CoNi2O4 Coated on Activated Carbon Wheat Husk (ACWH) as a Novel Nano-Electrocatalyst for Methanol and Ethanol Electro-Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Carbon Biomass
2.2. Synthesis of CoNi2O4 and CoNi2O4/ACWH
2.3. Preparation of Electrode
3. Results
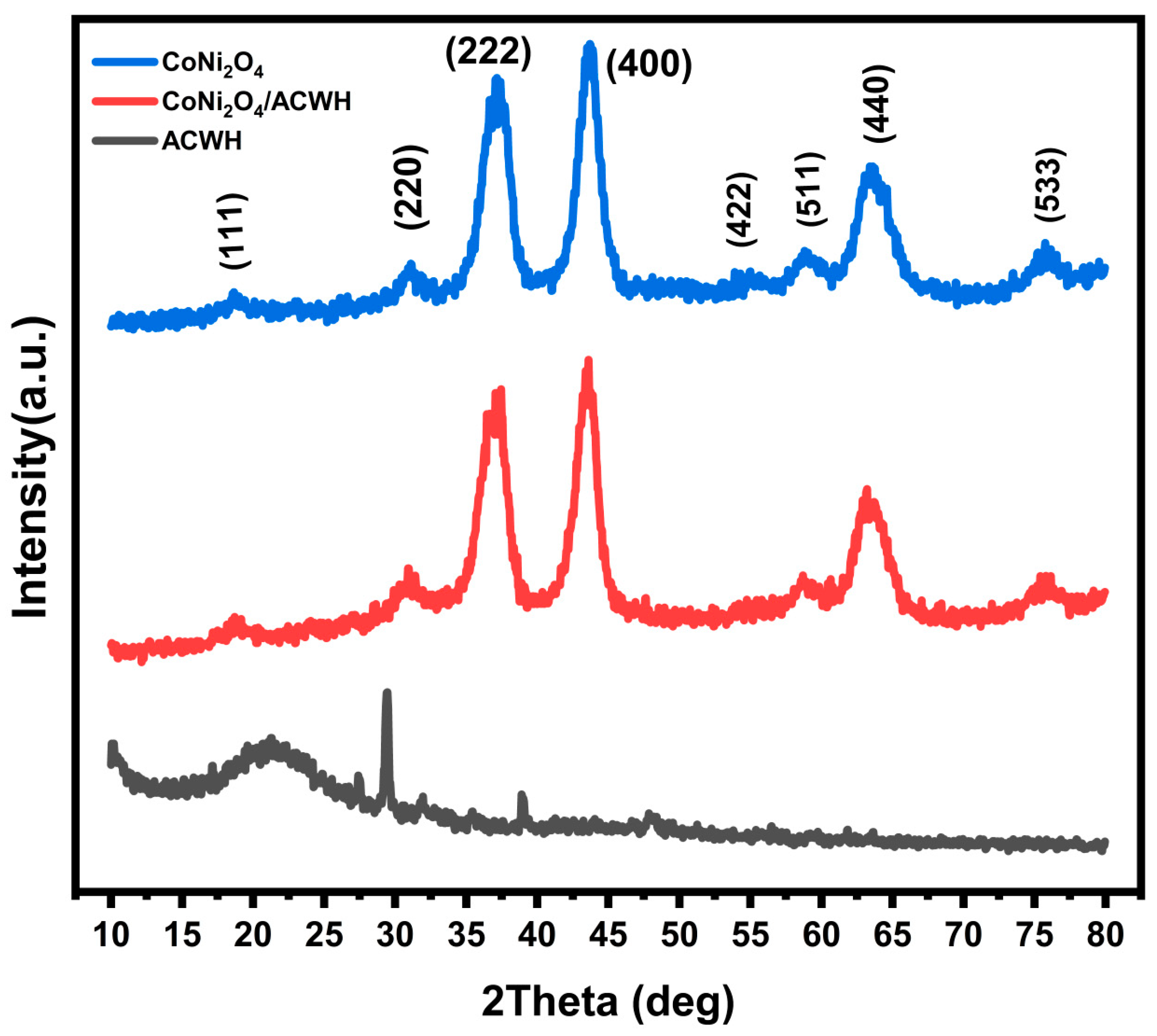
3.1. X-ray Diffraction (XRD)
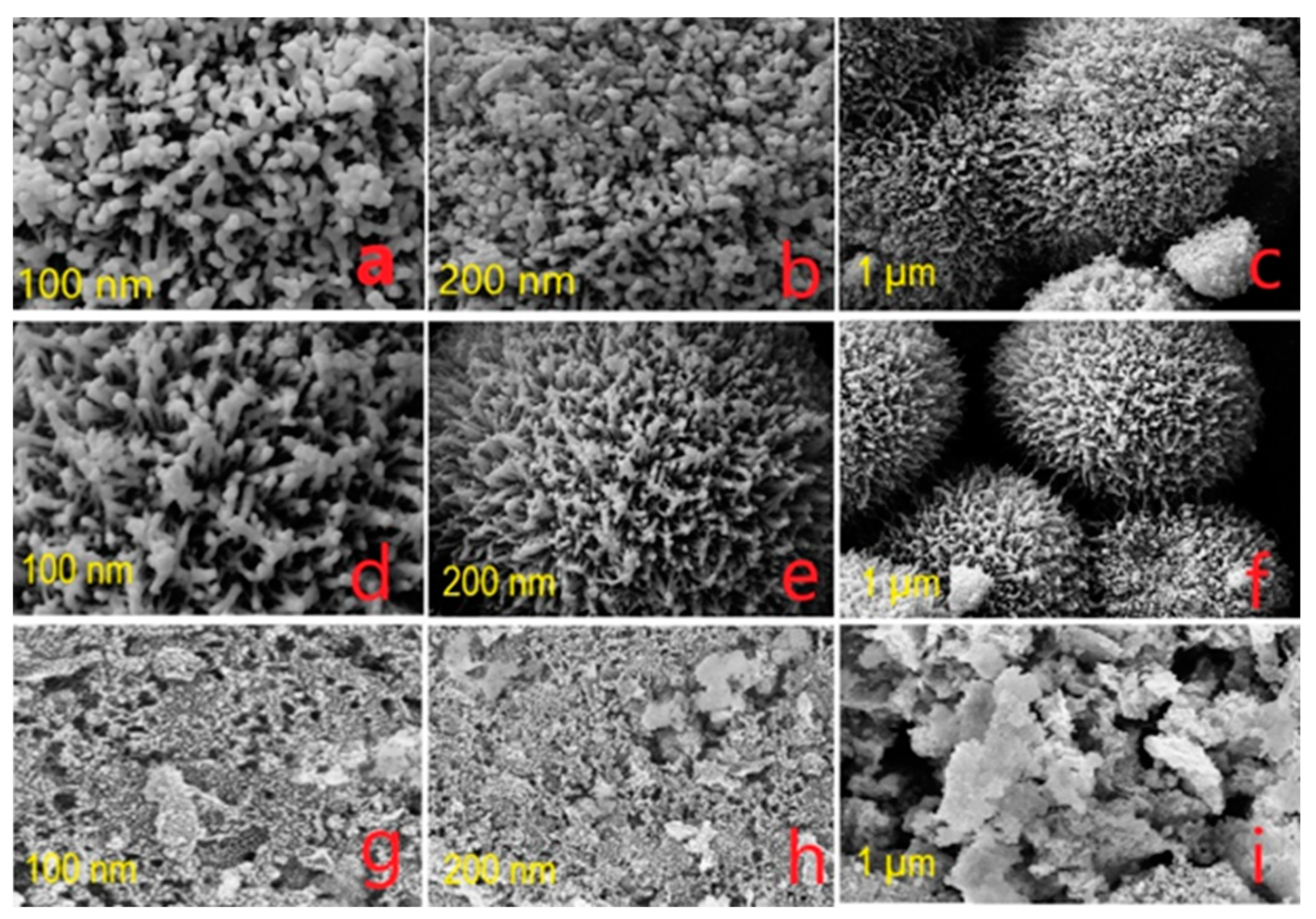
3.2. FE-SEM
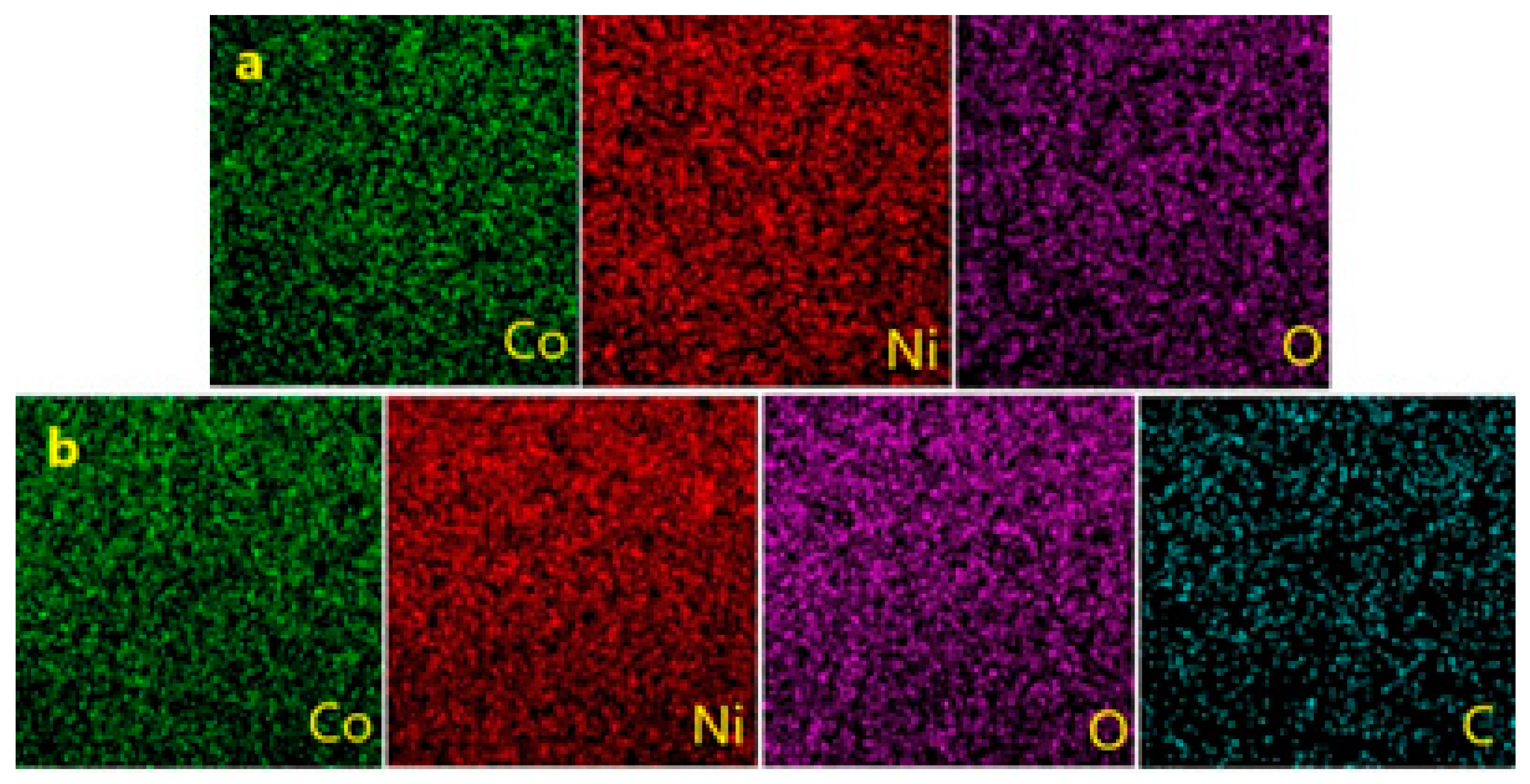
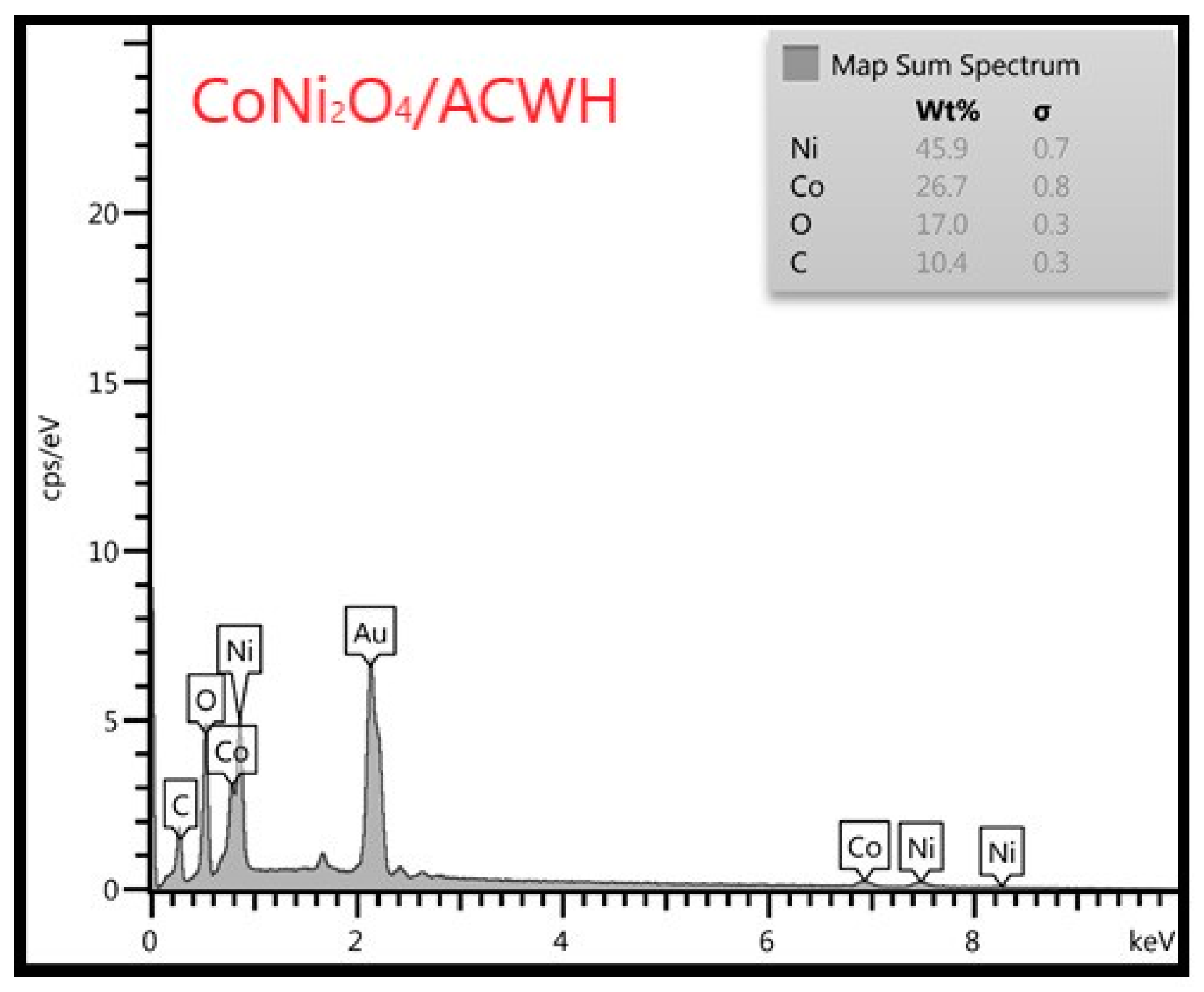
3.3. EDX-Mapping
3.4. Raman Spectroscopy
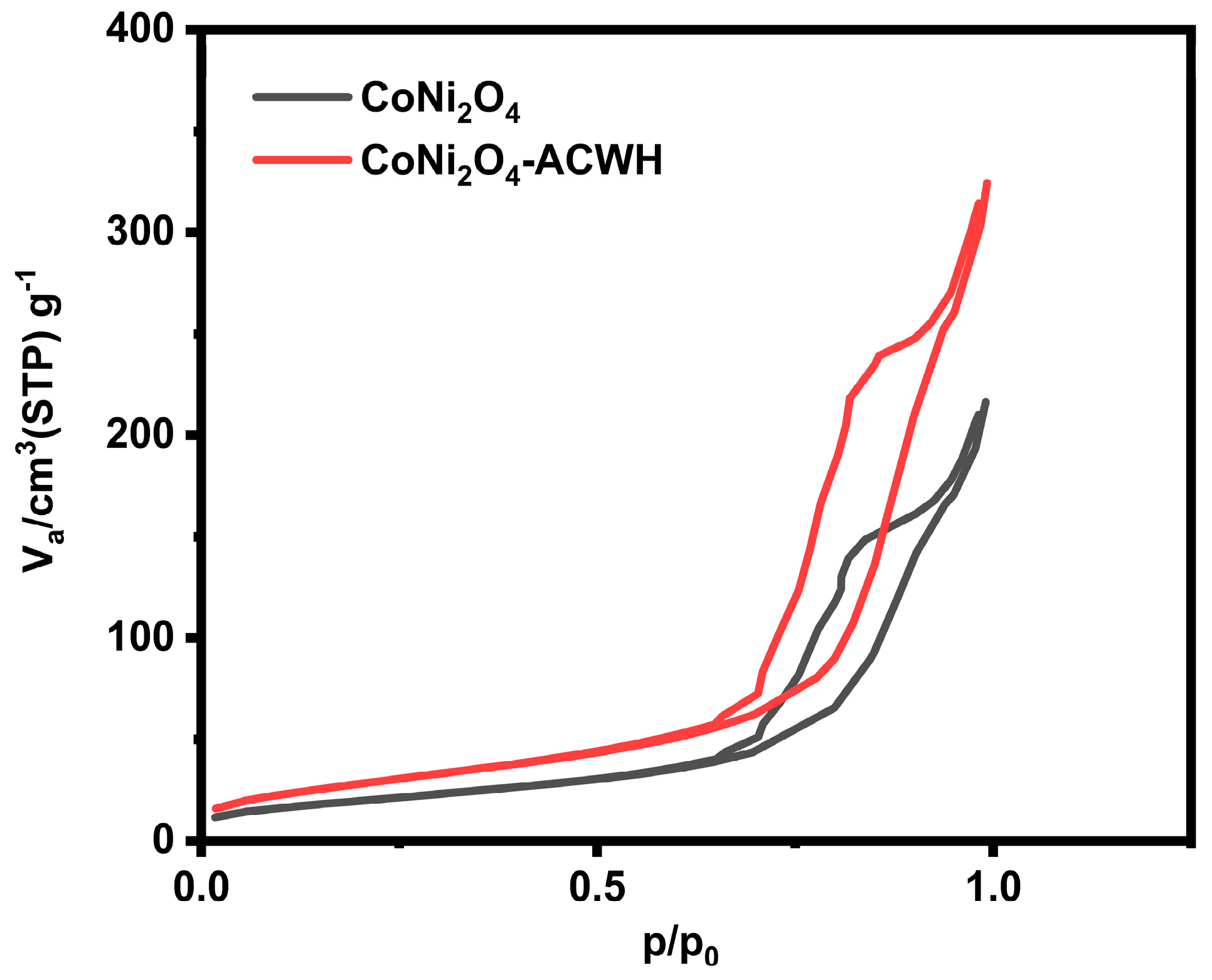
3.5. BET Method
3.6. Electrochemical Studies
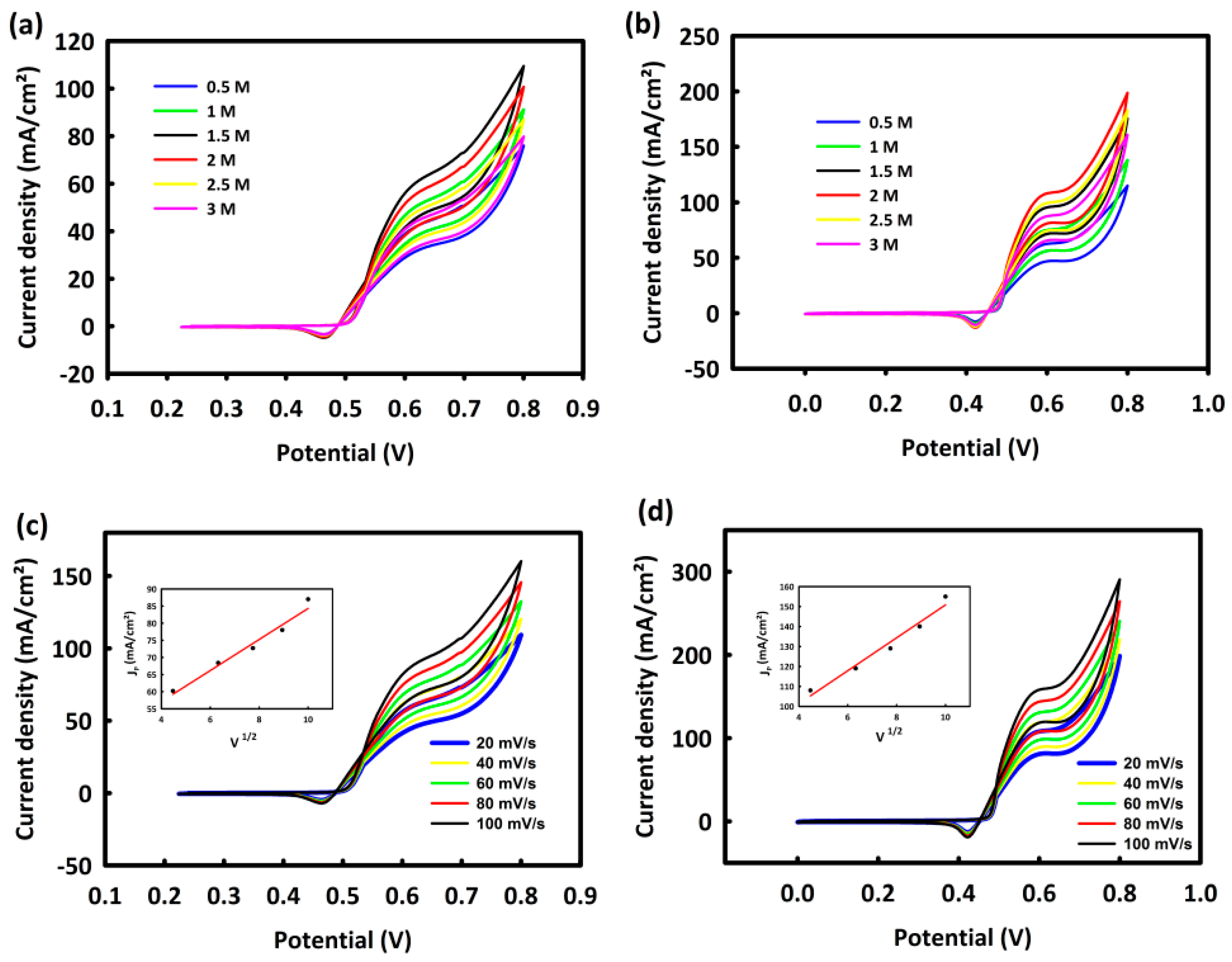
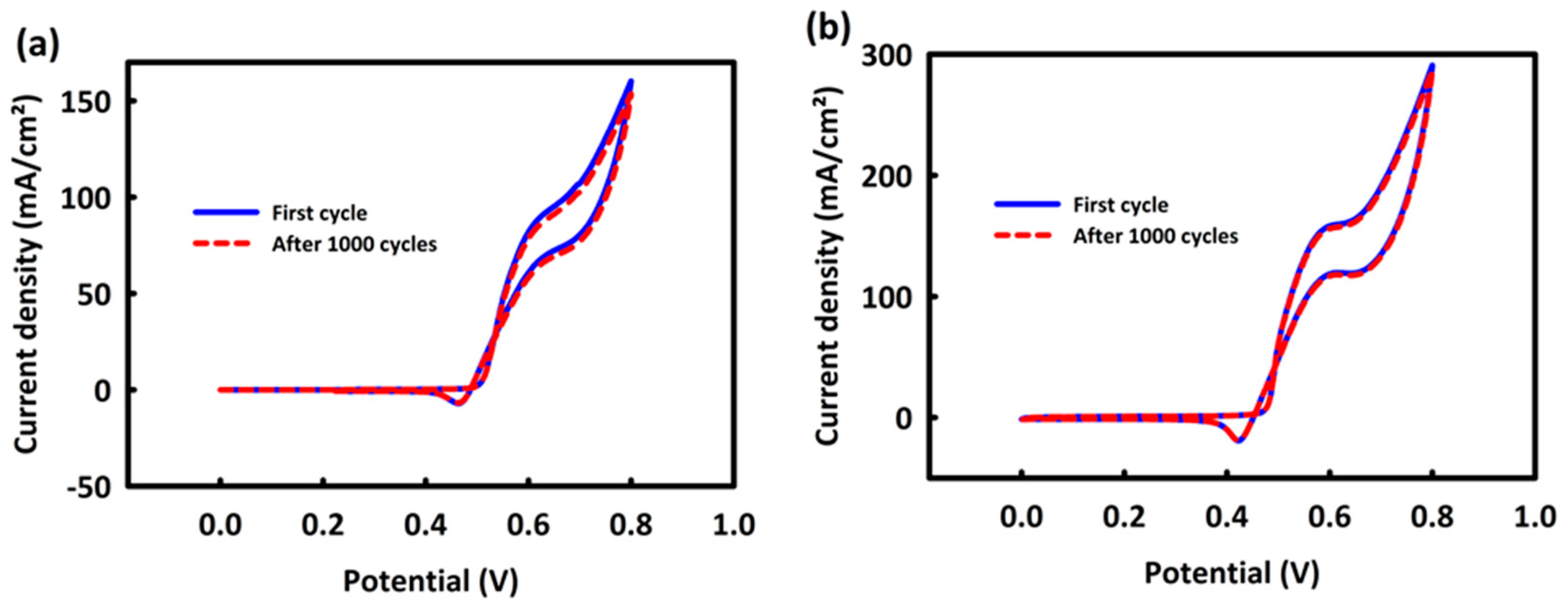
3.6.1. Investigation of CoNi2O4 and CoNi2O4/ACWH Nanocatalysts’ Abilities in Methanol Oxidation Process
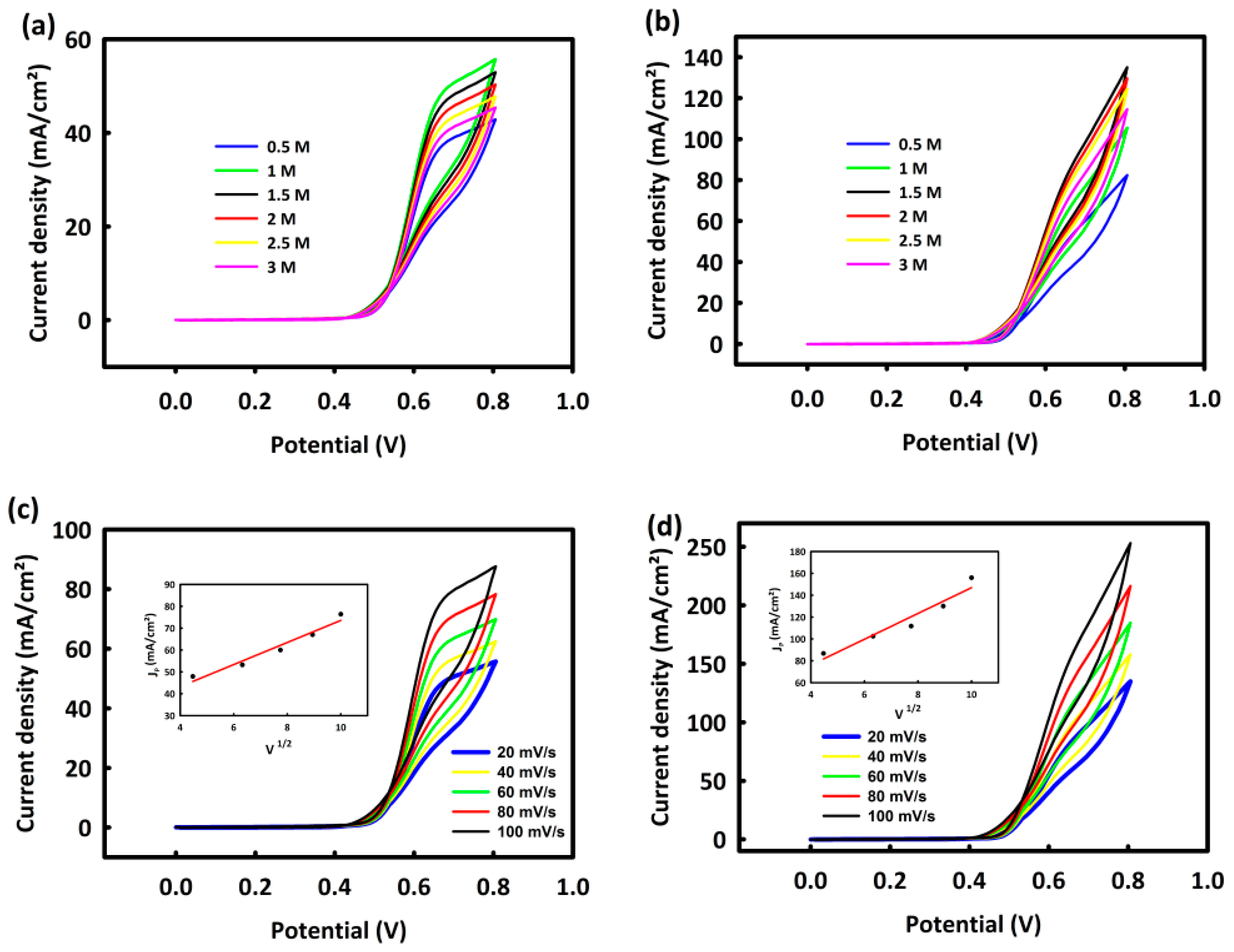
3.6.2. Investigation of CoNi2O4 and CoNi2O4/ACWH Nanocatalyst Capabilities in Ethanol Oxidation Process for Use in Ethanol Fuel Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bahrampour, H.; Marnani, A.K.B.; Askari, M.B.; Bahrampour, M.R. Evaluation of renewable energies production potential in the Middle East: Confronting the world’s energy crisis. Front. Energy 2017, 14, 42–56. [Google Scholar] [CrossRef]
- Singh, S. Energy Crisis and Climate Change: Global Concerns and Their Solutions, Energy: Crises, Challenges and Solutions; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 1–17. [Google Scholar]
- Shen, M.; Huang, W.; Chen, M.; Song, B.; Zeng, G.; Zhang, Y. (Micro)plastic crisis: Un-ignorable contribution to global greenhouse gas emissions and climate change. J. Clean. Prod. 2020, 254, 120138. [Google Scholar] [CrossRef]
- Shojaeifar, M.; Askari, M.B.; Hashemi, S.R.S.; Di Bartolomeo, A. MnO2–NiO–MWCNTs nanocomposite as a catalyst for methanol and ethanol electrooxidation. J. Phys. D Appl. Phys. 2022, 55, 355502. [Google Scholar] [CrossRef]
- Bhuiyan, M.R.A.; Mamur, H.; Begum, J. A brief review on renewable and sustainable energy resources in Bangladesh. Clean. Eng. Technol. 2021, 4, 100208. [Google Scholar] [CrossRef]
