Fluorine-Free Dual Superamphiphobic Cellulose Paper Coated with Mushroom-like Pillar Microstructure
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
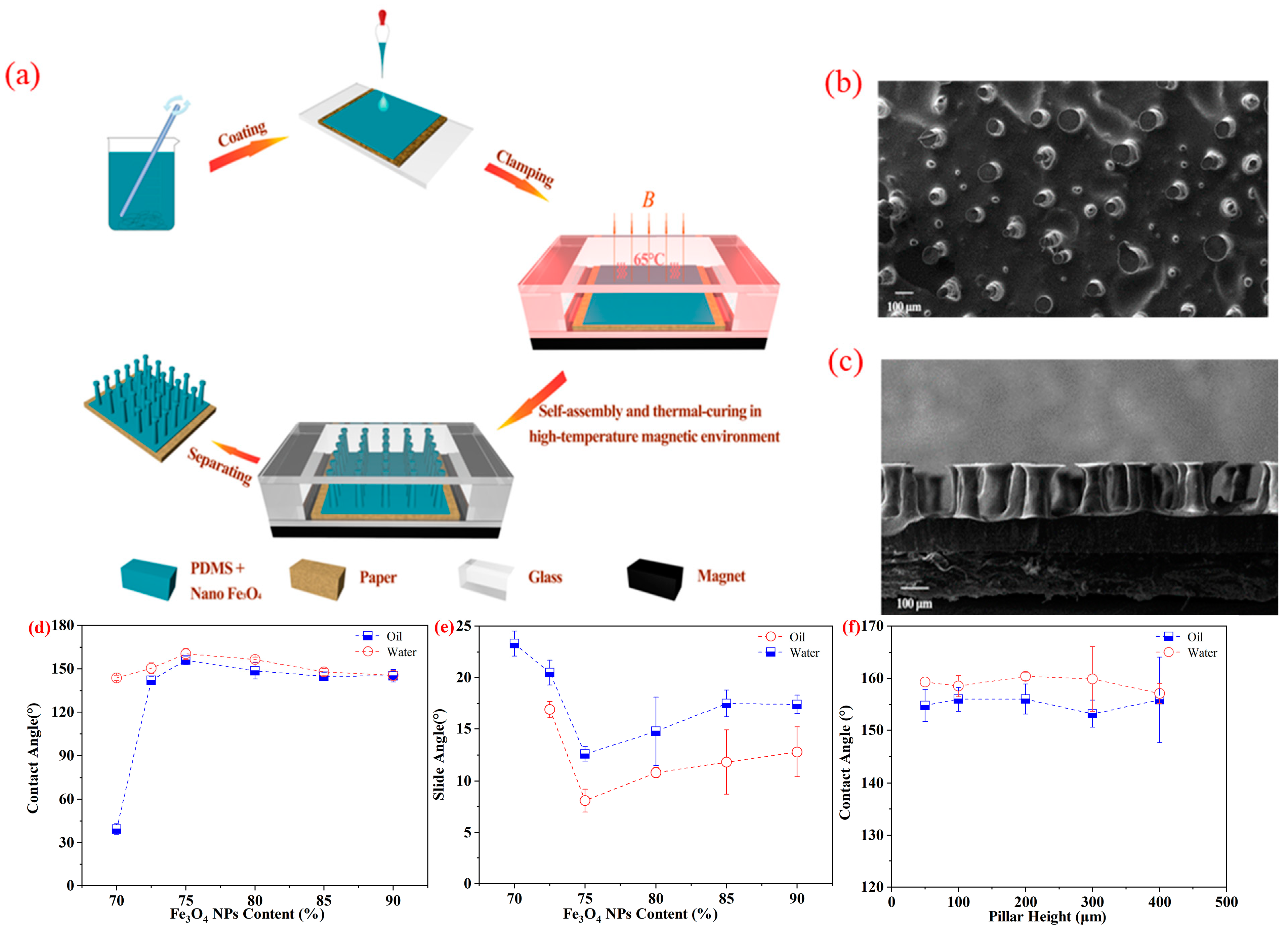
2.2. Fabrication of Superamphiphobic Paper
2.3. Characterization
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. Contact Angles (CAs) and Sliding Angles (SAs)
2.3.3. Grease Resistance and Grease Permeability Properties
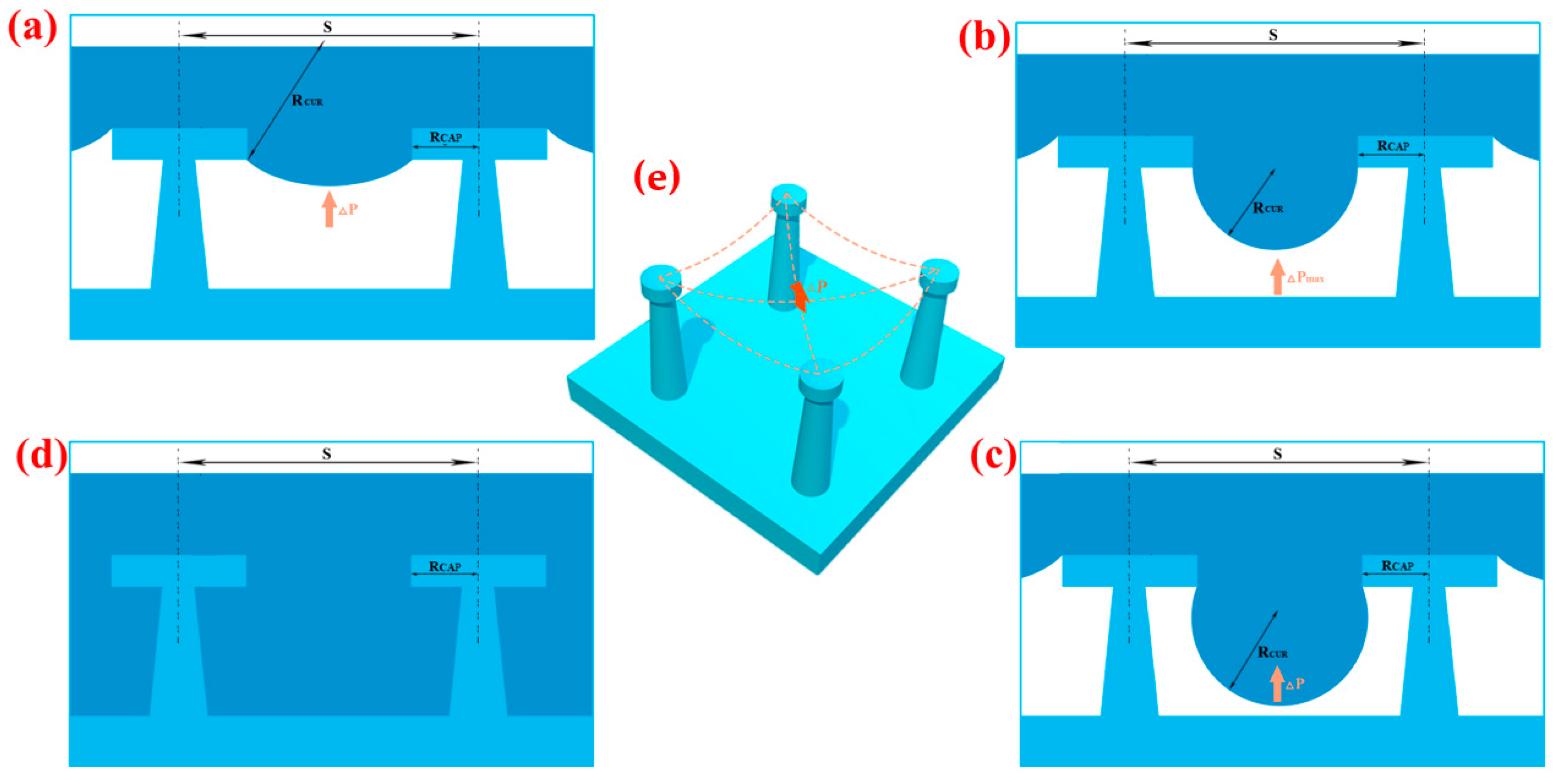
2.3.4. Caps Radius (RCAP) and Spacing between Two Pillars (S)
3. Result and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yong, J.; Chen, F.; Yang, Q.; Huo, J.; Hou, X. Superoleophobic surfaces. Chem. Soc. Rev. 2017, 46, 4168–4217. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, S.S.; Li, Z.; Sirinakbumrung, N.; Rabnawaz, M. Zein and PVOH-based bilayer approach for plastic-free, repulpable and biodegradable oil-and water-resistant paper as a replacement for single-use plastics. Ind. Eng. Chem. Res. 2020, 59, 17856–17866. [Google Scholar] [CrossRef]
- Zhang, Y.; Kan, L.; Feng, H.; Ling, H.; Wang, X. All-biomass-based eco-friendly waterproof coating for paper-based green packaging. Green Chem. 2022, 24, 7039–7048. [Google Scholar]
- Kansal, D.; Hamdani, S.S.; Ping, R.; Sirinakbumrung, N.; Rabnawaz, M. Food-safe Chitosan−Zein dual-layer coating for water- and oil-repellent paper substrates. ACS Sustain. Chem. Eng. 2020, 8, 6887–6897. [Google Scholar] [CrossRef]
- Ying, B.; Park, S.; Chen, L.; Dong, X.; Young, E.W.K.; Liu, X. NanoPADs and nanoFACEs: An optically transparent nanopaper-based device for biomedical applications. Lab. Chip. 2020, 20, 3322–3333. [Google Scholar] [CrossRef]
- Jalal, U.M.; Jin, G.J.; Shim, J.S. Paper-plastic hybrid microfluidic device for smartphone-based colorimetric analysis of urine. Anal. Chem. 2017, 89, 13160–13166. [Google Scholar] [CrossRef]
- Gao, B.; Li, X.; Yang, Y.; Chu, J.; He, B. Emerging paper microfluidic devices. Analyst 2019, 144, 6497–6511. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, X.; Jia, M.; Li, B.; Rong, J.; Yang, X. Deposition of CdTe quantum dots on microfluidic paper chips for rapid fluorescence detection of pesticide 2,4-D. Analyst 2019, 144, 1282–1291. [Google Scholar] [CrossRef]
