Abstract
Activated carbon (AC) is an effective adsorbent for creatinine removal in hemoperfusion. However, the hemocompatibility and adsorption capacity of AC was required to be improved further. Heparin has different anticoagulant mechanisms due to its different molecular weights. Thus, it was necessary to study the surface modification with unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH) on improvement of hemocompatibility and adsorption. In this study, UFH and LMWH were, respectively, grafted on AC through polyethyleneimine as an intermediate layer. The modification of AC regarding morphology, mechanical strength, and pore structure was characterized by X-ray photoelectron spectroscopy (XPS), scanning electron microscope (SEM), texture analyzer (TA), and surface area analyzer. It was found that, compared with AC, the morphology and mechanical strength of AC-UFH and AC-LMWH could be well maintained, but the specific surface area was decreased due to the grafting of macromolecules. Furthermore, AC-UFH and AC-LMWH showed better hemocompatibility on protein adsorption, clotting time, and platelet activation compared with AC, in which AC-LMWH had lower fibrinogen adsorption and longer clotting time than AC-UFH. In addition, it was found that AC, AC-UFH, and AC-LMWH had no significant effect on blood cell composition. Finally, the adsorption capacity of adsorbents for creatinine was evaluated. Although there was no significant difference between AC-UFH and AC-LMWH, it was found that heparin could be interacted with creatinine to enhance the adsorption capacity when compared with polyethyleneimine-modified AC. This study deepened the understanding of anticoagulation of heparinized surface and provided a theoretical basis for adsorption in hemoperfusion.
1. Introduction
Hemoperfusion, an extracorporeal blood purification technique, could remove endogenous or exogenous toxins through direct contact of porous adsorbents with blood drawn from the body to relieve symptoms and even cure diseases, which have been widely used in the treatment of acute poisoning [1], hyperlipidemia [2], acute hepatitis [3], sepsis [4], and uremia [5]. The technical core of hemoperfusion was adsorbents determining the treatment effect [6,7,8]. Activated carbon (AC), a kind of granular or powdery material obtained by high-temperature carbonization activation, is one of the commonly used broad-spectrum adsorbents for removal of various toxins, with high specific surface area and large pore volume [8]. As early as 1964, Yatzidis et al. used activated carbon as adsorbents with strong adsorption efficiency for creatinine, uric acid, phenols, indole, salicylic acid, barbiturates, and glutethimide [9]. Although AC has advantages, such as satisfactory adsorption performance, low cost, and wide application, poor blood compatibility limits its further clinical application [8,10]. The biocompatibility of AC could be improved by coating with good biocompatible biopolymers, such as collodion–albumin [11,12,13], dextran [14], zwitterionic hydrogel [15], poly(ether sulfone) [16], and so on. Coating could reduce the shedding of carbon particles into blood, thereby reducing damage to red blood cells and the occurrence of carbon thrombosis. However, the poor hemocompatibility and poor selectivity of AC absorbents needs to be improved.
Heparin, a linear polysaccharide, has been widely used as an anticoagulant. Unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH) can be administered by intravenous or subcutaneous injection to reduce the risk of coagulation in clinic [17,18]. UFH is a kind of aminodextran sulfate extracted and refined from porcine intestinal mucosa or bovine lung and is a mixture ranging in molecular weight from approximately 16,000 Da [19]. LMWH prepared by controlled chemical or enzymatic depolymerization of UFH is a fragment of aminodextran sulfate with a molecular weight range of 3000–5000 Da [18]. The anticoagulant mechanisms of heparins with different molecular weights are also different. Both UFH and LMWH can bind to antithrombin through the pentasaccharide sequence to play the role of anticoagulation factors [20,21], but the coagulation factors that can be inhibited are different due to the different lengths of the heparin chains [22,23]. After combining with antithrombin III, UFH with a larger molecular weight can inhibit coagulation factors Xa and IIa, while LMWH with a smaller molecular weight can increase the affinity with coagulation factor Xa and mainly play the role of anticoagulation factor Xa [24]. Compared with UFH, LMWH has lower binding to plasma proteins, platelets, and endothelial cells, longer half-life, and more predictable anticoagulant response, with lower number of side effects and incidence of bleeding complications [25,26,27]. Because of the anticoagulation and rich functional groups of heparin, surface heparinization has been paid much attention to the improvement of blood compatibility, aimed towards the amount of anticoagulation and some side effects. Heparin could be attached to materials by coating [28], LbL assembly [29], grafting [30], and mussel-inspired surface coating [31,32]. In addition, a great number of reports have proved that surface heparinization could improve the blood compatibility of blood-contact materials [33,34,35]. However, there was not much attention on the differences between different molecular weights of heparinized surfaces to improve hemocompatibility.
In addition, heparin as a linear polymer with a large negative charge could be used to improve the adsorption selectivity of target toxin [36,37]. Creatinine is a product of muscle metabolism in the human body, mainly excreted from the body through glomerular filtration. When the kidneys were in chronic or acute dysfunction, the concentration of creatinine in the blood was increased, ultimately leading to renal failure. So, the creatinine has been found to be a fairly reliable indicator of renal function. In clinic, the removal of creatinine by hemodialysis and hemoperfusion was one of the effective methods for treating renal failure. It was reported that heparin immobilized on graphene oxide presented outstanding removal of uremic toxins (urea, creatinine, and phosphorous) after 4 h by hemodialysis [31]. So, what is the kinetic adsorption mechanism of creatinine on heparinized surfaces and would the adsorption of creatinine be affected by the molecular weight of heparin?
