Effect of the Molybdenum Content on Wear and Corrosion Behavior of Fe-B-Based Surface-Alloyed Layer
Abstract
:1. Introduction
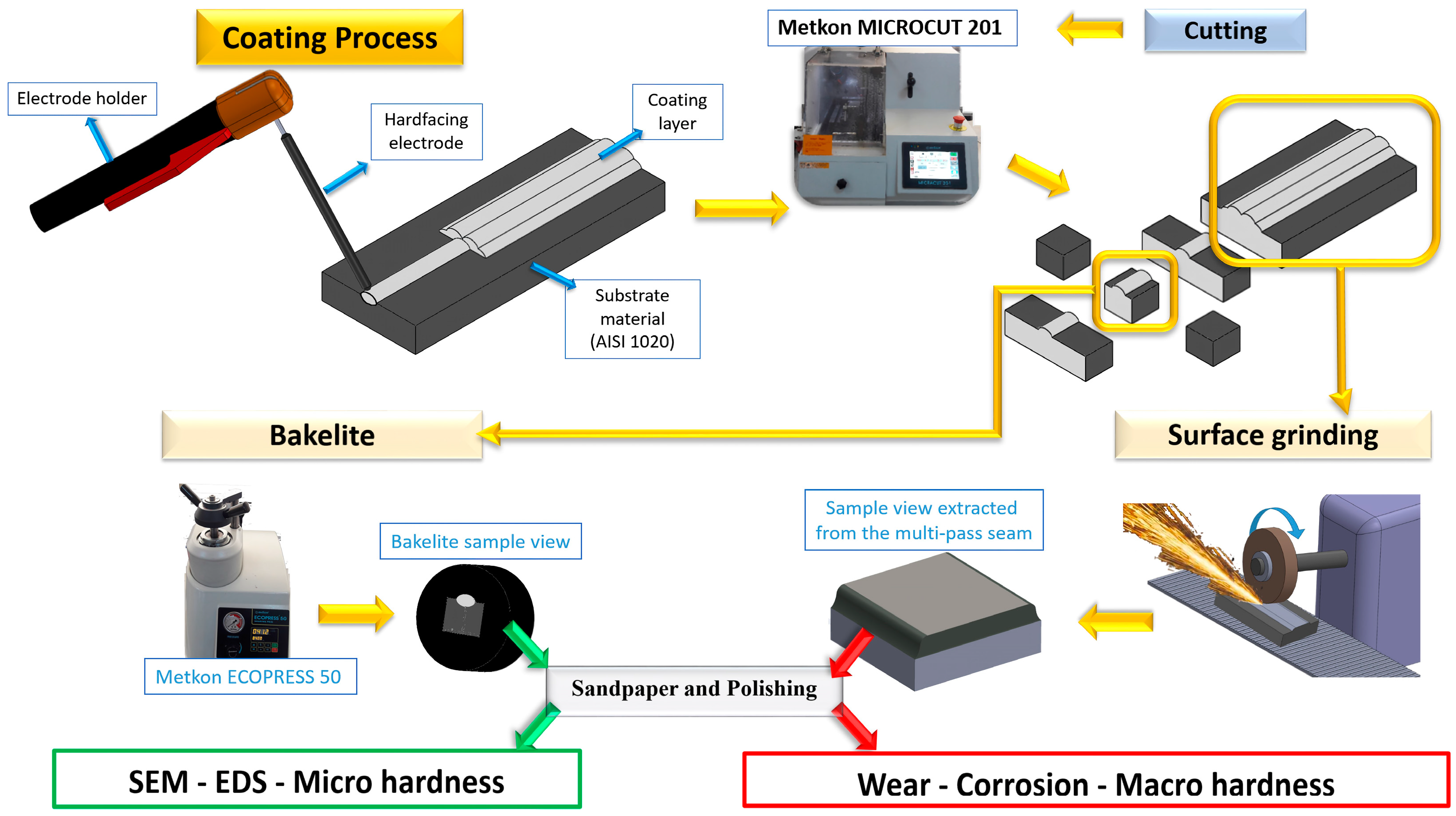
2. Experimental Procedure
3. Results and Discussion
