Study of Electrodeposition and Properties of Composite Nickel Coatings Modified with Ti3C2TX MXene
Abstract
1. Introduction
2. Materials and Methods
- (a)
- Ti3C2Tx MXene synthesis
- (b) Electrodeposition of nickel–Ti3C2Tx composite coatings
- (c) Investigation of structure and properties
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| The list of acronyms: | |
| CEC | composite electrochemical coating |
| PDP | potentiodinamic plot |
| SEM | scanning electron microscopy |
| XRD | X-ray diffraction |
References
- Orinakova, R.; Turonova, A.; Kladekova, D.; Galova, M.; Smith, R.M. Recent developments in the electrodeposition of nickel and some nickel-based alloys. J. Appl. Electrochem. 2006, 36, 957–972. [Google Scholar] [CrossRef]
- Dordsheikh Torkamani, A.; Velashjerdi, M.; Abbas, A.; Bolourchi, M.; Maji, P. Electrodeposition of nickel matrix composite coatings via various boride particles: A review. J. Compos. Compd. 2021, 3, 106–113. [Google Scholar] [CrossRef]
- Tseluikin, V.N. Composite coatings modified with nanoparticles: Structure and properties. Nanotechnologies Russ. 2014, 9, 1–14. [Google Scholar] [CrossRef]
