Solvent Effect on Small-Molecule Thin Film Formation Deposited Using the Doctor Blade Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
| (Z)-2-(4-bromophenyl)-3-(4-(diphenylamino)phenyl)acrylonitrile | (A), |
| (Z)-3-(4-(diphenylamino)phenyl)-2-phenylacrylonitrile | (B), |
| (Z)-2-(4-chlorophenyl)-3-(4-(diphenylamino)phenyl)acrylonitrile | (C), |
| (Z)-2-(4-(fluorophenyl)-3-(4-(diphenylamino)phenyl)acrylonitrile | (D) |
2.2. Film Deposition
2.3. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, S.; Duan, L.; Hou, L.; Qiao, J.; Zhang, D.; Dong, G.; Wang, L.; Qiu, Y. A Comparison Study of the Organic Small Molecular Thin Films Prepared by Solution Process and Vacuum Deposition: Roughness, Hydrophilicity, Absorption, Photoluminescence, Density, Mobility, and Electroluminescence. J. Phys. Chem. C 2011, 115, 14278–14284. [Google Scholar] [CrossRef]
- Mitzi, D.B. Thin-Film Deposition of Organic−Inorganic Hybrid Materials. Chem. Mater. 2001, 13, 3283–3298. [Google Scholar] [CrossRef]
- Diao, Y.; Shaw, L.; Bao, Z.; Mannsfeld, S.C.B. Morphology control strategies for solution-processed organic semiconductor thin films. Energy Environ. Sci. 2014, 7, 2145–2159. [Google Scholar] [CrossRef]
- Kan, B.; Li, M.; Zhang, Q.; Liu, F.; Wan, X.; Wang, Y.; Ni, W.; Long, G.; Yang, X.; Feng, H.; et al. A Series of Simple Oligomer-like Small Molecules Based on Oligothiophenes for Solution-Processed Solar Cells with High Efficiency. J. Am. Chem. Soc. 2015, 137, 3886–3893. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wan, X.; Wang, F.; Zhou, J.; Long, G.; Tian, J.; You, J.; Yang, Y.; Chen, Y. Spin-Coated Small Molecules for High Performance Solar Cells. Adv. Energy Mater. 2011, 1, 771–775. [Google Scholar] [CrossRef]