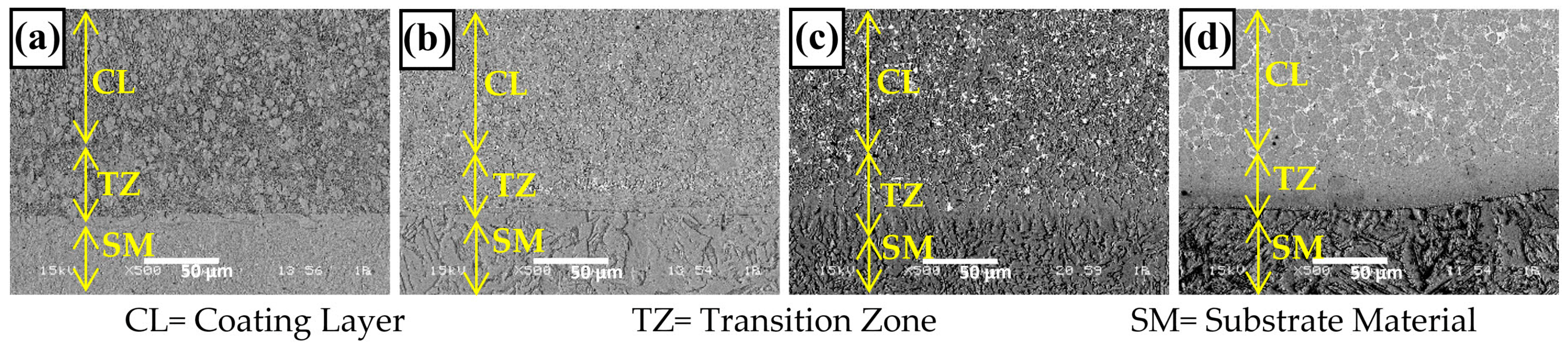
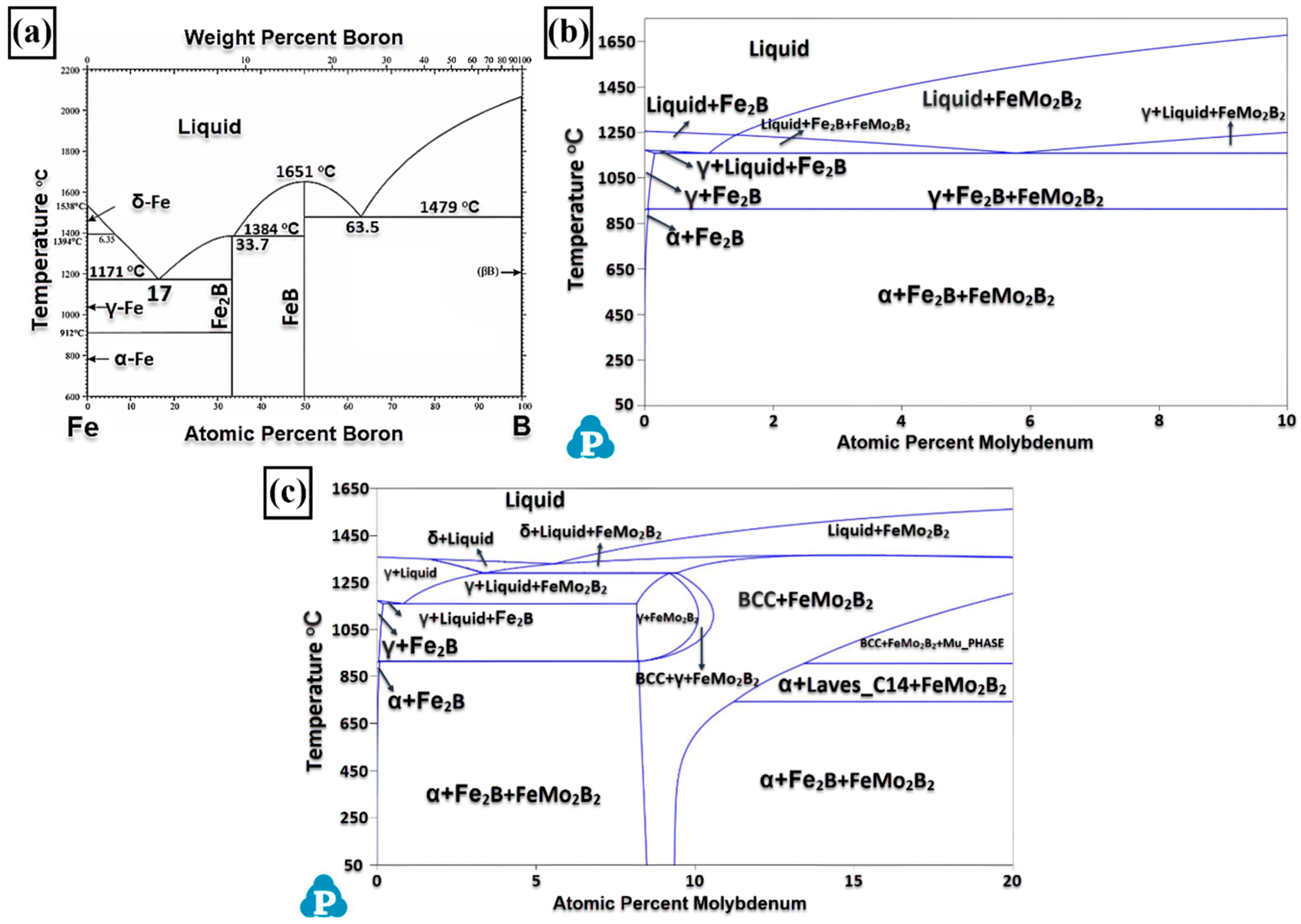