Based on the above, UFH or LMWH was covalently grafted on the surface of AC, aiming to improve the blood compatibility and creatinine adsorption of the adsorbent. The absorbents were then characterized by SEM, XPS, TA, and surface area analyzer to evaluate the immobilized process and retention of nano- and mesopores. Then, the protein adsorption, clotting time, platelet activation, and blood cell assay in vitro were evaluated to investigate the differences between UFH and LMWH modified surfaces in improving blood compatibility. Finally, the adsorption capacity of creatinine and the kinetic adsorption were calculated by UV-Vis spectrophotometer, respectively. This study was expected to provide a basis for better improving the blood compatibility and adsorption selectivity of AC in blood purification.
2. Materials and Methods
2.1. Materials
Activated carbon (AC) was provided by Shenzhen Global Green New Materials Co., Ltd., Shenzhen, China. Polyethyleneimine (PEI, MW = 600 Da), 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide (EDC), and N-Hydroxysuccinimide (NHS) were purchased from Aladdin Biochemical Technology Co., Ltd. in Shanghai, China. Unfractionated heparin and low-molecular-weight heparin (Enoxaparin sodium) were purchased from Hepalink Pharmaceutical Group Co., Ltd. in Shenzhen, China. Sodium dodecyl sulfate (SDS), sodium chloride (NaCl), sodium phosphate dibasic (Na2HPO4), citric acid, phosphate-buffered saline (PBS), bovine serum albumin (BSA), fibrinogen (FBG), and Micro BCA Protein Assay Reagent kit were obtained from Solarbio Science & Technology Co., Ltd. in Beijing, China. Creatinine was purchased from Sigma-Aldrich (Shanghai) Trading Co. Ltd. Disposable and negative pressure blood collection vessels containing 1:9 sodium citrate were purchased from KWS Medical Technology Co., Ltd. in Shandong, China.
2.2. Preparation of AC-UFH and AC-LMWH
A total of 50 g AC was immersed into 300 mL citrate buffer solution (pH = 4.7, 0.2 M Na2HPO4 and 0.1 M citric acid) containing EDC and NHS (molar ratio = 2:1) for 1 h at 37 °C to obtain the carboxyl-activated AC. Then, 3.0 g PEI (600 Da) was added into the above mixture with continuous reaction at 37 °C. After 24 h, the modified AC was filtered by vacuum and washed 3 times alternately with deionized water and PBS (pH = 7.0). Finally, the PEI-modified AC was dried at 65 °C for 24 h and named as AC-PEI.
UFH or LMWH was added, respectively, into citrate buffer solution (pH = 4.7) containing EDC and NHS (molar ratio = 2:1) for 1 h to activate carboxyl. Subsequently, AC-PEI was immersed into 3 mg/mL UFH or LMWH solution and shaken at 37 °C for 24 h to obtain UFH-modified AC (AC-UFH) or LMWH-modified AC (AC-LMWH). Then, heparinized AC was washed by 4 M NaCl solution to remove the ionically and physically adsorbed heparin, followed by washing 3 times alternately with deionized water and PBS (pH = 7.0). Finally, AC-UFH or AC-LMWH was dried by vacuum filtration and oven at 65 °C.
2.3. Characterization
X-ray photoelectron spectroscopy (XPS, K-Alpha+, Thermo Scientific, Waltham, MA, USA) was used to characterize the elemental composition of materials. The Al Kα excitation source (hυ = 1486.6 eV) was used, the area was 500 μm, and the pressure was <10−7 Pa. The morphologies of the fabricated materials were examined using a scanning electron microscope (SEM, JSM-7800F, Tokyo, Japan) at an accelerating voltage of 5.00 kV. The mechanical strength of absorbents was measured by Texture analyzer (TA, TA.XTC-20, Bosintech, Shanghai, China), with 10 tests for each sample by speed of 0.2 mm/s. Specific surface area and pore volume of absorbents were characterized by a surface area analyzer (BELSORP-max, MicrotracBEL, Tokyo, Japan).
2.4. Hemocompatibility
Samples of 100 mg were balanced in normal saline for 2 h and incubated with 1.0 mg/mL BSA or FBG solution at 37 °C. After 2 h, the samples were washed slightly with normal saline 3 times, followed by elution with 2% SDS at 37 °C for 2 h to make the adsorbed protein fall off into the solution. Finally, the protein concentration in the elution was determined using the Micro BCA Protein Assay Reagent Kit (Solarbio, Beijing, China) and microplate reader (Multiskan FC, Thermo Scientific, Waltham, MA, USA).
The blood sample was rabbit blood collected by blood collection vessels containing sodium citrate (1:9). Platelet-poor plasma (PPP) was obtained by centrifuging blood at 1500 rpm for 10 min. AC, AC-UFH, and AC-LMWH were immersed in PPP at 37 °C for 2 h, and the control was untreated PPP. Activated partial thromboplastin time (APTT) was measured by automated coagulation analyzer (CA620, Sysmex, Kobe, Japan).
All samples were, respectively, incubated with anticoagulant blood at 37 °C shaken with 60 r/min. The control was blood without contact with materials. After 1 h, the blood was centrifuged with 1500 rpm for 10min. The upper plasma was taken and measured according to rabbit blood platelet globulin (β-TG) ELISA test kit (Cusabio, Wuhan, China) operation. The absorbance was measured at 450 nm by microplate reader (Versa Max, Molecular Devices, Sunnyvale, CA, USA).
The samples were, respectively, incubated with anticoagulant blood at 37 °C. After incubation for 1 h, the white cells, red cells, and platelets were counted by blood cell analyzer (pocH 100iVD, Sysmex, Kobe, Japan).
2.5. Adsorption Experiments
AC, AC-PEI, AC-UFH, and AC-LMWH microspheres were balanced in PBS (50 mM, pH = 7.4) for 30 min at room temperature before the adsorption experiment.
