Preparation and Performance of a Self-Produced High-Molecular-Weight Waterborne Epoxy–Acrylic Emulsion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
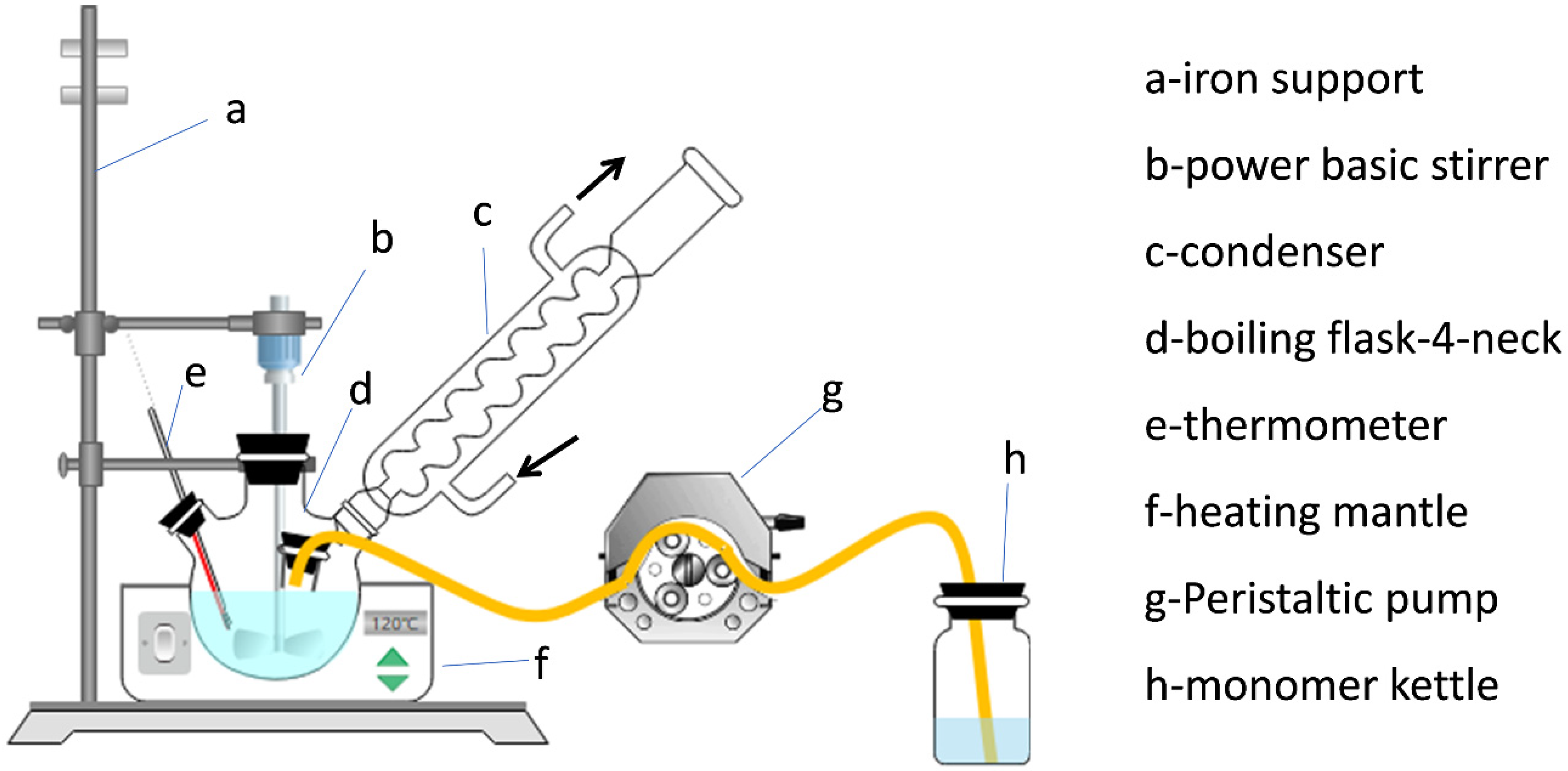
2.2. Preparation of Waterborne Epoxy–Acrylic Emulsion
2.3. Characterization
3. Results and Discussion
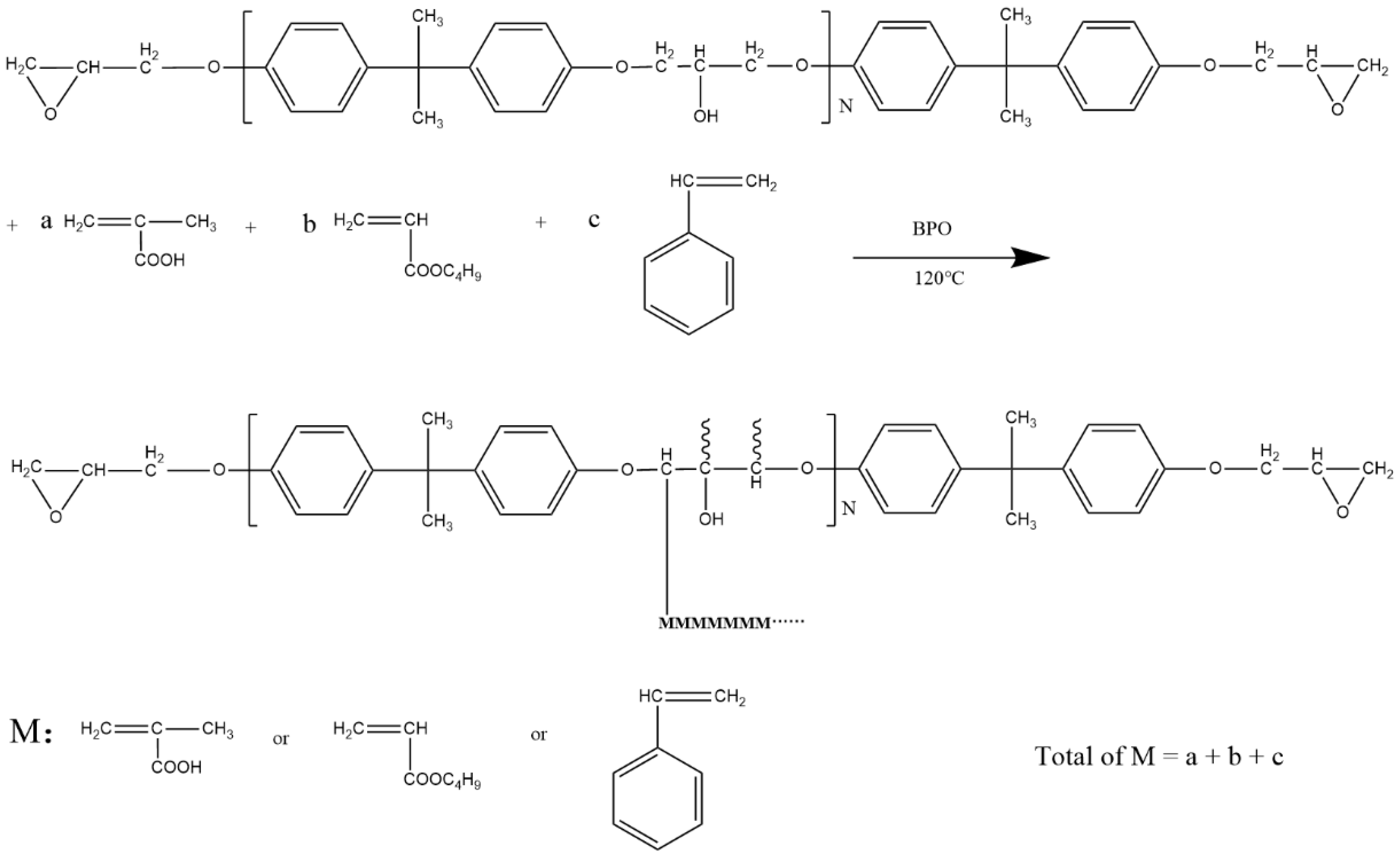
3.1. Chain Extension Reaction
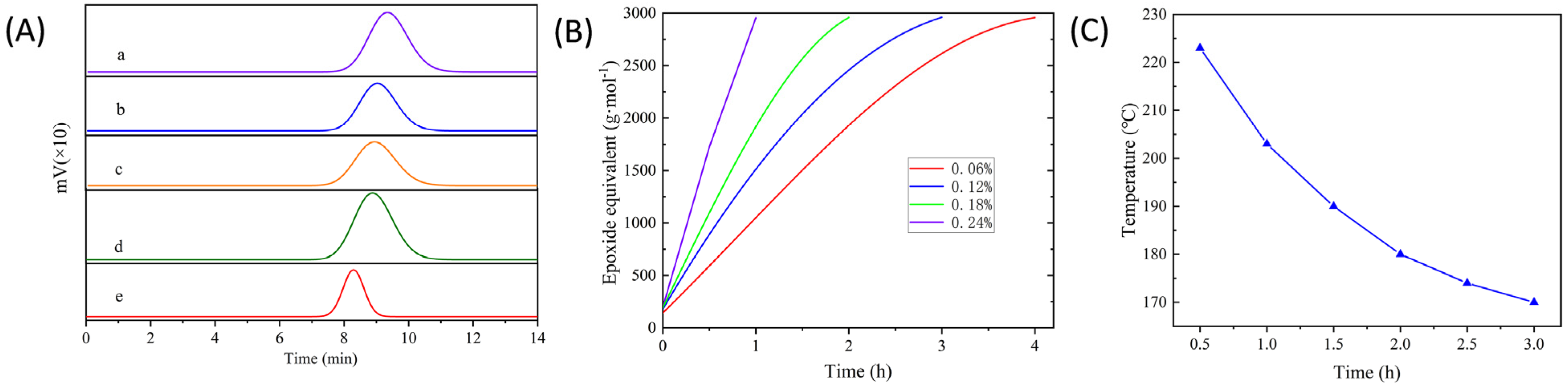
3.1.1. Selection of Optimal Molecular Weight
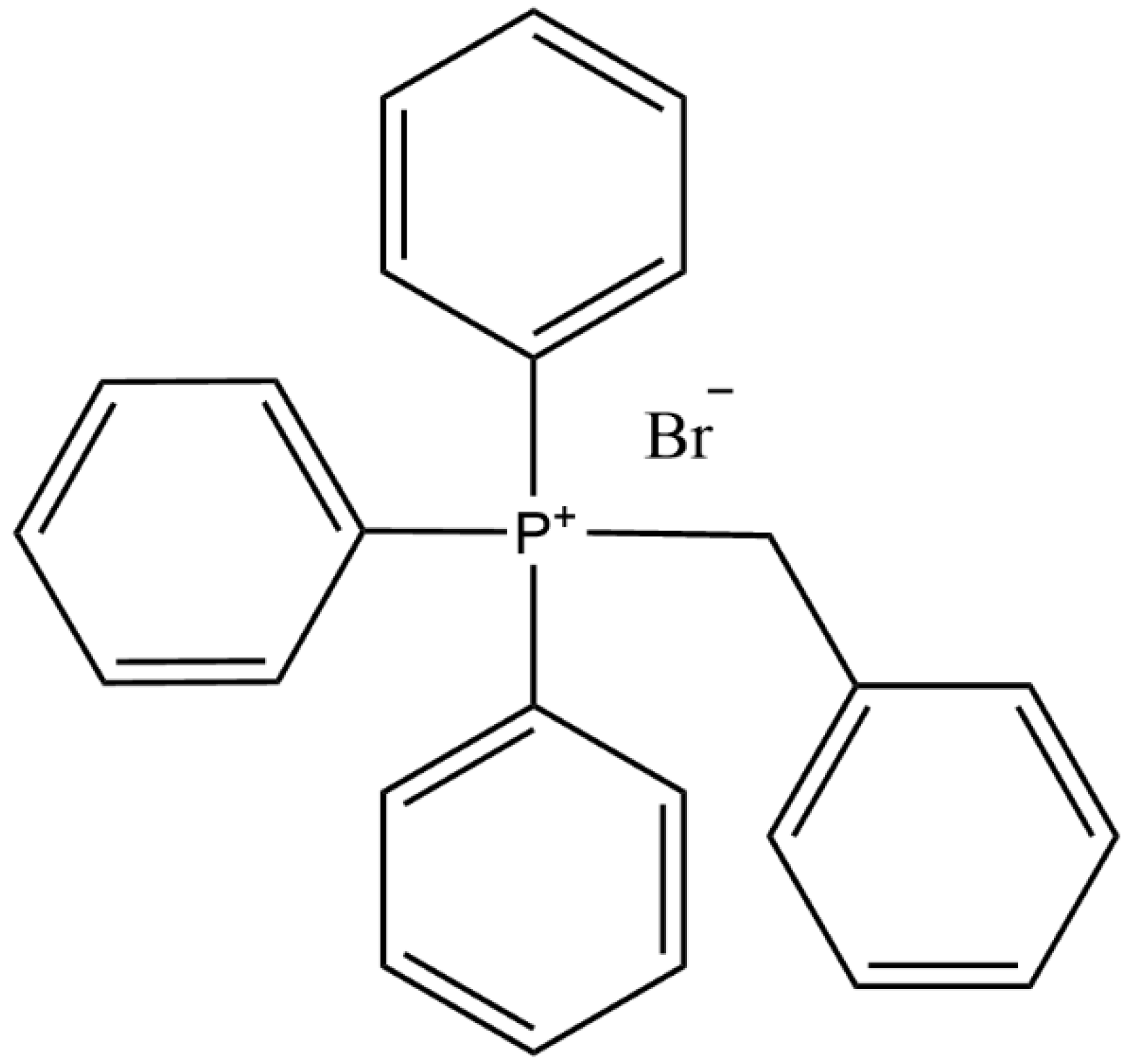
3.1.2. Determination of Catalyst Dosage
3.1.3. Reaction Temperature and Reaction Time
3.2. Grafting Reaction
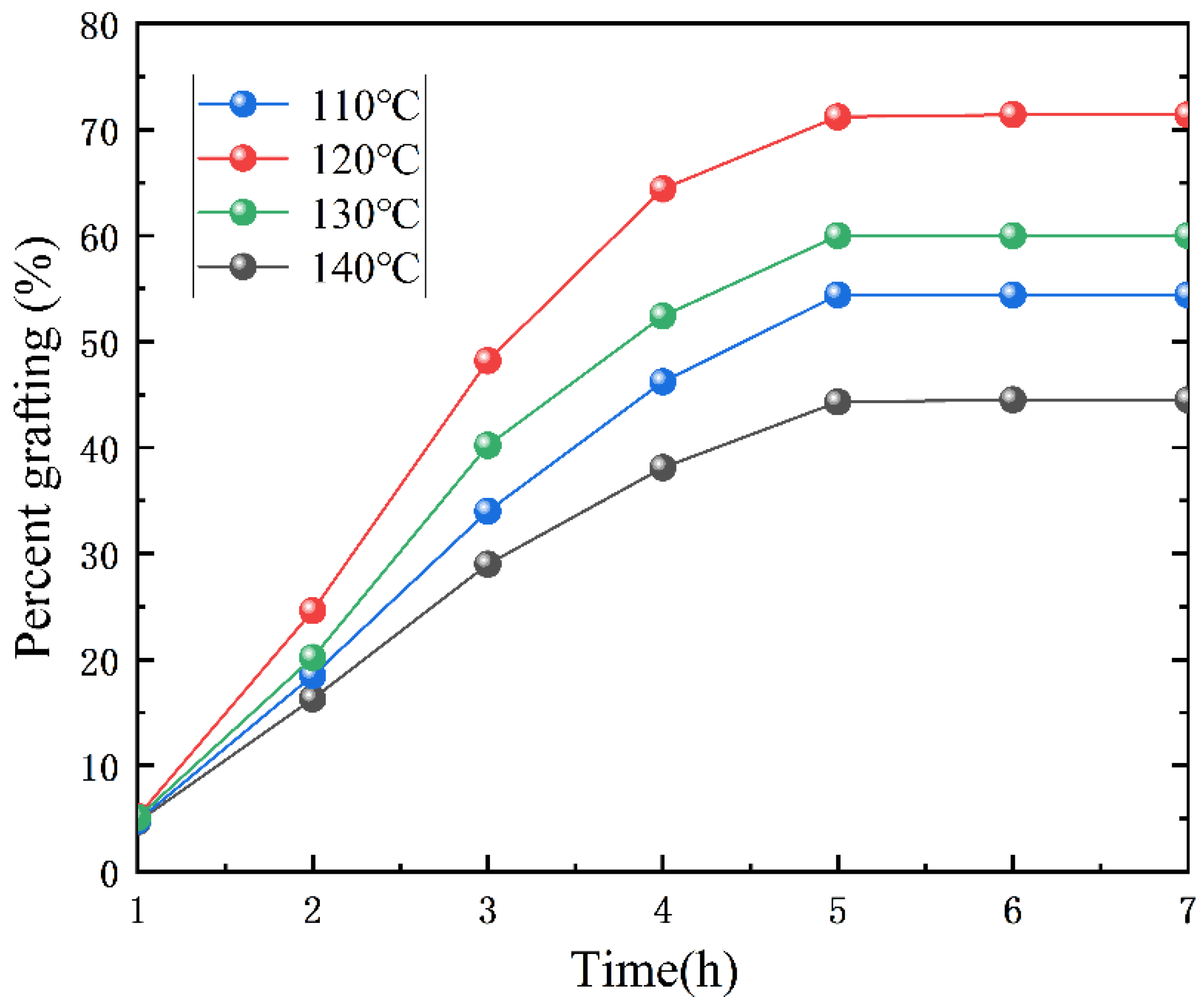
3.2.1. Determination of the Optimal Grafting Reaction Temperature
3.2.2. Effect of Initiator (BPO) Dosage on the Grafting Ratio
3.2.3. Influence of Epoxy Resin Dosage on the Coating Film Performance
3.2.4. Determination of MAA Dosage
3.2.5. Infrared Spectral Analysis of Graft Copolymers
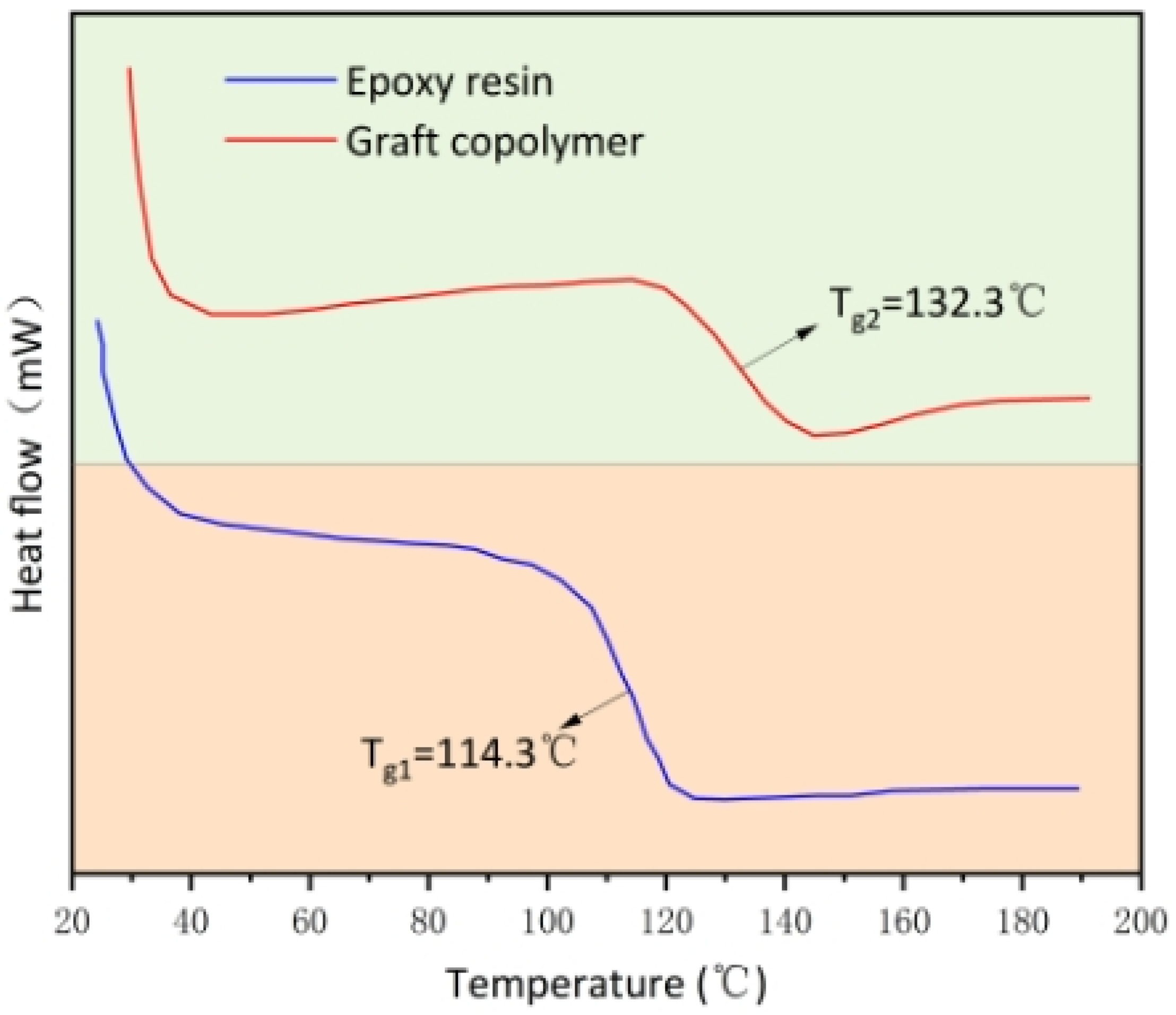
3.2.6. DSC Analysis of Graft Copolymers
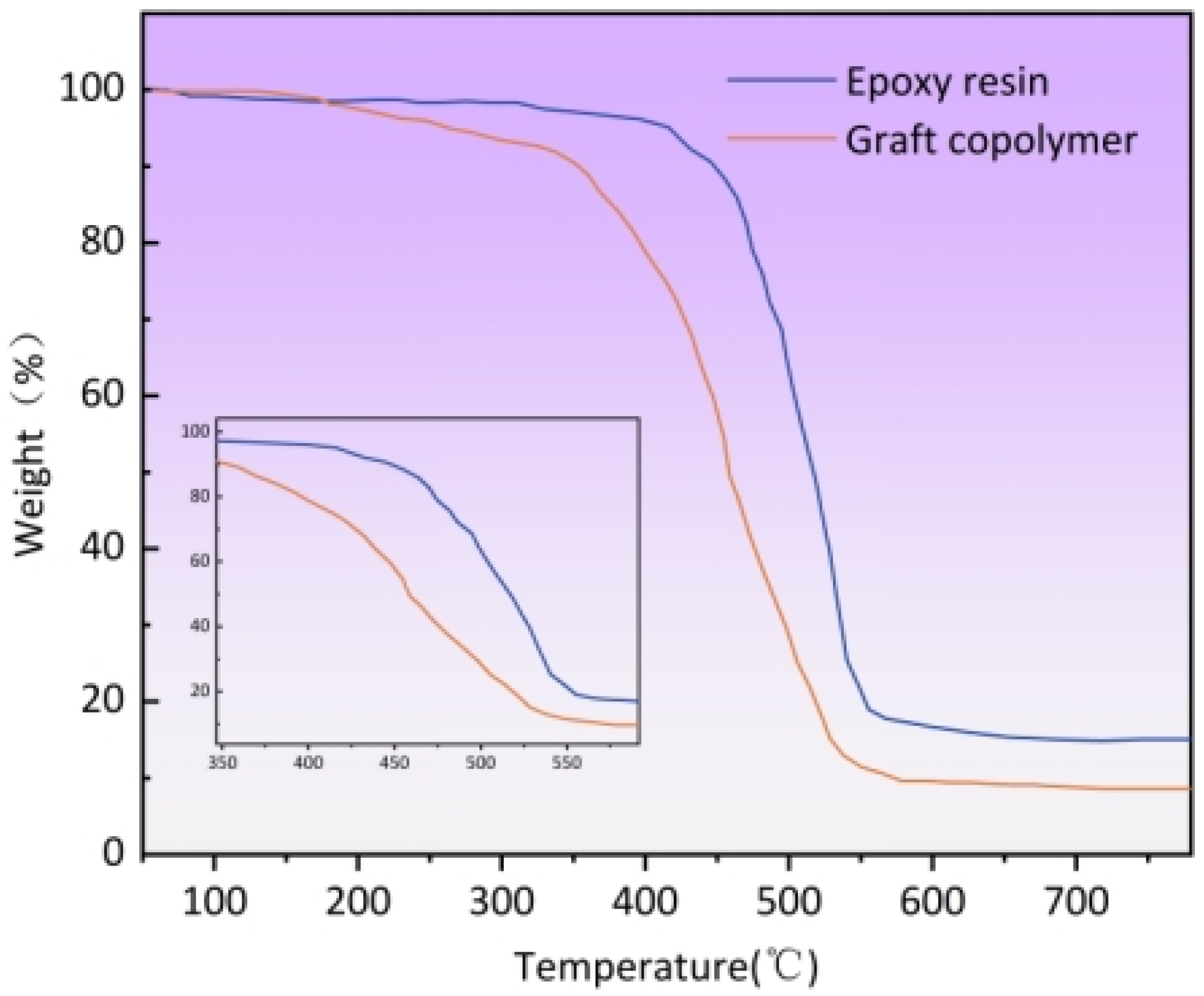
3.2.7. TGA of Graft Copolymers
3.3. Neutralization Reaction
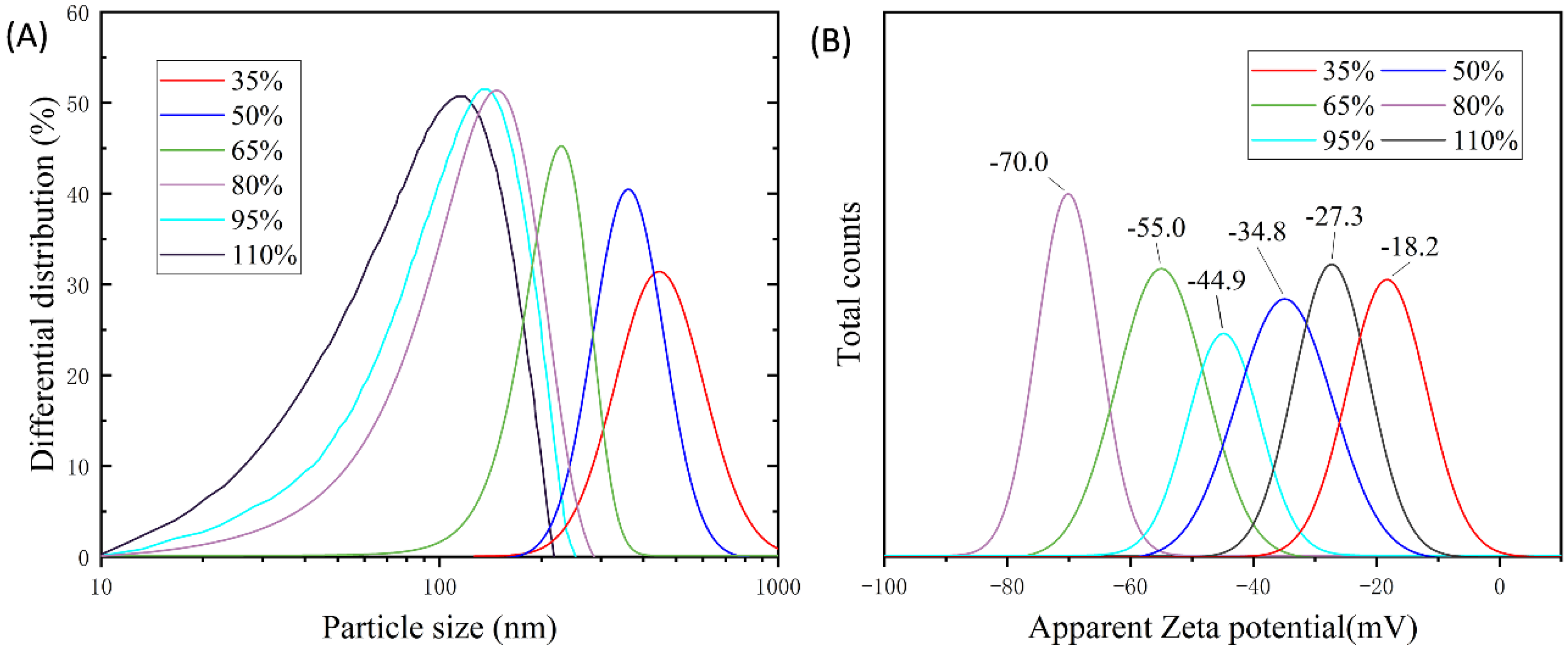
3.3.1. Influence of the Neutralization Degree on the Emulsion
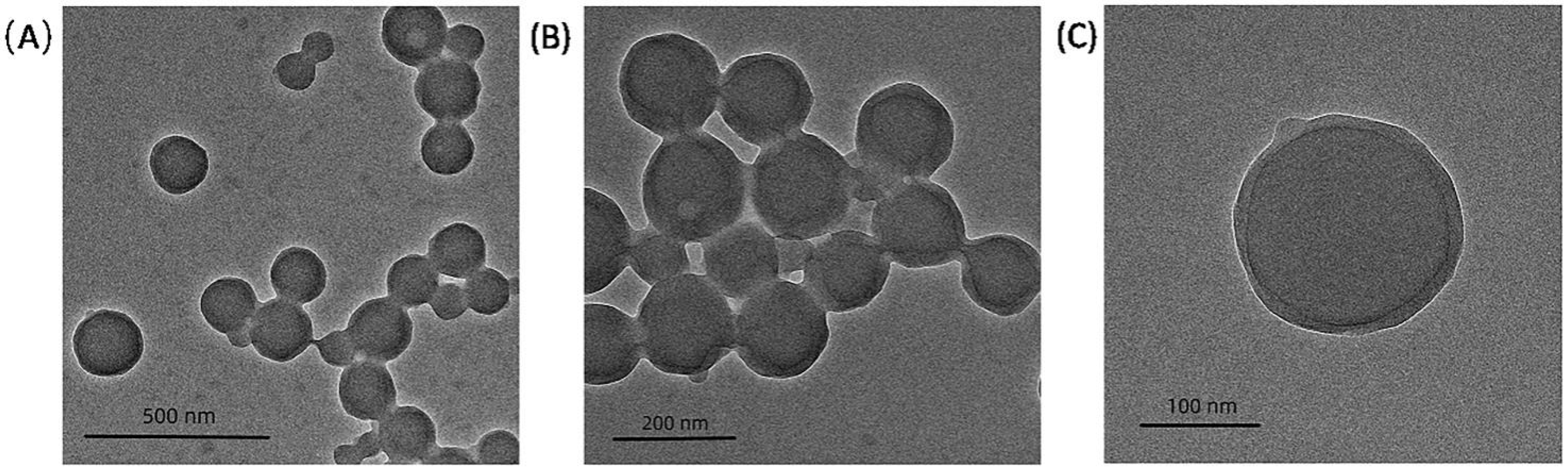
3.3.2. Morphology of Emulsion Particles
3.3.3. Emulsion Performance Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gündüz, G.; Khalid, A.H.; Mecidoğlu, I.A.; Aras, L. Water-Borne and Air-Drying Oil-Based Resins. Prog. Org. Coat. 2004, 49, 259–269. [Google Scholar] [CrossRef]
- Yu, J.; Pan, H.; Zhou, X. Preparation of Waterborne Phosphated Acrylate–Epoxy Hybrid Dispersions and Their Application as Coil Coating Primer. J. Coat. Technol. Res. 2014, 11, 361–369. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, F.L.; Yang, J.; Liu, X. Synthesis and Curing Kinetics of UV-Curable Waterborne Bisphenol-S Epoxy-Acrylate/Polyurethane-Acrylate/Methylacryloylpropyl-POSS Nanocomposites. J. Macromol. Sci. Part B 2014, 53, 1800–1813. [Google Scholar] [CrossRef]