- Huang, P.C.; Hou, K.H.; Hong, J.J.; Lin, M.H.; Wang, G.L. Study of fabrication and wear properties of Ni–SiC composite coatings on A356 aluminum alloy. Wear 2021, 477, 203772. [Google Scholar] [CrossRef]
- Xiang, Y.; He, Y.; Tang, W.; Li, H.; Zhang, Y.; Song, R.; Liu, B.; He, Y.; Guo, X.; He, Z. Fabrication of robust Ni-based TiO2 composite@TTOS superhydrophobic coating for wear resistance and anti-corrosion. Colloids Surf. 2021, 629, 127394. [Google Scholar] [CrossRef]
- Iacovetta, D.; Tam, J.; Erb, U. Synthesis, structure, and properties of superhydrophobic nickel–PTFE nanocomposite coatings made by electrodeposition. Surf. Coat. Technol. 2015, 279, 134–141. [Google Scholar] [CrossRef]
- Tseluikin, V.N.; Chubenko, I.S.; Gun’kin, I.F.; Pankst’yanov, A.Y. Colloidal dispersion of fullerene C60 free of organic solvents. Russ. J. Appl. Chem. 2006, 79, 325–326. [Google Scholar] [CrossRef]
- Tseluikin, V.N.; Solov’ova, N.D.; Gun’kin, I.F. Electrodeposition of nickel-fullerene C60 composition coatings. Prot. Met. 2007, 43, 388–390. [Google Scholar] [CrossRef]
- Yang, P.; Wang, N.; Zhang, J.; Lei, Y.; Shu, B. Investigation of the microstructure and tribological properties of CNTs/Ni composites prepared by electrodeposition. Mater. Res. Express 2022, 9, 036404. [Google Scholar] [CrossRef]