- Shang, Y.; Razzaq, A.; Chupradit, S.; An, N.B.; Abdul-Samad, Z. The role of renewable energy consumption and health expenditures in improving load capacity factor in ASEAN countries: Exploring new paradigm using advance panel models. Renew. Energy 2022, 191, 715–722. [Google Scholar] [CrossRef]
- Aslanturk, O.; Kıprızlı, G. The role of renewable energy in ensuring energy security of supply and reducing energy-related import. Int. J. Energy Econ. Policy 2020, 10, 354–359. [Google Scholar] [CrossRef]
- Hosseini, S.; Askari, M.B.; Beitollahi, H. MnNi2O4-MWCNTs as a nano-electrocatalyst for methanol oxidation reaction. Int. J. Hydrogen Energy 2022, 48, 21240–21248. [Google Scholar] [CrossRef]
- Azarpour, A.; Mohammadzadeh, O.; Rezaei, N.; Zendehboudi, S. Current status and future prospects of renewable and sustainable energy in North America: Progress and challenges. Energy Convers. Manag. 2022, 269, 115945. [Google Scholar] [CrossRef]
- Askari, M.B.; Beitollahi, H.; Di Bartolomeo, A. Methanol and Ethanol Electrooxidation on ZrO2/NiO/rGO. Nanomaterials 2023, 13, 679. [Google Scholar] [CrossRef]
- Askari, M.B.; Azizi, S.; Moghadam, M.T.T.; Seifi, M.; Rozati, S.M.; Di Bartolomeo, A. MnCo2O4/NiCo2O4/rGO as a Catalyst Based on Binary Transition Metal Oxide for the Methanol Oxidation Reaction. Nanomaterials 2022, 12, 4072. [Google Scholar] [CrossRef] [PubMed]
- Olabi, A.G.; Abbas, Q.; Al Makky, A.; Abdelkareem, M.A. Supercapacitors as next generation energy storage devices: Properties and applications. Energy 2022, 248, 123617. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Aziz, U. Supercapattery: Merging of battery-supercapacitor electrodes for hybrid energy storage devices. J. Energy Storage 2022, 46, 103823. [Google Scholar] [CrossRef]
- Kaur, A.; Kaur, G.; Singh, P.P.; Kaushal, S. Supported bimetallic nanoparticles as anode catalysts for direct methanol fuel cells: A review. Int. J. Hydrogen Energy 2021, 46, 15820–15849. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Otor, C. A review of gas diffusion layer properties and water management in proton exchange membrane fuel cell system. Int. J. Energy Res. 2020, 45, 3780–3800. [Google Scholar] [CrossRef]