- Zhaoying, Z.; Yuhui, H.; Bing, L.; Guangming, C. Studies on Particle Size of Waterborne Emulsions Derived from Epoxy Resin. Eur. Polym. J. 2001, 37, 1207–1211. [Google Scholar] [CrossRef]
- Shikha, D.; Kamani, P.K.; Shukla, M.C. Studies on Synthesis of Water-Borne Epoxy Ester Based on RBO Fatty Acids. Prog. Org. Coat. 2003, 47, 87–94. [Google Scholar] [CrossRef]
- Schauer, E.; Berglund, L.; PenÄa, G.; Marieta, C.; Mondragon, I. Morphological Variations in PMMA-Modified Epoxy Mixtures by PEO Addition. Polymer 2002, 43, 1241–1248. [Google Scholar] [CrossRef]
- Khurana, P.; Aggarwal, S.; Narula, A.; Choudhary, V. Curing and Thermal Behavior of Epoxy Resin in the Presence of Silicon-Containing Amide Amines. J. Appl. Polym. Sci. 2003, 87, 1345–1353. [Google Scholar] [CrossRef]
- Ma, C.; Deng, C.; Zheng, W. Waterborne Epoxy Resin Modified by AMPS. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 2007, 22, 649–652. [Google Scholar] [CrossRef]
- Choudhary, V.; Agarwal, N.; Varma, I.K. Evaluation of Bisacrylate Terminated Epoxy Resins as Coatings. Prog. Org. Coat. 2006, 57, 223–228. [Google Scholar] [CrossRef]
- Tong, L.; Akay, G. Process Intensification in Particle Technology: Flow Induced Phase Inversion in the Intensive Emulsification of Epoxy Polymer Melts in Water. J. Mater. Sci. 2002, 37, 4985–4992. [Google Scholar] [CrossRef]
- Wuzella, G.; Kandelbauer, A.; Mahendran, A.R.; Müller, U.; Teischinger, A. Influence of Thermo-Analytical and Rheological Properties of an Epoxy Powder Coating Resin on the Quality of Coatings on Medium Density Fibreboards (MDF) Using in-Mould Technology. Prog. Org. Coat. 2014, 77, 1539–1546. [Google Scholar] [CrossRef]
