway for the inhibition of toxic by-product generation. Chem. Eng. J. 2023, 454, 140084. [Google Scholar] [CrossRef]
- Liang, Y.; Zeng, Z.; Yang, J.; Yang, G.; Han, Y. Designing heterointerface in BiOBr/ g-C3N4 photocatalyst to enhance visible-light-driven photocatalytic performance in water purification. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126796. [Google Scholar] [CrossRef]
- Ni, Y.; Wang, M.; Liu, L.; Li, M.; Hu, S.; Lin, J.; Sun, J.; Yue, T.; Zhu, M.; Wang, J. Efficient and reusable photocatalytic river water disinfection by addictive graphitic carbon nitride/magnesium oxide nano-onions with particular “nano-magnifying glass effect”. J. Hazard. Mater. 2022, 439, 129533. [Google Scholar] [CrossRef] [PubMed]
- Rao, F.; Zhong, J.; Li, J. Improved visible light responsive photocatalytic hydrogen production over g-C3N4 with rich carbon vacancies. Ceram. Int. 2022, 48, 1439–1445. [Google Scholar] [CrossRef]
- Shi, Z.; Rao, L.; Wang, P.; Zhang, L. The photocatalytic activity and purification performance of g-C3N4/carbon nanotubes composite photocatalyst in underwater environment. Environ. Sci. Pollut. Res. 2022, 29, 83981–83992. [Google Scholar] [CrossRef]
- Cao, J.; Cai, J.; Li, R.; Han, J.; Liu, J.; Huang, M. A novel 3D yolk-double-shell Au@CdS/ g-C3N4 nanostructure with enhanced photoelectrochemical and photocatalytic properties. J. Phys. Chem. C 2022, 126, 4939–4947. [Google Scholar] [CrossRef]
- Saeidpour, S.; Khoshnevisan, B.; Boroumand, Z. Synthesis and characterization of a g-C3N4/TiO2-ZnO nanostructure for photocatalytic degradation of methylene blue. Nano Futures 2022, 6, 035001. [Google Scholar] [CrossRef]
- Liu, F.; Bi, S.; Wang, W.; Duan, Q.; Feng, Y.; Chen, J.; Luo, R.; Huang, Y.; Lee, J. Preparation of a modified g-C3N4 catalyst library and realization of a two-dimensional screening reaction. New J. Chem. 2021, 45, 2582–2588. [Google Scholar] [CrossRef]
- Deng, L.; Sun, J.; Sun, J.; Wang, X.; Shen, T.; Zhao, R.; Zhang, Y.; Wang, B. Improved performance of photosynthetic H2O2 and photodegradation by K-, P-, O-, and S-co-doped g-C3N4 with enhanced charge transfer ability under visible light. Appl. Surf. Sci. 2022, 597, 153586. [Google Scholar] [CrossRef]
- Ge, Y.; Guo, X.; Zhou, D.; Liu, J. Construction and excellent photoelectric synergistic anticorrosion performance of Z-scheme carbon nitride/tungsten oxide heterojunctions. Nanoscale 2022, 14, 12358–12376. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Lu, S.; Chen, X. Anionic donor-acceptor conjugated polymer dots/g-C3N4 nanosheets heterojunction: High efficiency and excellent stability for co-catalyst-free photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2022, 608, 912–921. [Google Scholar] [CrossRef]
- Yan, X.; Xie, M.; Pan, L.; Ai, T.; Li, Z.; Niu, Y. Piezo-photocatalytic activity of NaNbO3/g-C3N4 heterojunction for dye wastewater degradation. J. Mater. Sci. Mater. Electron. 2023, 34, 695. [Google Scholar] [CrossRef]
