Comparison of the Physical Properties and Electronic Structure of Nb2B3 and Ta2B3
Abstract
1. Introduction
2. Results and Discussion
2.1. Equilibrium Structure
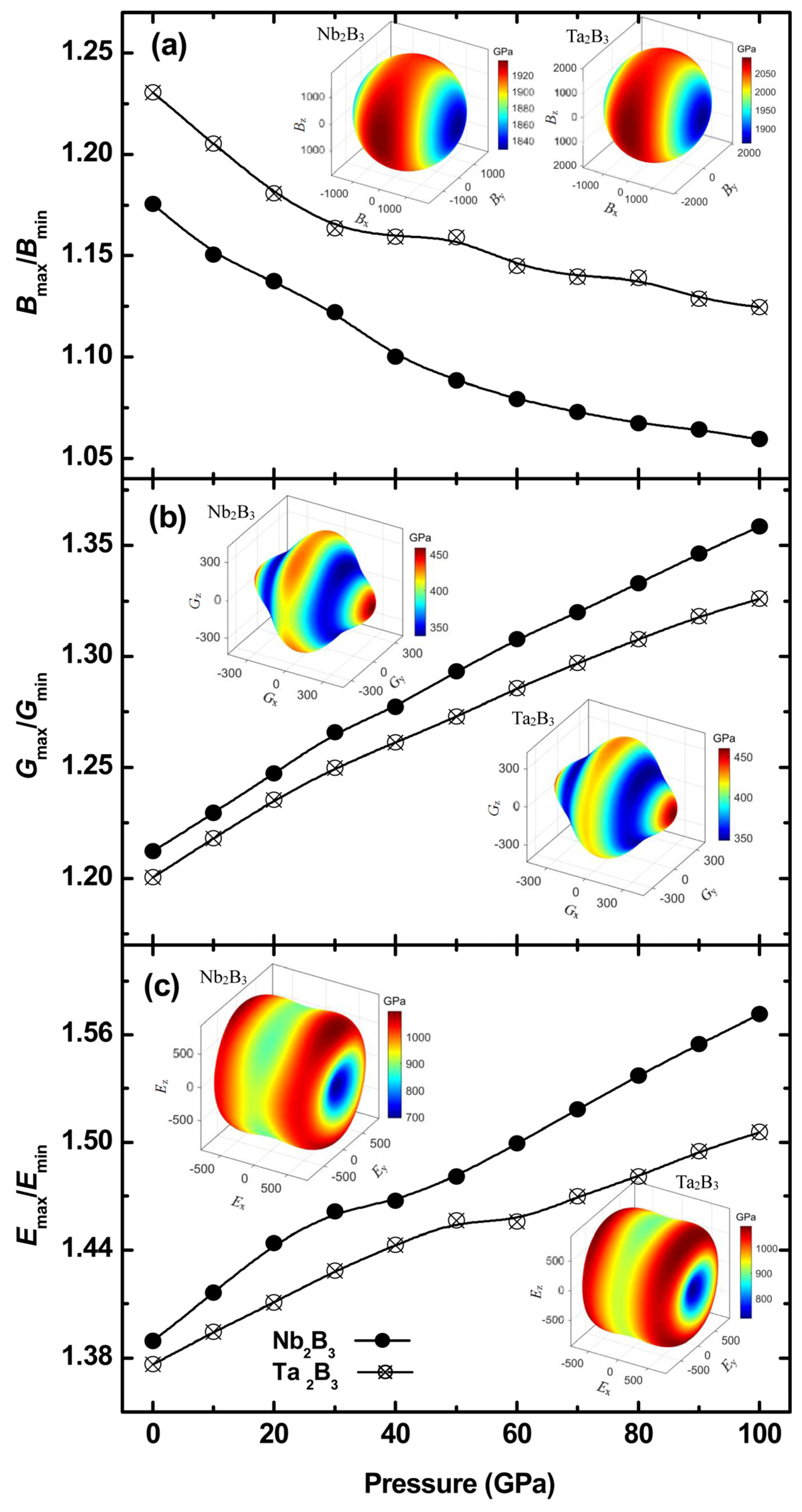
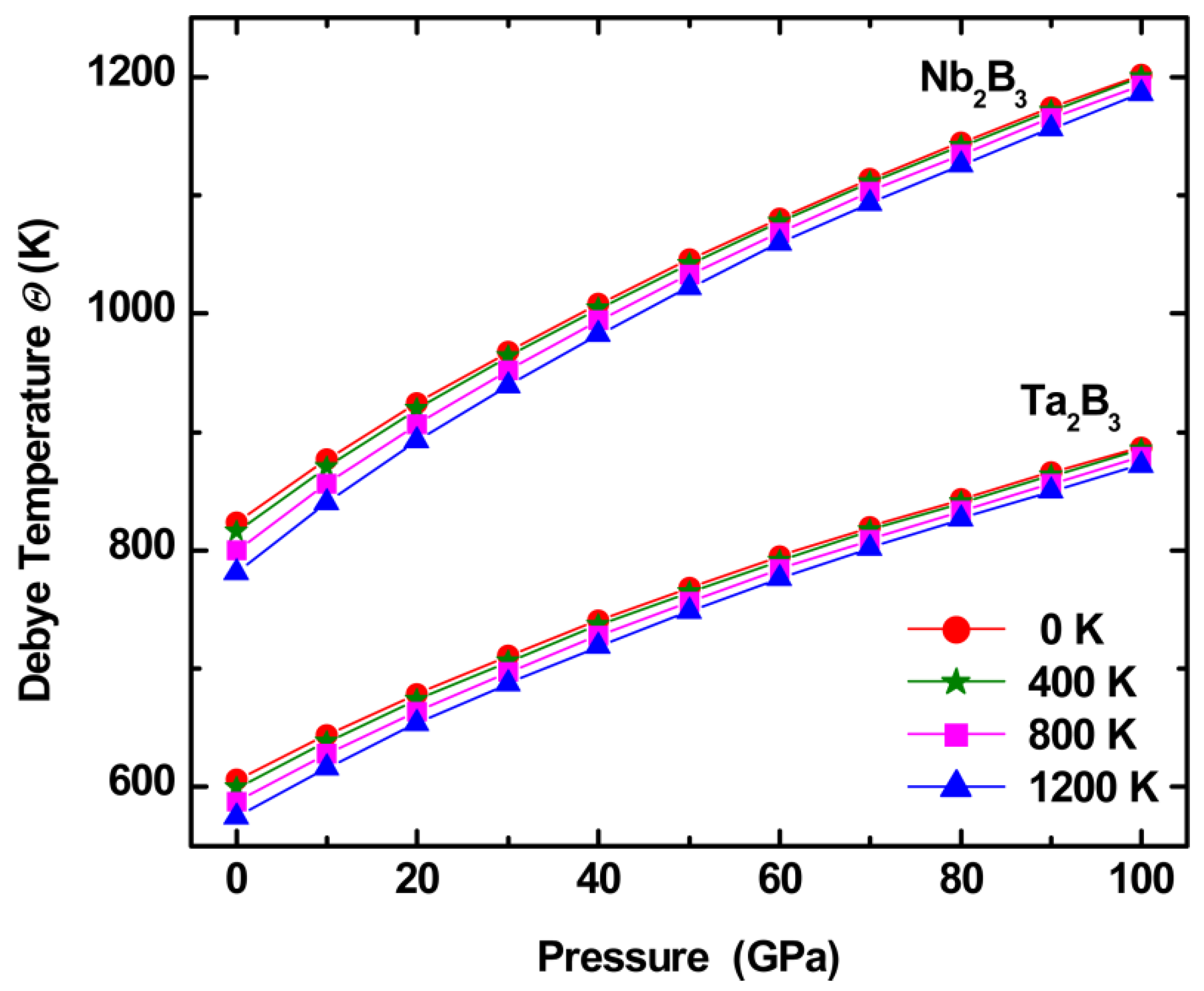
2.2. Physical Properties
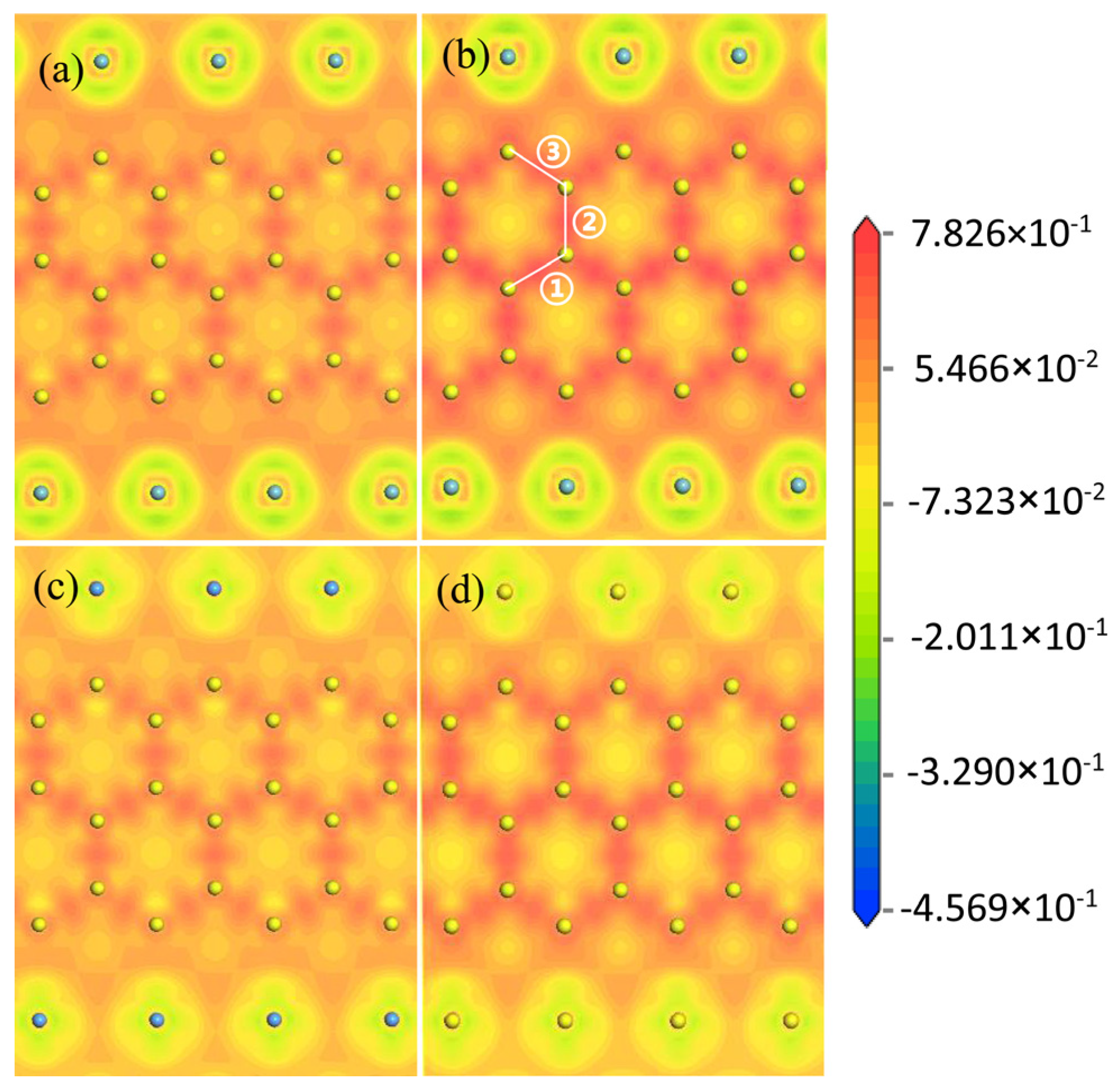
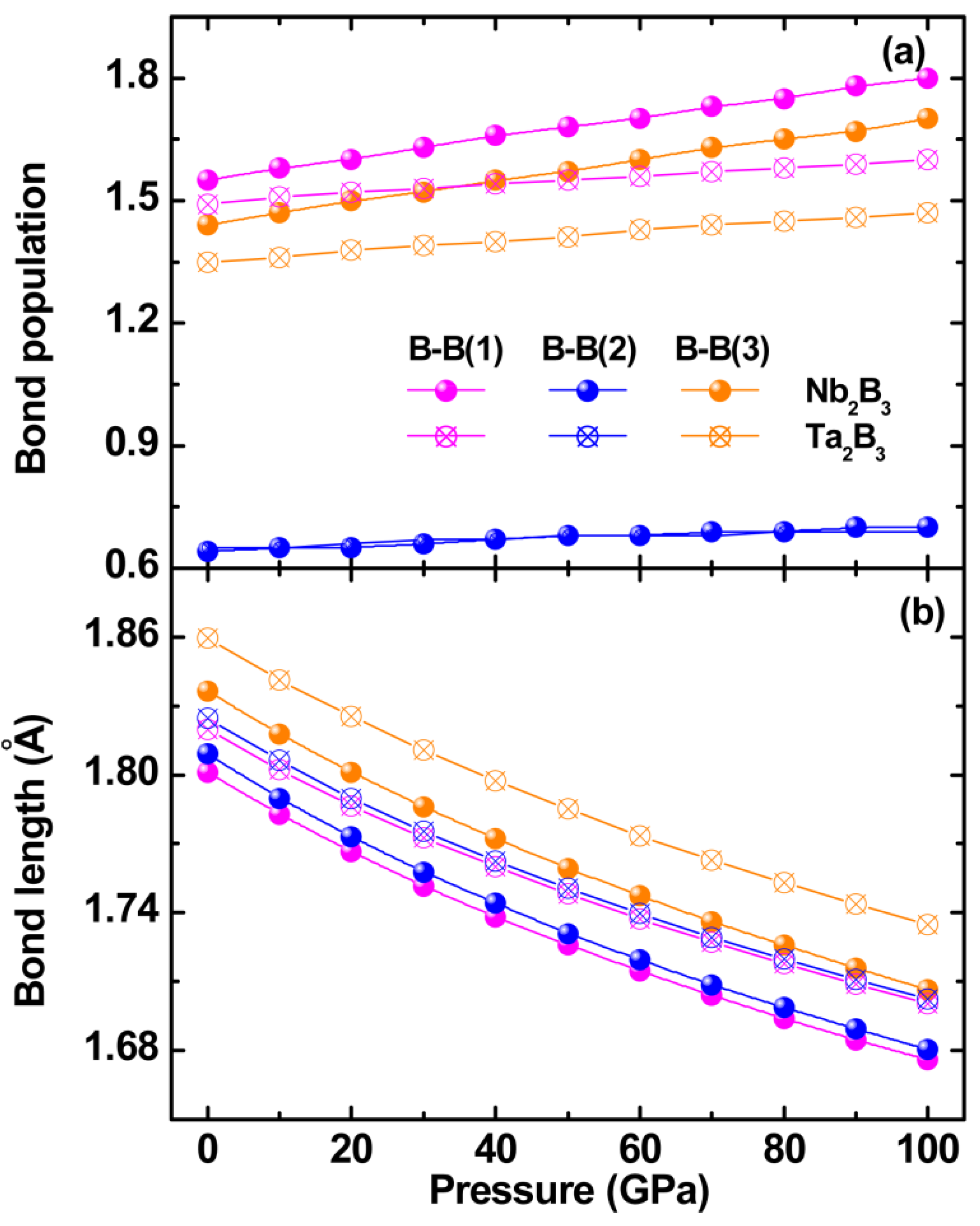
2.3. Electronic Structure
3. Methods and Computation Details
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeung, M.T.; Mohammadi, R.; Kaner, R.B. Ultraincompressible, superhard materials. Annu. Rev. Mater. Res. 2016, 46, 465–485. [Google Scholar] [CrossRef]
- Akopov, G.; Pangilinan, L.E.; Mohammadi, R.; Kaner, R.B. Perspective: Superhard metal borides: A look forward. APL Mater. 2018, 6, 070901. [Google Scholar] [CrossRef]
- Chung, H.Y.; Weinberger, M.B.; Levine, J.B.; Kavner, A.; Yang, J.M.; Tolbert, S.H.; Kaner, R.B. Synthesis of ultra-incompressible superhard rhenium diboride at ambient pressure. Science 2007, 306, 436–439. [Google Scholar] [CrossRef]
- Akopov, G.; Yeung, M.T.; Kaner, R.B. Rediscovering the Crystal Chemistry of Borides. Adv. Mater. 2017, 29, 1604506. [Google Scholar] [CrossRef]
