An Investigation into Electrodeposited Co−Ni−TiO2 Films with Improved Mechanical and Corrosion Properties
Abstract
1. Introduction
2. Experimental
2.1. TiO2 Sol Preparation
2.2. Electrodeposition of Co−Ni−TiO2 Films
2.3. Sample Characterization
3. Results and Discussion
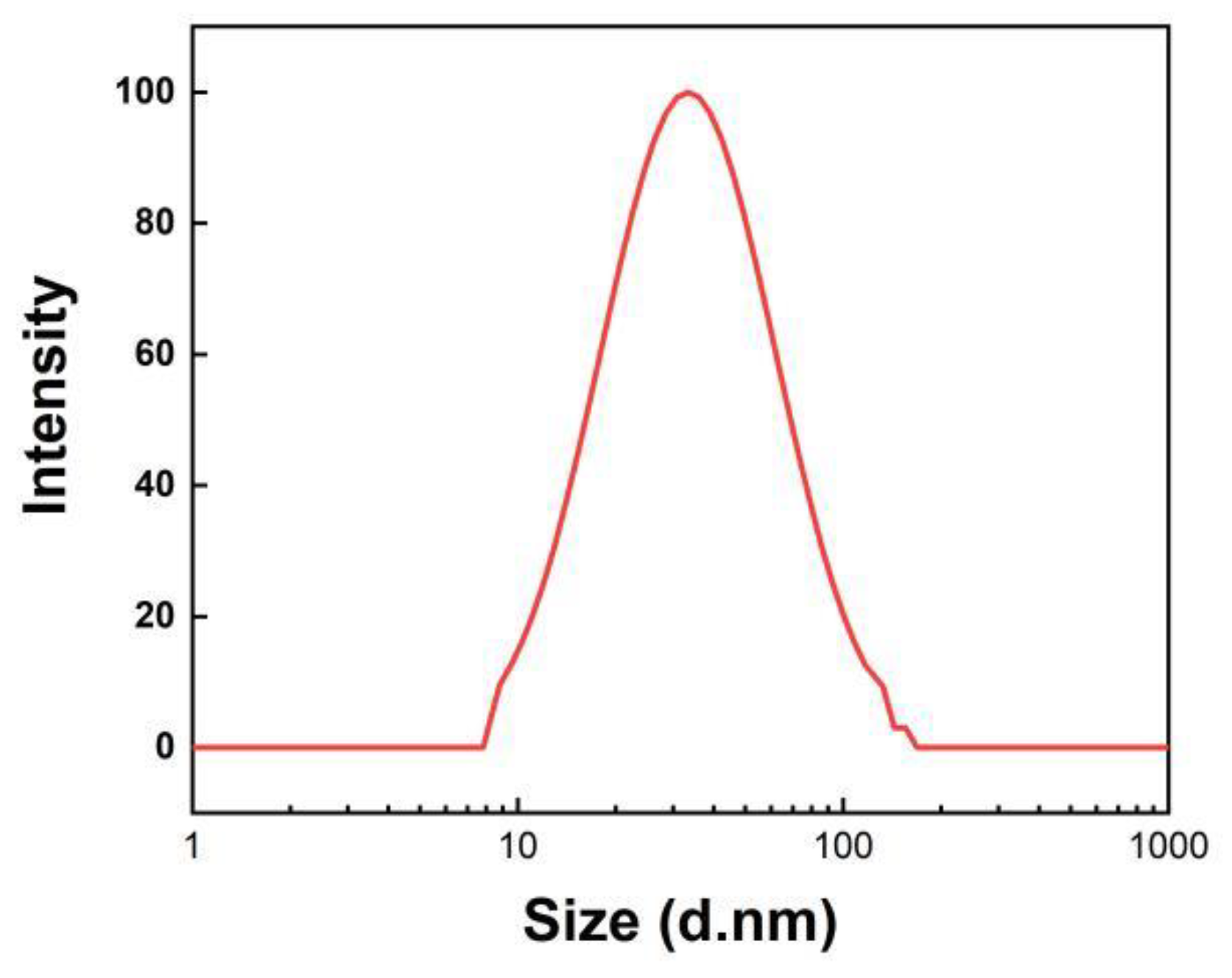
3.1. Characterization of TiO2 Sol
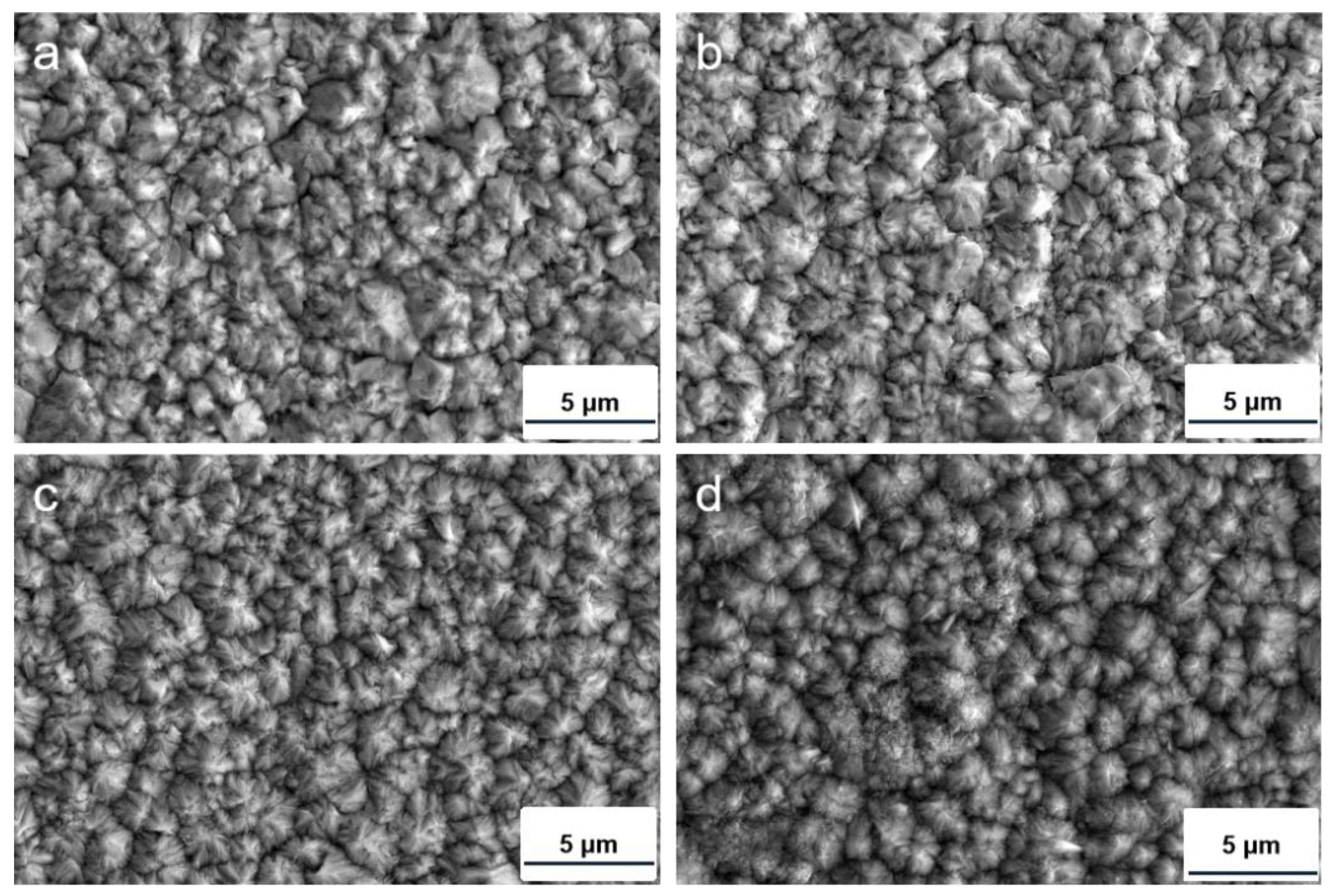
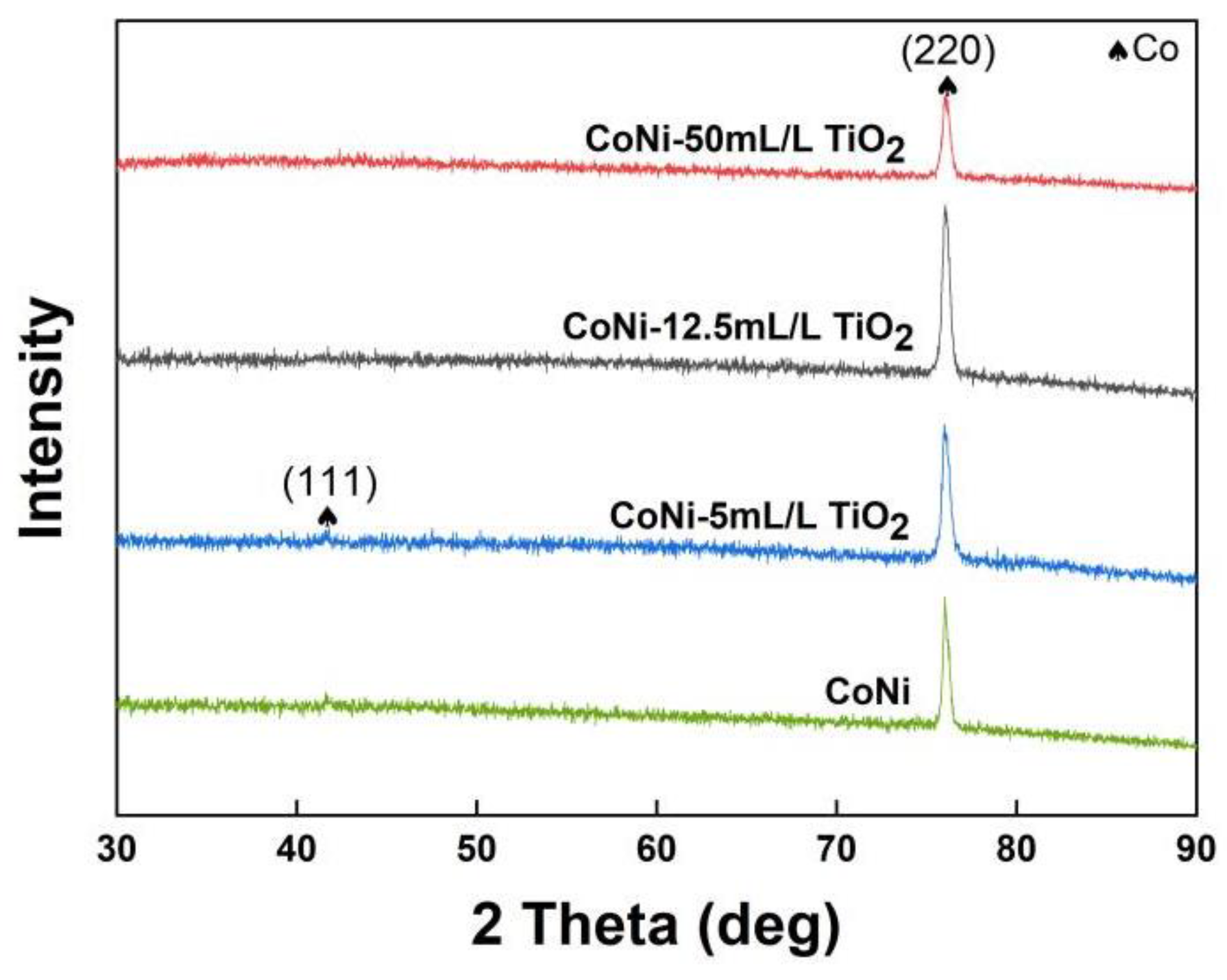
3.2. Structural Characterizations of Co−Ni−TiO2 Films
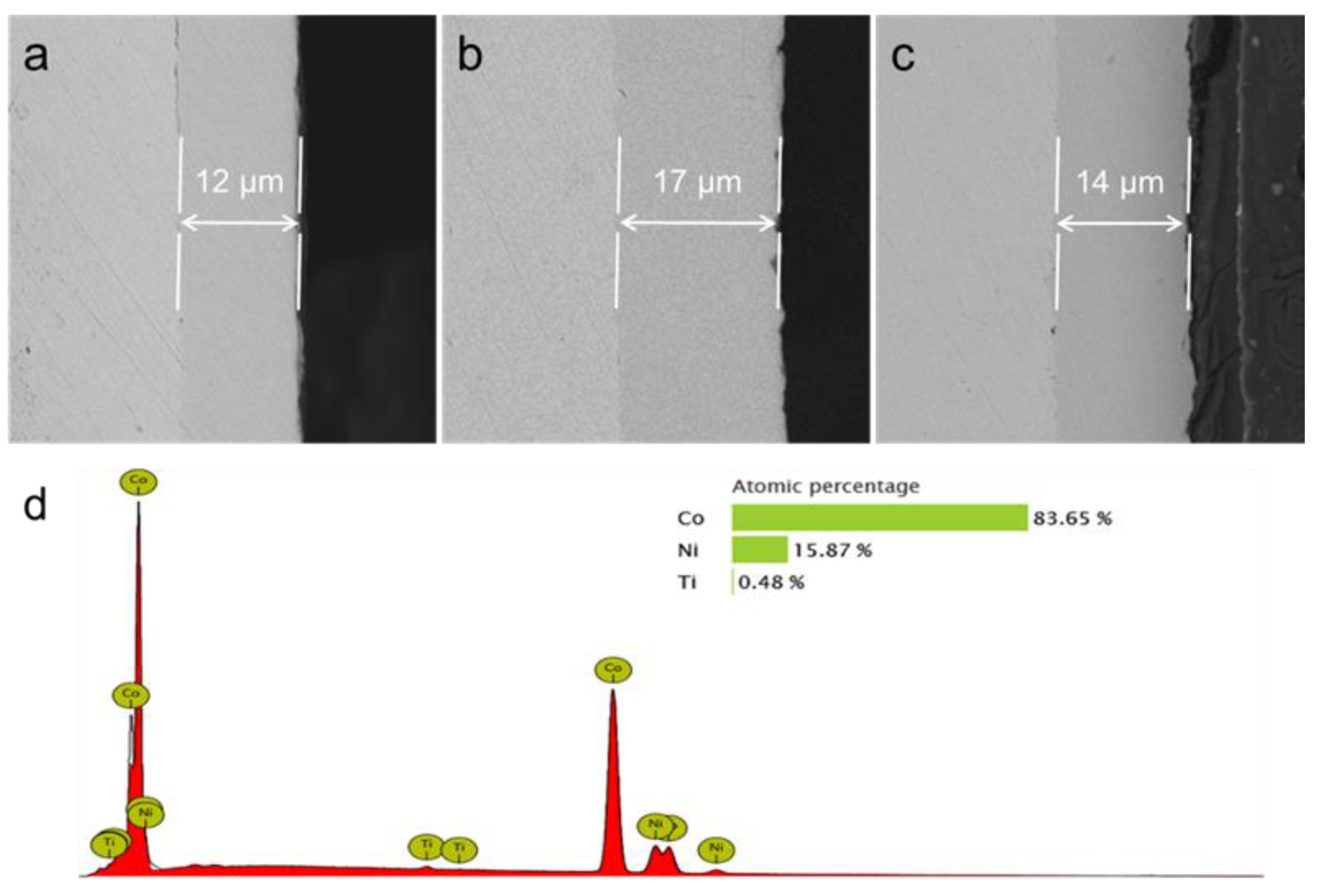
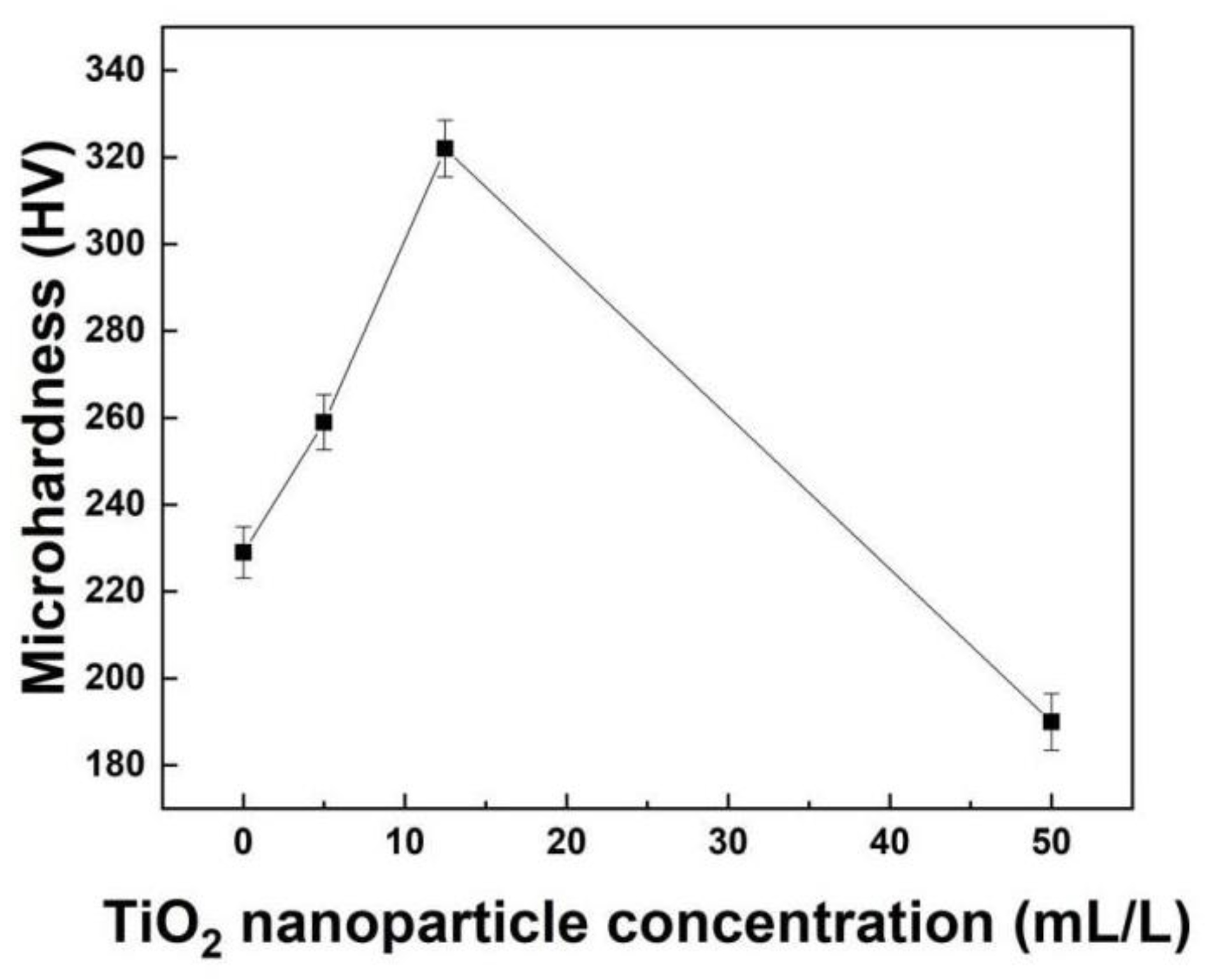
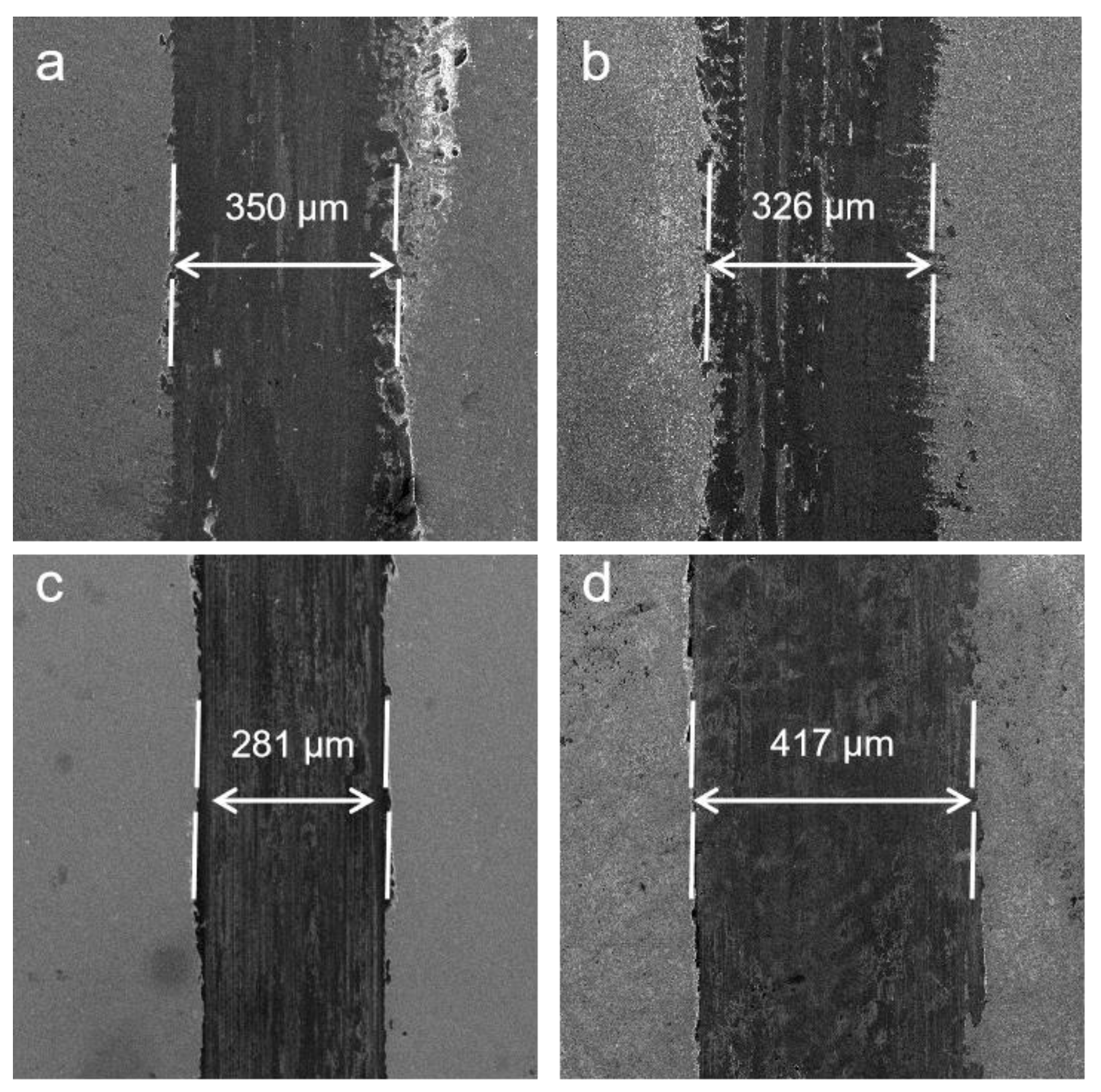
3.3. Mechanical Properties of Co−Ni−TiO2 Films
3.4. Corrosion Resistance of Co−Ni−TiO2 Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bouzit, F.Z.; Nemamcha, A.; Moumeni, H.; Rehspringer, J.L. Morphology and Rietveld analysis of nanostructured Co-Ni electrodeposited thin films obtained at different current densities. Surf. Coat. Technol. 2017, 315, 172–180. [Google Scholar] [CrossRef]