- Rodríguez-Seco, C.; Cabau, L.; Vidal-Ferran, A.; Palomares, E. Advances in the Synthesis of Small Molecules as Hole Transport Materials for Lead Halide Perovskite Solar Cells. Accounts Chem. Res. 2018, 51, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Zahid, S.; Rasool, A.; Shehzad, R.A.; Bhatti, I.A.; Iqbal, J. Tuning the optoelectronic properties of triphenylamine (TPA) based small molecules by modifying central core for photovoltaic applications. J. Mol. Model. 2021, 27, 237. [Google Scholar] [CrossRef]
- Sharif, A.; Jabeen, S.; Iqbal, S.; Iqbal, J. Tuning the optoelectronic properties of dibenzochrysene (DBC) based small molecules for organic solar cells. Mater. Sci. Semicond. Process. 2021, 127, 105689. [Google Scholar] [CrossRef]
- Pérez–Gutiérrez, E.; Percino, M.J.; Bernal, W.; Cerón, M.; Ceballos, P.; Rivadeneyra, M.S.; Siegler, M.A.; Thamotharan, S. Fluorescence tuning with a single dye embedded in a polymer matrix and its application on multicolor OLEDs. Dye. Pigment. 2020, 186, 108979. [Google Scholar] [CrossRef]
- You, J.-D.; Tseng, S.-R.; Meng, H.-F.; Yen, F.-W.; Lin, I.-F.; Horng, S.-F. All-solution-processed blue small molecular organic light-emitting diodes with multilayer device structure. Org. Electron. 2009, 10, 1610–1614. [Google Scholar] [CrossRef]
- Chang, H.-W.; Lee, Y.-T.; Tseng, M.-R.; Jang, M.-H.; Yeh, H.-C.; Luo, F.-T.; Meng, H.-F.; Chen, C.-T.; Chi, Y.; Qiu, Y.; et al. General application of blade coating to small-molecule hosts for organic light-emitting diode. Synth. Met. 2014, 196, 99–109. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chang, H.-W.; Chang, Y.-F.; Chang, B.-J.; Lin, Y.-S.; Jian, P.-S.; Yeh, H.-C.; Chien, H.-T.; Chen, E.-C.; Chao, Y.-C.; et al. Continuous blade coating for multi-layer large-area organic light-emitting diode and solar cell. J. Appl. Phys. 2011, 110, 094501. [Google Scholar] [CrossRef]