Creatinine was dissolved into PBS to prepare standard solution with the range of creatinine concentration of 0–0.1 mg/mL. The absorbance of creatinine solution was determined by a UV-Vis spectrophotometer (Nanodrop one, Thermo Scientific, Waltham, MA, USA) at the wavelength of 235 nm to obtain the calibration curve.
The microspheres were, respectively, immersed into 0.1 mg/mL creatinine solution and shaken for 2 h at room temperature (25 ± 2 °C). The amount of creatinine adsorption was evaluated by the initial concentration and final concentration. In addition, the amount of creatinine adsorption at different times was monitored by a UV-Vis spectrophotometer (Nanodrop one, Thermo Scientific, Waltham, MA, USA) at the wavelength of 235 nm. The concentration of creatinine adsorption at various stages was calculated by the calibration curve.
Kinetic adsorption equations of AC, AC-UFH, and AC-LMWH were established through the monitoring and recording of endotoxin concentrations at different times, and the adsorption curves were fitted and analyzed by pseudo-first-order kinetic equation and pseudo-second-order kinetic equation, respectively, as follows:
Pseudo-first-order kinetic equation:
Pseudo-second-order kinetic equation:
where was the adsorption time (min); was the adsorption amount of creatinine at some time (mg/g); was the adsorption amount of creatinine at equilibrium (mg/g); and were the rate constants of pseudo-first-order kinetic equation and pseudo-first-order kinetic equation, respectively.
2.6. Statistical Analysis
The data in the experimental results were expressed by mean ± SD and were statistically analyzed by GraphPad Prism 6 software with one-way ANOVA method. The data had a significant difference when p < 0.05, and * or # represented p < 0.05.
3. Results and Discussion
3.1. Characterization of AC
Although Shenzhen Global Green New Materials Co., Ltd. (Shenzhen, China) kept the specific method of preparing AC confidential, it was known that the AC was obtained from styrene divinylbenzene resin treated through high-temperature carbonization, impact removal, high-temperature activation, impact removal, and drying [38]. With the increase in carbonization treatment temperature, these organic structural groups would gradually transform to the direction of forming graphite microcrystals, leading to changes in the functional groups and composition of carbonized products [38]. Therefore, it was necessary to characterize its structural groups before the modification of AC, which was beneficial to provide a basis for subsequent modification.
The structure and composition of AC would be calculated by X-ray electron spectroscopy, as shown in Figure 1. Table 1 shows chemical valence distribution and content proportion of C1s and O 1s. The results indicated that the AC obtained from high-temperature carbonization and activation of styrene divinylbenzene resin had a certain amount of -COOH, which could provide the reactive sites for modification. However, the percentage of -COOH was limited, and the reactive sites could be amplified by the introduction of spacers with rich functional groups, which could decrease the steric hindrance during the grafting of heparin.
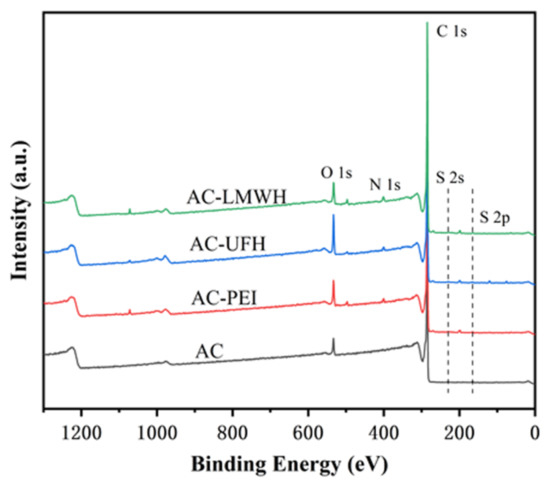
Figure 1.
XPS spectra of AC, AC-PEI, AC-UFH, and AC-LMWH.

Table 1.
Chemical valence distribution and content proportion of C 1s calculated by XPS.
3.2. Preparation and Characterization of AC-UFH and AC-LMWH
Surface modification is an effective way to endow materials with specific properties by adding or changing the chemical structure or composition of the surface. In order to increase the reactive sites, PEI was used to modify the AC through EDC/NHS chemical coupling reaction to provide rich amino group. In the same way, the UFH or LMWH was grafted onto AC-PEI to obtain the AC-UFH or AC-LMWH.
Figure 1 shows the XPS spectrum of each microsphere, and the analysis was carried out combined with the element percentage of each sample, as shown in Table 2. It could be seen that there were peaks at 285.1 eV and 532.1 eV of AC microspheres, corresponding to the binding energy of C 1s and O 1s, respectively. In addition, the C and O content of AC were 95.65% and 4.07%, respectively. The results indicated that the AC was composed of carbon skeleton (C-C/C=C) and a certain amount of oxygen-containing group such as -COOH, -OH, etc. (Table 1). The existence of S element in AC microspheres was due to the residual organic reagents in the synthesis of microspheres. After introduction of PEI, a new peak of AC-PEI appeared at 400.1 eV, corresponding to the binding energy of N 1s, and the N content was increased to 1.88%, demonstrating that the PEI was introduced on AC surface successfully. After the modification with heparin, the O and S content of AC-UFH was increased to 8.32% and 0.36%, indicating that heparin was modified with AC successfully. In addition, AC-LMWH was increased to 5.99% and 0.35%, which was not obviously changed.

Table 2.
Chemical composition of microspheres calculated from XPS survey scans.
In order to evaluate the composition of surface, three points were selected randomly to detect using EDS, as shown in Table 3. It could be seen that the O and S content of AC-UFH and AC-LMWH was significantly increased when compared with AC. In addition, it could be observed from Figure 2B that the signals of O and S on the surface of AC-UFH and AC-LMWH were slightly stronger than that on the surface of AC, which showed uniform dispersion. These results indicated that the UFH and LMWH were modified uniformly on AC surface successfully.