- Tseluikin, V.N.; Koreshkova, A.A. Electrochemical deposition and properties of composite coatings consisting of zinc and carbon nanotubes. Russ. J. Appl. Chem. 2015, 88, 272–274. [Google Scholar] [CrossRef]
- Tseluikin, V.N.; Koreshkova, A.A. Pulsed electrodeposition of composite coatings based on zinc–nickel alloy. Prot. Met. Phys. Chem. Surf. 2018, 54, 453–456. [Google Scholar] [CrossRef]
- Hong, Q.; Wang, D.; Yin, S. The microstructure, wear and electrochemical properties of electrodeposited Ni-diamond composite coatings: Effect of diamond concentration. Mater. Today Commun. 2023, 34, 105476. [Google Scholar] [CrossRef]
- Yasin, G.; Arif, M.; Nizam, N.M.; Shakeel, M.; Khan, M.A.; Khan, W.Q.; Hassan, T.M.; Abbas, Z.; Farahbakhsh, I.; Zuo, Y. Effect of surfactant concentration in electrolyte on the fabrication and properties of nickel-graphene nanocomposite coating synthesized by electrochemical co-deposition. RSC Adv. 2018, 8, 20039–20047. [Google Scholar] [CrossRef]
- Jyotheender, K.S.; Srivastava, C. Ni-graphene oxide composite coatings: Optimum graphene oxide for enhanced corrosion resistance. Compos. Part B 2019, 175, 107145. [Google Scholar] [CrossRef]