- Lasseter, R. Dynamic models for micro-turbines and fuel cells. In Proceedings of the 2001 Power Engineering Society Summer Meeting. Conference Proceedings (Cat. No. 01CH37262), IEEE, Vancouver, BC, Canada, 15–19 July 2001. [Google Scholar]
- Yang, B.C.; Koo, J.; Shin, J.W.; Go, D.; Shim, J.H.; An, J. Direct Alcohol-Fueled Low-Temperature Solid Oxide Fuel Cells: A Review. Energy Technol. 2018, 7, 5–19. [Google Scholar] [CrossRef]
- Salarizadeh, P.; Moghadam, M.T.T.; Askari, M.B. Comparison of methanol oxidation reaction process for NiCo2O4/X (X= rGO, MWCNTs, HCNs) nanocatalyst. Diam. Relat. Mater. 2023, 131, 109534. [Google Scholar] [CrossRef]
- Salarizadeh, P.; Askari, M.B.; Di Bartolomeo, A. MoS2/Ni3S2/Reduced graphene oxide nanostructure as an electrocatalyst for alcohol fuel cells. ACS Appl. Nano Mater. 2022, 5, 3361–3373. [Google Scholar] [CrossRef]
- Jinxi, W.; Aimin, W.; Ghasemi, A.K.; Lashkenari, M.S.; Pashai, E.; Karaman, C.; Niculina, D.E.; Karimi-Maleh, H. Tailoring of ZnFe2O4-ZrO2-based nanoarchitectures catalyst for supercapacitor electrode material and methanol oxidation reaction. Fuel 2023, 334, 26685. [Google Scholar] [CrossRef]
- Kim, S.; Ahn, C.; Karuppannan, M.; Sung, Y.; Kwon, O.J.; Cho, Y. Structural modification of electrode for anion exchange membrane fuel cell by controlling ionomer dispersion. Int. J. Energy Res. 2021, 46, 6471–6479. [Google Scholar] [CrossRef]
- Gong, C.; Zhao, S.; Tsen, W.-C.; Hu, F.; Zhong, F.; Zhang, B.; Liu, H.; Zheng, G.; Qin, C.; Wen, S. Hierarchical layered double hydroxide coated carbon nanotube modified quaternized chitosan/polyvinyl alcohol for alkaline direct methanol fuel cells. J. Power Sources 2019, 441, 227176. [Google Scholar] [CrossRef]
- Askari, M.B.; Rozati, S.M. Construction of Co3O4-Ni3S4-rGO ternary hybrid as an efficient nanoelectrocatalyst for methanol and ethanol oxidation in alkaline media. J. Alloys Compd. 2021, 900, 163408. [Google Scholar] [CrossRef]