Table 3.
Chemical composition of surface calculated by EDS point scanning.
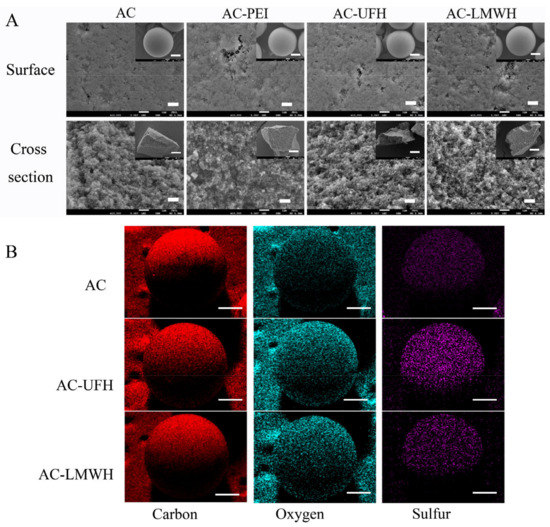
Figure 2.
(A) SEM images of surface and cross-section of AC, AC-PEI, AC-UFH, and AC-LMWH (scale bar = 1 μm and scale bar of inserted images = 300 μm). (B) EDS mapping of AC, AC-UFH, and AC-LMWH; scale bar = 250 μm (red = carbon, light blue = oxygen, purple = sulfur).
In addition, there was the difference between the O content of AC-UFH and AC-LMWH, and the O content of AC-UFH was higher than that of AC-LMWH (Table 3), which might be attributed to the steric hindrance of heparin grafting and the longer molecular chain of unfractionated heparin.
After a series of modification processes, whether the microspheres could maintain their original morphology and characteristics was important for practical application. Figure 2A shows the SEM images of the morphology of each sample. It could be seen that the particle size of microspheres was mainly focused on 500–600 μm, and AC was spherical and had rich internal channels. Compared with AC, the surface morphology of AC-PEI, AC-UFH, and AC-LMWH kept the original morphology and was unbroken after a series of reactions. The internal structure can be observed from the cross-section of the microsphere, and the modified microspheres maintained the abundant channels and pore structure inside. These results demonstrated that the microspheres could keep the integrity, original morphology, and rich pore structure after a series of modification.
The adsorbent with better mechanical strength could prevent the rupture and particle shedding during the blood perfusion process resulting in thrombosis, and the mechanical strength should be kept during the modification process. The mechanical strength of AC and modified AC was calculated by the texture curve, as shown in Figure 3, and Table 4 shows the average first peak force of materials. The average first peak force of AC was about 25.28 N and was not changed obviously during the modification, indicating that modified AC maintained the original mechanical strength. Carboxylic amine condensation reaction was EDC/NHS coupling reaction, which is a simple and mild modification method. The reaction could be completed in the buffer solution with pH = 4.7, which had no significant effect on the mechanical strength of the materials.
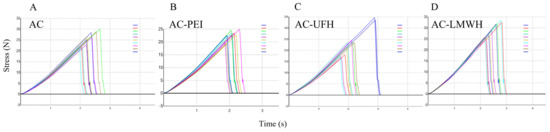
Figure 3.
Texture curve of AC (A), AC-PEI (B), AC-UFH (C), and AC-LMWH (D) with 10 scans per sample. The line colors from top to bottom in the legend were the 1st scan to the 10th scan.

Table 4.
The fracture strength of materials by texture profile analysis.
Figure 4A displayed the nitrogen adsorption/desorption isotherm of the samples. It could be seen that the isotherm of AC-PEI, AC-UFH, and AC-LMWH raised slower compared with AC in the area of low P/P0, indicating the decrease in the surface area of AC-PEI, AC-UFH, and AC-LMWH. In addition, the hysteresis loop on the isotherm of modified materials was not significantly changed compared with AC, indicating that grafting PEI or heparin did not significantly affect the mesopore volume of AC. The pore size distribution of AC, AC-PEI, AC-UFH, and AC-LMWH is displayed in Figure 4B. It could be seen that the reduction in pore volume after grafting PEI/heparin was mainly concentrated in micropores (<2 nm). The pore property of AC, AC-PEI, AC-UFH, and AC-LMWH was illustrated in Table 5, which is inserted in Figure 4B.
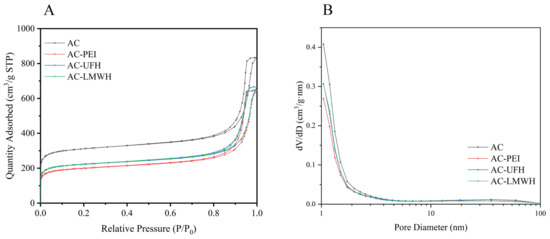
Figure 4.
(A) Nitrogen adsorption–desorption isotherms and (B) pore size distribution curves of AC, AC-PEI, AC-UFH, and AC-LMWH.

Table 5.
BET surface area and cumulative volume of pores of AC, AC-PEI, AC-UFH and AC-LMWH.
3.3. Hemocompatibility
Protein adsorption is considered to be the first event that occurs when blood contacts the surface of material, and the adsorbed protein will mediate the subsequent biological reaction, including the occurrence of platelet adhesion and coagulation cascade reaction. So, it is necessary to investigate the protein adsorption for the evaluation of the blood compatibility of heparinized surface. Figure 5 shows BSA and FBG adsorption of microspheres in normal saline. Compared with AC, the adsorption of BSA and FBG on modified microspheres was increased, which might be assigned to the surface charge and molecular freedom. It could be observed from Figure 5A,B that the FBG adsorption amount of microspheres in the same group was higher than that of BSA adsorption, implying that the surface of microspheres was more likely to cause FBG adsorption and presented the resistance to BSA. Interestingly, the BSA adsorption amount of AC-UFH and AC-LMWH had no obvious difference, but the FBG adsorption of AC-UFH was higher than that of AC-LMWH. It was reported that enoxaparin could improve the nature and structure of plasma fibrin clots and increased microvascular blood circulation by preventing microthrombosis [39].