- Triwulandari, E.; Ghozali, M.; Meliana, Y. Synthesis of 1, 4-Butanediol Monooleate and 1, 4-Butanediol, 9-Hydroxy-10-Methoxy-Monostearate from Palm Oil as Modifier of Epoxy Resin for Coating. Procedia Chem. 2015, 16, 495–502. [Google Scholar] [CrossRef]
- Chiang, T.H.; Hsieh, T.E. A Study of UV-Curable Epoxide Resins Containing Thermal Accelerator—Tertiary Amines. React. Funct. Polym. 2008, 68, 601–612. [Google Scholar] [CrossRef]
- Liu, H.; Chen, M.; Huang, Z.; Xu, K.; Zhang, X. The Influence of Silicon-Containing Acrylate as Active Diluent on the Properties of UV-Cured Epoxydiacrylate Films. Eur. Polym. J. 2004, 40, 609–613. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Yu, F. Preparation of Epoxy-Acrylic Latex Based on Bisphenol F Epoxy Resin. J. Macromol. Sci. Part A 2018, 55, 205–212. [Google Scholar] [CrossRef]
- Petit, H.; Henry, N.; Krebs, A.; Uytterhoeven, G.; Jong, F. Ambient Cure High Solids Acrylic Resins for Automotive Refinish Clear Coat Applications. Prog. Org. Coat. 2001, 43, 41–49. [Google Scholar] [CrossRef]
- Khallaf, M.K.; El-Midany, A.A.; El-Mofty, S.E. Influence of Acrylic Coatings on the Interfacial, Physical, and Mechanical Properties of Stone-Based Monuments. Prog. Org. Coat. 2011, 72, 592–598. [Google Scholar] [CrossRef]
- Jiao, C.; Sun, L.; Shao, Q.; Song, J.; Hu, Q.; Naik, N.; Guo, Z. Advances in Waterborne Acrylic Resins: Synthesis Principle, Modification Strategies, and Their Applications. ACS Omega 2021, 6, 2443–2449. [Google Scholar] [CrossRef]
- Zou, M.; Zhao, Q.; Nie, J.; Zhang, Z. Preparation and Characterization of Polysiloxane–Polyacrylates Composite Lattices by Two Seeded Emulsion Polymerization and Their Film Properties. J. Appl. Polym. Sci. 2007, 103, 1406–1411. [Google Scholar] [CrossRef]