- Young, A.F.; Sanloup, C.; Gregoryanz, E.; Scandolo, S.; Hemley, R.J.; Mao, H.K. Synthesis of Novel Transition Metal Nitrides IrN2 and OsN2. Phys. Rev. Lett. 2006, 96, 155501. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Li, Q.; Ma, Y.; Chen, C. Extraordinary indentation strain stiffening produces superhard tungsten nitrides. Phys. Rev. Lett. 2017, 119, 115503. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, J.; Liu, H. Theoretical research on novel orthorhombic tungsten dinitride from first principles calculations. RSC Adv. 2018, 8, 9272–9276. [Google Scholar] [CrossRef]
- Kaner, R.B.; Gilman, J.J.; Tolbert, S.H. Designing superhard materials. Science 2005, 308, 1268–1269. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Gao, Z.; Qin, P.; Gao, L.; Tang, C. The mechanism of anomalous hardening in transition-metal monoborides. Nanoscale 2017, 9, 9112–9118. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, D.; Zheng, W.; Ma, Y.; Chen, C. Anomalous Stress Response of Ultrahard WBn Compounds. Phys. Rev. Lett. 2015, 115, 185502. [Google Scholar] [CrossRef]
- Yeung, M.T.; Lei, J.; Mohammadi, R.; Turner, C.L.; Wang, Y.; Tolbert, S.H.; Kaner, R.B. Superhard Monoborides: Hardness Enhancement through Alloying in W1−xTaxB. Adv. Mater. 2016, 28, 6993–6998. [Google Scholar] [CrossRef]
