Abstract
Tissue engineering in the orofacial region with bioactive components by the activation of immune complexes or other proteins is the current focus of biomaterials research. Consequently, natural ground materials and tissue components are being created. Bioactive glass is one of the most promising biomaterials and has bioactive properties making it suited for a range of different clinical dental applications, including the regeneration of hard tissues in the craniofacial region. This narrative review provides a summary of the favorable properties and recent applications of bioactive glass materials for the management of periodontal lesions. Bioactive glass mimics natural calcified tissues in terms of composition and has a bioactive role in bone regeneration. The present review concluded that bioactive glass materials have a promising potential for various periodontal applications including the repair of infrabony defects, gingival recession, furcation defects, and guided tissue regeneration. However, further in vivo studies and clinical trials are warranted to advance and validate the potential of bioactive glass for periodontal applications and translate its usage in dental clinics for periodontology.
1. Introduction
Biomaterials researchers are now investigating bone regeneration and tissue engineering [1]. Tissue engineering has already been employed successfully in dental tissue regeneration, including dentine, pulp, and periodontium including alveolar bone, therefore it is not as new as we might believe [2,3,4]. Bioactive glass is one of the most used materials for this purpose because it interacts with living tissue in a way that promotes healing and regeneration [5]. Bioactive glass is generally produced by mixing and then heating at high temperature various elemental compounds (silica, calcium oxide, sodium oxide, and phosphorus pentoxide) [6]. There is strong evidence reported in the literature that ions released by bioactive glass support reduction in inflammation and infection, making it a valuable material for a range of medical applications, including bone grafts, dental implants, and wound healing [7]. The first bioactive glass material was developed by Hench et al. in 1969 which was later termed 45S5 (45% silica, 5% phosphate), and the commercial product was marketed as Bioglass® (trademarked by the University of Florida, USA) [8]. The 45S5 bioactive glass is a promising material for use in biomedical engineering and regenerative medicine applications due to its biocompatibility and excellent bioactivity. It is composed of glass that is composed of 45% SiO2, 24.5% Na2O, 24.5% CaO, and 6% P2O5 [9]. The high SiO2 content is critical to the material’s excellent bioactivity, which enables it to bond with surrounding tissues and promote the formation of hydroxyapatite, SiO2, Na2O, CaO, and P2O5 in specific weight percentages [10]. The high SiO2 content enables it to bond with surrounding tissues and promote the formation of hydroxyapatite, which is critical in bone and periodontal regeneration [9,11]. Therefore, 45S5 bioactive glass has been extensively studied for its potential applications in bone tissue engineering, wound healing, and drug delivery. Its unique composition and properties make it a promising material for many applications including periodontal bone regeneration [10,11,12].
Following the host rejection of several commonly used biomaterials for amputation (such as inert metals, and polymers), the trend set out to create a grafting biomaterial that is harmonic with human tissues [13]. This substance was found to be a glass that precipitated hydroxyapatite and could adhere to both hard and soft tissues without being rejected. Due to bioactive glass’ bioactive properties, the healthcare industry has seen a metamorphosis, and it is currently used in numerous clinical settings, including tissue regeneration in both dentistry (Figure 1) and medicine [13,14,15]. A possible mechanism by which bioactive glasses regenerate bone is demonstrated in Figure 2.
Rationale and Aim of the Review
Periodontal defects are caused by the breakdown of the supporting tissues around teeth leading to bone loss, tooth loss, and other complications. The goal of this review is to evaluate the potential for regenerative properties of bioactive glass in managing infrabony defects, gingival recession, and furcation defects in periodontology. Bioactive glass is being studied for its potential use in a range of clinical applications, including drug delivery, tissue engineering, and as a coating for medical devices owing to its unique properties that make it a promising material for a variety of medical and biotechnology applications. Therefore, this paper aims to provide an overview of bioactive glass in periodontology applications.
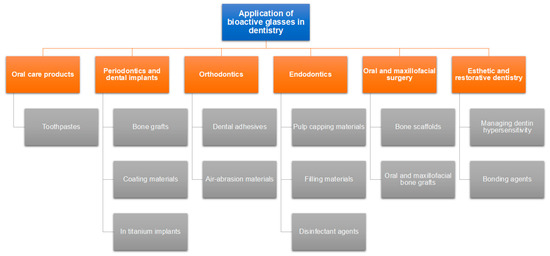
Figure 1.
Applications of bioactive glasses in dentistry [16].
Figure 1.
Applications of bioactive glasses in dentistry [16].
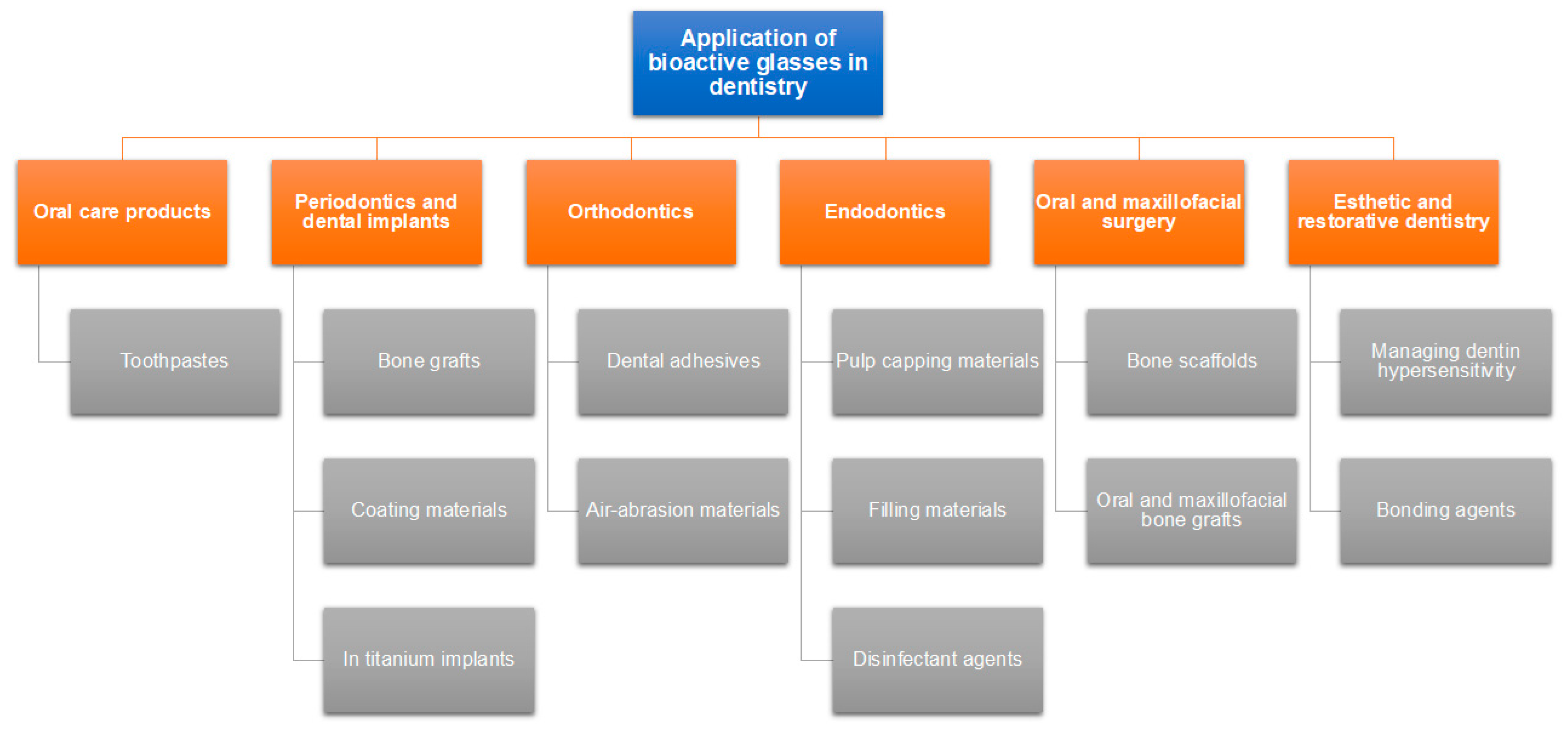
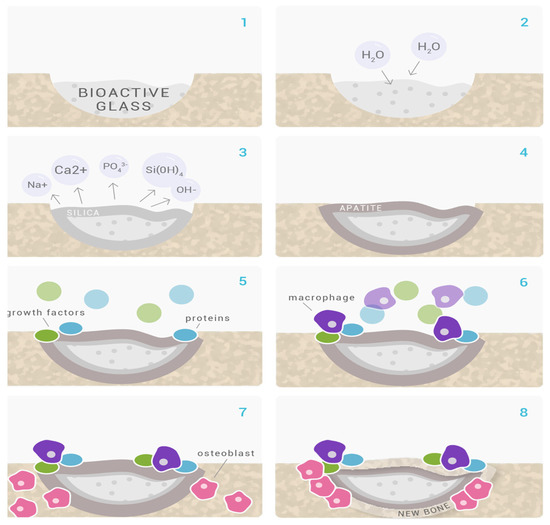
Figure 2.