- Vavilapalli, D.S.; Peri, R.G.; Muthuraaman, B.; Sridharan, K.; Rao, M.R.; Singh, S. Enhanced photocatalytic and photoelectrochemical performance of KBiFe2O5/g-C3N4 heterojunction photocatalyst under visible light. Phys. B Condens. Matter 2023, 648, 414411. [Google Scholar] [CrossRef]
- Huang, Y.; Li, B.; Wu, F.; Yang, B. Fabrication of novel flower-like Co3O4/g-C3N4 heterojunction for tetracycline degradation under visible light irradiation. Mater. Lett. 2022, 311, 131538. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Ji, X.; Wu, H.; Xu, X.; Zhan, J.; Shi, H.; Liu, W.; Tang, T. Construction of CdSe/ZnIn2S4 Z-Scheme heterojunction for enhanced photocatalytic degradation of tetracycline. Mater. Sci. Eng. B 2022, 286, 116065. [Google Scholar] [CrossRef]
- Yang, N.; Li, J. Construction of a 0D/2D heterojunction based on ZnO nanoparticles and ZnIn2S4 nanosheets to improve photocatalytic degradation efficiency. Opt. Mater. 2021, 115, 111040. [Google Scholar] [CrossRef]
- Wang, Z.; Su, B.; Xu, J.; Hou, Y.; Ding, Z. Direct Z-scheme ZnIn2S4/LaNiO3 nanohybrid with enhanced photocatalytic performance for H2 evolution. Int. J. Hydrogen. Energ. 2020, 45, 4113–4121. [Google Scholar] [CrossRef]
- Zhu, J.; Li, H.; Cui, X.; Yang, Z.; Chen, B.; Li, Y.; Zhang, P.; Li, J. Efficient photocathodic protection performance of ZnIn2S4 nanosheets/SnO2 quantum dots/TiO2 nanotubes composite for 316 SS under visible light. J. Alloys Compd. 2022, 926, 166901. [Google Scholar] [CrossRef]
- Cao, S.; Yu, J.; Wageh, S.; Al-Ghamdi, A.; Mousavi, M.; Ghasemi, J.; Xu, F. H2-production and electron-transfer mechanism of a noble-metal-free WO3@ZnIn2S4 S-scheme heterojunction photocatalyst. J. Mater. Chem. A 2022, 10, 17174–17184. [Google Scholar] [CrossRef]
- Wang, K.; Shao, X.; Cheng, Q.; Li, K.; Le, X.; Wang, G.; Wang, H. In situ-Illuminated X-Ray photoelectron spectroscopy investigation of S-scheme Ta2O5/ZnIn2S4 core-shell hybrid nanofibers for highly efficient solar-driven CO2 overall splitting. Solar RRL 2022, 6, 2200736. [Google Scholar] [CrossRef]
- Zhang, L.; Qiu, J.; Dai, D.; Zhou, Y.; Liu, X.; Yao, J. Cr-metal-organic framework coordination with ZnIn2S4 nanosheets for photocatalytic reduction of Cr(VI). J. Clean. Prod. 2022, 341, 130891. [Google Scholar] [CrossRef]
- Dang, X.; Xie, M.; Dai, F.; Guo, J.; Liu, J.; Lu, X. Ultrathin 2D/2D ZnIn2S4/g-C3N4 nanosheet heterojunction with atomic-level intimate interface for photocatalytic hydrogen evolution under visible light. Adv. Mater. Interfaces 2021, 8, 2196–7350. [Google Scholar] [CrossRef]
- Hou, L.; Li, W.; Wu, Z.; Wei, Q.; Yang, H.; Jiang, Y.; Wang, T.; Wang, Y.; He, Q. Embedding ZnCdS@ZnIn2S4 into thiazole-modified g-C3N4 by electrostatic self-assembly to build dual Z-scheme heterojunction with spatially separated active centers for photocatalytic H2 evolution and ofloxacin degradation. Sep. Purif. Technol. 2022, 290, 120858. [Google Scholar] [CrossRef]
- Zhang, G.P.; Zhu, X.W.; Chen, D.Y.; Li, N.J.; Xu, Q.F.; Li, H.; He, J.H.; Xu, H.; Lu, J.M. Hierarchical Z-scheme g-C3N4/Au/ZnIn2S4 photocatalyst for highly enhanced visible-light photocatalytic nitric oxide removal and carbon dioxide conversion. Environ. Sci. Nano 2020, 7, 676–687. [Google Scholar] [CrossRef]