- Zou, M.; Huang, F.; Nie, J.; Zhang, Z.; Ge, X. Preparation and Characterization of Polysiloxane-Polyacrylates Composite Latices and Their Film Properties. Polym. Int. 2005, 54, 861–869. [Google Scholar] [CrossRef]
- Brady, R.F., Jr. Properties Which Infuence Marine Fouling Resistance in Polymers Containing Silicon and Fuorine. Prog. Org. Coat. 1999, 35, 31–35. [Google Scholar] [CrossRef]
- Galliano, F.; Landolt, D. Evaluation of Corrosion Protection Properties of Additives for Waterborne Epoxy Coatings on Steel. Prog. Org. Coat. 2002, 44, 217–225. [Google Scholar] [CrossRef]
- Woo, J.T.K.; Toman, A. Water-Based Epoxy-Acrylic Graft Copolymer. Prog. Org. Coat. 1993, 21, 371–385. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, X.; Xiao, Y.; Liu, R.; Liu, J. Enhanced Anticorrosion Properties through Structured Particle Design of Waterborne Epoxy-Styrene-Acrylate Composite Emulsion. Coatings 2021, 11, 1422. [Google Scholar] [CrossRef]
- Hamouda, I.M.; Ahmed, S.A. Effect of Microwave Disinfection on Mechanical Properties of Denture Base Acrylic Resin. J. Mech. Behav. Biomed. Mater. 2010, 3, 480–487. [Google Scholar] [CrossRef]
- Guo, W.L.; Jin, Z.M.; Gao, C.H. Study on Formulation for Epoxy Acrylic Hybrid Emulsion. Adv. Mat. Res. 2012, 524, 1715–1718. [Google Scholar] [CrossRef]
- Kawahara, H.; Goto, T.; Ohnishi, K.; Ogura, H.; Kage, H.; Matsuno, Y. Preparation of Epoxy Resin/Acrylic Composite Latexes by Miniemulsion Polymerization Method. J. Appl. Polym. Sci. 2001, 81, 128–133. [Google Scholar] [CrossRef]
- Aggarwal, L.K.; Thapliyal, P.C.; Karade, S.R. Anticorrosive Properties of the Epoxy–Cardanol Resin Based Paints. Prog. Org. Coat. 2007, 59, 76–80. [Google Scholar] [CrossRef]