Mechanism of bone regeneration by BGs. (1,2) On the surface of the Glassbone, calcium and natrium ions quickly interact with body fluids. In addition to causing localized increases in pH and osmotic pressure, the reaction results in the hydrolysis of silica groups; (3) At the surface of Glassbone, soluble silica is converted to create a silica-gel layer; (4) A layer of carbonated hydroxyl apatite (HCA), which is a component of the mineral phase of natural bone, develops on top of Glassbone. (5,6) Growth factors adsorb to the surface of Glassbone due to its structural and chemical similarities to hydroxyapatite. Growth factors cause M2 macrophages to become activated, which aids in wound healing and starts progenitor cell migration to the area; (6,7) Mesenchymal stem cells and osteoprogenitor cells migrate to the Glassbone surface and adhere to the HCA layer as a result of M2 macrophage activation, where they undergo osteogenic cell differentiation to become osteoblasts; (7,8) Type I collagen, the primary protein constituent of bone, and other extracellular matrix (ECM) components are produced and deposited by the attached and developed osteoblasts; [9,10] Following these responses, the newly recruited cells continue to work and support tissue growth and repair, which results in the formation of new bone. Glassbone is still deteriorating and being changed into new ECM material [17].
Figure 2.
Mechanism of bone regeneration by BGs. (1,2) On the surface of the Glassbone, calcium and natrium ions quickly interact with body fluids. In addition to causing localized increases in pH and osmotic pressure, the reaction results in the hydrolysis of silica groups; (3) At the surface of Glassbone, soluble silica is converted to create a silica-gel layer; (4) A layer of carbonated hydroxyl apatite (HCA), which is a component of the mineral phase of natural bone, develops on top of Glassbone. (5,6) Growth factors adsorb to the surface of Glassbone due to its structural and chemical similarities to hydroxyapatite. Growth factors cause M2 macrophages to become activated, which aids in wound healing and starts progenitor cell migration to the area; (6,7) Mesenchymal stem cells and osteoprogenitor cells migrate to the Glassbone surface and adhere to the HCA layer as a result of M2 macrophage activation, where they undergo osteogenic cell differentiation to become osteoblasts; (7,8) Type I collagen, the primary protein constituent of bone, and other extracellular matrix (ECM) components are produced and deposited by the attached and developed osteoblasts; [9,10] Following these responses, the newly recruited cells continue to work and support tissue growth and repair, which results in the formation of new bone. Glassbone is still deteriorating and being changed into new ECM material [17].
2. Literature Search Strategies
Recent review studies have mostly focused on bioactive materials and how they are used in dental care. Research on the use of bioactive glass in periodontal applications is still in its infancy. To address this, the current narrative review’s objectives aim to give a thorough overview of the periodontal uses of bioactive glass materials. To facilitate understanding, particular attention is placed on the bioactive glass structure. This narrative review was produced following the standards established by the Scale for the Assessment of Narrative Review Articles (SANRA) [18]. With an emphasis on laboratory and clinical trial research, pertinent material was searched using the search engines Scopus, Google Scholar, and PubMed and the keywords “bioactive glass” and “periodontal defects” or “periodontal intrabony defects” or “furcation defects” or “gingival recession defects” with no time limitations. For this narrative review, the following inclusion criteria had to be met: (1) The use of bioactive glass materials in periodontology; and (2) Bioactive glass material research, including clinical trials, and laboratory and animal-based investigations. Papers that had not yet appeared in indexed journals or published in languages other than English were excluded from the study.
3. Bioactive Glass
3.1. History
Bioactive glasses are known as silicate-based amorphous biomaterials, which are biocompatible and stimulate bone regeneration while disintegrating over time. The original bioactive glass thought to create an interfacial bond with the host after the implantation was discovered by Larry Hench and colleagues in 1969 [19]. The first bioactive glass was designated 45S5 Bioglass® and comprised SiO2 (46.1%), CaO (26.9%), Na2O (24.4%), and P2O5 (2.6%). Hench selected this arrangement because it had a high CaO concentration and some P2O5 in a Na2O-SiO2 matrix. It quickly melted since it is extremely acquainted with a ternary eutectic. They were efficaciously eliminated after one and a half months. In animal studies, the qualities of the interfacial connection between the bioactive glass and cortical bone were similar to or better than the recipient bone [20,21,22].
3.2. Properties of Bioactive Glasses
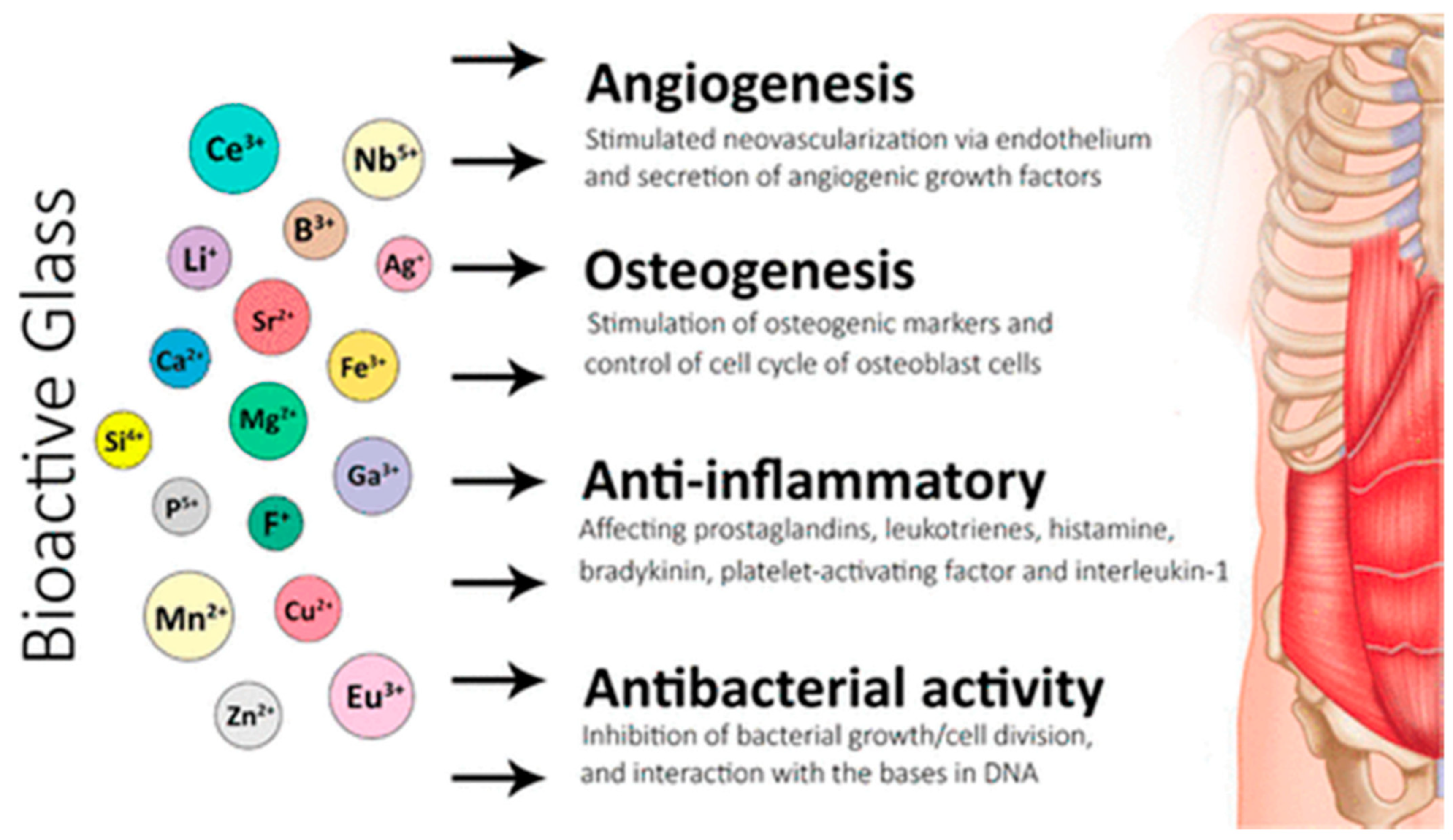
An interaction between a bioactive substance and the biological environment might result in a specific biological reaction, including the creation of a hydroxyapatite layer and a link between the substance and the tissue. This illustrates how bioactive glasses work in angiogenesis, osteogenesis, and anti-inflammatory and anti-bacterial activities (Figure 3). Calcified tissue composed of hydroxyapatite (Ca10(PO4)6(OH)2) [23], comprises most of the bone, enamel, and dentine. Conversely, bioinert materials do not affect the biological environment and do not elicit any specific reactions. However, they might trigger a foreign-body reaction, produce a fibrous capsule, and eventually cause a prosthetic to fail. Bioactive materials may be osteoconductive and may result in micromovements and eventual failure of a prosthesis. Bioactive materials may be osteoconductive or osteoinductive [24]. The bioactive properties are influenced by the structure and composition of the glass, manufacturing techniques, and the rate of ionic dissolution. This is clearly illustrated when comparing bioactive glasses to the traditional Bioglass® 45S5. Bioglass® 45S5 possesses several shortcomings, which include the possibility of gap formation between the material and host tissues due to a rapid rate of degradation. The configuration, method, and degree of particle accretion must be held responsible for the absence of porosity [25,26]. A pH rise brought on by a high Ca2+ and Na+ leakage in Bioglass® 45S5 can also cause cytotoxicity and delay the development of hydroxyapatite [25,26]. The composition of glass could not be appropriate for the creation of porous scaffolds due to its weak mechanical qualities [27,28]. Future study needs to enhance the bioactive glasses’ mechanical characteristics.