- Olvera, S.; Arce Estrada, E.M.; Sanchez-Marcos, J.; Palomares, F.J.; Vazquez, L.; Herrasti, P. Effect of the low magnetic field on the electrodeposition of CoxNi100−x alloys. Mater. Charact. 2015, 105, 136–143. [Google Scholar] [CrossRef]
- Li, D.; Levesque, A.; Franczak, A.; Wang, Q.; He, J.; Chopart, J.P. Evolution of morphology in electrodeposited nanocrystalline Co-Ni films by in-situ high magnetic field application. Talanta 2013, 110, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Karpuz, A.; Kockar, H.; Alper, M. Electrodeposited Co–Ni Films: Electrolyte pH—Property Relationships. J. Supercond. Nov. Magn. 2012, 26, 651–655. [Google Scholar] [CrossRef]
- Yu, M.M.; Li, H.Y.; Wang, Y. Study on Present Situation and New Trends of the Electrodeposition of Nickel-Cobalt Alloy. Adv. Mater. Res. 2012, 535–537, 973–976. [Google Scholar] [CrossRef]
- Sun, L.F.; Mao, J.M.; Pan, Z.W.; Chang, B.H.; Zhou, W.Y.; Wang, G.; Qian, L.X.; Xie, S.S. Growth of straight nanotubes with a cobalt–nickel catalyst by chemical vapor deposition. Appl. Phys. Lett. 1999, 74, 644–646. [Google Scholar] [CrossRef]
- Li, Y.; Xie, Y.; Huang, L.; Liu, X.; Zheng, X. Effect of physical vapor deposited Al2O3 film on TGO growth in YSZ/CoNiCrAlY coatings. Ceram. Int. 2012, 38, 5113–5121. [Google Scholar] [CrossRef]
- Cuglietta, M.; Kuhn, J.; Kesler, O. A Novel Hybrid Axial-Radial Atmospheric Plasma Spraying Technique for the Fabrication of Solid Oxide Fuel Cell Anodes Containing Cu, Co, Ni, and Samaria-Doped Ceria. J. Therm. Spray Technol. 2013, 22, 609–621. [Google Scholar] [CrossRef]
- Liu, C.; Su, F.; Liang, J. Nanocrystalline Co-Ni alloy coating produced with supercritical carbon dioxide assisted electrodeposition with excellent wear and corrosion resistance. Surf. Coat. Technol. 2016, 292, 37–43. [Google Scholar] [CrossRef]
- Hu, H.; Tan, M.; Liu, L. Anomalous codeposition mechanism of Co-Ni alloy nanowires. J. Alloys Compd. 2017, 715, 384–389. [Google Scholar] [CrossRef]