- Antosik, A.K.; Piątek, A.; Wilpiszewska, K. Carboxymethylated starch and cellulose derivatives-based film as human skin equivalent for adhesive properties testing. Carbohydr. Polym. 2019, 222, 115014–115020. [Google Scholar] [CrossRef]
- Liu, D.; Ju, C.; Han, C.; Shi, R.; Chen, X.; Duan, D.; Yan, J.; Yan, X. Nanozyme chemiluminescence paper test for rapid and sensitive detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2021, 173, 112817–112825. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Lee, J.-H.; Hong, S.-I. Water resistance and mechanical properties of biopolymer (alginate and soy protein) coated paperboards. LWT Food Sci. Technol. 2006, 39, 806–813. [Google Scholar] [CrossRef]
- Dinh, T.D.; Phan, M.N.; Nguyen, D.T.; Le, T.M.D.; Nadda, A.K.; Srivastav, A.L.; Pham, T.N.M.; Pham, T.D. Removal of beta-lactam antibiotic in water environment by adsorption technique using cationic surfactant functionalized nanosilica rice husk. Environ. Res. 2022, 210, 112943. [Google Scholar] [CrossRef]
- Vu, T.N.; Le, P.H.P.; Pham, D.N.P.; Hoang, T.H.; Nadda, A.K.; Le, T.S.; Pham, T.D. Highly adsorptive protein inorganic nanohybrid of Moringa seeds protein and rice husk nanosilica for effective adsorption of pharmaceutical contaminants. Chemosphere 2022, 307, 135856. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, C.; Wang, X.; Wang, J.; Zhang, H.; Liu, Y.; Huang, X.; Xu, K.; Bai, Y.; Wang, P. Fabrication of superhydrophobic and lyophobic paper for self-cleaning, moisture-proof and antibacterial activity. Appl. Surf. Sci. 2022, 598, 153639–153650. [Google Scholar] [CrossRef]
- Jiang, L.; Tang, Z.; Clinton, R.M.; Breedveld, V.; Hess, D.W. Two-step process to create “roll-off” superamphiphobic paper surfaces. ACS Appl. Mater. Interfaces 2017, 9, 9195–9203. [Google Scholar] [CrossRef]