Bioactive glass has been the subject of extensive research due to its potential to enhance tissue regeneration and healing. Its impact on different cell types, including fibroblasts, cementoblasts, osteoblasts, and immune cells, has been investigated in various studies [11,29,30,31]. Bioactive glass can stimulate the proliferation and migration of fibroblasts, increase the production of extracellular matrix proteins, promote the differentiation of osteoblasts, and enhance the mineralization of bone matrix. Moreover, it can interact with immune cells, such as macrophages and lymphocytes, and stimulate their activation and production of cytokines [32]. These effects are dependent on the specific cell type and culture conditions, as well as the composition and structure of the glass. Further research is necessary to elucidate the underlying mechanisms involved in the effects of bioactive glass on cells [33].
3.3. Long-Term Results of Bioactive Glasses
In order to treat degenerative spondylolisthesis, bioactive glass-S53P4 and autogenous bone were employed as bone graft substitutes in a prospective long-term follow-up study from 1996 to 1998. On the left side of the fusion bed, bioactive glass was implanted, and on the right, autogenous bone. X-rays, computed tomography (CT) scans, and a clinical examination were used to assess the surgical outcome. The 11-year follow-up involved 17 patients (12 women and 5 men). In comparison to the preoperative Oswestry Disability Index score of 49 (range 32 to 64), the mean Oswestry Disability Index score during the follow-up was 21 (range 0 to 52). On computed tomography images, a solid bony fusion was visible on the side with autogenous bone in all patients and on the side with bioactive glass in 12 individuals. The L4/5 level and the L5/S1 level both had a fusion rate of 88% for bioactive glass used as a bone substitute (n = 41). In the future, spine surgery may benefit from the use of bioactive glass as a bone graft extender [34].
In a prospective randomized 11-year follow-up study, the clinical and radiological results of bioactive glass-S53P4 and autogenous bone utilized as bone-graft substitutes in depressed tibial plateau fractures were assessed. All of the patients (n = 29) had tibial plateau fractures that were more than 3 mm depressed along the joint line. This long-term follow-up included fifteen patients—five in the bioactive glass group and 10 in the autogenous bone group. Preoperative, postoperative, and long-term follow-up X-rays as well as CT scans were performed to assess the bone substitute, osteoarthritis, the tibial-femoral angle, and mechanical axis deviation. In the bioactive glass group and the autogenous bone group, the mean articular surface depression on X-rays at the long-term follow-up was 1.4 mm, and on CT scans, the means were 2.2 mm and 2.1 mm, respectively. The tibial-femoral angle and mechanical axes’ deviation did not significantly differ between the two groups. With good functional and long-term radiological results, bioactive glass-S53P4 can be employed as a bone substitute in depressed lateral tibial plateau fractures [35].
In order to replace bone grafts in benign bone tumor surgeries performed between 1993 and 1997, a prospective randomized long-term follow-up research of bioactive glass-S53P4 and autogenous bone was conducted. Twenty-one patients took part in a 14-year follow-up (11 in the bioactive glass group and 10 in the autogenous bone group). In addition to getting X-rays and MRIs, the bioactive glass group also had CT scans taken. The filled cavity in the bioactive glass group appeared dense on X-ray. In the group of patients with significant bone tumors, MRI also revealed glass granule remnants and predominantly or partially fatty bone marrow. There was an increase in cortical thickness in enchondromas and non-ossifying fibromas. A safe and well-tolerated bone substitute with positive long-term outcomes is bioactive glass-S53P4. Bioactive glass-S53P4 does not interfere with a child’s ability to build bone [36].
3.4. Composition of Bioactive Glasses
Most Class A bioactive glasses are composed of SiO2 (4052%), Na2O (10-35%), and CaO (10–50%). Additionally, the composition of glass may include B2O3 (0–10%), CaF2 (0–25%), or P2O5 (2–8%). Class B glasses characteristically have a silica concentration of more than 60% and are bioinert [37,38]. Additionally, minerals including tricalcium phosphate, diopside, wollastonite, and fluorapatite may be included in the bioactive glass [39,40]. Fluorapatite (10%), tricalcium phosphate (20%), and diopside make up FastOs® bioactive glass, a commercially available alkali-free, especially Na-free (SiO2—38.49, CaO—36.07, P2O5—5.61, MgO—19.24, CaF2—0.59) bioactive glass.
Network modifiers including P2O5, CaO, and Na2O may be incorporated into the fundamental Na2O-CaO-SiO2 composition to increase the surface as well as the silica network’s reactivity [41]. Na has been thought of as a crucial component of bioactivity because it effectively disrupts the network of glass. However, Na has been eliminated as a necessary component after Na-free bioactive glass was created and shown to have the same bioactivity and dissolution as traditional Na-containing bioactive glass [42]. Apatite formation and degradation rate are also strongly impacted by the connectedness of the glass silica network and the amount of phosphate. Until now, it was believed that P2O5 is necessary for the material to have bioactive properties, but unfortunately, this hypothesis has not been confirmed. Bioactive phosphate-free glasses have shown this to be wrong [43]. Other ions such as CaO and Na2O can be exchanged for MgO and K2O, respectively. Additionally, ions such as F, Ag, Zn, Cu, P, Sr, and Si may be added to change the antimicrobial and bioactive properties and enhance angiogenesis when implanted in the bone [44], whereas Ag might enhance antibacterial qualities [45]; however, excessive amounts might be cytotoxic [46]. Silver or zinc might be beneficial and improve the antibacterial properties. In addition, Zn-doped alkali-free bioactive glass showed an improved apatite synthesis [47]. The glass’s bioactivity is enhanced by Sr, Cu, and Mg. It has been demonstrated that fluoride increases bioactivity for dental applications by producing acid-resistant fluorapatite compared to hydroxyapatite [48], and fluoride in combination with bioactive glass can increase dentine remineralization and lessen the likelihood of dentin-matrix deterioration [49].