- Liang, Y.; Qin, P.; Jiang, H.; Zhang, L.; Zhang, J.; Tang, C. Designing superhard metals: The case of low borides. AIP Adv. 2018, 8, 045305. [Google Scholar] [CrossRef]
- Yao, T.; Wang, Y.; Li, H.; Lian, J.; Zhang, J.; Gou, H. A universal trend of structural, mechanical and electronic properties in transition metal (M = V, Nb, and Ta) borides: First-principle calculations. Comput. Mater. Sci. 2012, 65, 302–308. [Google Scholar] [CrossRef]
- Fan, C.-Z.; Zeng, S.-Y.; Li, L.-X.; Zhan, Z.-J.; Liu, R.-P.; Wang, W.-K.; Zhang, P.; Yao, Y.-G. Potential superhard osmium dinitride with fluorite and pyrite structure: First-principles calculations. Phys. Rev. B 2006, 74, 125118. [Google Scholar] [CrossRef]
- Grimvall, G.; Magyari-Köpe, B.; Ozoliņš, V.; Persson, K.A. Lattice instabilities in metallic elements. Rev. Mod. Phys. 2012, 84, 945–986. [Google Scholar] [CrossRef]
- Hill, R. The elastic behaviour of a crystalline aggregate. Proc. Phys. Soc. A 1952, 65, 349–354. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Hou, H.; Wen, Z.; Duan, M. Comparison of mechanical and thermodynamic properties of fcc and bcc titanium under high pressure. Mater. Res. Express 2019, 6, 1065c4. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Niu, H.; Li, D.; Li, Y. Modeling hardness of polycrystalline materials and bulk metallic glasses. Intermetallics 2011, 19, 1275–1281. [Google Scholar] [CrossRef]