- Liu, M.; Mao, X.; Zhu, H.; Lin, A.; Wang, D. Water and Corrosion Resistance of Epoxy–Acrylic–Amine Waterborne Coatings: Effects of Resin Molecular Weight, Polar Group and Hydrophobic Segment. Corros. Sci. 2013, 75, 106–113. [Google Scholar] [CrossRef]
- Woo, J.T.; Ting, V.; Evans, J.; Ortiz, C.; Carlson, G.; Marcinko, R. Water Dispersible Epoxy-g-Acrylic Copolymer for Container Coating; Bauer, R.S., Ed.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1983; Volume 221, pp. 283–300. ISBN 978-0-8412-0777-6. [Google Scholar]
- Zafar, S.; Riaz, U.; Ahmad, S. Water-Borne Melamine–Formaldehyde-Cured Epoxy–Acrylate Corrosion Resistant Coatings. J. Appl. Polym. Sci. 2008, 107, 215–222. [Google Scholar] [CrossRef]
- Zhang, K.; Fu, H.; Huang, H.; Chen, H. Waterborne Epoxy—Acrylic Dispersions Modified by Siloxane. J. Dispers. Sci. Technol. 2007, 28, 1209–1217. [Google Scholar] [CrossRef]
- Guo, W.L.; Zhou, H.C.; Lu, H.Q.; Hu, W. Preparation and Characterization of Fluorinated Epoxy Resin Emulsion for Waterborne Coatings. AMR 2011, 347, 4065–4068. [Google Scholar] [CrossRef]
- Sun, S.; Sun, P.; Liu, D. The Study of Esterifying Reaction between Epoxy Resins and Carboxyl Acrylic Polymers in the Presence of Tertiary Amine. Eur. Polym. J. 2005, 41, 913–922. [Google Scholar] [CrossRef]
- Feng, Z.; Yang, G.; Rong, M.; Ou, H.; Fu, R. Synthesis and characterization of solid bisphenol A type epoxy resins. Thermosetting Resin. 2010, 6, 33–36. [Google Scholar] [CrossRef]
- Wu, Z.; Li, S.; Lu, J. Improved method for determining epoxy value. J. Wuhan Inst. Chem. Tech. 2006, 1, 5–7. (In Chinese) [Google Scholar]
- Deng, A.; Dang, J.; Mu, R. Study on epoxy resin graft modified by acrylic acid. Chem. Adhesion. 2013, 1, 22–25. (In Chinese) [Google Scholar]



| Formulation | Raw Materials | Mass/g |
|---|---|---|
| 1 | Epoxy resin | 48 |
| 2 | Ethylene glycol monobutyl ether | 15 |
| 3 | n-Butanol | 20 |