- Liu, X.M.; Wang, S.Q.; Yang, F.; Zhang, Y.C.; Yan, L.S.; Li, K.X.; Guo, H.Q.; Yan, J.J.; Lin, J. Construction of Au/g-C3N4/ZnIn2S4 plasma photocatalyst heterojunction composite with 3D hierarchical microarchitecture for visible-light-driven hydrogen production. Int. J. Hydrogen Energy 2022, 47, 2900–2913. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Wang, Y.; Dong, L.; Chao, M.; Sun, J.; Zhao, Z.; Zhang, J. One-step synthesis of high photocatalytic graphitic carbon nitride porous nanosheets. Nanotechnology 2020, 31, 464001. [Google Scholar] [CrossRef]
- Ai, L.; Fan, H. CTAB-melamine molecular crystals as precursor for synthesis of layered carbon nitride porous nanostructures with enhanced photocatalytic activity for hydrogen production. Mater. Today Commun. 2021, 29, 102780. [Google Scholar] [CrossRef]
- Zhu, K.; Luan, X.; Matras-Postolek, K.; Yang, P. 2D/2D MoS2/g-C3N4 layered heterojunctions with enhanced interfacial electron coupling effect. J. Electroanal. Chem. 2021, 893, 115350. [Google Scholar] [CrossRef]
- Kesir, M.; Yildiz, I.; Bilgen, S.; Sokmen, M. Role of a novel cationic gemini surfactant (CGS) on a one-step sol-gel process and photocatalytic properties of TiO2 powders. J. Water Health 2022, 20, 1629–1643. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Thomas, A.; Fu, X.; Antonietti, M. Metal-containing carbon nitride compounds: A new functional organic-metal hybrid material. Adv. Mater. 2009, 21, 1609–1612. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.; Natarajan, T. Influence of TiO2 morphology on the photocatalytic efficiency of direct Z-scheme g-C3N4/TiO2 photocatalysts for isoniazid degradation. Chem. Eng. J. 2015, 281, 549–565. [Google Scholar] [CrossRef]
- Guo, F.; Huang, X.; Chen, Z.; Cao, L.; Cheng, X.; Chen, L.; Shi, W. Construction of Cu3P-ZnSnO3-g-C3N4 p-n-n heterojunction with multiple built-in electric fields for effectively boosting visible-light photocatalytic degradation of broad-spectrum antibiotics. Sep. Purif. Technol. 2021, 265, 118477. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, W.; Sun, H.; Shi, Y.; Luo, H.; Jing, S.; Fan, Y.; Guo, F.; Lu, C. Fabrication of ternary CoO/g-C3N4/Co3O4 nanocomposite with p-n-p type heterojunction for boosted visible-light photocatalytic performance. J. Chem. Technol. Biotechnol. 2021, 96, 1854–1863. [Google Scholar] [CrossRef]
- Ni, T.; Yang, Z.; Cao, Y.; Lv, H.; Liu, Y. Rational design of MoS2/g-C3N4/ZnIn2S4 hierarchical heterostructures with efficient charge transfer for significantly enhanced photocatalytic H2 production. Ceram. Int. 2021, 47, 22985–22993. [Google Scholar] [CrossRef]
- Ye, X.; Zhu, T.; Hui, Z.; Wang, X.; Wei, J.; Chen, S. Revealing the transfer mechanisms of photogenerated charge carriers over g-C3N4/ZnIn2S4 composite: A model study for photocatalytic oxidation of aromatic alcohols with visible light. J. Catal. 2021, 401, 149–159. [Google Scholar] [CrossRef]