- Barzegar, M.; Allahkaram, S.R.; Naderi, R.; Ghavidel, N. Effect of phosphorous content and heat treatment on the structure, hardness and wear behavior of Co-P coatings. Wear 2019, 422–423, 35–43. [Google Scholar] [CrossRef]
- Tebbakh, S.; Messaoudi, Y.; Azizi, A.; Fenineche, N.; Schmerber, G.; Dinia, A. The influence of saccharin on the electrodeposition and properties of Co–Ni alloy thin films. Trans. IMF 2015, 93, 196–204. [Google Scholar] [CrossRef]
- Altamirano-Garcia, L.; Vazquez-Arenas, J.; Pritzker, M.; Luna-Sánchez, R.; Cabrera-Sierra, R. Effects of saccharin and anions (SO4 2−, Cl−) on the electrodeposition of Co–Ni alloys. J. Solid State Electrochem. 2014, 19, 423–433. [Google Scholar] [CrossRef]
- Vazquez-Arenas, J.; Treeratanaphitak, T.; Pritzker, M. Formation of Co–Ni alloy coatings under direct current, pulse current and pulse-reverse plating conditions. Electrochim. Acta 2012, 62, 63–72. [Google Scholar] [CrossRef]
- Wang, S. Influence of 2-butyne-1, 4-diol on the structure and performance of the Co-Ni alloy plated by electrodeposition. Acta Metall. Sin. (Engl. Lett.) 2008, 21, 50–56. [Google Scholar] [CrossRef]
- Bu, A.; Zhang, Y.; Zhang, Y.; Chen, W.; Cheng, H.; Wang, L.; Wang, Y. A Novel Electrolytic Plasma Spraying Preparation SiO2/SiC Coating on Carbon Fiber Fabric. Coatings 2018, 8, 344. [Google Scholar] [CrossRef]
- Xiong, C.; Xiao, J.; Zhao, Y.; Wang, Y.; Tay, S.L.; Xu, W. Properties of Ni–ZrO2 nanocomposite coatings by electroplating. Int. J. Mod. Phys. B 2019, 33, 1940024. [Google Scholar] [CrossRef]
- Zhang, W.; Du, S.; Li, B.; Mei, T.; Miao, Y.; Chu, H.; Wang, J. Synthesis and characterization of TiN nanoparticle reinforced binary Ni-Co alloy coatings. J. Alloys Compd. 2021, 865, 158722. [Google Scholar] [CrossRef]
- Elkhoshkhany, N.; Hafnway, A.; Khaled, A. Electrodeposition and corrosion behavior of nano-structured Ni-WC and Ni-Co-WC composite coating. J. Alloys Compd. 