- Peters, K.; Raupp, S.; Hummel, H.; Bruns, M.; Scharfer, P.; Schabel, W. Formation of blade and slot die coated small molecule multilayers for OLED applications studied theoretically and by XPS depth profiling. AIP Adv. 2016, 6, 065108. [Google Scholar] [CrossRef]
- Yu, Y.; Sun, R.; Wang, T.; Yuan, X.; Wu, Y.; Wu, Q.; Shi, M.; Yang, W.; Jiao, X.; Min, J. Improving Photovoltaic Performance of Non-Fullerene Polymer Solar Cells Enables by Fine-Tuning Blend Microstructure via Binary Solvent Mixtures. Adv. Funct. Mater. 2020, 31, 2008767. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, C.; Deng, W.; Achille, M.T.; Jie, J.; Zhang, X. Meniscus-guided coating of organic crystalline thin films for high-performance organic field-effect transistors. J. Mater. Chem. C 2020, 8, 9133–9146. [Google Scholar] [CrossRef]
- Ding, L.; Zhao, J.; Huang, Y.; Tang, W.; Chen, S.; Guo, X. Flexible-Blade Coating of Small Molecule Organic Semiconductor for Low Voltage Organic Field Effect Transistor. IEEE Electron Device Lett. 2017, 38, 338–340. [Google Scholar] [CrossRef]
- Pierre, A.; Sadeghi, M.; Payne, M.M.; Facchetti, A.; Anthony, J.E.; Arias, A.C. All-Printed Flexible Organic Transistors Enabled by Surface Tension-Guided Blade Coating. Adv. Mater. 2014, 26, 5722–5727. [Google Scholar] [CrossRef] [PubMed]