- Tudu, B.K.; Kumar, A.; Bhushan, B. Fabrication of superoleophobic cotton fabric for multi-purpose applications. Philos. Trans. R. Soc. A 2019, 377, 20190129. [Google Scholar] [CrossRef]
- Huan, Z.; Huan, C.; Huan, K.; Qiu, L.; Lv, P.; Wu, P.; Yang, Y. Toxic effects of fluoride on organisms. Life Sci. Res. Rep. 2018, 198, 18–24. [Google Scholar]
- Araya, D.; Podgorski, J.; Kumi, M.; Mainoo, P.A.; Berg, M. Fluoride contamination of groundwater resources in Ghana: Country-wide hazard modeling and estimated population at risk. Water Res. 2022, 212, 118083–118092. [Google Scholar] [CrossRef]
- Kansal, D.; Hamdani, S.S.; Ping, R.; Rabnawaz, M. Starch and zein biopolymers as a sustainable replacement for PFAS, silicone oil, and plastic-coated paper. Ind. Eng. Chem. Res. 2020, 59, 12075–12084. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, L.; He, B. Chitosan/montmorillonite coatings for the fabrication of food-safe greaseproof paper. Polymers 2021, 13, 1607. [Google Scholar] [CrossRef]
- Cheng, Q.-Y.; An, X.-P.; Li, Y.-D.; Huang, C.-L.; Zeng, J.-B. Sustainable and biodegradable superhydrophobic coating from epoxidized soybean oil and ZnO nanoparticles on cellulosic substrates for efficient oil/water separation. ACS Sustain. Chem. Eng. 2017, 5, 11440–11450. [Google Scholar] [CrossRef]
- Hamdani, S.S.; Li, Z.; Rabnawaz, M.; Kamdem, D.P.; Khan, B.A. Chitosan-graft-poly(dimethylsiloxane)/zein coatings for the fabrication of environmentally friendly oil- and water-resistant paper. ACS Sustain. Chem. Eng. 2020, 8, 5147–5155. [Google Scholar] [CrossRef]
- Zhang, F.; Tao, H.; Li, Y.; Wang, Y.; Zhou, Y.; Xu, Q.; Ma, J. Enhanced pickering emulsion stabilization of cellulose nanocrystals and application for reinforced and hydrophobic coatings. Coatings 2022, 12, 1594. [Google Scholar] [CrossRef]
- Gu, F.; Yang, W.; Song, J.; Xiao, H.; Wang, W.; Cai, Z. Crosslinked PVA/nanoclay hydrogel coating for improving water vapor barrier of cellulose-based packaging at high temperature and humidity. Coatings 2022, 12, 1562. [Google Scholar] [CrossRef]