- Lou, G.; Shen, L.; Qian, Y.; Chen, Y.; Bai, H.; Cheng, H.; Xu, J.; Yang, Y. Study on the antibacterial and anti-corrosion properties of Ni-GO/Ni-rGO composite coating on manganese steel. Surf. Coat. Technol. 2021, 424, 127681. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Xu, W.; Shen, X.; Gao, F.; Zhu, J.; Wan, X.; Lian, X.; Xu, J.; Tong, Y. Chemical Preparation of New Ti3C2MXene and the Performance and Mechanism of Memristor Based on MXene. Acta Phys. Chim. Sin. 2022, 38, 1907076. [Google Scholar]
- Huang, Y.; Wang, C.; Wang, R.; Zhang, Y.; Li, D.; Zhu, H.; Wang, G.; Zhang, X. Ethanol Solution Plasma Loads Carbon Dots onto 2D HNb3O8 for Enhanced Photocatalysis. ACS Appl. Mater. Interfaces 2023, 15, 1157–1166. [Google Scholar] [CrossRef]
- Cheng, S.; Xiong, Q.; Zhao, C.; Yang, X. Synergism of 1D CdS/2D Modified Ti3C2Tx MXene Heterojunctions for Boosted Photocatalytic Hydrogen Production. Chin. J. Struct. Chem. 2022, 41, 2208058–2208064. [Google Scholar]
- Chang, C.; Chen, W.; Chen, Y.; Chen, Y.; Chen, Y.; Feng, D.; Fan, C.; Fan, H.J.; Fan, Z.; Gong, C.; et al. Recent progress on Two-Dimensional Materials. Acta Phys. Chim. Sin. 2021, 37, 2108017. [Google Scholar] [CrossRef]
- Cao, X.; Hou, C.; Li, Y.; Li, K.; Zhang, Q.; Wang, H. MXenes-Based Functional Fibers and Their Applications in the Intelligent Wearable Field. Acta Phys. Chim. Sin. 2022, 38, 2204058. [Google Scholar] [CrossRef]