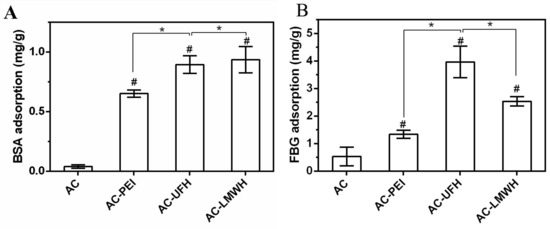
Figure 5.
BSA (A) and FBG (B) adsorption capacity of absorbents. * p < 0.05, # p < 0.05 vs. AC.
To evaluate the anticoagulation of AC-UFH and AC-LMWH, APTT was measured after PPP incubated with materials. It could be seen from Figure 6A that APTT of AC was not changed obviously compared with the control, showing no effect on blood coagulation. In addition, APTT of AC-UFH and AC-LMWH was longer than that of AC, indicating that whether UFH or LMWH modified on the surface, it could improve the anticoagulation performance of materials. Although LMWH partially lost the ability to promote AT inactivation of thrombin (IIa) due to the lower molecular weight of heparin (enoxaparin), it still had the ability to inactivate Xa, which could result in the prolongation of APTT. Furthermore, APTT of AC-LMWH was longer than that of AC-UFH, consistent with the literature [40]. However, the prolongation of clotting time was lower than that of some previous reports [30,40], which might be attributed to the lower grafting amount of heparin. Although the focus was the molecular weight of heparin on hemocompatibility, the grafting density could be improved in further research through developing efficient grafting methods.
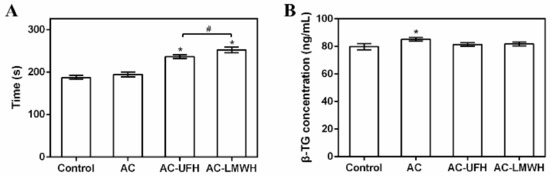
Figure 6.
APTT (A) and (B) β-TG concentration of plasma incubated with absorbents, and control was untreated plasma. # p < 0.05, * p < 0.05 vs. control.
When the medical device/material contacted with human blood, the increase in particulate matter in blood might be the inducement for the medical device/material to cause or promote bleeding or thrombosis [41]. If the platelet granules were significantly higher than the control, it indicated that the medical device/material had the potential to activate platelets [42]. β-TG was one of the platelet granules and could represent the activation of platelets. Figure 6B shows the β-TG concentration of materials incubated with blood. It could be seen that the β-TG concentration of AC was higher than that of the control, indicating that AC could activate platelets. After grafting heparin, the β-TG concentration of AC-UFH and AC-LMWH had no significant difference with the control, demonstrating that AC-UFH and AC-LMWH could reduce the activation level of platelets and heparin modification could decrease the platelet activation of AC. Although there was no difference between AC-UFH and AC-LMWH, UFH and LMWH showed the ability of antiplatelet activation.
The loss of cell components in blood during the hemoperfusion was a problem that could not be ignored. In addition to high adsorption capacity, the ideal adsorbents for hemoperfusion also needed to have adsorption specificity and good biocompatibility to achieve less nonspecific adsorption of other blood components. In blood, blood cells account for about 45% of the blood volume, including red cells, white cells, and platelets. The main function of red cells is to transport oxygen. White blood cells mainly play the role of immunity. When bacteria invade the human body, white cells can pass through the capillary wall and are concentrated on the invasion site of the bacteria, surrounding the bacteria and swallowing them. Platelets play an important role in hemostasis. Figure 7 shows the amount of white cells, red cells, and platelets of blood incubated with AC, AC-UFH, and AC-LMWH. It could be seen that the amount of white cells, red cells, and platelets of AC, AC-UFH, and AC-LMWH had no significant difference from the control, indicating that AC, AC-UFH, and AC-LMWH had no significant effect on blood cell composition. This was attributed to the micropores of AC preventing blood cells from entering and being damaged.
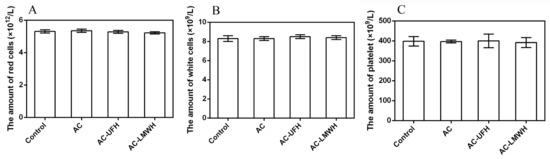
Figure 7.
The amount of red cells (A), white cells (B), and platelets (C) of blood incubated with absorbents, and control was untreated blood.
3.4. Adsorption of Creatinine
As shown in Figure 8A, the adsorption capacity of creatinine was decreased after the immobilization when compared with that of AC. After the immobilization, molecules would block the nano- and mesopores of AC, causing the reduction in specific surface area, which resulted in the decrease in adsorption capacity. Then, the adsorption capacity of AC-UFH and AC-LMWH for creatinine was increased when compared with that of AC-PEI, which might be assigned to two reasons: one was the decline of specific surface area and the other was the positive charge of AC-PEI caused by rich amino. These results implied that the adsorption of creatinine could be affected by surface charges, and the heparin with negative functional groups would enhance the interaction with creatinine, which was consistent with a previous report [31]. In addition, heparin grafted on the surface could enhance the side-chain flexibility to improve the ability of capturing the target toxins [43]. For heparin with different molecular weights, it could be found that there was no significant difference between the adsorption capacity of AC-UFH and AC-LMWH. Different molecular weights of heparin chains have different flexibility, which should have had different effects on the adsorption of creatinine, but the results were not the same. It was attributed to the fixation on the surface of activated carbon resulting in a change in the molecular chain conformation of heparin, with no significant improvement in surface flexibility. In addition, the grafting amount of heparin was not sufficient to produce a difference in adsorption capacity between AC-UFH and AC-LMWH.