- Tuteja, A.; Choi, W.; Ma, M.; Mabry, J.M.; Mazzella, S.A.; Rutledge, G.C.; McKinley, G.H.; Cohen, R.E. Designing superoleophobic surfaces. Science 2007, 318, 1618–1622. [Google Scholar] [CrossRef]
- Cao, P.; Tao, L.; Gong, J.; Wang, T.; Wang, Q.; Ju, J.; Zhang, Y. 4D printing of a sodium alginate hydrogel with step-wise shape deformation based on variation of crosslinking density. ACS Appl. Polym. Mater. 2021, 3, 6167–6175. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, M.-J.; Ouyang, X.; Zhang, A.P.; Tam, H.-Y. 3D μ-printing of polytetrafluoroethylene microstructures: A route to superhydrophobic surfaces and devices. Appl. Mater. Today 2020, 19, 110580–110589. [Google Scholar] [CrossRef]
- Shams, H.; Basit, K.; Khan, M.A.; Saleem, S.; Mansoor, A. Realizing surface amphiphobicity using 3D printing techniques: A critical move towards manufacturing low-cost reentrant geometries. Addit. Manuf. 2021, 38, 101777–101814. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, Z. Recent advances of bioinspired functional materials with specific wettability: From nature and beyond nature. Nanoscale Horiz. 2019, 4, 52–76. [Google Scholar] [CrossRef]
- Kim, J.H.; Shim, T.S.; Kim, S.-H. Lithographic design of overhanging microdisk arrays toward omniphobic surfaces. Adv. Mater. 2016, 28, 291–298. [Google Scholar] [CrossRef]
- Jiao, L.; Tong, J.; Wu, Y.; Hu, Y.; Wu, H.; Li, D.; Chen, R. Self-assembly of supraparticles on a lubricated-superamphiphobic patterned surface. Appl. Surf. Sci. 2022, 576, 151684–151698. [Google Scholar] [CrossRef]
- Ganesh, V.A.; Dinachali, S.S.; Nair, A.S.; Ramakrishna, S. Robust superamphiphobic film from electrospun TiO2 nanostructures. ACS Appl. Mater. Interfaces 2013, 5, 1527–1532. [Google Scholar] [CrossRef]
- Yu, J.; Wang, H.; Yin, N.; Xu, X. HF-assisted one-step synthesis of pompon-like/chip-like FeSe2 particles and their superamphiphobic/antireflective property. RSC Adv. 2014, 4, 24163–24169. [Google Scholar] [CrossRef]
- Hyväluoma, J.; Raiskinmäki, P.; Jäsberg, A.; Koponen, A.; Kataja, M.; Timonen, J. Simulation of liquid penetration in paper. Phys. Rev. E 2006, 73, 36705–36713. [Google Scholar] [CrossRef]