| 4 | MAA | 8 |
| 5 | ST | 6 |
| 6 | BA | 6 |
| 7 | BPO | 1.6 |
| 8 | n-Butanol | 3.8 |
| 9 | DMEA | 6.4 |
| 10 | Ethylene glycol monobutyl ether | 3.7 |
| 11 | Deionized water | 140 |
| Number | Mn | Mw | Mz | Mw/Mn | Mz/Mw |
|---|---|---|---|---|---|
| a | 3980 | 5508 | 7716 | 1.384 | 1.401 |
| b | 4905 | 6681 | 9540 | 1.362 | 1.428 |
| c | 6004 | 8286 | 11,683 | 1.380 | 1.410 |
| d | 7084 | 9974 | 14,213 | 1.408 | 1.425 |
| e | 7861 | 10,667 | 14,806 | 1.357 | 1.388 |
| Epoxy Resin | Pencil Hardness | Adhesion /Grade | Flexibility /mm | Water Resistance/d | Salt Water Resistance/d | Acid Resistance/d | Alkali Resistance/d |
|---|---|---|---|---|---|---|---|
| a | 4H | 1 | 1 | >60 | <20 | <7 | <3 |
| b | 4H | 1 | 1 | >60 | <20 | <10 | <5 |
| c | 5H | 1 | 1 | >60 | >30 | ≥10 | ≥5 |
| d | 4H | 1 | 1 | >60 | >30 | <5 | <3 |
| e | 5H | 1 | 1 | >60 | >30 | <5 | <3 |
| Grafting Temperature (°C) | Resin Water Dispersibility | Storage Stability |
|---|---|---|
| 110 | rather poor | Stratification, a small amount of light yellow precipitate |
| 120 | Good | Stable white emulsion with a slight blue tint |
| 130 | rather poor | Delamination, a small amount of brown gel |
| 140 | poor | Delamination, adhesion appeared, reddish-brown gel |
| Epoxy Resin Dosage/% | 10 | 12 | 14 | 16 | 18 | 20 | 22 |
|---|---|---|---|---|---|---|---|
| Pencil hardness | 2H | 2H | 3H | 4H | 5H | 4H | 4H |
| Adhesion/grade | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| Flexibility/mm | 1 | 1 | 1 | 1 | 1 | 1 | 2 |
| MAA Dosage/% | Water Dispersibility | Pencil Hardness | Adhesion/Grade | Flexibility/mm |
|---|---|---|---|---|
| 1 | Poor | — | — | — |
| 2 | Rather poor | 3H | 2 | 2 |
| 3 | Good | 5H | 1 | 1 |
| 4 | Good | 4H | 1 | 1 |
| 5 | Good | 3H | 2 | 2 |
| 6 | Good | 3H | 3 | 2 |