- Bu, F.; Zagho, M.M.; Ibrahim, Y.; Ma, B.; Elzatahry, A.; Zhao, D. Porous MXenes: Synthesis, structures, and applications. Nano Today 2020, 30, 100803. [Google Scholar] [CrossRef]
- Naguib, M.; Barsoum, M.W.; Gogotsi, Y. Ten Years of Progress in the Synthesis and Development of MXenes. Adv. Mater. 2021, 33, 2103393. [Google Scholar] [CrossRef] [PubMed]
- Salim, O.; Mahmoud, K.A.; Pant, K.K.; Joshi, R.K. Introduction to MXenes: Synthesis and characteristics. Mater. Today Chem. 2019, 14, 100191. [Google Scholar] [CrossRef]
- Ronchi, R.M.; Arantes, J.T.; Santos, S.F. Synthesis, structure, properties and applications of MXenes: Current status and perspectives. Ceram. Int. 2019, 45, 18167–18188. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Cao, Y.; Wang, G. Functional Mxene Materials: Progress of Their Applications. Chem. Asian J. 2018, 13, 2742. [Google Scholar] [CrossRef]
- Rosenkranz, A.; Righi, M.C.; Sumant, A.V.; Anasori, B.; Mochalin, V.N. Perspectives of 2D MXene Tribology. Adv. Mater. 2023, 35, 2207757. [Google Scholar] [CrossRef]
- Miao, X.; Li, Z.; Liu, S.; Wang, J.; Yang, S. MXenes in tribology: Current status and perspectives. Adv. Powder Mater. 2023, 2, 100092. [Google Scholar] [CrossRef]
