Effect of Bath Composition on Titanium Anodization Using the Constant-Current Approach: A Crystallographic and Morphological Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
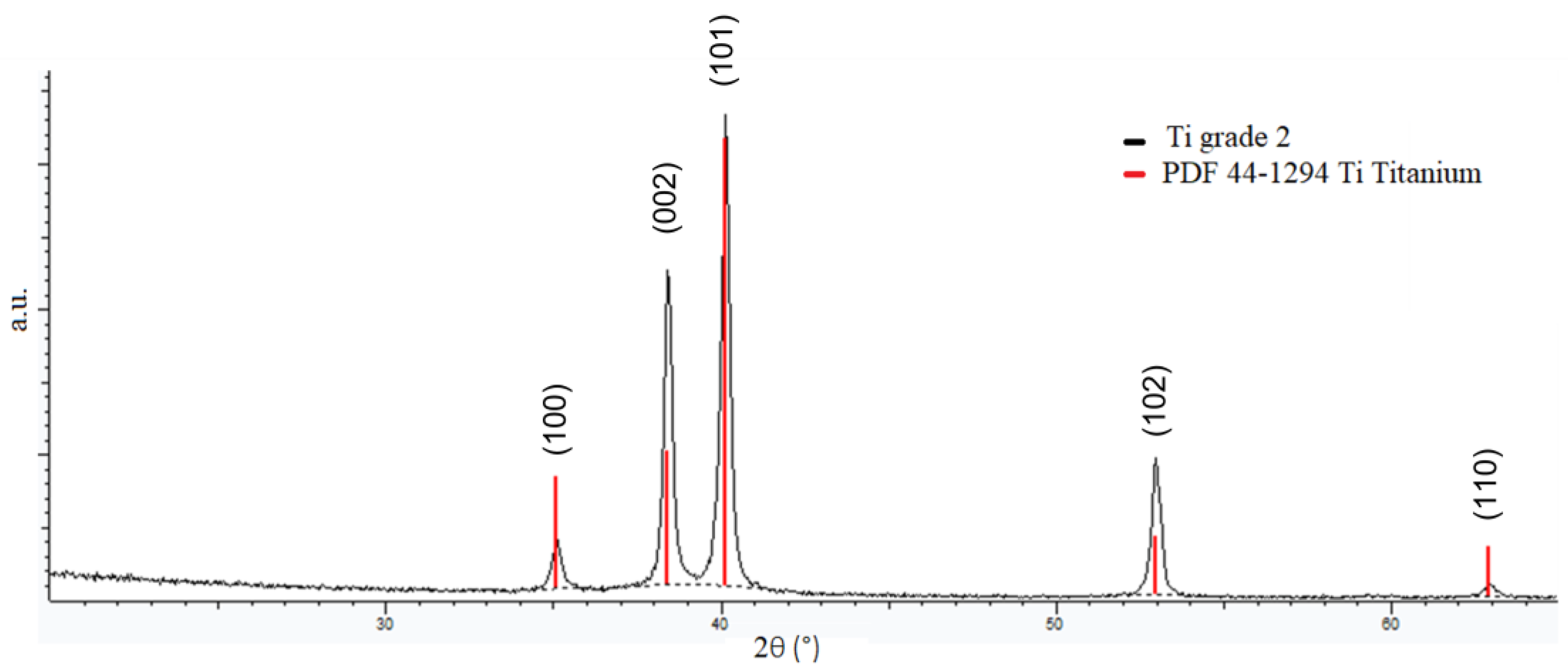
3.1. Substrate Characterization
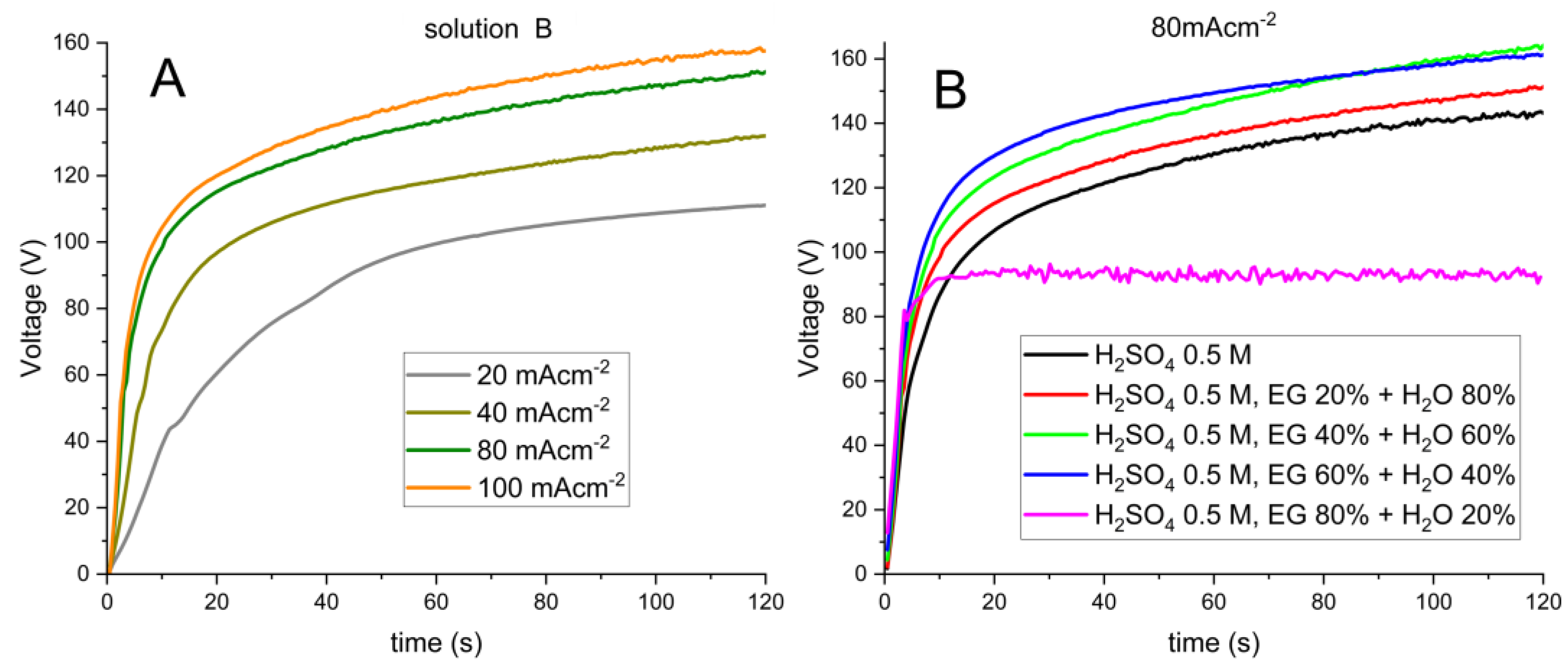
3.2. Anodizing Treatment Conditions
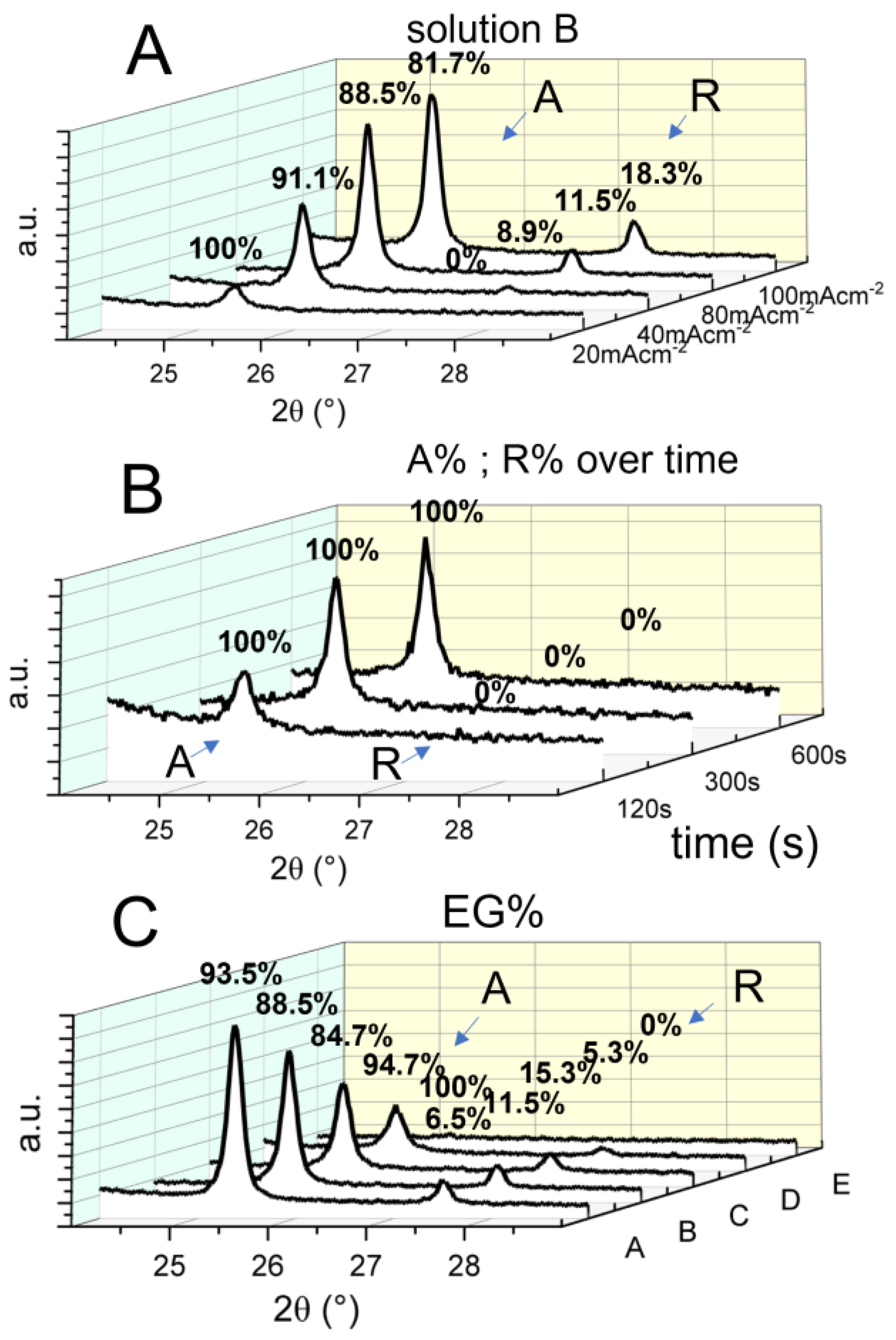
3.3. Mineralogical Composition
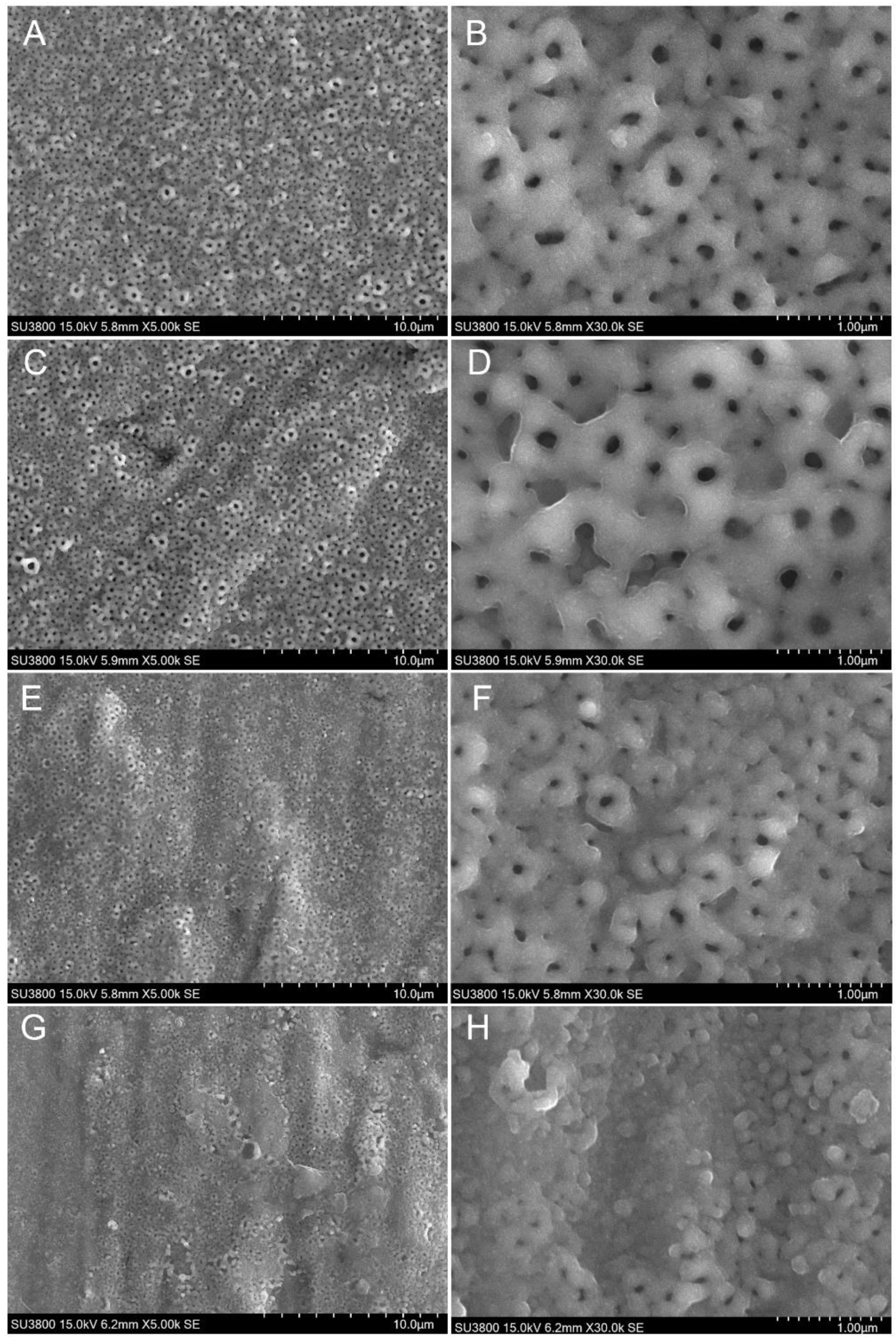
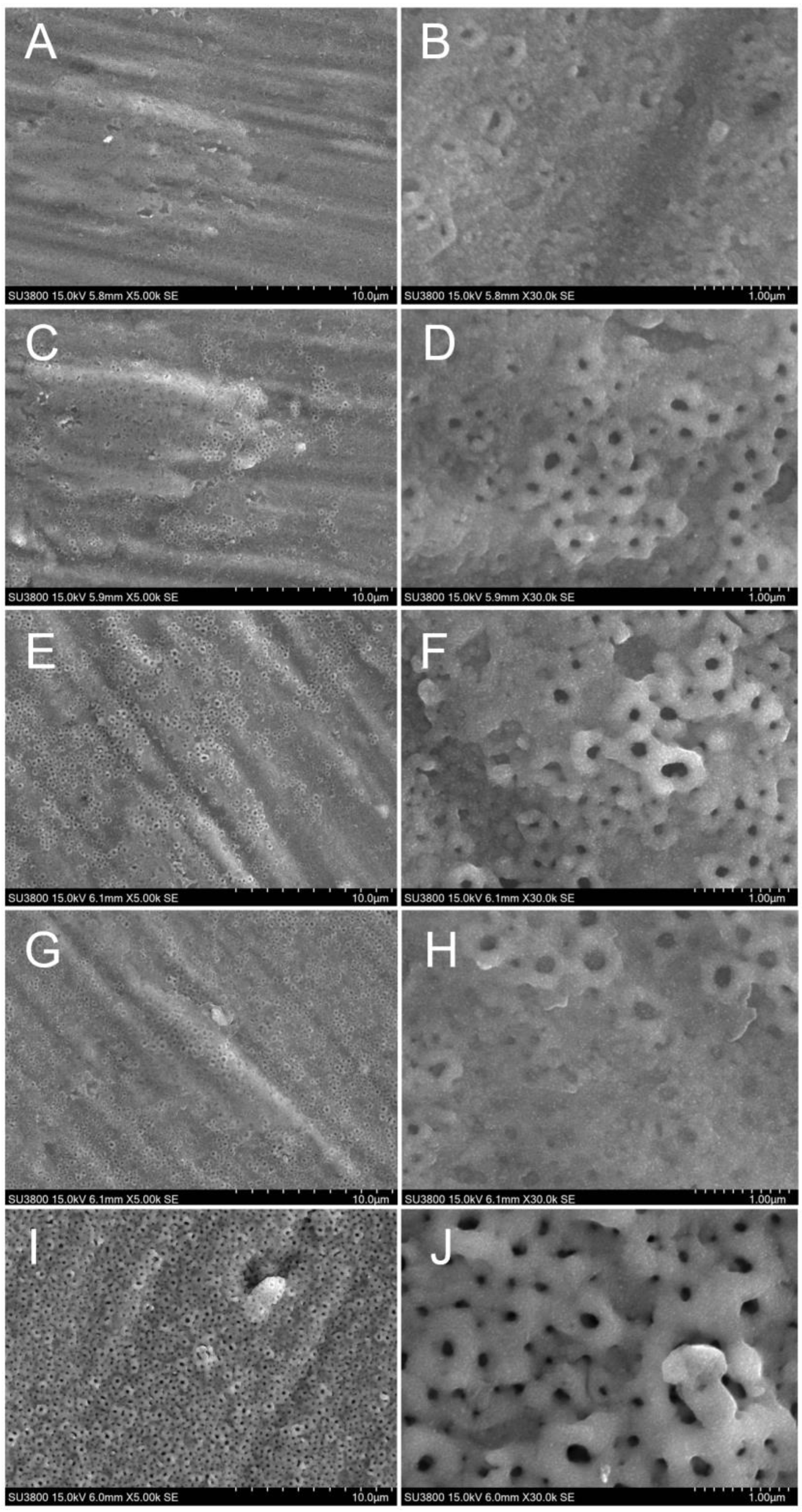
3.4. Morphological Investigation
3.5. Bandgap and Chemical Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, K.T.; Eo, M.Y.; Nguyen TT, H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant Dent. 2019, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Julkapli, N.M.; Bagheri, S.; Bee Abd, S.H. Recent Advances in Heterogeneous Photocatalytic Decolorization of Synthetic Dyes. Sci. World J. 2014, 2014, 692307. [Google Scholar] [CrossRef] [PubMed]
- Vydianathan, K.; Nuesca, G.; Peterson, G.; Eisenbraun, E.; Kaloyeros, A.; Sullivan, J.; Han, B. Metalorganic chemical vapor deposition of titanium oxide for microelectronics applications. J. Mater. Res. 2001, 16, 1838–1849. [Google Scholar] [CrossRef]
- De Nardo, L.; Raffaini, G.; Ebramzadeh, E.; Ganazzoli, F. Titanium oxide modeling and design for innovative biomedical surfaces: A concise review. Int. J. Artif. Organs 2012, 35, 629–641. [Google Scholar] [CrossRef]
- Eswaramoorthi, I.; Lian-Pin, H. Anodic titanium oxide: A new template for the synthesis of larger diameter multi-walled carbon nanotubes. Diam. Relat. Mater. 2007, 16, 1571–1578. [Google Scholar] [CrossRef]