2017, 695, 1505–1514. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, B.; Tay, S.L.; Hou, F.; Gao, W.; Xiong, C. The microstructure and properties of sol-enhanced Sn–TiO2 nanocomposite coatings. Int. J. Mod. Phys. B 2017, 31, 1744025. [Google Scholar] [CrossRef]
- He, Z.; Cao, D.; Wang, Y.; Yin, L.; Hayat, M.D.; Singh, H. Preparation of Co–P–TiO2 nanocomposite coatings via a pulsed electrodeposition process. Surf. Eng. 2019, 36, 975–981. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, D.; Qiao, Y.; He, Z.; Wang, Y.; Li, X.; Gao, W. Microstructure and Properties of Duplex Ni-P-TiO2/Ni-P Nanocomposite Coatings. Mater. Res. 2019, 22 (Suppl. 2), e20180478. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Lu, G.-L.; Han, Z.-W.; Liu, J.-D.; Yu, S.-R. Fractal characteristics and wettability of Nano-Al2O3/Ni-Co composite coating prepared by electrodeposition. Trans. Nonferrous Met. Soc. China 2011, 21, s380–s383. [Google Scholar] [CrossRef]
- Rasooli, A.; Safavi, M.S.; Kasbkar Hokmabad, M. Cr2O3 nanoparticles: A promising candidate to improve the mechanical properties and corrosion resistance of Ni-Co alloy coatings. Ceram. Int. 2018, 44, 6466–6473. [Google Scholar] [CrossRef]
- Bakhit, B.; Akbari, A.; Nasirpouri, F.; Hosseini, M.G. Corrosion resistance of Ni–Co alloy and Ni–Co/SiC nanocomposite coatings electrodeposited by sediment codeposition technique. Appl. Surf. Sci. 2014, 307, 351–359. [Google Scholar] [CrossRef]
- Gómez, E.; Pané, S.; Alcobe, X.; Vallés, E. Influence of a cationic surfactant in the properties of cobalt–nickel electrodeposits. Electrochim. Acta 2006, 51, 5703–5709. [Google Scholar] [CrossRef]



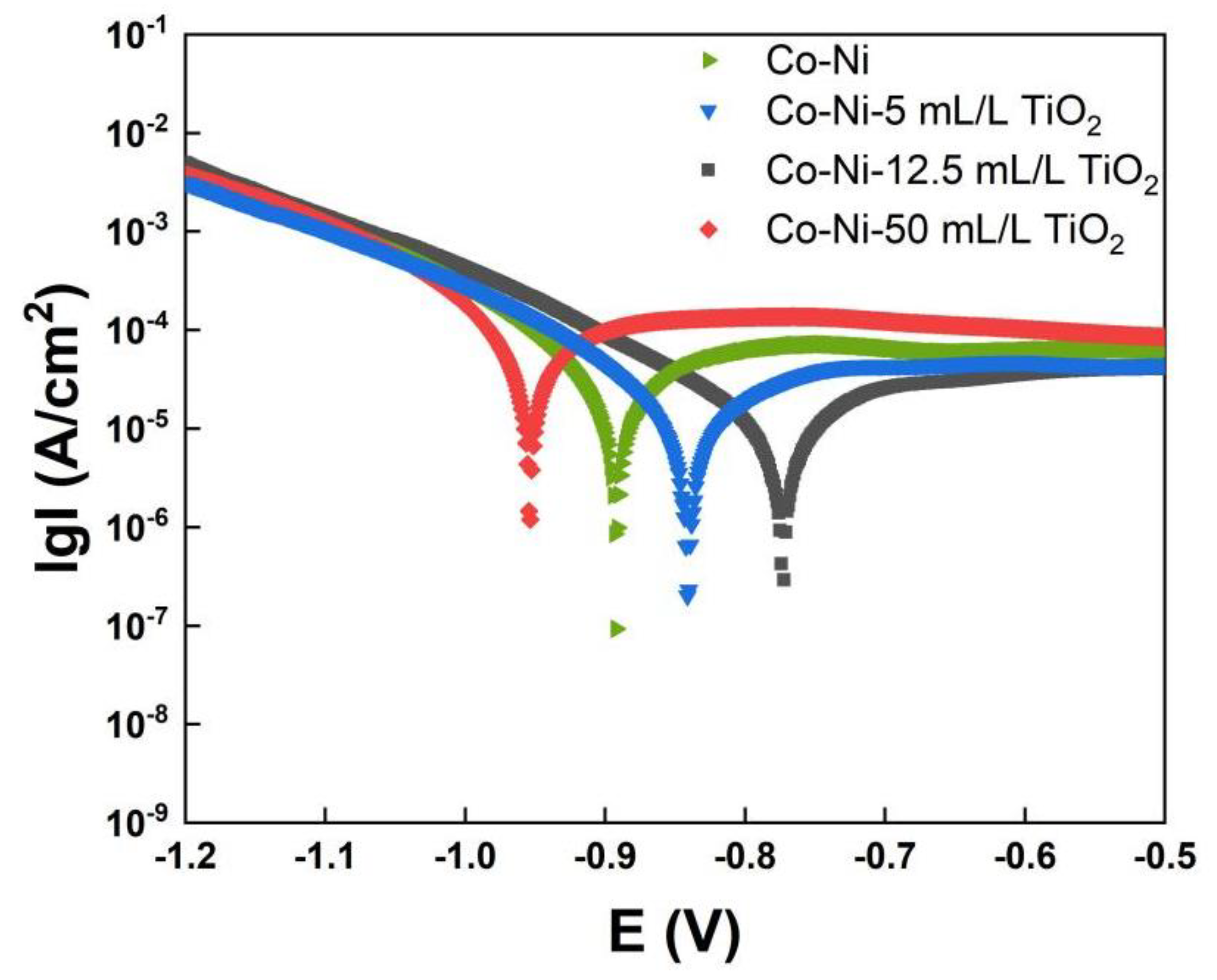
| Sample | Ecorr (V) | Icorr (μA/cm2) | Corrosion Rate (mm/a) |
|---|---|---|---|
| Co−Ni | −0.88 | 6.28 | 0.074 |
| Co−Ni−5 mL/L TiO2 | −0.84 | 3.69 | 0.054 |
| Co−Ni−12.5 mL/L TiO2 | −0.76 | 3.09 | 0.036 |