- Zhao, G.; Fang, C.; Hu, J.; Zhang, D. Platinum-Based Electrocatalysts for Direct Alcohol Fuel Cells: Enhanced Performances toward Alcohol Oxidation Reactions. Chempluschem 2021, 86, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Yu, Y.; Wang, Q.; Li, J.; Rao, P.; Li, R.; Du, Y.; Jia, C.; Luo, J.; Deng, P.; et al. Recent advances in two-dimensional Pt based electrocatalysts for methanol oxidation reaction. Int. J. Hydrogen Energy 2021, 46, 31202–31215. [Google Scholar] [CrossRef]
- Yang, C.; Jiang, Q.; Huang, H.; He, H.; Yang, L.; Li, W. Polyelectrolyte-Induced Stereoassembly of Grain Boundary-Enriched Platinum Nanoworms on Ti3C2T x MXene Nanosheets for Efficient Methanol Oxidation. ACS Appl. Mater. Interfaces 2020, 12, 23822–23830. [Google Scholar] [CrossRef]
- Wei, S.; Zhan, W.; Ma, L.; Gan, M. NiMoO4 nanorods derives carbon layers wrapped NiCo alloy decorated with Mo2C platinum-based catalyst for efficient methanol electrooxidation. J. Alloys Compd. 2022, 927, 166963. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Ren, Y.; Chen, D.; Wei, J.; Wang, L.; Xie, A.; Luo, S. Electrochemical synthesis of Pt nanoparticles on ZrO2/MWCNTs hybrid with high electrocatalytic performance for methanol oxidation. J. Electroanal. Chem. 2021, 898, 115641. [Google Scholar] [CrossRef]
- Vulcu, A.; Radu, T.; Porav, A.; Berghian-Grosan, C. Low-platinum catalyst based on sulfur doped graphene for methanol oxidation in alkaline media. Mater. Today Energy 2020, 19, 100588. [Google Scholar] [CrossRef]
- Kianfar, S.; Golikand, A.N.; ZareNezhad, B. Bimetallic-metal oxide nanoparticles of Pt-M (M: W, Mo, and V) supported on reduced graphene oxide (rGO): Radiolytic synthesis and methanol oxidation electrocatalysis. J. Nanostructure Chem. 2020, 11, 287–299. [Google Scholar] [CrossRef]
- Askari, M.B.; Salarizadeh, P.; Beitollahi, H.; Tajik, S.; Eshghi, A.; Azizi, S. Electro-oxidation of hydrazine on NiFe2O4-rGO as a high-performance nano-electrocatalyst in alkaline media. Mater. Chem. Phys. 2021, 275, 125313. [Google Scholar] [CrossRef]