- Eswaramoorthi, I.; Lian-Pin, H. Synthesis and characterisation of larger diameter multi-walled carbon nanotubes over anodic titanium oxide template. Carbon 2006, 44, 2341–2344. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Chen, J.; Wu, H.; Lu, S.; Wang, K.; Li, H.; Harris, C.J.; Xi, K.; Kumar, R.V.; et al. Enhancing Catalytic Activity of Titanium Oxide in Lithium–Sulfur Batteries by Band Engineering. Adv. Energy Mater. 2019, 9, 1900953. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Ormellese, M.; Marin, E.; Lanzutti, A.; Mele, A.; Pedeferri, M.P. Anodic titanium oxide as immobilized photocatalyst in UV or visible light devices. J. Hazard. Mater. 2011, 186, 2103–2109. [Google Scholar] [CrossRef]
- Kanna, M.; Wongnawa, S.; Buddee, S.; Dilokkhunakul, K.; Pinpithak, P. Amorphous titanium dioxide: A recyclable dye remover for water treatment. J. Sol-Gel Sci. Technol. 2010, 53, 162–170. [Google Scholar] [CrossRef]
- Brunella, M.F.; Diamanti, M.V.; Pedeferri, M.P.; Di Fonzo, F.; Casari, C.S.; Li Bassi, A. Photocatalytic behavior of different titanium dioxide layers. Thin Solid Films 2007, 515, 6309–6313. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Ormellese, M.; Pedeferri, M.P. Application-wise nanostructuring of anodic films on titanium: A review. J. Exp. Nanosci. 2015, 10, 1285–1308. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Ormellese, M.; Pedeferri, M.P. Tuning of titanium oxide morphology at micro and nano scale by alternating current anodising. J. Nano Res. 2009, 6, 61–66. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Ormellese, M.; Pedeferri, M.P. Alternating current anodizing of titanium in halogen acids combined with anodic spark deposition: Morphological and structural variations. Corros. Sci. 2010, 52, 1824–1829. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Pedeferri, M.P. Effect of anodic oxidation parameters on the titanium oxides formation. Corros. Sci. 2007, 49, 939–948. [Google Scholar] [CrossRef]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Chacón-Nava, J.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Almeraya-Calderón, F. Electrochemical corrosion of titanium and titanium alloys anodized in H2SO4 and H3PO4 solutions. Coatings 2022, 12, 325. [Google Scholar] [CrossRef]
- Kuromoto, N.K.; Simão, R.A.; Soares, G.A. Titanium oxide films produced on commercially pure titanium by anodic oxidation with different voltages. Mater. Charact. 2007, 58, 114–121. [Google Scholar] [CrossRef]
- Maytorena-Sánchez, A.; Hernández-Torres, J.; Orozco-Cruz, R.; Zamora-Peredo, L.; López-Huerta, F.; Pacio-Castillo, M.; Serrano-de la Rosa, L.E.; Garcia-Gonzalez, L. Morphological and structural study of anodized titanium grade 2, using HCl in aqueous solution. Mater. Res. 2022, 25, e20210497. [Google Scholar] [CrossRef]
- Ohtsu, N.; Komiya, S.; Kodama, K. Effect of electrolytes on anodic oxidation of titanium for fabricating titanium dioxide photocatalyst. Thin Solid Films 2013, 534, 70–75. [Google Scholar] [CrossRef]
- Chelliah, N.M.; Saxena, A.; Sharma, K.; Singh, H.; Surappa, M.K. Surface characterization of nanoporous aluminium oxide films synthesized by single-step DC and AC anodization. Surf. Interfaces 2017, 7, 139–145. [Google Scholar] [CrossRef]
- Kudelko, K.; Rozhdestvenskaya, L.; Ogenko, V.; Chmilenko, V. Formation and characterisation of porous anodized aluminum oxide, synthesized electrochemically in the presence of graphene oxide. Appl. Nanosci. 2022, 12, 1967–1976. [Google Scholar] [CrossRef]