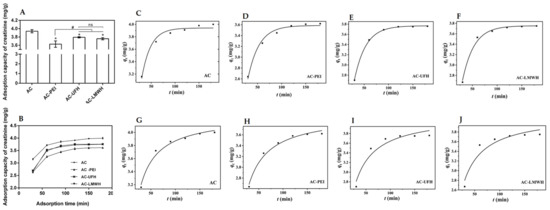
Figure 8.
(A) Creatinine adsorption capacity of AC, AC-PEI, AC-UFH, and AC-LMWH (n = 3), * p < 0.05 vs. AC, # p < 0.05. (B) Creatinine adsorption on AC, AC-PEI, AC-UFH, and AC-LMWH at different times. Fitting curve of pseudo-first-order kinetic model of AC (C), AC-PEI (D), AC-UFH (E), and AC-LMWH (F) and fitting curve of pseudo-second-order kinetic model of AC (G), AC-PEI (H), AC-UFH (I), and AC-LMWH (J).
Furthermore, the influence of adsorption time on adsorption of creatinine was investigated as shown in Figure 8B. It could be seen that there was rapid adsorption for creatinine at the initial 60 min and the adsorption capacity of adsorbents could reach equilibrium within about 120 min. In addition, it was found from the adsorption curves that the adsorption rate of heparinized AC at the initial 60 min was higher than that of AC and AC-PEI, which might be attributed to the functional groups of heparin.
Further, the adsorption kinetics of the adsorbent were nonlinearly fitted by pseudo-first-order kinetics model and pseudo-second-order kinetics model according to the data of Figure 8B, as shown in Figure 8C–J. In addition, Table 6 presents the corresponding kinetic adsorption parameters calculated from the fitting equations. was the correlation coefficients, which represented the degree of fitting between kinetic adsorption curve and the model equation. The kinetic adsorption of AC was more in line with the pseudo-second-order kinetics model ( < ), and the kinetic adsorption of AC-UFH and AC-LMWH was more in line with the pseudo-first-order kinetics model ( > ), suggesting that the introduction of heparin resulted in more strong Van der Waals force between adsorbents and creatinine when compared with AC. Although the value of AC-UFH and AC-LMWH was lower than that of AC and AC-PEI, the obtained by fitting was higher than that of AC-PEI, once again proving that heparin immobilized on AC could enhance the adsorption capacity of creatinine through the intermolecular force.

Table 6.
Parameters for pseudo-first-order and pseudo-second-order kinetic model of adsorbents.
4. Conclusions
In summary, based on the different anticoagulant properties and flexibility of the macromolecular chain, UFH or LMWH was grafted on AC to investigate the heparin with different molecular weights on anticoagulation and creatinine adsorption. After the modification, AC-UFH and AC-LMWH could maintain the original morphology and mechanical strength of AC, and the specific surface area was found to be decreased due to the occupancy of heparin molecules. The anticoagulation of AC-UFH and AC-LMWH was found to be increased compared with AC, in which AC-LMWH had lower FBG adsorption and longer APTT. These results demonstrated that modification with LMWH could decrease the influence on FBG, which had great potential in antithrombosis. In future, heparin with different molecular weights could be selected for modification according to different anticoagulant properties required by materials, which could have more targeted effects. In addition, although creatinine adsorption capacity of materials after the modification was decreased, the introduction of heparin could enhance adsorption capacity compared with AC-PEI, which was attributed to the interaction between heparin chain and creatinine via Van der Waals force. In addition, the heparin with different molecular weights had no effect on the adsorption of creatinine. In addition, the kinetic adsorption of adsorbents could reach equilibrium within 120 min, and the kinetic adsorption of AC-UFH and AC-LMWH was more in line with the pseudo-first-order kinetics model. This research provided the theoretical basis for the widespread application of heparin in hemoperfusion. In this experiment, we found the adsorption capacity of heparin to creatinine interestingly. In the next experiment, we will firstly increase the grafting density of heparin with different molecular weights and then study the Van der Waals force between heparin with different molecular weights and the target toxin at the atomic level.