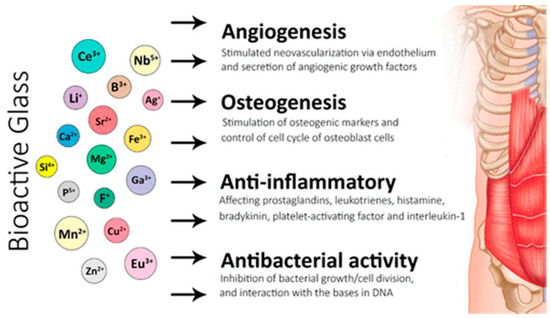
Figure 3.
Applications of bioactive glasses in angiogenesis, osteogenesis, and anti-inflammatory and anti-bacterial activities [38].
Figure 3.
Applications of bioactive glasses in angiogenesis, osteogenesis, and anti-inflammatory and anti-bacterial activities [38].
3.5. Methods for Preparing Bioactive Glasses
To obtain a bioactive glass of any composition, two techniques are mainly used: the melting process of the components and the sol-gel technique. The first method is an older process of obtaining a glass of any composition, which consists of mixing the precursors and melting them at high temperatures, followed by cooling and grinding the obtained glass. This method is still applied today to obtain bioactive glass or other types of glass. The sol-gel method consists of the transformation of precursors, such as tetraethyl orthosilicate, triethyl orthophosphate, and calcium nitrate into a colloidal solution (gel), followed by solvent removal by heating, then crushing of the obtained glass. This can eliminate certain disadvantages that are present in the first process. The sol-gel process allows the attainment of bioactive glass with different compositions and biological properties. Furthermore, using the sol-gel technique, bioactive glasses with different porosities can be obtained [50].
3.6. Bioactive Glasses’ Effect on Periodontal Pathogens
Several studies have investigated the antimicrobial properties of bioactive glass against periodontal pathogens and have provided information on the effect of bioactive glass on periodontal pathogens. Esfahanizadeh et al. [51] investigated the antibiofilm activity of zinc-doped bioactive glass compared with 45S5 Bioglass® on putative periodontal pathogens. In this in vitro experimental study, the nano BG doped with 5-mol% zinc and BG® were synthesized by the sol-gel method. Mono-species biofilms of Aggregatibacter actinomycetemcomitans (A. a), Porphyromonas gingivalis (P. g), and Prevotella intermedia (P. i) were prepared separately in a well-containing microplate. Both doped zinc-doped bioactive glass and 45S5 Bioglass® significantly reduced the biofilm formation ability of all examined strains after 48 h of incubation. Moreover, the anti-biofilm activity of zinc-doped bioactive glass was significantly stronger than 45S5 Bioglass®, which resulted in the formation of a weak biofilm compared with a moderately adhered biofilm observed with 45S5 Bioglass®. Zinc-doped bioactive glass showed a significant inhibitory effect on the biofilm formation of all examined periodontal pathogens. Given the enhanced regenerative and anti-biofilm properties of this novel biomaterial, further investigations are required for its implementation in clinical situations [51].
Similarly, Hiltunen et al. [52] explored the anti-biofilm effects that could result from combining bioactive glass with bisphosphonates, particularly in a dental biofilm model. The experiments were performed with an oral cavity single-specie (Aggregatibacter actinomycetemcomitans) biofilm assay, which was optimized in this contribution. Risedronate displayed an intrinsic anti-biofilm effect, and all bisphosphonates, except clodronate, reduced biofilm formation when combined with bioactive glass. In particular, the anti-biofilm activity of risedronate was significantly increased by the combination with bioactive glass. Overall, these results do provide further support for the promising use of bisphosphonate-bioactive glass combinations in dental applications. These findings are particularly relevant for patients undergoing cancer chemotherapy, or osteoporotic patients, who are known to be more vulnerable to periodontitis. In such cases, bisphosphonate treatment could play a double positive effect: local treatment of periodontitis (in combination with bioactive glass) and systemic treatment of osteoporosis, and prevention of hypercalcemia and metastases [52].
4. Bioactive Glass in Clinical Applications of Periodontal Diseases
There is significant evidence that using bioactive glasses in conjunction with or instead of other biomaterials has great potential to treat periodontal infrabony, furcation, and gingival recession defects. Barrier membranes [53], enamel matrix derivatives (EMDs) [54,55,56], various bone grafts [57,58,59], and growth factors [60,61] have been used to treat periodontal hard and soft tissue problems utilizing bioactive glass.
4.1. Infrabony or Intraosseous Defects
Bioactive glass and autologous platelet-rich fibrin (PRF) were compared by Apine et al. (2020) to treat 30 infrabony deformities. Preoperatively, at 3, 6, and 9 months, clinical and radiographic outcomes (increase in clinical attachment level (CAL), decrease in probing pocket depth (PPD), change in gingival recession, and fill and depth of defects) were assessed. Results at defects treated with bioactive glass were better than those treated with platelet-rich fibrin; however, the differences were not statistically significant. The study’s conclusions show that bioactive glass’s regenerating capability was foreseen and superior to PRF [62]. Similarly, to speed healing, Gupta et al. (2019) sanitized periodontal pockets with a diode laser before injecting bone biomaterial. Twelve people with bilateral infrabony abnormalities participated in a clinical trial research study. Diode laser pocket sanitization had a limited impact on the healing of infrabony defects treated with bioactive glass, according to the corresponding decline in results in both hard and soft tissues in the two groups [63].
To treat infrabony defects, Saravanan et al. (2019) investigated the additive application of PRF and bioactive glass. The periodontal defects were randomly divided into test (PRF) and control (no PRF) groups. The results of this study showed that at six months following periodontal surgery, postoperative evaluations showed a significant PPD decline and CAL increase. Bone formation was also evident in radiographs. When PRF and bioactive glass are combined, periodontal regeneration occurs quickly and effectively, and intraosseous defects repair more efficiently. The authors proposed that this combined approach might serve as a possible alternative therapeutic option for intraosseous periodontal defects [64].
In a recent meta-analysis, Sohrabi et al. (2012) examined the effectiveness of bioactive glass in correcting intraosseous deformities. According to the authors, bioactive glass was superior to the control for both CAL gain and PPD decrease. The studies that compared bioactive glass to open flap debridement (OFD) showed that the bioactive glass efficacy was significant in the subgroup analysis (p < 0.0001). In comparison to both OFD and active controls, infrabony defects repaired with bioactive glass significantly enhance both CAL and PPD [65]. Infrabony lesions were studied by Yadav et al. to determine the clinical effects of guided tissue regeneration (GTR) with collagen membrane (CM) alone (control), CM augmented with autogenous bone graft (group 1), or autogenous bone mixed with a bioactive glass (group 2). (2011). The results showed that both groups showed considerable gains in CAL gain, PPD reduction, and defect resolution after six months, with the test groups performing significantly better. However, there was no discernible difference between the two test groups [66]. Additionally, Demir et al. (2007) examined the clinical outcomes of infrabony defects using bioactive glass and platelet-rich plasma (PRP). The outcomes indicated that both therapeutic modalities were efficient. The CAL gain, PPD decrease, and defect fill for the combo group were 3.3 (1.77) mm, 3.60 (0.51) mm, and 3.47 (0.53) mm, respectively, while for the bioactive glass, these improvements were 2.86 (1.56), 3.29 (1.68), and 3.36 (0.55) mm. The authors suggested that successful therapies for infrabony malformations include both PRP/bioactive glass and bioactive glass alone [67].
Mengel et al. (2006) investigated the efficiency of GTR (test) and bioactive glass (control) for the treatment of intraosseous defects for long-term clinical outcomes. Clinical outcomes for the periodontal surgery patients’ gingival index (GI), plaque index (PI), CAL, PPD, gingival recession, bleeding on probing (BOP), and tooth mobility were noted before the procedure as well as at 6 months and annually for the following five years. Radiographically, the bioactive glass group’s defects were much more filled; yet, after five years, both materials’ CAL and PPD showed notable improvements. The treatment result was enhanced in the postoperative phase by an uninflamed periodontium and appropriate dental hygiene [68]. Sculean et al. compared the management of deep infrabony defects with EMD + bioactive glass to EMD for 12 months. Results showed that the EMD + bioactive glass combination does not appear to improve clinical outcomes. There were statistically insignificant differences between the groups at the start of the study, but after a 12-month review, both groups had significant increases in CAL and decreases in PPD. Nevertheless, there were no intergroup differences in terms of CAL gain and PPD reduction after 12 months [69].