- Winiarski, J.; Tylus, W.; Pawlyta, M.; Szczygieł, B. Titanium anodization in deep eutectic solvents: The effect of anodizing time on the morphology and structure of anodic layers. Appl. Surf. Sci. 2022, 577, 151892. [Google Scholar] [CrossRef]
- Vega, V.; Prida, V.M.; Hernandez-Vélez, M.; Mantova, E.; Aranda, P.; Ruiz-Hitzky, E.; Vazquez, M. Influence of anodic conditions on self-ordered growth of highly aligned titanium oxide nanopores. Nanoscale Res. Lett. 2007, 2, 355–363. [Google Scholar] [CrossRef]
- Jain, S.; Williamson, R.S.; Roach, M.D. Surface characterization, shear strength, and bioactivity of anodized titanium prepared in mixed-acid electrolytes. Surf. Coat. Tech. 2017, 325, 594–603. [Google Scholar] [CrossRef]
- Roach, M.D.; Williamson, R.S.; Blakely, I.P.; Didier, L.M. Tuning anatase and rutile phase ratios and nanoscale surface features by anodization processing onto titanium substrate surfaces. Mater. Sci. Eng. C 2016, 58, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Delplancke, J.-L.; Winand, R. Galvanostatic anodization of titanium—I. Structures and compositions of the anodic films. Electrochim. Acta 1988, 33, 1539–1549. [Google Scholar] [CrossRef]
- Santos, E., Jr.; de Souza, G.B.; Serbena, F.C.; Santos, H.L.; de Lima, G.G.; Szesz, E.M.; Kuromoto, N.K. Effect of anodizing time on the mechanical properties of porous titania coatings formed by micro-arc oxidation. Surf. Coat. Tech. 2017, 309, 203–211. [Google Scholar] [CrossRef]