| Neutralizing Agent | Resin Water Dispersibility | Emulsion State | Film-Forming Properties |
|---|---|---|---|
| Ammonia | Poor | Higher viscosity | Poor |
| Triethylamine | Good | Moderate viscosity, stable emulsion | Poor |
| DMEA | Good | Moderate viscosity, stable emulsion | Good |
| Degree of Neutralization (%) | Particle Size (nm) | Viscosity (mPa·s) | Dispersion Surface Condition | Stability (d) |
|---|---|---|---|---|
| 35 | 467.6 | 10.8 | Small viscosity, white, with a small amount of granularity | ≤30 |
| 50 | 368.2 | 12.2 | Low viscosity, white | ≤90 |
| 65 | 242.3 | 141.5 | Low viscosity, milky white | ≤240 |
| 80 | 144.5 | 438.3 | Moderate viscosity, milky white | ≥360 |
| 95 | 128 | 3644.8 | High viscosity, milky white | ≤180 |
| 110 | 119 | 13,381.6 | Very viscous, white-yellowish | ≤30 |
| 125 | — | — | Gel | — |
| Neutralization Temperature (°C) | Water Dispersibility of Grafting Products |
|---|---|
| 30 | Large viscosity, poor flowability, semi-solid, and difficult to disperse |
| 40 | Large viscosity, poor flowability, semi-solid, and difficult to disperse |
| 50 | Moderate viscosity, turned light yellow after neutralization, and white stable emulsion appeared after water dispersion |
| 60 | Moderate viscosity, turned yellow after neutralization, and a small amount of granular material appeared after water dispersion |
| 70 | Small viscosity, turned into a brown color after neutralization, lumpy precipitation appeared after water dispersion, and poor water solubility |
| 80 | Small viscosity, turned into a brown color after neutralization, lumpy precipitation appeared after water dispersion, and poor water solubility |
| Test Items | Test Results | Test Method |
|---|---|---|
| Emulsion appearance | Milky white, slightly blue | Visual assessment |
| Solid content/% | 29.4% | Weighing method |