The clinical and radiological outcomes of broad infrabony periodontal lesions treated with EMD alone (group 1) or EMD plus bioactive glass (group 2) for 8 months were examined in a clinical study by Kuru et al. (2006). Clinical and radiological outcomes improved in each group. Due to the strong clinical and radiological improvements that were observed in both groups, the intergroup comparison revealed significant variances in each outcome, indicating a better outcome for the combination therapy. Nonetheless, a combination strategy seemed to have better results in the treatment of wide intraosseous lesions [70]. In a study including 12 people with aggressive periodontitis, Mengel et al. (2003) investigated the effectiveness of bioactive glass and a bioabsorbable membrane to correct intraosseous abnormalities. The clinical parameters GI, PI, CAL, PPD, gingival recession, BOP, and mobility were calculated both before and after surgery (at 6 and 12 months). After 6 months and a year, respectively, both biomaterials produced highly significant outcomes in terms of PPD, crestal resorption, and CAL [71]. Additionally, Sculean et al. (2002) examined the repair of deep infrabony lesions with bioactive glass alone versus EMD + bioactive glass in 28 patients. Soft tissue measurements were undertaken both at the start of treatment and a year thereafter. There were no statistically significant changes between the two intervention groups for any of the examined parameters (p > 0.05). The clinical outcomes under study were significantly improved by both therapies, while the bioactive glass and EMD combination did not demonstrate any improvements in terms of clinical outcomes. [72].
Those with chronic periodontitis were investigated by Park et al. (2001) for the therapeutic advantages of bioactive glass implantation in infrabony periodontal defects 6 months following surgery. In contrast to the 17 control lesions that were simply treated with OFD (control), 21 defects were implanted with a bioactive glass (test). The criteria for comparative observation were gingival recession, CAL, PPD, and bone PPD before and after surgery. There were significant correlations in both groups between CAL and PPD alterations as well as between CAL and changes in bone PPD. In cases with severe pre-surgery CAL and bone PPD, the use of bioactive glass appeared to have a positive impact on post-surgery CAL, PPD, and bone PPD. It also produced noticeably better results in CAL and bone PPD than the OFD alone [73].
A clinical experiment by Ong et al. (1998) explored bioactive glass use for repairing periodontal intraosseous deficiencies. Defects were either corrected using OFD (control) or OFD plus bioactive glass (test). Pre- and postoperative measurements were taken for PI, GI, CAL, PPD, and mobility. Both interventions raised CAL while reducing PPD. Even though the test group had a higher CAL gain and PPD decline than the OFD, no remarkable differences were observed between the groups for the outcomes examined. Whether bioactive glass alloplast is effective in treating periodontal infrabony lesions needs more research [74].
4.2. Furcation Defects
Bioactive glass has been investigated for its potential use in repairing furcation defects, which refers to bone loss in the area between the roots of multi-rooted teeth that may occur due to periodontal disease. The use of BG stimulates the growth of new bone and soft tissue. Biswas et al. (2016) investigated PRF to the 2nd generation bioactive glass (group 1) for the management of grade-II furcation defects (group 2). The clinical measurements were performed for GI, PI, vertical PPD, CAL, and horizontal PPD of furcation involvement. Bioactive glass demonstrated better clinical outcomes, including better PPD reduction, when compared to group 2, indicating the clinical significance of BG biomaterials’ ease of use and higher biological implementation in furcation defects [75].
El-haddad et al. (2014) evaluated the effectiveness of bioactive glass grafting with autogenous bone for treating class II furcation defects. Group 1 included 30 sites treated with bioactive glass grafting, group 2 included 30 sites treated with autogenous bone grafting, and group 3 included 10 sites treated non-surgically only after flap reflection. Following 3 and 6 months of follow-up, groups 1 and 3, and 2 and 3 demonstrated statistically significant differences while groups 1 and 2 experienced a statistically significant decrease in furcation defects. Better results are seen with bioactive glass combined with autologous bone transplants than with OFD alone. The success rate of bioactive glass is equivalent to that of autogenous bone transplants; however, it is less stressful for patients, according to reports [76].
Humagain et al. (2007) examined the influence of horizontal and vertical bone fill in terms of CAL gain with OFD with bioactive glass versus solely OFD in the management of class II furcation defects. Both groups showed notable variations in hard and soft tissue outcomes at 6 months as compared to baseline. Gingival recession, CAL gain, and PPD reduction were soft tissue improvements between groups that were not statistically different from one another. Vertical defect fill was substantially higher at the bioactive glass locations when compared to the control sites. After 6 months, the horizontal PPD dramatically diminished. Clinical outcomes for bioactive glass over OFD were much better, including the filling of horizontal and vertical defects in class II furcation [77]. Fernandes et al. (2005) also investigated the effectiveness of EMD in combination with bioactive glass and GTR in the treatment of class III furcation defects in dogs using histological/histometric methods. Furcation defects were randomly distributed to three groups: EMD + bioactive glass + GTR, test 1, and control. When EMD was combined with both GTR and bioactive glass, as well as when GTR was used alone, the results were comparable to those obtained with bioactive glass mixed with membrane. Cementum and bone regeneration were limited to the apical area of the defect as a result of the three treatment techniques, resulting in partial furcation filling [78].
4.3. Gingival Recession Defects
Gingival recession defects refer to the exposure of the root surface due to the loss of gum tissue. It can be caused by factors such as periodontal disease, aggressive brushing, and tooth malposition. Bioactive glass is a material that has been investigated for its potential use in repairing these defects. The effectiveness of bioactive glass, PRF, and a combination of the two in treating 31 maxillary gingival recession abnormalities were investigated by Swarupa et al. (2019). Over the course of 6 months, clinical parameters such as PPD, recession height, CAL, recession breadth, keratinized tissue height (KTH), and the width of associated gingiva were measured. The biotype changes, mean root coverage, visual analog scale, and root coverage aesthetic score (RES) were calculated biannually. At baseline to 6 months, recession width and recession height were reduced in each group. The intergroup evaluation revealed a negligible difference in parameters between the groups at any given period. Treatment of gingival recession abnormalities was successful with each therapy approach [79].
Bansal et al. (2016) investigated the effects of bioactive glass for the coronally advanced flap (CAF) in terms of increasing KTH, and RES. Clinical indicators pre- and postoperatively at 6 months included CAL, PPD, gingival recession, KTH, and RES. Each evaluated outcome had significant reductions 6 months after surgery. KTH, CAL, RES, and root coverage did not differ significantly between CAF with bioactive glass and CAF alone. Nevertheless, both groups’ measurements of each metric significantly improved from the starting point. To verify these findings, long-term clinical trials are required [80]. Overall, bioactive glass shows promise in the repair of gingival recession defects. However, more research is required to establish the ideal dosage and use of bioactive glass to ascertain long-term outcomes. Table 1 shows different commercially used bioactive glasses in periodontal regeneration.

Table 1.
Commercially available bioactive glasses for periodontal regeneration.
5. Challenges, Future Directions, and Recommendations
The unique characteristics of bioactive glass make it a promising material for application in periodontology, and further research in this field is ongoing to enhance our understanding. However, several challenges must be addressed before they can be widely used in this field. For example, one of the most challenging areas of bioactive glass is to retain and transform the shape according to the periodontal defect unique to each patient’s need, particularly for larger defects, where the material may need to be packed into place and then stabilized until it sets. There are ample in vivo studies and clinical trials published on using bioactive glass in the form of ‘granular filler, combined with resorbable and non-resorbable membrane’ to achieve periodontal bone repair and regeneration [86,87,88,89]. However, it is challenging to determine which commercial formulation and experimental composition authorize the best experimental model and promising clinical results because of the clinical outcomes due to the variations in specific periodontal defects and selected surgical intervention strategies [86,90,91].