- Galvis, O.A.; Quintero, D.; Castaño, J.G.; Liu, H.; Thompson, G.E.; Skeldon, P.; Echeverría, F. Formation of grooved and porous coatings on titanium by plasma electrolytic oxidation in H2SO4/H3PO4 electrolytes and effects of coating morphology on adhesive bonding. Surf. Coat. Tech. 2015, 269, 238–249. [Google Scholar] [CrossRef]
- Liu, Z.; Thompson, G.E. Formation of porous anodic oxide film on titanium in phosphoric acid electrolyte. J. Mater. Eng. Perform. 2015, 24, 59–66. [Google Scholar] [CrossRef]
- Root, N.V.; Kultin, Y.D.; Kustov, L.M.; Kudryavtsev, I.K.; Lebedeva, O.K. Effect of the conditions of anodizing on the morphology of nanotitania. Russ. J. Phys. Chem. 2017, 91, 213–216. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Konaka, Y.; Ohtsu, N. Effects of alcoholic solvents on the structure of anodized TiO2 layer grown in nitrate electrolyte. Surf. Coat. Tech. 2020, 387, 125469. [Google Scholar] [CrossRef]
- Ohtsu, N.; Ishikawa, D.; Komiya, S.; Sakamoto, K. Effect of phosphorous incorporation on crystallinity, morphology, and photocatalytic activity of anodic oxide layer on titanium. Thin Solid Films 2014, 556, 247–252. [Google Scholar] [CrossRef]
- Zaniolo, K.M.; Biaggio, S.R.; Bocchi, N.; Rocha-Filho, R.C. Properties of colored oxide films formed electrochemically on titanium in green electrolytes under ultrasonic stirring. J. Mater. Sci. 2018, 53, 7294–7304. [Google Scholar] [CrossRef]
- Regonini, D.; Bowen, C.R.; Jaroenworaluck, A.; Stevens, R. A review of growth mechanism, structure and crystallinity of anodized TiO2 nanotubes. Mater. Sci. Eng. R Rep. 2013, 74, 377–406. [Google Scholar] [CrossRef]
- Razzaboni, L.; Altomare, M.; Pedeferri, M.; Diamanti, M.V.; Schmuki, P. Hierarchical Anodic TiO2 Nanostructures Formed in Ethylene Glycol/o-H3PO4 Electrolytes for Direct Photocatalysis. ChemElectroChem 2020, 7, 2859–2863. [Google Scholar] [CrossRef]