| pH | 7–8 | Precision pH test paper |
| Viscosity/mPa·s | 438.3 | Rotary Viscometer |
| Storage stability | >360 days | Leave at room temperature |
| Dilution stability | Stable, infinitely dilutable | Deionized water dilution |
| Mechanical stability | No stratification within 30 min | Centrifuge at 4000 r/min |
| Freeze–thaw stability | Repeat 10 times if any stable emulsion forms | Frozen for 18 h, thawed at room temperature for 6 h |
| Alkali stability | Good, still stable emulsion at pH 11 | Ammonia |
| Acid stability | Poor, adding a small amount will destroy the emulsion state | Acetic acid |
| Test Items | Coating Film Properties of Homemade Emulsions | Technical Specifications of IPN8710 |
|---|---|---|
| Pencil hardness | 5H | 2H |
| Adhesion/grade | 1 | 2 |
| Flexibility/mm | 1 | 1 |
| Salt water resistance (5%NaCl)/d | 30 | 3 |
| Acid resistance (5%H2SO4)/d | 10 | 3 |
| Alkali resistance (5%KOH)/d | 5 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhang, Y.; Sun, J. Preparation and Performance of a Self-Produced High-Molecular-Weight Waterborne Epoxy–Acrylic Emulsion. Coatings 2023, 13, 595. https://doi.org/10.3390/coatings13030595
Liu J, Zhang Y, Sun J. Preparation and Performance of a Self-Produced High-Molecular-Weight Waterborne Epoxy–Acrylic Emulsion. Coatings. 2023; 13(3):595. https://doi.org/10.3390/coatings13030595
Chicago/Turabian StyleLiu, Jianbao, Yifu Zhang, and Jianping Sun. 2023. "Preparation and Performance of a Self-Produced High-Molecular-Weight Waterborne Epoxy–Acrylic Emulsion" Coatings 13, no. 3: 595. https://doi.org/10.3390/coatings13030595
APA StyleLiu, J., Zhang, Y., & Sun, J. (2023). Preparation and Performance of a Self-Produced High-Molecular-Weight Waterborne Epoxy–Acrylic Emulsion. Coatings, 13(3), 595. https://doi.org/10.3390/coatings13030595