Another challenge is that the bioactivity of the glass may be limited in the oral environment. Saliva and other oral fluids can interfere with the ability of the glass to release ions and stimulate tissue regeneration, reducing its effectiveness. Additionally, the material can be brittle and prone to fracture if not handled carefully. Finally, there is the issue of long-term stability as bioactive glass has shown good short-term results in periodontal defects, but it is not yet clear how well the material will hold up over the long term, particularly in a dynamic oral environment. Despite these challenges, researchers continue to explore the potential of bioactive glass for use in periodontology, and new formulations and techniques are being developed to address these issues. Moreover, a comprehensive understanding of the current state of research in this field, including the advantages and limitations of using Bioglass, the different formulations, and delivery methods should be further explored by considering several experiments.
6. Conclusions
The chemistry of bioactive glass closely mimics the composition of natural calcified tissues, which has significance in the regeneration of bony tissues. Furthermore, bioactive glasses have demonstrated promising outcomes for the management of various periodontal lesions including infrabony defects, gingival recession, and furcation defects. However, further clinical research and evidence are essential to further validate the regenerative potential of bioactive glass materials for periodontal applications.
Author Contributions
Conceptualization, M.S.S. and M.S.Z.; methodology, M.S.S.; validation, M.S.Z.; formal analysis, M.A.F.; resources, M.S.S.; data curation, M.S.S.; writing—original draft preparation, M.S.S.; writing—review and editing, M.S.Z. and M.A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fernandes, H.R.; Gaddam, A.; Rebelo, A.; Brazete, D.; Stan, G.E.; Ferreira, J.M. Bioactive glasses and glass-ceramics for healthcare applications in bone regeneration and tissue engineering. Materials 2018, 11, 2530. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Khurshid, Z.; Ghabbani, H.; Zafar, M.S.; Sefat, F. Nano glass ionomer cement: Modification for biodental applications. In Advanced Dental Biomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 217–227. [Google Scholar]
- Najeeb, S.; Khurshid, Z.; Zafar, M.S.; Khan, A.S.; Zohaib, S.; Martí, J.M.N.; Sauro, S.; Matinlinna, J.P.; Rehman, I.U. Modifications in glass ionomer cements: Nano-sized fillers and bioactive nanoceramics. Int. J. Mol. Sci. 2016, 17, 1134. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S.; Khurshid, Z.; Almas, K. Oral tissue engineering progress and challenges. Tissue Eng. Regen. Med. 2015, 12, 387–397. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R.; Brauer, D.S.; Hupa, L.; Greenspan, D.C. Bioglass and bioactive glasses and their impact on healthcare. Int. J. Appl. Glass Sci. 2016, 7, 423–434. [Google Scholar] [CrossRef]
- Cheah, C.W.; Al-Namnam, N.M.; Lau, M.N.; Lim, G.S.; Raman, R.; Fairbairn, P.; Ngeow, W.C. Synthetic material for bone, periodontal, and dental tissue regeneration: Where are we now, and where are we heading next? Materials 2021, 14, 6123. [Google Scholar] [CrossRef]
- Hench, L.L. Chronology of bioactive glass development and clinical applications. New J. Glass Ceram. 2013, 3, 67–73. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef]
- Raszewski, Z.; Chojnacka, K.; Mikulewicz, M. Preparation and characterization of acrylic resins with bioactive glasses. Sci. Rep. 2022, 12, 16624. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive glasses: Where are we and where are we going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef]
- Lu, X.; Kolzow, J.; Chen, R.R.; Du, J. Effect of solution condition on hydroxyapatite formation in evaluating bioactivity of B2O3 containing 45S5 bioactive glasses. Bioact. Mater. 2019, 4, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Mocquot, C.; Attik, N.; Pradelle-Plasse, N.; Grosgogeat, B.; Colon, P. Bioactivity assessment of bioactive glasses for dental applications: A critical review. Dent. Mater. 2020, 36, 1116–1143. [Google Scholar] [CrossRef] [PubMed]
- Jafari, N.; Habashi, M.S.; Hashemi, A.; Shirazi, R.; Tanideh, N.; Tamadon, A. Application of bioactive glasses in various dental fields. Biomater. Res. 2022, 26, 31. [Google Scholar] [CrossRef] [PubMed]
- Noraker. BIOACTIVE GLASS “The Key Technology to Regenerate Your Bones”. Available online: https://noraker.com/en/bioactive-glass/ (accessed on 28 March 2023).
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Hench, L.L.; Splinter, R.J.; Allen, W.; Greenlee, T. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Hench, L.; Pantano, C., Jr.; Buscemi, P.; Greenspan, D. Analysis of bioglass fixation of hip prostheses. J. Biomed. Mater. Res. 1977, 11, 267–282. [Google Scholar] [CrossRef]
- Piotrowski, G.; Hench, L.; Allen, W.; Miller, G. Mechanical studies of the bone bioglass interfacial bond. J. Biomed. Mater. Res. 1975, 9, 47–61. [Google Scholar] [CrossRef]
- Weinstein, A.; Klawitter, J.; Cook, S. Implant-bone interface characteristics of bioglass dental implants. J. Biomed. Mater. Res. 1980, 14, 23–29. [Google Scholar] [CrossRef]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [PubMed]
- Damen, J.; Ten Cate, J. Silica-induced precipitation of calcium phosphate in the presence of inhibitors of hydroxyapatite formation. J. Dent. Res. 1992, 71, 453–457. [Google Scholar] [CrossRef]
- Skallevold, H.E.; Rokaya, D.; Khurshid, Z.; Zafar, M.S. Bioactive Glass Applications in Dentistry. Int. J. Mol. Sci. 2019, 20, 5960. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Thompson, I.D.; Boccaccini, A.R. 45S5 Bioglass®-derived glass–ceramic scaffolds for bone tissue engineering. Biomaterials 2006, 27, 2414–2425. [Google Scholar] [CrossRef]
- Chen, Q.; Baino, F.; Spriano, S.; Pugno, N.M.; Vitale-Brovarone, C. Modelling of the strength–porosity relationship in glass-ceramic foam scaffolds for bone repair. J. Eur. Ceram. Soc. 2014, 34, 2663–2673. [Google Scholar] [CrossRef]
- Baino, F.; Novajra, G.; Miguez-Pacheco, V.; Boccaccini, A.R.; Vitale-Brovarone, C. Bioactive glasses: Special applications outside the skeletal system. J. Non-Cryst. Solids 2016, 432, 15–30. [Google Scholar] [CrossRef]
- Carvalho, S.M.; Oliveira, A.A.; Jardim, C.A.; Melo, C.B.; Gomes, D.A.; de Fátima Leite, M.; Pereira, M.M. Characterization and induction of cementoblast cell proliferation by bioactive glass nanoparticles. J. Tissue Eng. Regen. Med. 2012, 6, 813–821. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, C.; Xiao, Y. The stimulation of proliferation and differentiation of periodontal ligament cells by the ionic products from Ca7Si2P2O16 bioceramics. Acta Biomater. 2012, 8, 2307–2316. [Google Scholar] [CrossRef]
- Qiu, G.; Shi, Z.; Xu, H.H.; Yang, B.; Weir, M.D.; Li, G.; Song, Y.; Wang, J.; Hu, K.; Wang, P. Bone regeneration in minipigs via calcium phosphate cement scaffold delivering autologous bone marrow mesenchymal stem cells and platelet-rich plasma. J. Tissue Eng. Regen. Med. 2018, 12, e937–e948. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Frantzén, J.; Rantakokko, J.; Aro, H.T.; Heinänen, J.; Kajander, S.; Gullichsen, E.; Kotilainen, E.; Lindfors, N.C. Instrumented spondylodesis in degenerative spondylolisthesis with bioactive glass and autologous bone: A prospective 11-year follow-up. Clin. Spine Surg. 2011, 24, 455–461. [Google Scholar] [CrossRef]