- Raja, K.S.; Gandhi, T.; Misra, M. Effect of water content of ethylene glycol as electrolyte for synthesis of ordered titania nanotubes. Electrochem. Commun. 2007, 9, 1069–1076. [Google Scholar] [CrossRef]
- Xie, Z.B.; Blackwood, D.J. Effects of anodization parameters on the formation of titania nanotubes in ethylene glycol. Electrochim. Acta 2010, 56, 905–912. [Google Scholar] [CrossRef]
- Macak, J.M.; Schmuki, P. Anodic growth of self-organized anodic TiO2 nanotubes in viscous electrolytes. Electrochim. Acta 2006, 52, 1258–1264. [Google Scholar] [CrossRef]
- Guo, T.; Ivanovski, S.; Gulati, K. Fresh or aged: Short time anodization of titanium to understand the influence of electrolyte aging on titania nanopores. J. Mater. Sci. Technol. 2022, 119, 245–256. [Google Scholar] [CrossRef]
- Lutterotti, L.; Matthies, S.; Wenk, H.R. MAUD: A friendly Java program for material analysis using diffraction. IUCr Newsl. CPD 1999, 21, 14–15. [Google Scholar]
- Razaee, M.; Kohie, S.M.; Liu, K.H. The role of brookite in mechanical activation of anatase-to-rutile transformation of nanocrystalline TiO2: An XRD and Raman spectroscopy investigation. Cryst. Eng. Comm. 2011, 13, 5055–5061. [Google Scholar] [CrossRef]
- Spektor, K.; Tran, D.T.; Leinenweber, K.; Häussermann, U. Transformation of rutile to TiO2-II in a high pressure hydrothermal environment. J. Solid State Chem. 2013, 206, 209–216. [Google Scholar] [CrossRef]