- Masimukku, S.; Hu, Y.-C.; Lin, Z.-H.; Chan, S.-W.; Chou, T.-M.; Wu, J.M. High efficient degradation of dye molecules by PDMS embedded abundant single-layer tungsten disulfide and their antibacterial performance. Nano Energy 2018, 46, 338–346. [Google Scholar] [CrossRef]
- Lv, C.; Wang, J.; Li, Z.; Zhao, K.; Zheng, J. Degradable, reprocessable, self-healing PDMS/CNTs nanocomposite elastomers with high stretchability and toughness based on novel dual-dynamic covalent sacrificial system. Compos. Part B Eng. 2019, 177, 107270. [Google Scholar] [CrossRef]
- Beltran, F.O.; Houk, C.J.; Grunlan, M.A. Bioactive siloxane-containing shape-memory polymer (SMP) scaffolds with tunable degradation rates. ACS Biomater. Sci. Eng. 2021, 7, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, H.; Zhang, J.; Zhu, L.; Zhu, X.; Yu, S.; Xue, J.; Wang, R.; Gan, S.; Wei, B.; et al. Durable superhydrophobic engineered mesh with self-healing and anti-corrosive capabilities for efficient oil/water separation. J. Environ. Chem. Eng. 2022, 10, 108823–108832. [Google Scholar] [CrossRef]
- Lu, A.-H.; Salabas, E.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Anik, M.I.; Hossain, M.K.; Hossain, I.; Mahfuz, A.M.U.B.; Rahman, M.T.; Ahmed, I. Recent progress of magnetic nanoparticles in biomedical applications: A review. Nano Sel. 2021, 2, 1146–1186. [Google Scholar] [CrossRef]
- Huang, Y.; Stogin, B.B.; Sun, N.; Wang, J.; Yang, S.; Wong, T.S. A switchable cross-species liquid repellent surface. Adv. Mater. 2017, 29, 1604641. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, M.; Yang, Y.; Huang, Q.; Fukuda, T.; Wang, Z.; Shen, Y. A bioinspired multilegged soft millirobot that functions in both dry and wet conditions. Nat. Commun. 2018, 9, 3944. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Z.; Wang, Z.; Liang, Y.; Cui, Z.; Zhao, J.; Li, X.; Ren, L. Multistimuli-responsive microstructured superamphiphobic surfaces with large-range, reversible switchable wettability for oil. ACS Appl. Mater. Interfaces 2019, 11, 28478–28486. [Google Scholar] [CrossRef] [PubMed]