- Pernaa, K.; Koski, I.; Mattila, K.; Gullichsen, E.; Heikkila, J.; Aho, A.; Lindfors, N. Bioactive glass S53P4 and autograft bone in treatment of depressed tibial plateau fractures-a prospective randomized 11-year follow-up. J. Long-Term Eff. Med. Implant. 2011, 21, 139–148. [Google Scholar] [CrossRef]
- Lindfors, N.C.; Koski, I.; Heikkilä, J.T.; Mattila, K.; Aho, A.J. A prospective randomized 14-year follow-up study of bioactive glass and autogenous bone as bone graft substitutes in benign bone tumors. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 94, 157–164. [Google Scholar] [CrossRef] [PubMed]
- El-Meliegy, E.; Van Noort, R. Glasses and Glass Ceramics for Medical Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Al-Harbi, N.; Mohammed, H.; Al-Hadeethi, Y.; Bakry, A.S.; Umar, A.; Hussein, M.A.; Abbassy, M.A.; Vaidya, K.G.; Al Berakdar, G.; Mkawi, E.M. Silica-based bioactive glasses and their applications in hard tissue regeneration: A review. Pharmaceuticals 2021, 14, 75. [Google Scholar] [CrossRef]
- Ferreira, M.M.; Brito, A.F.; Brazete, D.; Pereira, I.C.; Carrilho, E.; Abrantes, A.M.; Pires, A.S.; Aguiar, M.J.; Carvalho, L.; Botelho, M.F. Doping β-TCP as a strategy for enhancing the regenerative potential of composite β-TCP—Alkali-free bioactive glass bone grafts. Experimental study in rats. Materials 2018, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Lowe, B.; Ottensmeyer, M.P.; Xu, C.; He, Y.; Ye, Q.; Troulis, M.J. The regenerative applicability of bioactive glass and beta-tricalcium phosphate in bone tissue engineering: A transformation perspective. J. Funct. Biomater. 2019, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, O.; Alhalawani, A.; Arshad, S.; Towler, M.R. Rapidly-dissolving silver-containing bioactive glasses for cariostatic applications. J. Funct. Biomater. 2018, 9, 28. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Brauer, D.S.; Wilson, R.M.; Law, R.V.; Hill, R.G.; Karpukhina, N. Sodium is not essential for high bioactivity of glasses. Int. J. Appl. Glass Sci. 2017, 8, 428–437. [Google Scholar] [CrossRef]
- Hill, R.G.; Brauer, D.S. Predicting the bioactivity of glasses using the network connectivity or split network models. J. Non-Cryst. Solids 2011, 357, 3884–3887. [Google Scholar] [CrossRef]
- Hoppe, A.; Jokic, B.; Janackovic, D.; Fey, T.; Greil, P.; Romeis, S.; Schmidt, J.; Peukert, W.; Lao, J.; Jallot, E. Cobalt-releasing 1393 bioactive glass-derived scaffolds for bone tissue engineering applications. ACS Appl. Mater. Interfaces 2014, 6, 2865–2877. [Google Scholar] [CrossRef]
- Rokaya, D.; Srimaneepong, V.; Sapkota, J.; Qin, J.; Siraleartmukul, K.; Siriwongrungson, V. Polymeric materials and films in dentistry: An overview. J. Adv. Res. 2018, 14, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.H.; Xiao, W.; Wei, X.J.; Jia, W.T.; Zhang, C.Q.; Huang, W.H.; Jin, D.X.; Rahaman, M.N.; Day, D.E. In vitro evaluation of cytotoxicity of silver-containing borate bioactive glass. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 95, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, S.; Goel, A.; Tilocca, A.; Dhuna, V.; Bhatia, G.; Dhuna, K.; Ferreira, J.M. Role of glass structure in defining the chemical dissolution behavior, bioactivity and antioxidant properties of zinc and strontium co-doped alkali-free phosphosilicate glasses. Acta Biomater. 2014, 10, 3264–3278. [Google Scholar] [CrossRef] [PubMed]
- Thuy, T.T.; Nakagaki, H.; Kato, K.; Hung, P.A.; Inukai, J.; Tsuboi, S.; Nakagaki, H.; Hirose, M.N.; Igarashi, S.; Robinson, C. Effect of strontium in combination with fluoride on enamel remineralisation in vitro. Arch. Oral Biol. 2008, 53, 1017–1022. [Google Scholar] [CrossRef]
- Groh, D.; Döhler, F.; Brauer, D.S. Bioactive glasses with improved processing. Part 1. Thermal properties, ion release and apatite formation. Acta Biomater. 2014, 10, 4465–4473. [Google Scholar] [CrossRef]
- Maximov, M.; Maximov, O.-C.; Craciun, L.; Ficai, D.; Ficai, A.; Andronescu, E. Bioactive Glass—An Extensive Study of the Preparation and Coating Methods. Coatings 2021, 11, 1386. [Google Scholar] [CrossRef]
- Esfahanizadeh, N.; Nourani, M.R.; Bahador, A.; Akhondi, N.; Montazeri, M. The Anti-biofilm Activity of Nanometric Zinc doped Bioactive Glass against Putative Periodontal Pathogens: An in vitro Study. Biomed. Glasses 2018, 4, 95–107. [Google Scholar] [CrossRef]
- Hiltunen, A.K.; Skogman, M.E.; Rosenqvist, K.; Juvonen, H.; Ihalainen, P.; Peltonen, J.; Juppo, A.; Fallarero, A. Bioactive glass combined with bisphosphonates provides protection against biofilms formed by the periodontal pathogen Aggregatibacter actinomycetemcomitans. Int. J. Pharm. 2016, 501, 211–220. [Google Scholar] [CrossRef]
- Needleman, I.; Worthington, H.V.; Giedrys-Leeper, E.; Tucker, R. Guided tissue regeneration for periodontal infra-bony defects. Cochrane Database Syst. Rev. 2006. [Google Scholar] [CrossRef]
- Koop, R.; Merheb, J.; Quirynen, M. Periodontal regeneration with enamel matrix derivative in reconstructive periodontal therapy: A systematic review. J. Periodontol. 2012, 83, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, M.S.; Lone, M.A.; Matabdin, H.; Lone, M.A.; Soomro, A.H.; Zafar, M.S. Regenerative potential of enamel matrix protein derivative and acellular dermal matrix for gingival recession: A systematic review and meta-analysis. Proteomes 2021, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, M.S.; Pisani, F.; De Vito, D.; Lone, M.A.; Almasri, M. Long-term Clinical Performance of Regeneration versus Conservative Surgery in the Treatment of Infra-bony Defects: A systematic review. J. Int. Acad. Periodontol 2021, 23, 31–56. [Google Scholar] [PubMed]
- Shaikh, M.S.; Zafar, M.S.; Alnazzawi, A. Comparing Nanohydroxyapatite Graft and Other Bone Grafts in the Repair of Periodontal Infrabony Lesions: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2021, 22, 12021. [Google Scholar] [CrossRef]
- Shaikh, M.S.; Husain, S.; Lone, M.A.; Lone, M.A.; Akhlaq, H.; Zafar, M.S. Clinical effectiveness of anorganic bovine-derived hydroxyapatite matrix/cell-binding peptide grafts for regeneration of periodontal defects: A systematic review and meta-analysis. Regen. Med. 2020, 15, 2379–2395. [Google Scholar] [CrossRef]
- Shaikh, M.S.; Zafar, M.S.; Alnazzawi, A.; Javed, F. Nanocrystalline hydroxyapatite in regeneration of periodontal intrabony defects: A systematic review and meta-analysis. Ann. Anat. Anat. Anz. 2022, 240, 151877. [Google Scholar] [CrossRef]
- Lynch, S.; Williams, R.; Poison, A.; Howell, T.; Reddy, M.; Zappa, U.; Antoniades, H. A combination of platelet-derived and insulin-like growth factors enhances periodontal regeneration. J. Clin. Periodontol. 1989, 16, 545–548. [Google Scholar] [CrossRef]
- Darby, I.B.; Morris, K.H. A systematic review of the use of growth factors in human periodontal regeneration. J. Periodontol. 2013, 84, 465–476. [Google Scholar] [CrossRef]
- Apine, A.A.; Bilichodmath, S.; Janardhanan, N. Comparison of Bioactive Glass Bone Graft (Putty) with Autologous Platelet-rich Fibrin in the Treatment of Intrabony Defects. J. Health Sci. Res. 2020, 11, 42–52. [Google Scholar] [CrossRef]