- Makula, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis spectra. J. Fis. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Rudnev, V.S.; Medkov, M.A.; Lukiyanchuk, I.V.; Steblevskaya, N.I.; Kilin, K.N.; Belobeletskaya, M.V. Ta-containing coatings formed on titanium and stainless steel by plasma electrolytic oxidation and/or extraction pyrolysis. Surf. Coat. Technol. 2014, 258, 1232–1238. [Google Scholar] [CrossRef]
- Guo, H.; An, M. Effect of surfactants on surface morphology of ceramic coatings fabricated on magnesium alloys by micro-arc oxidation. Thin Solid Films 2006, 500, 186–189. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, L.; Song, Y.; Jia, H.; Yu, H.; Xiao, X.; Yang, X. Oxygen bubble mould effect: Serrated nanopore formation and porous alumina growth. Monatsh. Chem. 2008, 139, 999–1003. [Google Scholar] [CrossRef]
- Berger, S.; Kunze, J.; Schmuki, P.; Valota, A.T.; LeClere, D.J.; Skeldon, P.; Thompson, G.E. Influence of water content on the growth of anodic TiO2 nanotubes in fluoride-containing ethylene glycol electrolytes. J. Electrochem. Soc. 2009, 157, C18. [Google Scholar] [CrossRef]

| Solution | EG% | H2O% |
|---|---|---|
| A 1 | 0 | 100 |
| B 1 | 20 | 80 |
| C 1 | 40 | 60 |
| D 1 | 60 | 40 |
| E 1 | 80 | 20 |
| Solution | Current (mA·cm−2) | % (A/ R) | End Potential (V) |
|---|---|---|---|
| A | 80 | (93.5/ 6.5) | 142 |
| B | 80 | (88.5/ 11.5) | 151 |
| C | 80 | (84.7/ 15.3) | 162 |
| D | 80 | (94.7/ 5.3) | 160 |
| E | 80 | (100/ 0) | 93 |
| Solution (Process Time) | Current (mA·cm−2) | % (A/ R) | End Potential (V) |
|---|---|---|---|
| B (120 s) | 20 | (100/ 0) | 109 |
| 40 | (91.1/ 8.9) | 130 | |
| 80 | (88.5/ 11.5) | 151 | |
| 100 | (81.7/ 15.3) | 157 | |
| B (300 s) | 20 | (100/ 0) | 123 |
| B (600 s) | 20 | (100/ 0) | 125 |
| Bath Type | Current (mA·cm−2) | % S (Weight) | End Potential (V) |
|---|---|---|---|