| Co−Ni−50 mL/L TiO2 | −0.99 | 14.6 | 0.171 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Miao, Z.; Zheng, S.; Chen, J.; He, Z. An Investigation into Electrodeposited Co−Ni−TiO2 Films with Improved Mechanical and Corrosion Properties. Coatings 2023, 13, 783. https://doi.org/10.3390/coatings13040783
Wang Y, Miao Z, Zheng S, Chen J, He Z. An Investigation into Electrodeposited Co−Ni−TiO2 Films with Improved Mechanical and Corrosion Properties. Coatings. 2023; 13(4):783. https://doi.org/10.3390/coatings13040783
Chicago/Turabian StyleWang, Yuxin, Zengcheng Miao, Songlin Zheng, Jiahuan Chen, and Zhen He. 2023. "An Investigation into Electrodeposited Co−Ni−TiO2 Films with Improved Mechanical and Corrosion Properties" Coatings 13, no. 4: 783. https://doi.org/10.3390/coatings13040783
APA StyleWang, Y., Miao, Z., Zheng, S., Chen, J., & He, Z. (2023). An Investigation into Electrodeposited Co−Ni−TiO2 Films with Improved Mechanical and Corrosion Properties. Coatings, 13(4), 783. https://doi.org/10.3390/coatings13040783