Author Contributions
Conceptualization, J.L. and Q.D.; methodology, J.L., H.W. and Q.D.; software, J.H. (Jing Huang); validation, X.W.; formal analysis, H.W.; investigation, J.L., H.W. and Q.D.; resources, J.H. (Jingzhou Hou); data curation, Q.D. and K.F.; writing—original draft preparation and writing—review and editing, Q.D. and C.L.; visualization, X.W.; supervision, X.W. and S.Z.; project administration, Q.D.; funding acquisition, J.L. and X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant number. 12172071), Project of Science and Technology Research Program of Chongqing Education Commission of China (grant number. KJQN201903312), Natural Science Foundation of Chongqing (grant number. cstc2021jcyj-bsh0268), and Scientific Research Foundation for High-level Talents of Chongqing City Management College (grant number. 2023KYQD02 and 2017KYQD01).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, T.G.; Yan, Y.; Wang, N.N.; Zhao, M. Acute carbamazepine poisoning treated with resin hemoperfusion successfully. Am. J. Emerg. Med. 2011, 29, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Bambauer, R.; Bambauer, C.; Lehmann, B.; Latza, R.; Schiel, R. LDL-apheresis: Technical and clinical aspects. Sci. World J. 2012, 2012, 314283. [Google Scholar] [CrossRef] [PubMed]
- Plotz, P.H.; Berk, P.D.; Scharschmidt, B.F.; Gordon, J.K.; Vergalla, J. Removing substances from blood by affinity chromatography. I. Removing bilirubin and other albumin-bound substances from plasma and blood with albumin-conjugated agarose beads. J. Clin. Investig. 1974, 53, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, R.; Subramanian, S.; Kellum, J.A. Clinical review: Extracorporeal blood purification in severe sepsis. Crit. Care 2003, 7, 139–145. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, X.; Hu, Z.; Yu, M.; Fu, J.; Huang, Y. Fabrication of a novel nitrogen-containing porous carbon adsorbent for protein-bound uremic toxins removal. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111879. [Google Scholar] [CrossRef]
- Li, Z.; Yan, X.; Wu, K.; Jiao, Y.; Zhou, C.; Yang, J. Surface Modification of Reduced Graphene Oxide Beads: Integrating Efficient Endotoxin Adsorption and Improved Blood Compatibility. ACS Appl. Bio Mater. 2021, 4, 4896–4906. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, S.; He, C.; Wu, X.; Yin, J.; Zhang, J.; Ma, L.; Zhao, W.; Cheng, C.; Zhao, C. Construction of Kevlar nanofiber/graphene oxide composite beads as safe, self-anticoagulant, and highly efficient hemoperfusion adsorbents. J. Mater. Chem. B 2020, 8, 1960–1970. [Google Scholar] [CrossRef]
- Dou, W.; Wang, J.; Yao, Z.; Xiao, W.; Huang, M.; Zhang, L. A critical review of hemoperfusion adsorbents: Materials, functionalization and matrix structure selection. Mater. Adv. 2022, 3, 918–930. [Google Scholar] [CrossRef]
- Yatzidis, H. Research on Extrarenal Purification with the Aid Of Activated Charcoal. Nephron 1964, 1, 310–312. [Google Scholar] [CrossRef]
- Barnes, J.; Cowgill, L.D.; Diaz Auñon, J. Activated Carbon Hemoperfusion and Plasma Adsorption. Adv. Small Anim. Care 2021, 2, 131–142. [Google Scholar] [CrossRef]
- Chang, T.M. Microencapsulated adsorbent hemoperfusion for uremia, intoxication and hepatic failure. Kidney Int. Suppl. 1975, 3, 387–392. [Google Scholar]
- Chang, T.M. Biodegradable semipermeable microcapsules containing enzymes, hormones, vaccines, and other biologicals. J. Bioeng. 1976, 1, 25–32. [Google Scholar]
- Chang, T.M. Semipermeable Microcapsules. Science 1964, 146, 524–525. [Google Scholar] [CrossRef]
- Howell, C.A.; Sandeman, S.R.; Zheng, Y.; Mikhalovsky, S.V.; Nikolaev, V.G.; Sakhno, L.A.; Snezhkova, E.A. New dextran coated activated carbons for medical use. Carbon 2016, 97, 134–146. [Google Scholar] [CrossRef]
- Cai, N.; Li, Q.; Zhang, J.; Xu, T.; Zhao, W.; Yang, J.; Zhang, L. Antifouling zwitterionic hydrogel coating improves hemocompatibility of activated carbon hemoadsorbent. J. Colloid. Interface Sci. 2017, 503, 168–177. [Google Scholar] [CrossRef]
- Deng, X.; Wang, T.; Zhao, F.; Li, L.; Zhao, C. Poly(ether sulfone)/activated carbon hybrid beads for creatinine adsorption. J. Appl. Polym. Sci. 2007, 103, 1085–1092. [Google Scholar] [CrossRef]
- Rodriguez-Fernandez, A.; Sanchez-Dominguez, M.; Torrado-Espanol, I.; Noguerado-Mellado, B.; Rojas-Perez-Ezquerra, P. Clinical Patterns of Heparin Allergy: Cross-reactivity Between Low-Molecular-Weight Heparins and Unfractionated Heparins. J. Investig. Allergol. Clin. Immunol. 2019, 29, 132–134. [Google Scholar] [CrossRef]
- Banik, N.; Yang, S.B.; Kang, T.B.; Lim, J.H.; Park, J. Heparin and Its Derivatives: Challenges and Advances in Therapeutic Biomolecules. Int. J. Mol. Sci. 2021, 22, 10524. [Google Scholar] [CrossRef]
- Ingle, R.G.; Agarwal, A.S. A world of low molecular weight heparins (LMWHs) enoxaparin as a promising moiety—A review. Carbohydr. Polym. 2014, 106, 148–153. [Google Scholar] [CrossRef]
- Brinkhous, K.M.; Smith, H.P., Jr.; Warner, E.D.; Seegers, W.H. Heparin And Blood Clotting. Science 1939, 90, 539. [Google Scholar] [CrossRef]