| B | 20 | 0.39 | 109 |
| 40 | 0.36 | 130 | |
| 80 | 0.61 | 151 | |
| 100 | 0.63 | 157 | |
| B (300 s) | 20 | 0.44 | 123 |
| B (600 s) | 20 | 0.36 | 125 |
| A | 80 | 0.50 | 142 |
| C | 80 | 0.99 | 162 |
| D | 80 | 0.82 | 160 |
| E | 80 | 0.36 | 93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabellini, L.; Calisi, N.; Martinuzzi, S.M.; Taurino, R.; Innocenti, M.; Bacci, T.; Borgioli, F.; Galvanetto, E.; Caporali, S. Effect of Bath Composition on Titanium Anodization Using the Constant-Current Approach: A Crystallographic and Morphological Study. Coatings 2023, 13, 1284. https://doi.org/10.3390/coatings13071284
Gabellini L, Calisi N, Martinuzzi SM, Taurino R, Innocenti M, Bacci T, Borgioli F, Galvanetto E, Caporali S. Effect of Bath Composition on Titanium Anodization Using the Constant-Current Approach: A Crystallographic and Morphological Study. Coatings. 2023; 13(7):1284. https://doi.org/10.3390/coatings13071284
Chicago/Turabian StyleGabellini, Lapo, Nicola Calisi, Stefano Mauro Martinuzzi, Rosa Taurino, Massimo Innocenti, Tiberio Bacci, Francesca Borgioli, Emanuele Galvanetto, and Stefano Caporali. 2023. "Effect of Bath Composition on Titanium Anodization Using the Constant-Current Approach: A Crystallographic and Morphological Study" Coatings 13, no. 7: 1284. https://doi.org/10.3390/coatings13071284
APA StyleGabellini, L., Calisi, N., Martinuzzi, S. M., Taurino, R., Innocenti, M., Bacci, T., Borgioli, F., Galvanetto, E., & Caporali, S. (2023). Effect of Bath Composition on Titanium Anodization Using the Constant-Current Approach: A Crystallographic and Morphological Study. Coatings, 13(7), 1284. https://doi.org/10.3390/coatings13071284