- Hernández, J.; Solla-Gullón, J.; Herrero, E.; Aldaz, A.; Feliu, J.M. Methanol oxidation on gold nanoparticles in alkaline media: Unusual electrocatalytic activity. Electrochim. Acta 2006, 52, 1662–1669. [Google Scholar] [CrossRef]
- Chrzanowski, W.; Wieckowski, A. Surface structure effects in platinum/ruthenium methanol oxidation electrocatalysis. Langmuir 1998, 14, 1967–1970. [Google Scholar] [CrossRef]
- Ali, A.; Shen, P.K. Recent advances in graphene-based platinum and palladium electrocatalysts for the methanol oxidation reaction. J. Mater. Chem. A 2019, 7, 22189–22217. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Kim, H.; Jung, W.; Zhou, W.; Shao, Z. Electrochemical water splitting: Bridging the gaps between fundamental research and industrial applications. Energy Environ. Mater. 2023, e12441. [Google Scholar] [CrossRef]
- Song, S.; Mu, L.; Jiang, Y.; Sun, J.; Zhang, Y.; Shi, G.; Sun, H. Turning Electrocatalytic Activity Sites for the Oxygen Evolution Reaction on Brownmillerite to Oxyhydroxide. ACS Appl. Mater. Interfaces 2022, 14, 47560–47567. [Google Scholar] [CrossRef]
- Sun, H.; Li, L.; Chen, Y.; Kim, H.; Xu, X.; Guan, D.; Hu, Z.; Zhang, L.; Shao, Z.; Jung, W. Boosting Ethanol Oxidation by NiOOH-CuO Nano-Heterostructure for Energy-Saving Hydrogen Production and Biomass Upgrading. Appl. Catalysis B Environ. 2023, 325, 122388. [Google Scholar] [CrossRef]
- Zhang, G.; Huang, C.; Qin, R.; Shao, Z.; An, D.; Zhang, W.; Wang, Y. Uniform Pd–Pt alloy nanoparticles supported on graphite nanoplatelets with high electrocatalytic activity towards methanol oxidation. J. Mater. Chem. A 2015, 3, 5204–5211. [Google Scholar] [CrossRef]
- Hu, Y.; Mei, T.; Li, J.; Wang, J.; Wang, X. Porous SnO2 hexagonal prism-attached Pd/rGO with enhanced electrocatalytic activity for methanol oxidation. RSC Adv. 2017, 7, 29909–29915. [Google Scholar] [CrossRef]
- Ye, J.; Cheng, B.; Yu, J.; Ho, W.; Wageh, S.; Al-Ghamdi, A.A. Hierarchical Co3O4-NiO hollow dodecahedron-supported Pt for room-temperature catalytic formaldehyde decomposition. Chem. Eng. J. 2021, 430, 132715. [Google Scholar] [CrossRef]