- Gupta, R.K.; Singh, B.; Goyal, S.; Rani, N. Effect of laser application in the healing of intrabony defects treated with bioactive glass. J. Indian Soc. Periodontol. 2019, 23, 124–130. [Google Scholar] [CrossRef]
- Saravanan, D.; Rethinam, S.; Muthu, K.; Thangapandian, A. The Combined Effect of Bioactive Glass and Platelet-Rich Fibrin in Treating Human Periodontal Intrabony Defects—A Clinicoradiographic Study. Contemp. Clin. Dent. 2019, 10, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, K.; Saraiya, V.; Laage, T.A.; Harris, M.; Blieden, M.; Karimbux, N. An evaluation of bioactive glass in the treatment of periodontal defects: A meta-analysis of randomized controlled clinical trials. J. Periodontol. 2012, 83, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.S.; Narula, S.C.; Sharma, R.K.; Tewari, S.; Yadav, R. Clinical evaluation of guided tissue regeneration combined with autogenous bone or autogenous bone mixed with bioactive glass in intrabony defects. J. Oral. Sci. 2011, 53, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Demir, B.; Şengün, D.; Berberoğlu, A. Clinical evaluation of platelet-rich plasma and bioactive glass in the treatment of intra-bony defects. J. Clin. Periodontol. 2007, 34, 709–715. [Google Scholar] [CrossRef]
- Mengel, R.; Schreiber, D.; Flores-de-Jacoby, L. Bioabsorbable membrane and bioactive glass in the treatment of intrabony defects in patients with generalized aggressive periodontitis: Results of a 5-year clinical and radiological study. J. Periodontol. 2006, 77, 1781–1787. [Google Scholar] [CrossRef]
- Sculean, A.; Pietruska, M.; Schwarz, F.; Willershausen, B.; Arweiler, N.B.; Auschill, T.M. Healing of human intrabony defects following regenerative periodontal therapy with an enamel matrix protein derivative alone or combined with a bioactive glass: A controlled clinical study. J. Clin. Periodontol. 2005, 32, 111–117. [Google Scholar] [CrossRef]
- Kuru, B.; Yılmaz, S.; Argın, K.; Noyan, Ü. Enamel matrix derivative alone or in combination with a bioactive glass in wide intrabony defects. Clin. Oral Investig. 2006, 10, 227–234. [Google Scholar] [CrossRef]
- Mengel, R.; Soffner, M.; Flores-de-Jacoby, L. Bioabsorbable membrane and bioactive glass in the treatment of intrabony defects in patients with generalized aggressive periodontitis: Results of a 12-month clinical and radiological study. J. Periodontol. 2003, 74, 899–908. [Google Scholar] [CrossRef]
- Sculean, A.; Barbé, G.; Chiantella, G.C.; Arweiler, N.B.; Berakdar, M.; Brecx, M. Clinical evaluation of an enamel matrix protein derivative combined with a bioactive glass for the treatment of intrabony periodontal defects in humans. J. Periodontol. 2002, 73, 401–408. [Google Scholar] [CrossRef]
- Park, J.S.; Suh, J.J.; Choi, S.H.; Moon, I.S.; Cho, K.S.; Kim, C.K.; Chai, J.K. Effects of pretreatment clinical parameters on bioactive glass implantation in intrabony periodontal defects. J. Periodontol. 2001, 72, 730–740. [Google Scholar] [CrossRef]
- Ong, M.M.; Eber, R.M.; Korsnes, M.I.; MacNeil, R.L.; Glickman, G.N.; Shyr, Y.; Wang, H.L. Evaluation of a bioactive glass alloplast in treating periodontal intrabony defects. J. Periodontol. 1998, 69, 1346–1354. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Sambashivaiah, S.; Kulal, R.; Bilichodmath, S.; Kurtzman, G.M. Comparative Evaluation of Bioactive Glass (Putty) and Platelet Rich Fibrin in Treating Furcation Defects. J. Oral Implantol. 2016, 42, 411–415. [Google Scholar] [CrossRef] [PubMed]
- El-Haddad, S.; Abd-El Razzak, M.; Saudi, H.; El Ghorab, N. Evaluation of bioactive glass and autogenous bone in the treatment of Grade II furcation involvement: A randomized controlled trial. J. Interdiscip. Dent. 2014, 4, 13–23. [Google Scholar] [CrossRef]
- Humagain, M.; Nayak, D.G.; Uppoor, A.S. A clinical evaluation of bioactive glass particulate in the treatment of mandibular class II furcation defects. Braz. J. Oral Sci. 2007, 6, 1450–1456. [Google Scholar]
- Fernandes, J.M.A.; Rego, R.O.C.C.; Spolidorio, L.C.; Marcantonio, R.A.C.; Marcantonio Júnior, E.; Cirelli, J.A. Enamel matrix proteins associated with GTR and bioactive glass in the treatment of class III furcation in dogs. Braz. Oral Res. 2005, 19, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Swarupa, K.; Tadepalli, A.; Parthasarathy, H.; Ponnaiyan, D. Clinical efficacy of bioactive glass in combination with platelet rich fibrin in management of gingival recession defects: A prospective comparative study. J. Clin. Case Rep. 2019, 9, 2. [Google Scholar]
- Bansal, A.; Kulloli, A.; Kathariya, R.; Shetty, S.; Jain, H.; Raikar, S. Comparative evaluation of coronally advanced flap with and without bioactive glass putty in the management of gingival recession defects: A randomized controlled clinical trial. J. Int. Acad. Periodontol. 2016, 18, 7–15. [Google Scholar]
- Yajamanya, S.R.; Chatterjee, A.; Hussain, A.; Coutinho, A.; Das, S.; Subbaiah, S. Bioactive glass versus autologous platelet-rich fibrin for treating periodontal intrabony defects: A comparative clinical study. J. Indian Soc. Periodontol. 2017, 21, 32. [Google Scholar] [CrossRef]
- Fetner, A.; Hartigan, M.; Low, S. Periodontal repair using PerioGlas in nonhuman primates: Clinical and histologic observations. Compendium 1994, 15, 932, 935–938. [Google Scholar]
- Chacko, N.L.; Abraham, S.; Rao, H.S.; Sridhar, N.; Moon, N.; Barde, D.H. A clinical and radiographic evaluation of periodontal regenerative potential of PerioGlas®: A synthetic, resorbable material in treating periodontal infrabony defects. J. Int. Oral Health 2014, 6, 20–26. [Google Scholar]
- Wang, K.C.; Yang, S.S. Clinical Application of Unigraft® In the Treatment Of Human Periodontal Defects. Available online: https://www.unicarebiomedical.com/pdf/UnigraftClinicalStudy.pdf (accessed on 20 March 2023).
- Shobha, K.; Mani, R.; Deshpande, A.; Seshan, H.; Kranti, K. Clinical and radiographic evaluation of demineralized bone matrix (grafton) as a bone graft material in the treatment of human periodontal intraosseous defects. J. Indian Soc. Periodontol. 2013, 17, 495. [Google Scholar]
- Cannio, M.; Bellucci, D.; Roether, J.A.; Boccaccini, D.N.; Cannillo, V. Bioactive glass applications: A literature review of human clinical trials. Materials 2021, 14, 5440. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, A.L.; Kotsakis, G.A.; Kumar, T.; Hinrichs, J.E.; Romanos, G. Evaluation of the bone regeneration potential of bioactive glass in implant site development surgeries: A systematic review of the literature. Clin. Oral Investig. 2015, 19, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.-M.G.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Gritsch, L.; Perrin, E.; Chenal, J.-M.; Fredholm, Y.; Maçon, A.L.; Chevalier, J.; Boccaccini, A.R. Combining bioresorbable polyesters and bioactive glasses: Orthopedic applications of composite implants and bone tissue engineering scaffolds. Appl. Mater. Today 2021, 22, 100923. [Google Scholar] [CrossRef]
- Cannillo, V.; Salvatori, R.; Bergamini, S.; Bellucci, D.; Bertoldi, C. Bioactive Glasses in Periodontal Regeneration: Existing Strategies and Future Prospects—A Literature Review. Materials 2022, 15, 2194. [Google Scholar] [CrossRef]
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Wang, H.L.; Giannobile, W.V. Biologics-based regenerative technologies for periodontal soft tissue engineering. J. Periodontol. 2020, 91, 147–154. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