- Bian, H.; Du, Y.; Ren, Y.; Wu, H.; Ma, Y.; Yang, B.; Tang, S.; Bin, D.; Lu, H.; Meng, X. One-step electrodeposition of polypyrrole/Ti3C2Tx MXene composite coating for 304SS bipolar plates in PEMFC. Surf. Coat. Technol. 2023, 462, 129460. [Google Scholar] [CrossRef]
- Du, Y.; Wang, D.; Si, P.; Wei, L.; Wang, Y.; Yu, B.; Zhang, X.; Ye, S. Electrodeposition of a Ni-P-Ti3C2Tx/MoS2 coating incorporating MoS2 intercalated Ti3C2Tx particles. Surf. Coat. Technol. 2018, 354, 119–125. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, S.; Weng, Y.; Li, J.; Han, P.; Ye, S.; Zhang, X. Preparation of Ni-P-Ti3C2Tx-Ce composite coating with enhanced wear resistance and electrochemical corrosion behavior on the surface of low manganese steel. Surf. Coat. Technol. 2022, 441, 128508. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, B.; Xu, B.; Yang, B. Ambient-stable polyethyleneimine functionalized Ti3C2Tx nanohybrid corrosion inhibitor for copper in alkaline electrolyte. Mater. Lett. 2023, 337, 133979. [Google Scholar] [CrossRef]
- Galvin, T.; Hyatt, N.C.; Rainforth, W.M.; Reaney, I.M.; Shepherd, D. Molten salt synthesis of MAX phases in the Ti-Al-C system. J. Eur. Ceram. Soc. 2018, 38, 4585–4589. [Google Scholar] [CrossRef]