- Niu, H.; Niu, S.; Oganov, A.R. Simple and accurate model of fracture toughness of solids. J. Appl. Phys. 2019, 125, 065105. [Google Scholar] [CrossRef]
- Pugh, S.F. XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Philos. Mag. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Frantsevich, I.N.; Voronov, F.F.; Bokuta, S.A. Elastic Constants and Elastic Moduli of Metals and Insulators: Handbook; Naukova Dumka: Kiev, Ukraine, 1983; p. 60. [Google Scholar]
- Li, X.H.; Yong, Y.L.; Cui, H.L.; Zhang, R.Z. Mechanical behavior, electronic and phonon properties of ZrB12 under pressure. J. Phys. Chem. Solids 2018, 117, 173–179. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, X.; Li, S.; Li, Y.; Wen, M. DFT prediction of a novel molybdenum tetraboride superhard material. RSC Adv. 2018, 8, 18008–18015. [Google Scholar] [CrossRef]
- Liang, Y.; Zhong, Z.; Zhang, W. A thermodynamic criterion for designing superhard transition-metal borides with ultimate boron content. Comput. Mater. Sci. 2013, 68, 222–228. [Google Scholar] [CrossRef]
- Blanco, M.A.; Francisco, E.; Luanan, V. GIBBS: Isothermal-isobaric thermodynamics of solids from energy curves using a quasi-harmonic Debye model. Comput. Phys. Commun. 2004, 158, 57–72. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, J.; Li, J.; Hu, Z.; Wang, J. Theoretical study on crystal structures, elastic stiffness, and intrinsic thermal conductivities of β-, γ-, and δ-Y2Si2O7. J. Mater. Res. 2015, 30, 493–502. [Google Scholar] [CrossRef]
- Pan, Y.; Lin, Y.H.; Guo, J.M.; Wen, M. Correlation between hardness and bond orientation of vanadium borides. RSC Adv. 2014, 4, 47377–47382. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]



| a | b | c | ||
|---|---|---|---|---|
| Nb2B3 | present | 3.310 | 19.507 | 3.159 |
| Cal. [13] | 3.310 | 19.504 | 3.128 | |
| Ta2B3 | present | 3.337 | 19.687 | 3.159 |
| Cal. [13] | 3.337 | 19.685 | 3.159 | |
| 0 GPa | 100 GPa | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Bond | P | Bond Length (Å) | Atom | Charge (e) | P | Bond Length (Å) | Atom | Charge (e) | |
| Nb2B3 | B5--Nb4 | −0.09 | 2.36768 | −0.45 | 2.20505 | ||||
| B2--Nb2 | −0.09 | 2.36768 | −0.45 | 2.20505 | |||||
| B6--Nb2 | 0.77 | 2.39143 | B1 | −0.53 | 0.21 | 2.21945 | B1 | −0.65 | |
| B3--Nb4 | 0.77 | 2.39143 | B2 | −0.53 | 0.21 | 2.21945 | B2 | −0.65 | |
| B4--Nb3 | 0.03 | 2.43817 | B3 | −0.54 | −0.11 | 2.26022 | B3 | −0.67 | |