| Category | Chemical Agent | WCA/° | OCA/° | Ref. |
|---|---|---|---|---|
| fluorinated | (heptadecafluoro-1,1,2,2-tetradecyl) trimethoxysilane | 154 | 138 | Zhang et al. (2022) [14] |
| (heptadecafluoro-1,1,2,2-tetradecyl) trimethoxysilane | 163 | >150 | Jiang et al. (2017) [15] | |
| Perfluorodecyltriethoxysilane | 171 | 152 | Balraj et al. (2019) [16] | |
| fluorine-free | chitosan-graft-PDMS, zein | 120 | 62.7 | Hamdani et al. (2020) [19] |
| chitosan, montmorillonite | 92.5 | 37.8 | Wang et al. (2021) [21] | |
| soybean oil, stearic acid, and ZnO | 145 | - | Cheng et al. (2017) [22] | |
| butyl acrylate grafted cellulose nanocrystal | 97.8 | - | Zhang et al. (2022) [24] | |
| crosslinked polyvinylalcohol, nanoclay | 108 | - | Gu et al. (2022) [25] |
| Fe3O4 NPs Content | 70% | 72.5% | 75% | 80% | 85% | 90% |
|---|---|---|---|---|---|---|
| RCAP (μm) | 32 ± 17 | 35 ± 13 | 37 ± 18 | 48 ± 23 | 51 ± 20 | 54 ± 24 |
| S (μm) | 318 ± 78 | 283 ± 73 | 237 ± 38 | 231 ± 45 | 217 ± 36 | 175 ± 39 |
| RCAP/S | 0.10 | 0.12 | 0.16 | 0.21 | 0.23 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, X.; Wang, K.; Zhao, L.; Wang, Z.; Wu, M. Fluorine-Free Dual Superamphiphobic Cellulose Paper Coated with Mushroom-like Pillar Microstructure. Coatings 2023, 13, 323. https://doi.org/10.3390/coatings13020323
Ke X, Wang K, Zhao L, Wang Z, Wu M. Fluorine-Free Dual Superamphiphobic Cellulose Paper Coated with Mushroom-like Pillar Microstructure. Coatings. 2023; 13(2):323. https://doi.org/10.3390/coatings13020323
Chicago/Turabian StyleKe, Xun, Kaipeng Wang, Lihong Zhao, Zhiwei Wang, and Min Wu. 2023. "Fluorine-Free Dual Superamphiphobic Cellulose Paper Coated with Mushroom-like Pillar Microstructure" Coatings 13, no. 2: 323. https://doi.org/10.3390/coatings13020323
APA StyleKe, X., Wang, K., Zhao, L., Wang, Z., & Wu, M. (2023). Fluorine-Free Dual Superamphiphobic Cellulose Paper Coated with Mushroom-like Pillar Microstructure. Coatings, 13(2), 323. https://doi.org/10.3390/coatings13020323