- Nie, Y.; Wang, Y.; Zheng, X.; Yang, T.; Wen, Q.; Fang, Y.; Cheng, X.; Li, R.; Li, L. Minutely surficial functionalization of Ce-O-Pt linkages on Pt/C for enhanced electrocatalytic methanol oxidation. Appl. Surf. Sci. 2022, 602, 154194. [Google Scholar] [CrossRef]
- Khalafallah, D.; Alothman, O.Y.; Fouad, H.; Khalil, K.A. Hierarchical Co3O4 decorated PPy nanocasting core-shell nanospheres as a high performance electrocatalysts for methanol oxidation. Int. J. Hydrogen Energy 2018, 43, 2742–2753. [Google Scholar] [CrossRef]
- Askari, M.B.; Salarizadeh, P.; Di Bartolomeo, A.; Zadeh, M.H.R.; Beitollahi, H.; Tajik, S. Hierarchical nanostructures of MgCo2O4 on reduced graphene oxide as a high-performance catalyst for methanol electro-oxidation. Ceram. Int. 2021, 47, 16079–16085. [Google Scholar] [CrossRef]
- Zaiman, N.F.H.N.; Shaari, N. Review on flower-like structure nickel based catalyst in fuel cell application. J. Ind. Eng. Chem. 2023, 119, 1–76. [Google Scholar] [CrossRef]
- Sheikhi, S.; Jalali, F. Hierarchical NiCo2O4/CuO-C nanocomposite derived from copper-based metal organic framework and Ni/Co hydroxides: Excellent electrocatalytic activity towards methanol oxidation. J. Alloys Compd. 2022, 907, 164510. [Google Scholar] [CrossRef]
- Subramanian, V.; Luo, C.; Stephan, A.M.; Nahm, K.S.; Thomas, S.; Wei, B. Supercapacitors from Activated Carbon Derived from Banana Fibers. J. Phys. Chem. C 2007, 111, 7527–7531. [Google Scholar] [CrossRef]
- Soltani, N.; Bahrami, A.; Pech-Canul, M.; González, L. Review on the physicochemical treatments of rice husk for production of advanced materials. Chem. Eng. J. 2015, 264, 899–935. [Google Scholar] [CrossRef]
- Shen, Z.; Feng, J. Preparation of thermally conductive polymer composites with good electromagnetic interference shielding efficiency based on natural wood-derived carbon scaffolds. ACS Sustain. Chem. Eng. 2019, 7, 6259–6266. [Google Scholar] [CrossRef]
- Askari, N.; Askari, M.B.; Di Bartolomeo, A. Electrochemical Alcohol Oxidation and Biological Properties of Mn3O4-Co3O4-rGO. J. Electrochem. Soc. 2022, 169, 106511. [Google Scholar] [CrossRef]