| B1--Nb1 | 0.03 | 2.43817 | B4 | −0.53 | −0.11 | 2.26022 | B4 | −0.65 | |
| B4--Nb1 | 0.18 | 2.44751 | B5 | −0.53 | −0.53 | 2.26572 | B5 | −0.65 | |
| B1--Nb3 | 0.18 | 2.44751 | B6 | −0.54 | 0.53 | 2.26572 | B6 | −0.67 | |
| B2--Nb3 | 0.32 | 2.45273 | Nb1 | 1.03 | −0.21 | 2.27156 | Nb1 | 1.25 | |
| B5--Nb1 | 0.32 | 2.45273 | Nb2 | 0.58 | −0.21 | 2.27156 | Nb2 | 0.72 | |
| B6--Nb3 | 0.16 | 2.50015 | Nb3 | 1.03 | 0.19 | 2.31677 | Nb3 | 1.25 | |
| B3--Nb1 | 0.16 | 2.50015 | Nb4 | 0.58 | 0.19 | 2.31677 | Nb4 | 0.72 | |
| B6--Nb4 | 0.12 | 2.58615 | 0.02 | 2.37148 | |||||
| B3--Nb2 | 0.12 | 2.58615 | 0.02 | 2.37148 | |||||
| Ta2B3 | B5--Ta4 | −0.05 | 2.39181 | −0.35 | 2.23146 | ||||
| B2--Ta2 | −0.05 | 2.39181 | −0.35 | 2.23146 | |||||
| B6--Ta2 | 1.14 | 2.41141 | B1 | −0.61 | 0.98 | 2.24347 | B1 | −0.63 | |
| B3--Ta4 | 1.14 | 2.41141 | B2 | −0.59 | 0.98 | 2.24347 | B2 | −0.60 | |
| B4--Ta3 | −0.01 | 2.46156 | B3 | −0.64 | −0.35 | 2.28940 | B3 | −0.69 | |
| B1--Ta1 | −0.01 | 2.46156 | B4 | −0.61 | −0.35 | 2.28940 | B4 | −0.63 | |
| B4--Ta1 | 0.54 | 2.46975 | B5 | −0.59 | 0.42 | 2.29339 | B5 | −0.60 | |
| B1--Ta3 | 0.54 | 2.46975 | B6 | −0.64 | 0.42 | 2.29339 | B6 | −0.69 | |
| B2--Ta3 | 0.62 | 2.47436 | Ta1 | 1.23 | 0.45 | 2.29746 | Ta1 | 1.30 | |