- Yang, F.; Kang, H.; Guo, E.; Li, R.; Chen, Z.; Zeng, Y. The role of nickel in mechanical perfopmance and corrosion behaviour of nickel-aluminium bronze in 3.5 wt.% NaCl solution. Corros. Sci. 2018, 139, 333–345. [Google Scholar] [CrossRef]
- Rekha, M.Y.; Srivastava, C. Microstructural evolution and corrosion behavior of Zn-Ni-graphene oxide composite coatings. Met. Mater. Trans. A 2019, 50, 5896–5913. [Google Scholar] [CrossRef]
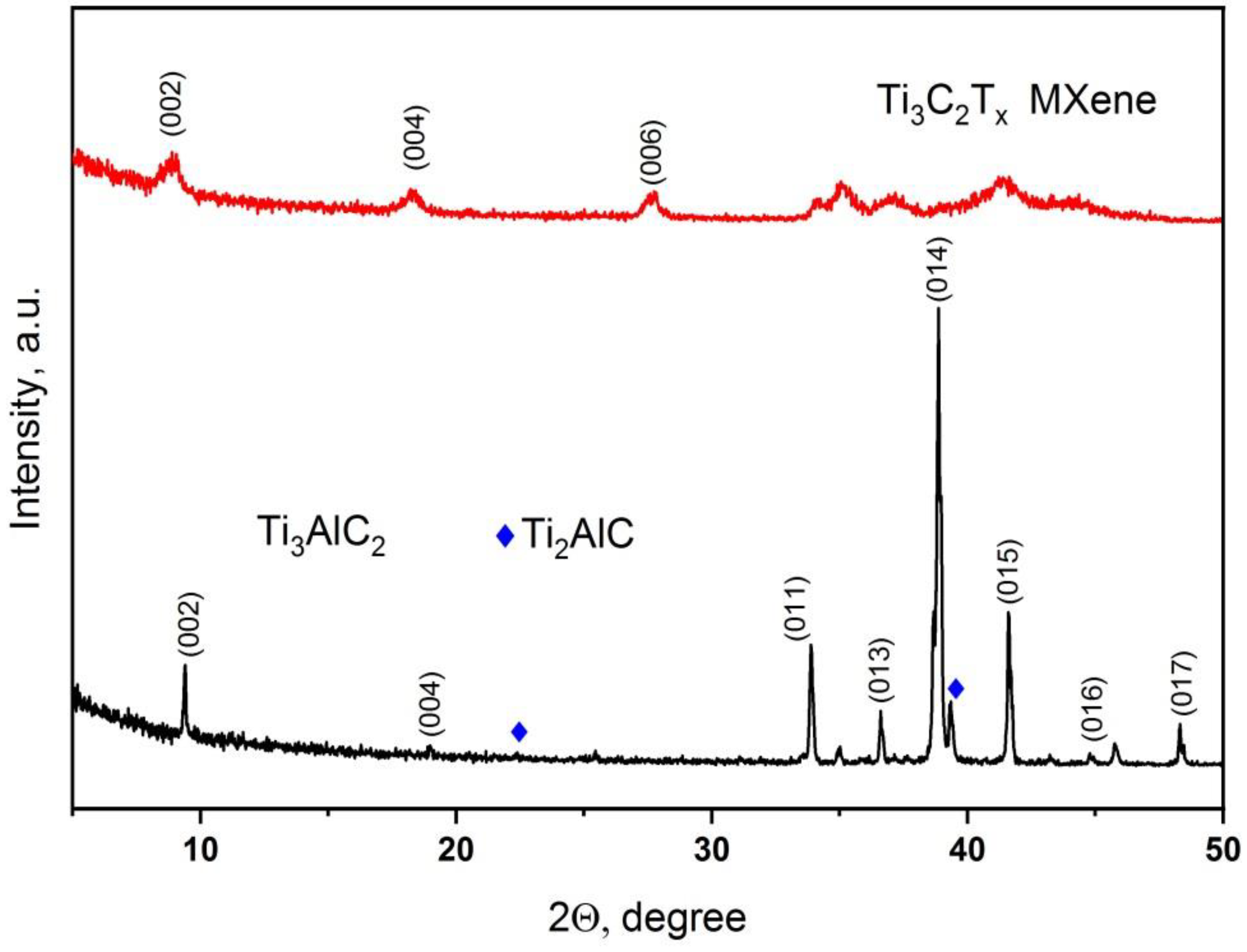
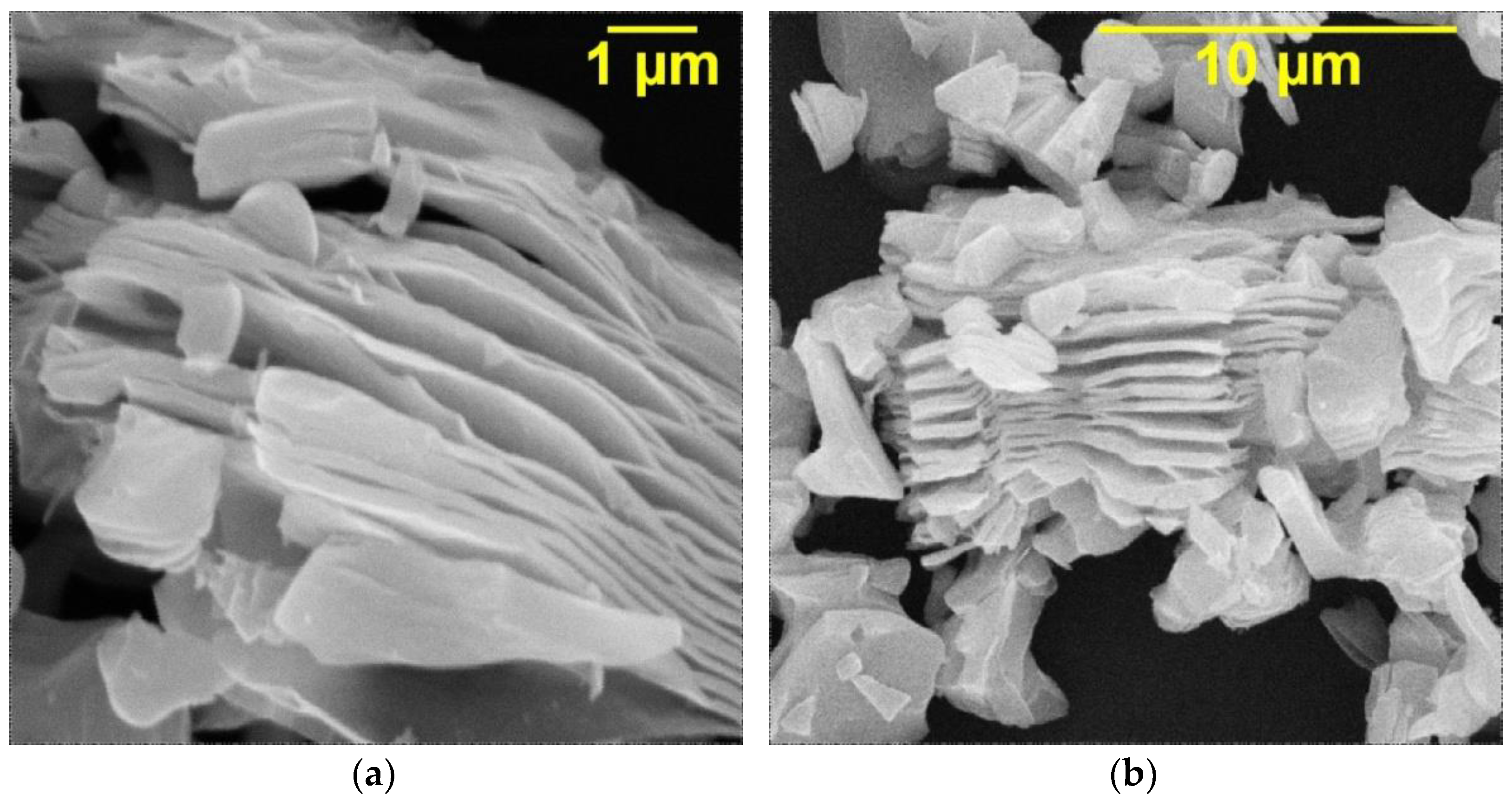
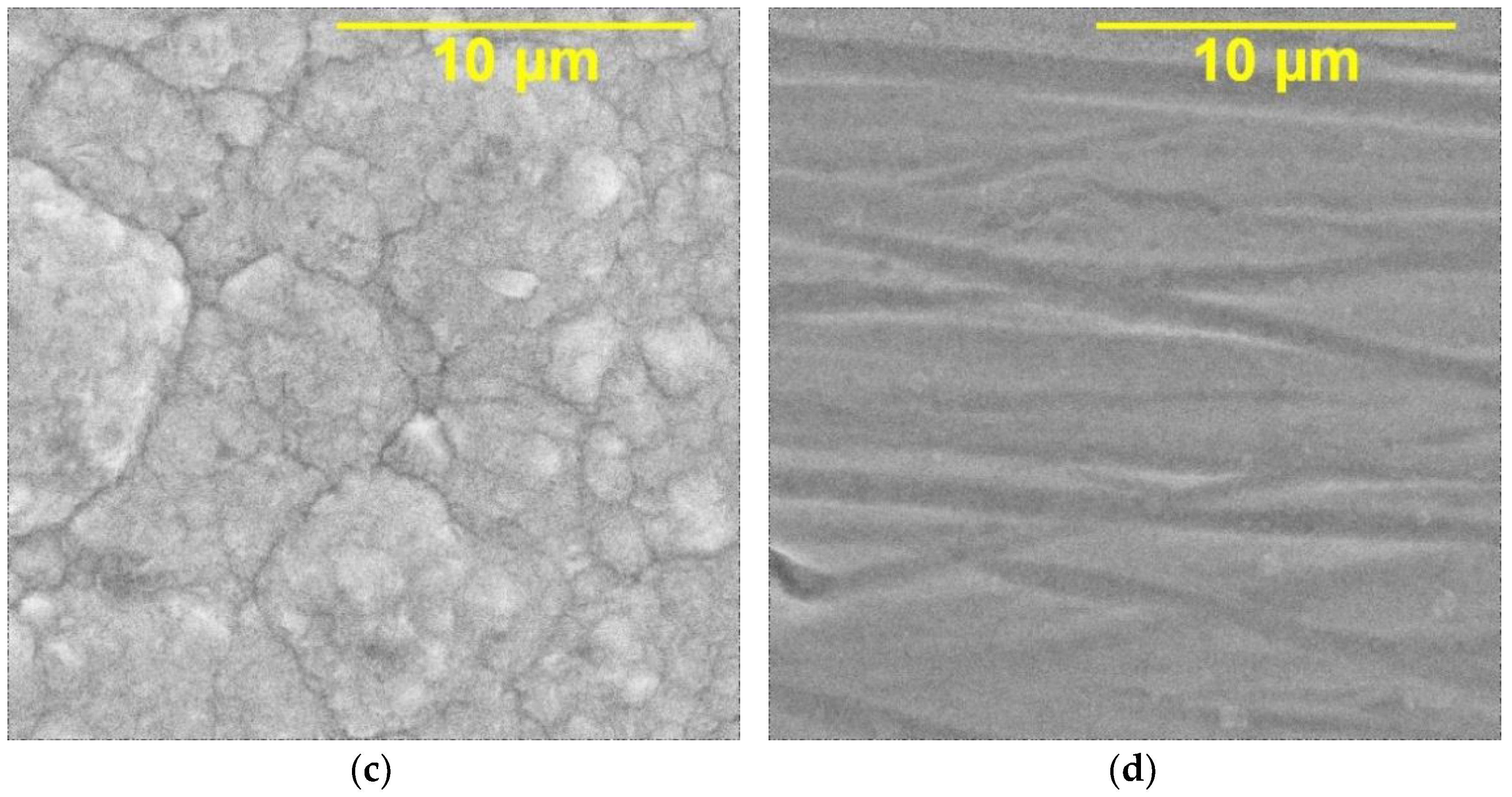
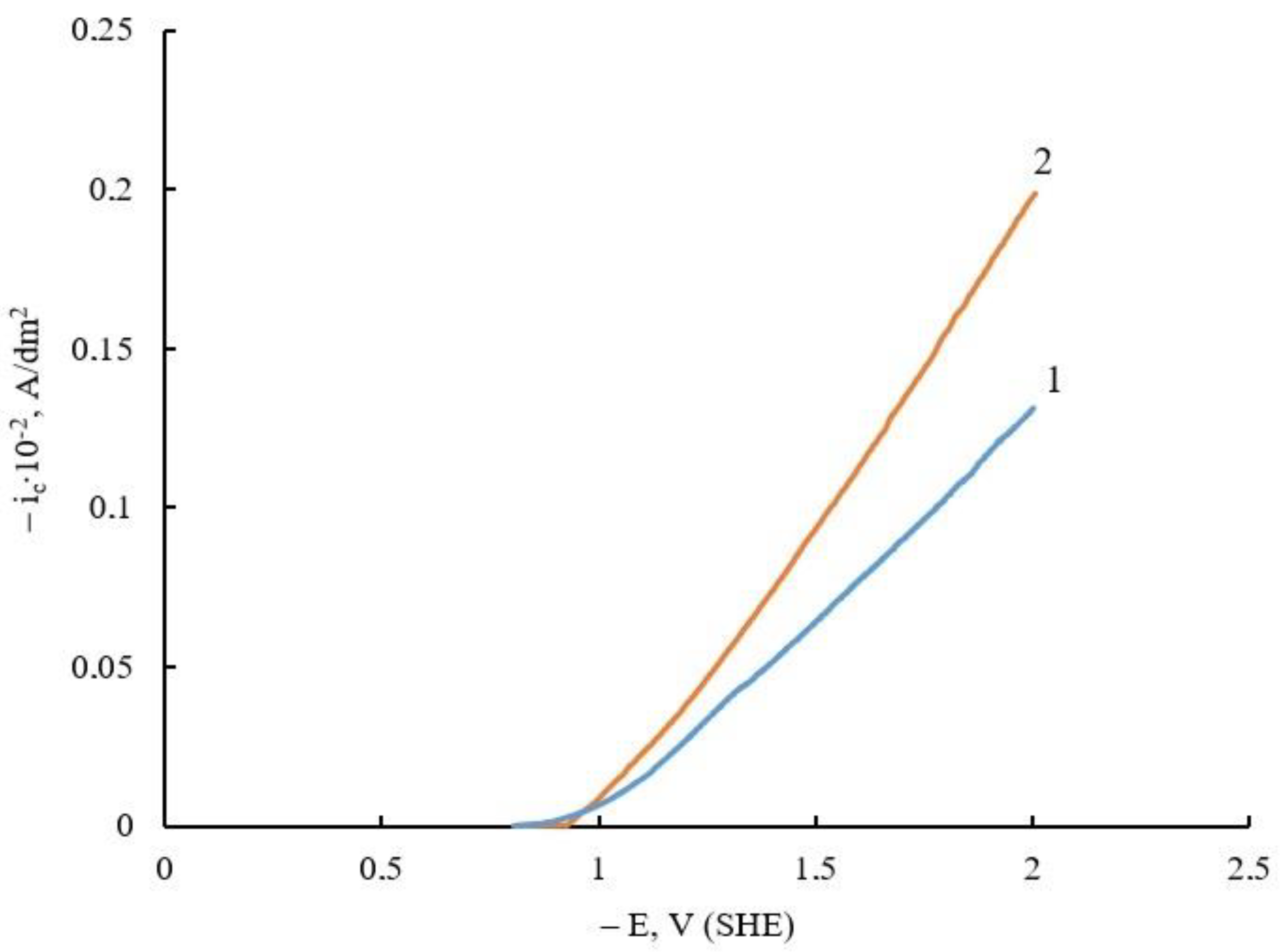
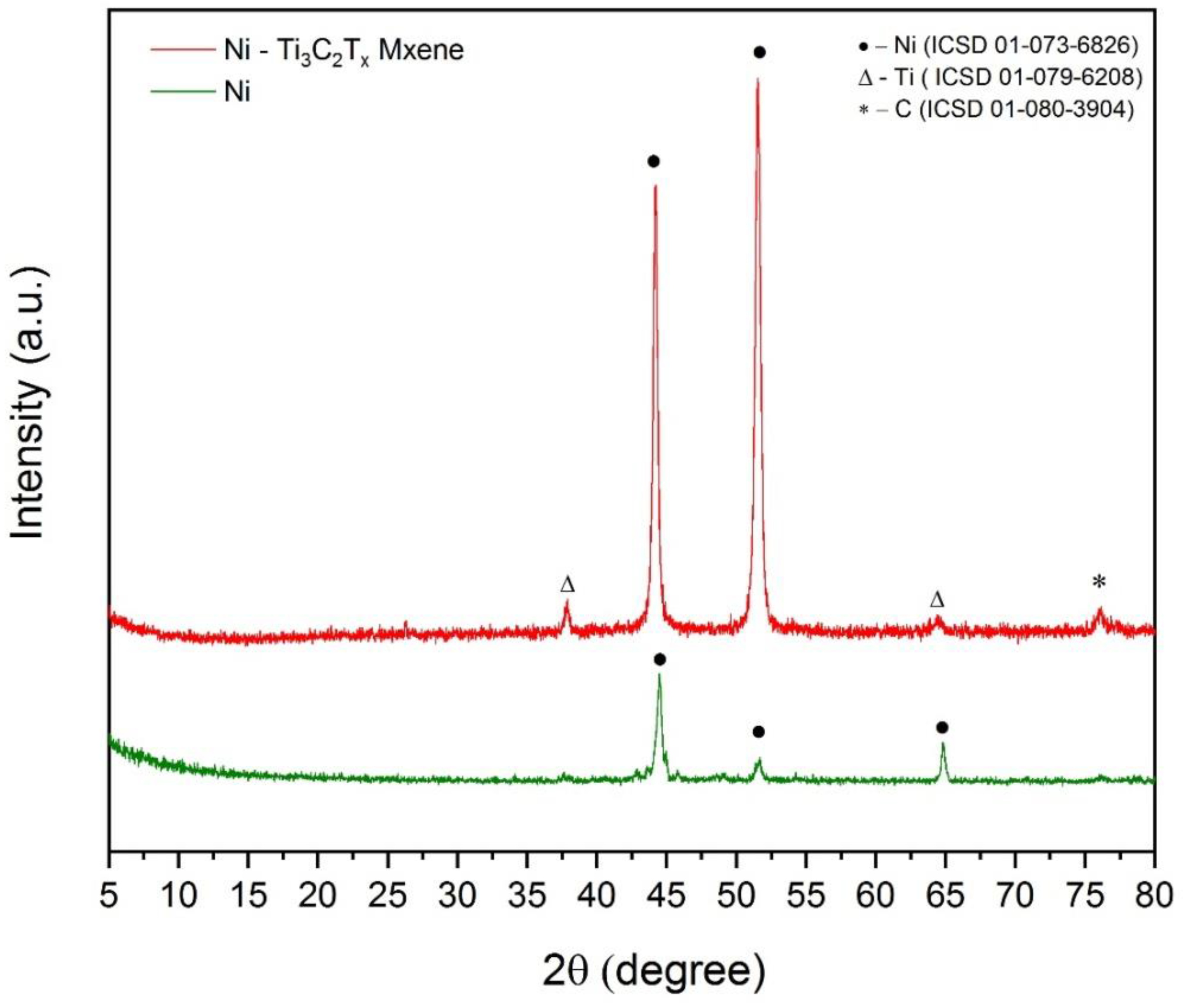
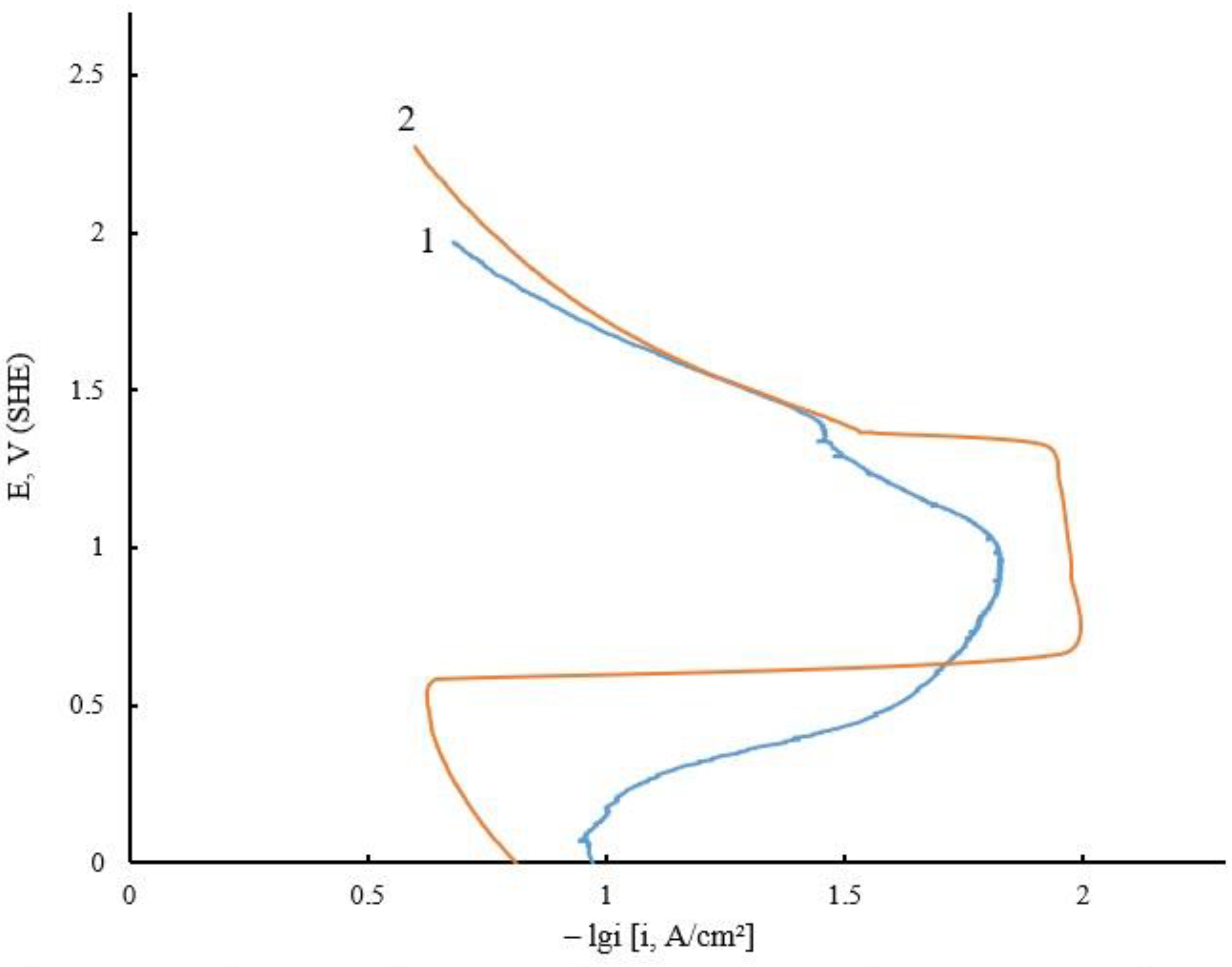
| Electrolyte Composition | Concentration, g/L | Deposition Parameters |
|---|---|---|
| NiSO4·7H2O | 220 | Temperature t = 45 °C, pH ≈ 4.5 |
| NiCl2·6H2O | 40 | Constant stirring |
| CH3COONa | 30 | Cathode current densities |
| Ti3C2Tx MXene | 1 | ic = 7, 8, 9, 10 A/dm2 |
| Cathode Current Density ic, A/dm2 | Ni | Ni–Ti3C2Tx |
|---|---|---|
| 7 | 1938 | 3563 |
| 8 | 2150 | 3894 |
| 9 | 2350 | 4189 |
| 10 | 2459 | 4565 |
| Cathode Current Density ic, A/dm2 | Ni | Ni–Ti3C2Tx |
|---|---|---|
| 7 | 0.656 | 0.410 |