- Fu, D.; Han, G.; Liu, F.; Xiao, Y.; Wang, H.; Liu, R.; Liu, C. Visible-light enhancement of methylene blue photodegradation by graphitic carbon nitride-titania composites. Mater. Sci. Semicond. Process. 2014, 27, 966–974. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, Y.; Zhang, M.; Wu, J.; Liu, G.; He, P.; Qi, Y.; Li, X.; Zhou, Y.; Li, J. Bimetallic sulfides ZnIn2S4 modified g-C3N4 adsorbent with wide temperature range for rapid elemental mercury uptake from coal-fired flue gas. Chem. Eng. J. 2021, 426, 131343. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, J.; Wang, Z.; Wang, L.; Zhao, Z.; Li, S.; Li, G.; Cai, Z. Facile construction of g-C3N4/ZnIn2S4 nanocomposites for enhance Cr(VI) photocatalytic reduction. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 276, 121184. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Liu, Y.; Zhou, Y.; Zhao, D.; Wang, D.; Yun, L.; Zhang, D.; Zhang, L. Improved photocathodic protection performance of g-C3N4/rGO/ZnS for 304 stainless steel. J. Phys. Chem. Solids 2021, 148, 109672. [Google Scholar] [CrossRef]
- Mishra, N.; Kuila, A.; Saravanan, P.; Bahnemann, D.; Jang, M.; Routu, S. Simultaneous S-scheme promoted Ag@AgVO3/g-C3N4/CeVO4 heterojunction with enhanced charge separation and photo redox ability towards solar photocatalysis. Chemosphere 2023, 326, 138496. [Google Scholar] [CrossRef]
- Rajendran, R.; Vignesh, S.; Sasireka, A.; Priya, P.; Suganthi, S.; Raj, V.; Sundar, J.K.; Srinivasan, M.; Shkir, M.; AlFaify, S. Investigation on novel Cu2O modified g-C3N4/ZnO heterostructures for efficient photocatalytic dye degradation performance under visible-light exposure. Colloid Interface Sci. Commun. 2021, 44, 100480. [Google Scholar] [CrossRef]
- Wu, B.; Shan, C.; Zhang, X.; Zhao, H.; Ma, S.; Shi, Y.; Yang, J.; Bai, H.; Liu, Q. CeO2/Co3O4 porous nanosheet prepared using rose petal as biotemplate for photo-catalytic degradation of organic contaminants. Appl. Surf. Sci. 2021, 543, 148677. [Google Scholar] [CrossRef]
- Sivanandan, V.; Prasad, A. Lactose monohydrate (C12H22O11 center dot H2O) mediated synthesis and spectral analysis of nanocrystalline Ni0.5Cu0.5Fe2O. Int. J. Mater. Res. 2021, 112, 980–984. [Google Scholar]
- Huang, X.; Xu, X.; Yang, R.; Fu, X. Synergetic adsorption and photocatalysis performance of g-C3N4/Ce-doped MgAl-LDH in degradation of organic dye under LED visible light. Colloid Surface A Physicochem. Eng. Asp. 2022, 643, 128738. [Google Scholar] [CrossRef]
- Hoang, T.; Nguyen, P.; Shin, E. Effect of morphological modification over g-C3N4 on photocatalytic hydrogen evolution performance of g-C3N4-Pt photocatalysts. Catalysts 2023, 13, 10092. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Y.; Liang, J.; Wang, D. Enhancement of photoelectrochemical and photocathodic protection properties of TiO2 nanotube arrays by simple surface UV treatment. Appl. Surf. Sci. 2017, 394, 440–445. [Google Scholar] [CrossRef]
- Tian, J.; Chen, Z.; Ma, L.; Hou, J.; Feng, C.; Jing, J.; Sun, M.; Chen, D. Fabrication of flower-like WO3/ZnIn2S4 composite with special electronic transmission channels to improve carrier separation for photoinduced cathodic protection and electron storage. Appl. Surf. Sci. 2023, 607, 155019. [Google Scholar] [CrossRef]