| B5--Ta1 | 0.62 | 2.47436 | Ta2 | 0.61 | 0.45 | 2.29746 | Ta2 | 0.62 | |
| B6--Ta3 | −0.00 | 2.52862 | Ta3 | 1.23 | −0.30 | 2.34801 | Ta3 | 1.30 | |
| B3--Ta1 | −0.00 | 2.52862 | Ta4 | 0.61 | −0.30 | 2.34801 | Ta4 | 0.62 | |
| B6--Ta4 | 0.25 | 2.59587 | 0.28 | 2.40293 | |||||
| B3--Ta2 | 0.25 | 2.59587 | 0.28 | 2.40293 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, H.; Wang, X.; Zhang, X.; Gao, Y. Comparison of the Physical Properties and Electronic Structure of Nb2B3 and Ta2B3. Coatings 2023, 13, 1302. https://doi.org/10.3390/coatings13081302
Zhang Y, Wang H, Wang X, Zhang X, Gao Y. Comparison of the Physical Properties and Electronic Structure of Nb2B3 and Ta2B3. Coatings. 2023; 13(8):1302. https://doi.org/10.3390/coatings13081302
Chicago/Turabian StyleZhang, Yongmei, Hongxia Wang, Xiaona Wang, Xiuqing Zhang, and Yanqin Gao. 2023. "Comparison of the Physical Properties and Electronic Structure of Nb2B3 and Ta2B3" Coatings 13, no. 8: 1302. https://doi.org/10.3390/coatings13081302
APA StyleZhang, Y., Wang, H., Wang, X., Zhang, X., & Gao, Y. (2023). Comparison of the Physical Properties and Electronic Structure of Nb2B3 and Ta2B3. Coatings, 13(8), 1302. https://doi.org/10.3390/coatings13081302