- Azizi, S.; Askari, M.B.; Moghadam, M.T.T.; Seifi, M.; Di Bartolomeo, A. Ni3S4/NiS/rGO as a promising electrocatalyst for methanol and ethanol electro-oxidation. Nano Futur. 2023, 7, 015002. [Google Scholar] [CrossRef]
- Das, A.K.; Jena, S.; Sahoo, S.; Kuchi, R.; Kim, D.; Aljohani, T.; Nayak, G.C.; Jeong, J.-R. Facile synthesis of NiCo2O4 nanorods for electrocatalytic oxidation of methanol. J. Saudi Chem. Soc. 2020, 24, 434–444. [Google Scholar] [CrossRef]
- El-Deeb, M.M.; El Rouby, W.M.; Abdelwahab, A.; Farghali, A.A. Effect of pore geometry on the electrocatalytic performance of nickel cobaltite/ carbon xerogel nanocomposite for methanol oxidation. Electrochim. Acta 2018, 259, 77–85. [Google Scholar] [CrossRef]
- Zhang, K.; Han, Y.; Qiu, J.; Ding, X.; Deng, Y.; Wu, Y.; Zhang, G.; Yan, L. Interface engineering of Ni/NiO heterostructures with abundant catalytic active sites for enhanced methanol oxidation electrocatalysis. J. Colloid Interface Sci. 2023, 630, 570–579. [Google Scholar] [CrossRef]
- Li, W.; Song, Z.; Deng, X.; Fu, X.-Z.; Luo, J.-L. Decoration of NiO hollow spheres composed of stacked nanosheets with CeO2 nanoparticles: Enhancement effect of CeO2 for electrocatalytic methanol oxidation. Electrochim. Acta 2020, 337, 135684. [Google Scholar] [CrossRef]
- Yang, B.; Yu, Y.; Qiao, J.; Yuan, L.; Shen, X.; Hu, X. Solution plasma method for the preparation of Cu-Ni/CuO-NiO with excellent methanol electrocatalytic oxidation performance. Appl. Surf. Sci. 2020, 513, 145808. [Google Scholar] [CrossRef]
- Luo, Q.; Peng, M.; Sun, X.; Asiri, A.M. Hierarchical nickel oxide nanosheet@nanowire arrays on nickel foam: An efficient 3D electrode for methanol electro-oxidation. Catal. Sci. Technol. 2015, 6, 1157–1161. [Google Scholar] [CrossRef]
- Baruah, B.; Kumar, A. PEDOT: PSS/MnO2/rGO ternary nanocomposite based anode catalyst for enhanced electrocatalytic activity of methanol oxidation for direct methanol fuel cell. Synth. Met. 2018, 245, 74–86. [Google Scholar] [CrossRef]



| Electrocatalyst | Electrolyte Composition | Peak Potential (V) | Current Density (mA cm−2) | Scan Rate (mV/s) | Reference |