- Percino, J.; Cerón, M.; Venkatesan, P.; Ceballos, P.; Bañuelos, A.; Rodríguez, O.; Siegler, M.A.; Robles, F.; Chapela, V.M.; Soriano-Moro, G.; et al. Two Different Emissions of (2Z)-2-(4-Bromophenyl)-3-[4-(dimethylamino)phenyl]prop-2-enenitrile Due to Crystal Habit and Size: Synthesis, Optical, and Supramolecular Characterization. Cryst. Growth Des. 2017, 17, 1679–1694. [Google Scholar] [CrossRef]
- Yeh, H.-C.; Meng, H.-F.; Lin, H.-W.; Chao, T.-C.; Tseng, M.-R.; Zan, H.-W. All-small-molecule efficient white organic light-emitting diodes by multi-layer blade coating. Org. Electron. 2012, 13, 914–918. [Google Scholar] [CrossRef]
- Temiño, I.; Basiricò, L.; Fratelli, I.; Tamayo, A.; Ciavatti, A.; Mas-Torrent, M.; Fraboni, B. Morphology and mobility as tools to control and unprecedentedly enhance X-ray sensitivity in organic thin-films. Nat. Commun. 2020, 11, 2136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, H.; Lin, B.; Yuan, J.; Xu, X.; Wu, J.; Zhou, K.; Guo, X.; Zhang, M.; Ma, W. A blade-coated highly efficient thick active layer for non-fullerene organic solar cells. J. Mater. Chem. A 2019, 7, 22265–22273. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Lin, B.; Bi, Z.; Zhou, X.; Naveed, H.B.; Zhou, K.; Yan, H.; Tang, Z.; Ma, W. Achieving Balanced Crystallization Kinetics of Donor and Acceptor by Sequential-Blade Coated Double Bulk Heterojunction Organic Solar Cells. Adv. Energy Mater. 2020, 10, 2000826. [Google Scholar] [CrossRef]
- Chang, Y.-F.; Chiu, Y.-C.; Chang, H.-W.; Wang, Y.-S.; Shih, Y.-L.; Wu, C.-H.; Liu, Y.-L.; Lin, Y.-S.; Meng, H.-F.; Chi, Y.; et al. Interface and thickness tuning for blade coated small-molecule organic light-emitting diodes with high power efficiency. J. Appl. Phys. 2013, 114, 123101. [Google Scholar] [CrossRef]