- Gong, D.; Xu, S.; Zhang, K.; Du, L.; Qiu, P. Enhancing photoelectrochemical cathodic protection performance by facile tuning sulfur redox state in sacrificial agents. Chem. Eng. J. 2023, 451, 138552. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Cui, X.; Zhu, J.; Li, Y.; Zhang, P.; Li, J. Direct Z-scheme nanoporous BiVO4/CdS quantum dots heterojunction composites as photoanodes for photocathodic protection of 316 stainless steel under visible light. Appl. Surf. Sci. 2022, 603, 154394. [Google Scholar] [CrossRef]
- Kathiravan, S.; Kaliaraj, G.; Kumar, R.; Kirubaharan, A. A novel experimental setup for in situ oxidation behavior study of Nb/Hf/Ti (C-103) alloy for high temperature environments. Mater. Lett. 2021, 302, 130336. [Google Scholar] [CrossRef]
- Igbari, F.; Essien, E.; Abdulwahab, K.; Nejo, A.; Adetona, A.; Adams, L.A. Bipolar conductivity in amorphous Cu–Al–O thin films prepared by r.f. magnetron sputtering. Mater. Sci. Semicond. Process. 2021, 123, 105557. [Google Scholar] [CrossRef]
- Duan, Z.; Zhao, X.; Chen, L. BiVO4/Cu0.4V2O5 composites as a novel Z-scheme photocatalyst for visible-light-driven CO2 conversion. J. Environ. Chem. Eng. 2021, 9, 104628. [Google Scholar] [CrossRef]
- Wang, W.; Feng, X.; Chen, L.; Zhang, F. Z-Scheme Cu2O/Bi/BiVO4 nanocomposite photocatalysts: Synthesis, characterization, and application for CO2 photoreduction. Ind. Eng. Chem. Res. 2021, 60, 18384–18396. [Google Scholar] [CrossRef]



| Samples | Ecorr (V vs. SCE) | icorr (μA cm−2) |
|---|---|---|
| CNNs | −0.271 | 4.08 |
| 20% ZIS@CNNs | −0.332 | 6.46 |
| 30% ZIS@CNNs | −0.62 | 13.54 |
| 40% ZIS@CNNs | −0.434 | 10.93 |
| Rs (Ω cm−2) | Qf (F·cm−2·10−2) | Rf (Ω cm−2) | Cdl (F·cm−2·10−2) | Rct (Ω cm−2) | |
|---|---|---|---|---|---|
| CNNs | 4.605 | 5.411 × 10−2 | 948.7 | 0.01807 | 934.1 |
| 20% ZIS@CNNs | 4.574 | 4.983 × 10−2 | 976.3 | 0.02 | 874.2 |
| 30% ZIS@CNNs | 4.278 | 1.803 × 10−3 | 1590 | 3.916 × 10−4 | 426.5 |
| 40% ZIS@CNNs | 2.709 | 4.235 × 10−3 | 1135.3 | 2.721 × 10−4 | 556.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Yang, Z.; Li, Y.; Zhang, P.; Li, H. Highly Efficient Photocathodic Protection Performance of ZIS@CNNs Composites under Visible Light. Coatings 2023, 13, 1479. https://doi.org/10.3390/coatings13091479
Li W, Yang Z, Li Y, Zhang P, Li H. Highly Efficient Photocathodic Protection Performance of ZIS@CNNs Composites under Visible Light. Coatings. 2023; 13(9):1479. https://doi.org/10.3390/coatings13091479
Chicago/Turabian StyleLi, Weitao, Zhanyuan Yang, Yanhui Li, Pengfei Zhang, and Hong Li. 2023. "Highly Efficient Photocathodic Protection Performance of ZIS@CNNs Composites under Visible Light" Coatings 13, no. 9: 1479. https://doi.org/10.3390/coatings13091479
APA StyleLi, W., Yang, Z., Li, Y., Zhang, P., & Li, H. (2023). Highly Efficient Photocathodic Protection Performance of ZIS@CNNs Composites under Visible Light. Coatings, 13(9), 1479. https://doi.org/10.3390/coatings13091479