|---|---|---|---|---|---|
| CoNi2O4/ACWH | 2 M methanol/0.5 M KOH | 0.58 | 160 | 60 | This work |
| CoNi2O4/ACWH | 1.5 M ethanol/0.5 M KOH | 0.63 | 150 | 60 | This work |
| ZrO2/NiO/rGO | 0.7 M methanol/0.5 M KOH | 0.52 | 26.6 | 20 | [10] |
| ZrO2/NiO/rGO | 0.5 M ethanol/0.5 M KOH | 0.52 | 17.3 | 20 | [10] |
| Mn3O4-CeO2-rGO | 0.8 M methanol/1 M KOH | 0.51 | 17.7 | 90 | [49] |
| Ni3S4–NiS-rGO | 0.7 M methanol/1 M KOH | 0.54 | 55 | 80 | [50] |
| Ni3S4–NiS-rGO | 0.5 M ethanol/1 M KOH | 0.59 | 11 | 60 | [50] |
| NiCo2O4 | 0.5 M methanol/1 M KOH | 0.7 | 129 | 10 | [51] |
| NiCo2O4/carbon xerogel | 0.5 M methanol/0.5 M KOH | 0.29 | 98 | 50 | [52] |
| 3D Ni/NiO/RG | 1 M methanol/1 M KOH | About 0.6 | 79.21 | 50 | [53] |
| NiO 2.5CeO2–NiO | 1 M methanol/1 M KOH | About 0.55 | 159.62 | 50 | [54] |
| Cu-Ni/CuO-NiO/GNs | 0.5 M methanol/1 M KOH | 0.7 | 150 | 50 | [55] |
| NiO NS@NW/NF | 0.5 M methanol/1 M KOH | 1.62 | 89 | 10 | [56] |
| PEDOT:PSS/MnO2/rGO | 0.5 M methanol/1 M NaOH | 0.32 | 56.38 | 50 | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamali, F.; Seifi, M.; Askari, M.B. CoNi2O4 Coated on Activated Carbon Wheat Husk (ACWH) as a Novel Nano-Electrocatalyst for Methanol and Ethanol Electro-Oxidation. Coatings 2023, 13, 1124. https://doi.org/10.3390/coatings13061124
Jamali F, Seifi M, Askari MB. CoNi2O4 Coated on Activated Carbon Wheat Husk (ACWH) as a Novel Nano-Electrocatalyst for Methanol and Ethanol Electro-Oxidation. Coatings. 2023; 13(6):1124. https://doi.org/10.3390/coatings13061124
Chicago/Turabian StyleJamali, Fatemeh, Majid Seifi, and Mohammad Bagher Askari. 2023. "CoNi2O4 Coated on Activated Carbon Wheat Husk (ACWH) as a Novel Nano-Electrocatalyst for Methanol and Ethanol Electro-Oxidation" Coatings 13, no. 6: 1124. https://doi.org/10.3390/coatings13061124
APA StyleJamali, F., Seifi, M., & Askari, M. B. (2023). CoNi2O4 Coated on Activated Carbon Wheat Husk (ACWH) as a Novel Nano-Electrocatalyst for Methanol and Ethanol Electro-Oxidation. Coatings, 13(6), 1124. https://doi.org/10.3390/coatings13061124