- Basu, A.; Niazi, M.R.; Scaccabarozzi, A.D.; Faber, H.; Fei, Z.; Anjum, D.H.; Paterson, A.F.; Boltalina, O.; Heeney, M.; Anthopoulos, T.D. Impact of p-type doping on charge transport in blade-coated small-molecule:polymer blend transistors. J. Mater. Chem. C 2020, 8, 15368–15376. [Google Scholar] [CrossRef]
- Xue, R.; Zhang, M.; Luo, D.; Chen, W.; Zhu, R.; Yang, Y.; Li, Y.; Li, Y. Dopant-free hole transporting materials with supramolecular interactions and reverse diffusion for efficient and modular p-i-n perovskite solar cells. Sci. China Chem. 2020, 63, 987–996. [Google Scholar] [CrossRef]
- Strawhecker, K.E.; Kumar, S.K.; Douglas, J.F.; Karim, A. The Critical Role of Solvent Evaporation on the Roughness of Spin-Cast Polymer Films. Macromolecules 2001, 34, 4669–4672. [Google Scholar] [CrossRef]
- Fowler, P.D.; Ruscher, C.; McGraw, J.D.; Forrest, J.A.; Dalnoki-Veress, K. Controlling Marangoni-induced instabilities in spin-cast polymer films: How to prepare uniform films. Eur. Phys. J. E 2016, 39, 90. [Google Scholar] [CrossRef]
- Birnie, D.P. Rational solvent selection strategies to combat striation formation during spin coating of thin films. J. Mater. Res. 2001, 16, 1145–1154. [Google Scholar] [CrossRef]
- Feng, S.; Ma, D.; Qiu, Y.; Duan, L. Deep insights into the viscosity of small molecular solutions for organic light-emitting diodes. RSC Adv. 2018, 8, 4153–4161. [Google Scholar] [CrossRef]
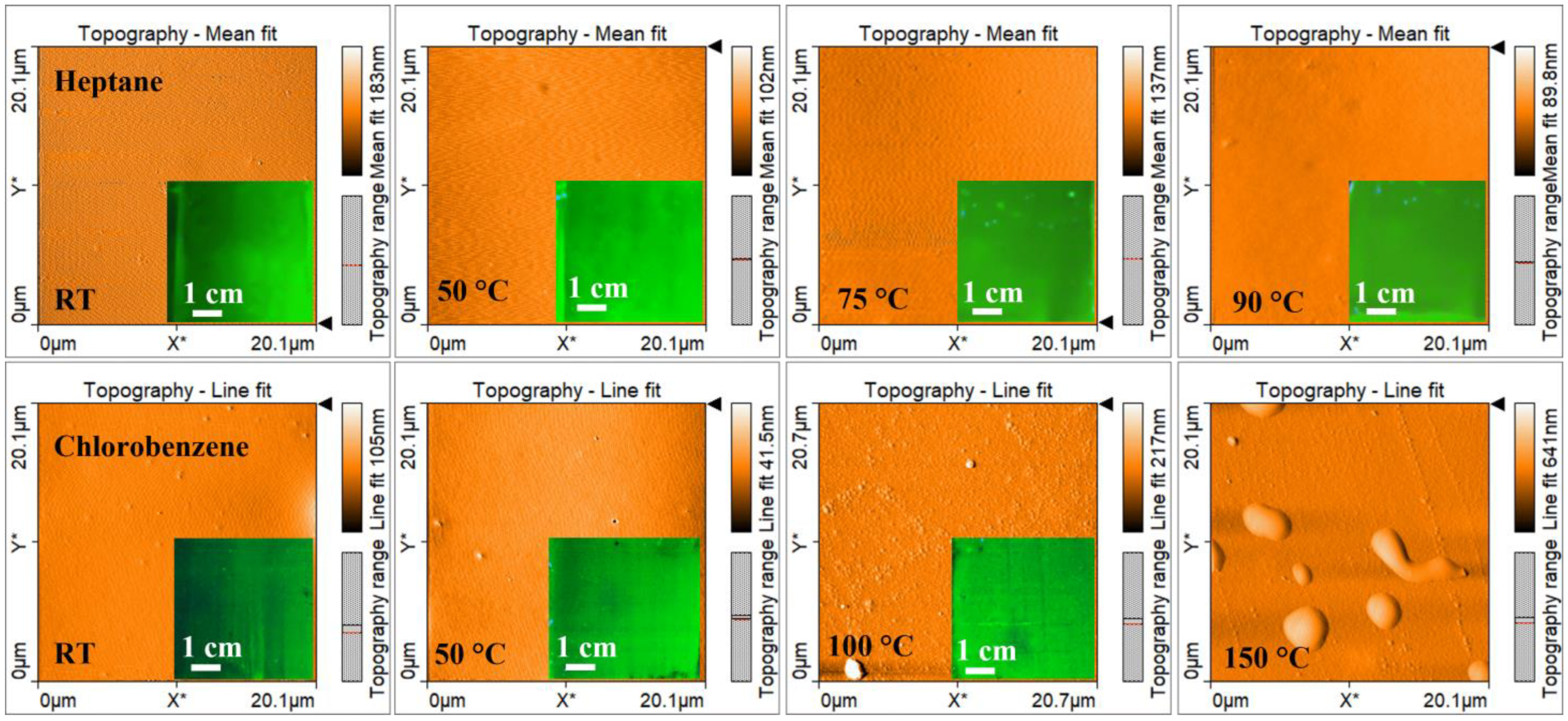

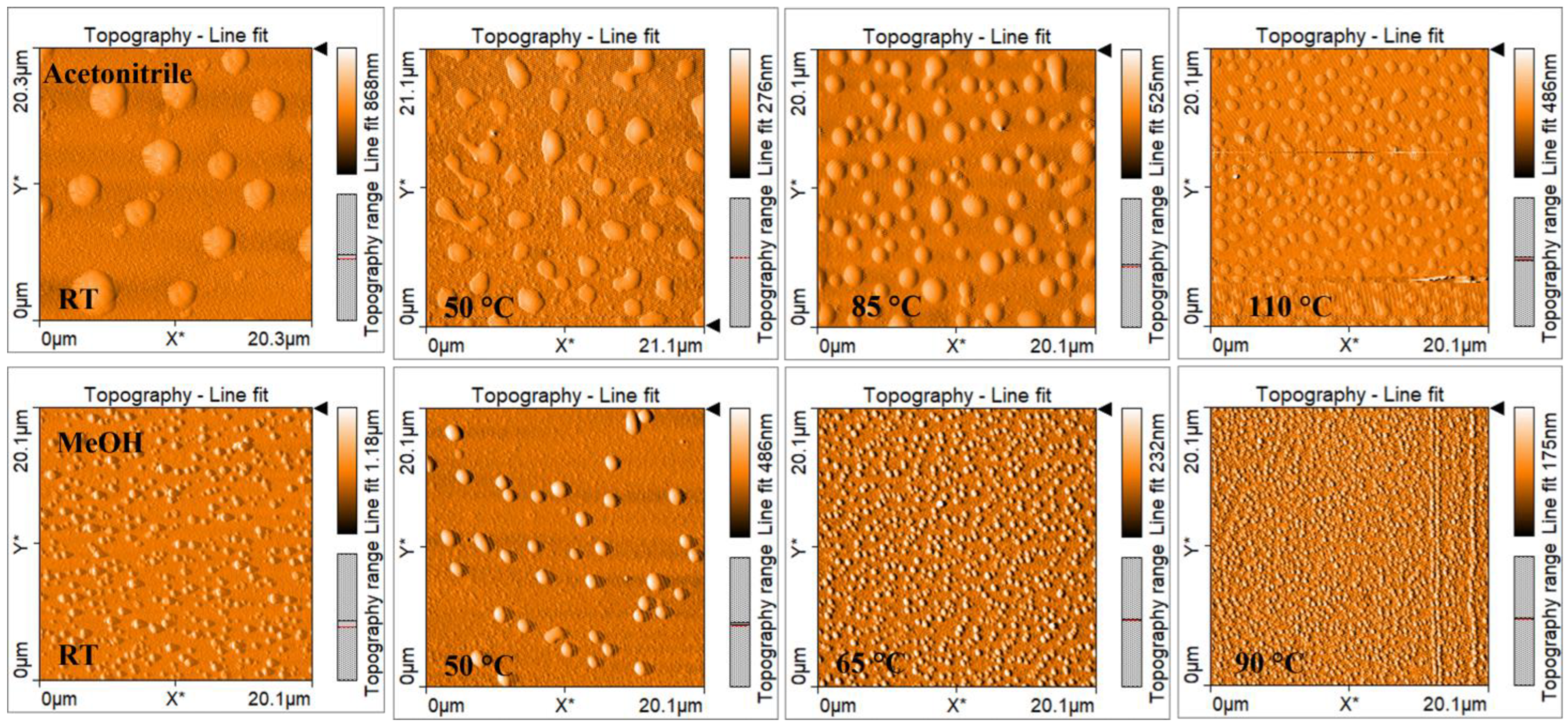
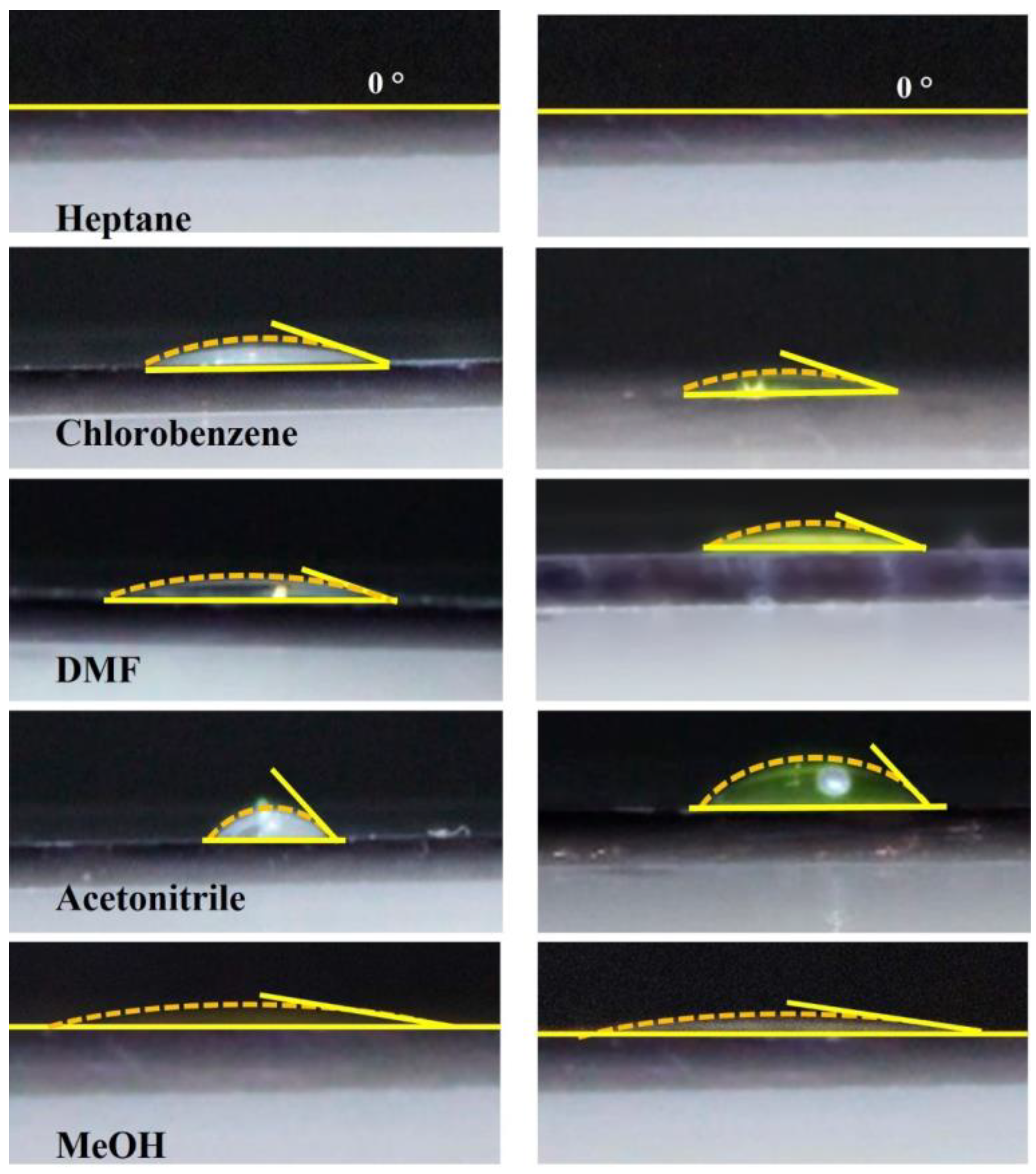
| Solvent | Relative Polarity | Vapor Pressure (hPa) | Boiling Point (°C) | Concentration (mg/mL) | Deposition Temperature (°C) | Deposit Features |
|---|---|---|---|---|---|---|
| Heptane | 0.012 | 48 | 98 | 2.5 | RT | Dense and homogeneous film |
| 50 | ||||||
| 75 | ||||||