| 8 | 0.574 | 0.328 |
| 9 | 0.451 | 0.287 |
| 10 | 0.328 | 0.205 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseluikin, V.; Dzhumieva, A.; Tribis, A.; Tikhonov, D.; Tsyganov, A.; Gorshkov, N.; Lopukhova, M. Study of Electrodeposition and Properties of Composite Nickel Coatings Modified with Ti3C2TX MXene. Coatings 2023, 13, 1042. https://doi.org/10.3390/coatings13061042
Tseluikin V, Dzhumieva A, Tribis A, Tikhonov D, Tsyganov A, Gorshkov N, Lopukhova M. Study of Electrodeposition and Properties of Composite Nickel Coatings Modified with Ti3C2TX MXene. Coatings. 2023; 13(6):1042. https://doi.org/10.3390/coatings13061042
Chicago/Turabian StyleTseluikin, Vitaly, Asel Dzhumieva, Alena Tribis, Denis Tikhonov, Alexey Tsyganov, Nikolay Gorshkov, and Marina Lopukhova. 2023. "Study of Electrodeposition and Properties of Composite Nickel Coatings Modified with Ti3C2TX MXene" Coatings 13, no. 6: 1042. https://doi.org/10.3390/coatings13061042
APA StyleTseluikin, V., Dzhumieva, A., Tribis, A., Tikhonov, D., Tsyganov, A., Gorshkov, N., & Lopukhova, M. (2023). Study of Electrodeposition and Properties of Composite Nickel Coatings Modified with Ti3C2TX MXene. Coatings, 13(6), 1042. https://doi.org/10.3390/coatings13061042