- Lindahl, U.; Backstrom, G.; Hook, M.; Thunberg, L.; Fransson, L.A.; Linker, A. Structure of the antithrombin-binding site in heparin. Proc. Natl. Acad. Sci. USA 1979, 76, 3198–3202. [Google Scholar] [CrossRef] [PubMed]
- Oosta, G.M.; Gardner, W.T.; Beeler, D.L.; Rosenberg, R.D. Multiple functional domains of the heparin molecule. Proc. Natl. Acad. Sci. USA 1981, 78, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Thunberg, L.; Lindahl, U.; Tengblad, A.; Laurent, T.C.; Jackson, C.M. On the molecular-weight-dependence of the anticoagulant activity of heparin. Biochem. J. 1979, 181, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Howard, P.A. Low molecular weight heparins in special populations. J. Infus. Nurs. Off. Publ. Infus. Nurses Soc. 2003, 26, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Chong, B.H.; Ismail, F. The mechanism of heparin-induced platelet aggregation. Eur. J. Haematol. 1989, 43, 245–251. [Google Scholar] [CrossRef]
- Burgess, J.K.; Chong, B.H. The platelet proaggregating and potentiating effects of unfractionated heparin, low molecular weight heparin and heparinoid in intensive care patients and healthy controls. Eur. J. Haematol. 1997, 58, 279–285. [Google Scholar] [CrossRef]
- Garcia, D.A.; Baglin, T.P.; Weitz, J.I.; Samama, M.M. Parenteral anticoagulants: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e24S–e43S. [Google Scholar] [CrossRef]
- Biran, R.; Pond, D. Heparin coatings for improving blood compatibility of medical devices. Adv. Drug Deliv. Rev. 2017, 112, 12–23. [Google Scholar] [CrossRef]
- Taniguchi, T.; Kyung, K.-H.; Shiratori, S. Layer-by-layer self-assembled thin films of chitin fibers and heparin with anti-thrombus characteristics. RSC Adv. 2015, 5, 107488–107496. [Google Scholar] [CrossRef]
- Dang, Q.; Li, C.G.; Jin, X.X.; Zhao, Y.J.; Wang, X. Heparin as a molecular spacer immobilized on microspheres to improve blood compatibility in hemoperfusion. Carbohydr. Polym. 2019, 205, 89–97. [Google Scholar] [CrossRef]
- Jacob Kaleekkal, N. Heparin immobilized graphene oxide in polyetherimide membranes for hemodialysis with enhanced hemocompatibility and removal of uremic toxins. J. Membr. Sci. 2021, 623, 119068. [Google Scholar] [CrossRef]
- Li, C.G.; Yang, Q.; Chen, D.; Zhu, H.; Chen, J.; Liu, R.; Dang, Q.; Wang, X. Polyethyleneimine-assisted co-deposition of polydopamine coating with enhanced stability and efficient secondary modification. RSC Adv. 2022, 12, 34837–34849. [Google Scholar] [CrossRef]
- Wei, H.; Han, L.; Ren, J.; Jia, L. Anticoagulant surface coating using composite polysaccharides with embedded heparin-releasing mesoporous silica. ACS Appl. Mater. Interfaces 2013, 5, 12571–12578. [Google Scholar] [CrossRef]
- Shan, L.; Sun, Y.; Shan, F.; Li, L.; Xu, Z.P. Recent advances in heparinization of polymeric membranes for enhanced continuous blood purification. J. Mater. Chem. B 2020, 8, 878–894. [Google Scholar] [CrossRef]
- Zhang, M.; Chan, C.H.H.; Pauls, J.P.; Semenzin, C.; Ainola, C.; Peng, H.; Fu, C.; Whittaker, A.K.; Heinsar, S.; Fraser, J.F. Investigation of heparin-loaded poly(ethylene glycol)-based hydrogels as anti-thrombogenic surface coatings for extracorporeal membrane oxygenation. J. Mater. Chem. B 2022, 10, 4974–4983. [Google Scholar] [CrossRef]
- Wang, L.; Fang, F.; Liu, Y.; Li, J.; Huang, X. Facile preparation of heparinized polysulfone membrane assisted by polydopamine/polyethyleneimine co-deposition for simultaneous LDL selectivity and biocompatibility. Appl. Surf. Sci. 2016, 385, 308–317. [Google Scholar] [CrossRef]
- Chao, Z.; Li, J.; Jiang, W.; Zhang, C.; Ji, J.; Hua, X.; Xu, L.; Han, L.; Jia, L. Hemocompatible MOF-decorated pollen hemoperfusion absorbents for rapid and highly efficient removal of protein-bound uremic toxins. Mater. Chem. Front. 2021, 5, 7617–7627. [Google Scholar] [CrossRef]
- Chang, M. High-Performance Spherical Activated Carbon, Preparation Method Therefor and Use Thereof. U.S. Patent US201816652858, 30 March 2018. [Google Scholar]
- Zabczyk, M.; Natorska, J.; Malinowski, K.P.; Undas, A. Effect of enoxaparin on plasma fibrin clot properties and fibrin structure in patients with acute pulmonary embolism. Vasc. Pharmacol. 2020, 133–134, 106783. [Google Scholar] [CrossRef]
- Meher, M.K.; Poluri, K.M. Bifunctional Dalteparin/Enoxaparin coated nanosilver formulation to prevent bloodstream infections during hemodialysis. Carbohydr. Polym. 2022, 291, 119546. [Google Scholar] [CrossRef]
- Egan, K.; van Geffen, J.P.; Ma, H.; Kevane, B.; Lennon, A.; Allen, S.; Neary, E.; Parsons, M.; Maguire, P.; Wynne, K.; et al. Effect of platelet-derived beta-thromboglobulins on coagulation. Thromb. Res. 2017, 154, 7–15. [Google Scholar] [CrossRef]
- Gerling, K.; Herrmann, L.M.; Salewski, C.; Wolf, M.; Mullerbader, P.; Siegel-Axel, D.; Wendel, H.P.; Schlensak, C.; Avci-Adali, M.; Stoppelkamp, S. Synthetic Material Abdominal Swabs Reduce Activation of Platelets and Leukocytes Compared to Cotton Materials. Biomolecules 2021, 11, 1023. [Google Scholar] [CrossRef] [PubMed]
- Ganazzoli, F.; Raffaini, G. Classical atomistic simulations of protein adsorption on carbon nanomaterials. Curr. Opin. Colloid. Interface Sci. 2019, 41, 11–26. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).