| 90 | ||||||
| Chlorobenzene | 0.188 | 12 | 132 | 10 | RT | Dense and homogeneous film |
| 50 | ||||||
| 100 | Agglomerates | |||||
| 150 | ||||||
| DMF | 0.386 | 3.5 | 153 | 5 | RT | Dense and homogeneous film |
| 50 | Non total coverage | |||||
| 100 | Small agglomerates | |||||
| 150 | Dense and homogeneous film | |||||
| Acetonitrile | 0.46 | 97 | 81.6 | 4 | RT | Small agglomerates |
| 50 | ||||||
| 85 | ||||||
| 110 | ||||||
| Methanol | 0.762 | 128 | 64.6 | 2 | RT | Small agglomerates |
| 50 | ||||||
| 65 | ||||||
| 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Hernández, R.; Pérez-Gutiérrez, E.; Calvo, F.D.; Beristain, M.F.; Cerón, M.; Percino, M.J. Solvent Effect on Small-Molecule Thin Film Formation Deposited Using the Doctor Blade Technique. Coatings 2023, 13, 425. https://doi.org/10.3390/coatings13020425
Ramos-Hernández R, Pérez-Gutiérrez E, Calvo FD, Beristain MF, Cerón M, Percino MJ. Solvent Effect on Small-Molecule Thin Film Formation Deposited Using the Doctor Blade Technique. Coatings. 2023; 13(2):425. https://doi.org/10.3390/coatings13020425
Chicago/Turabian StyleRamos-Hernández, Rodrigo, Enrique Pérez-Gutiérrez, Francisco Domingo Calvo, Miriam Fatima Beristain, Margarita Cerón, and Maria Judith Percino. 2023. "Solvent Effect on Small-Molecule Thin Film Formation Deposited Using the Doctor Blade Technique" Coatings 13, no. 2: 425. https://doi.org/10.3390/coatings13020425
APA StyleRamos-Hernández, R., Pérez-Gutiérrez, E., Calvo, F. D., Beristain, M. F., Cerón, M., & Percino, M. J. (2023). Solvent Effect on Small-Molecule Thin Film Formation Deposited Using the Doctor Blade Technique. Coatings, 13(2), 425. https://doi.org/10.3390/coatings13020425