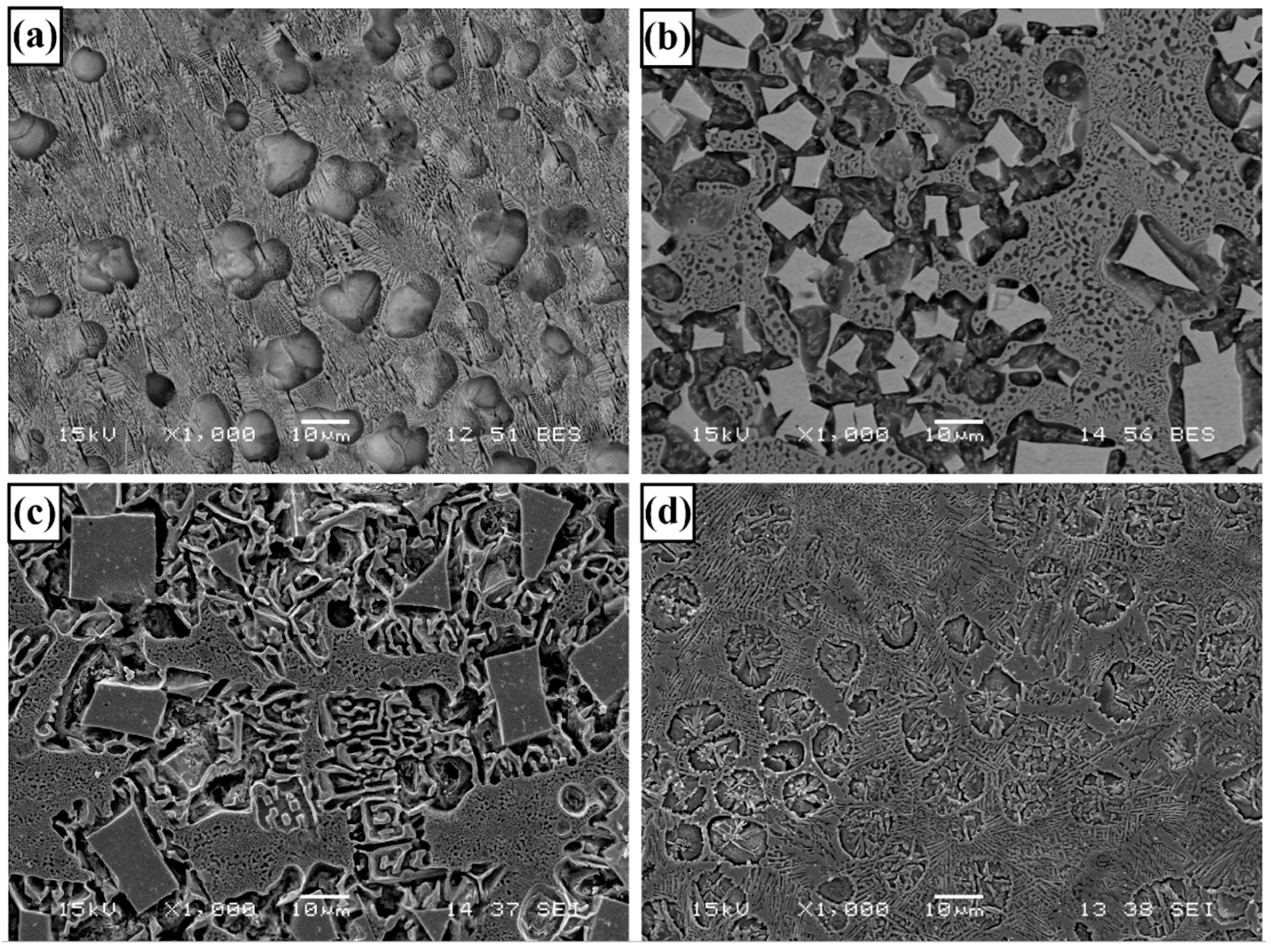
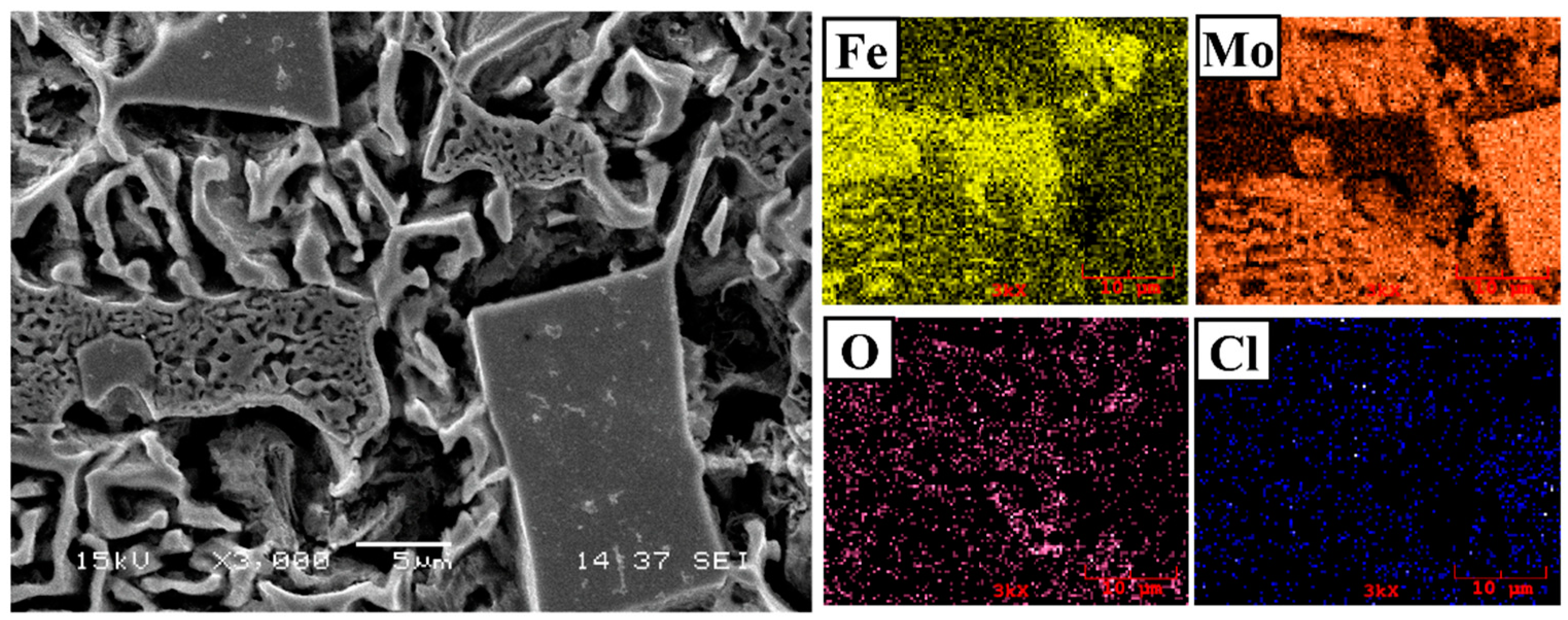
3.1. Microstructure and Phases Analysis
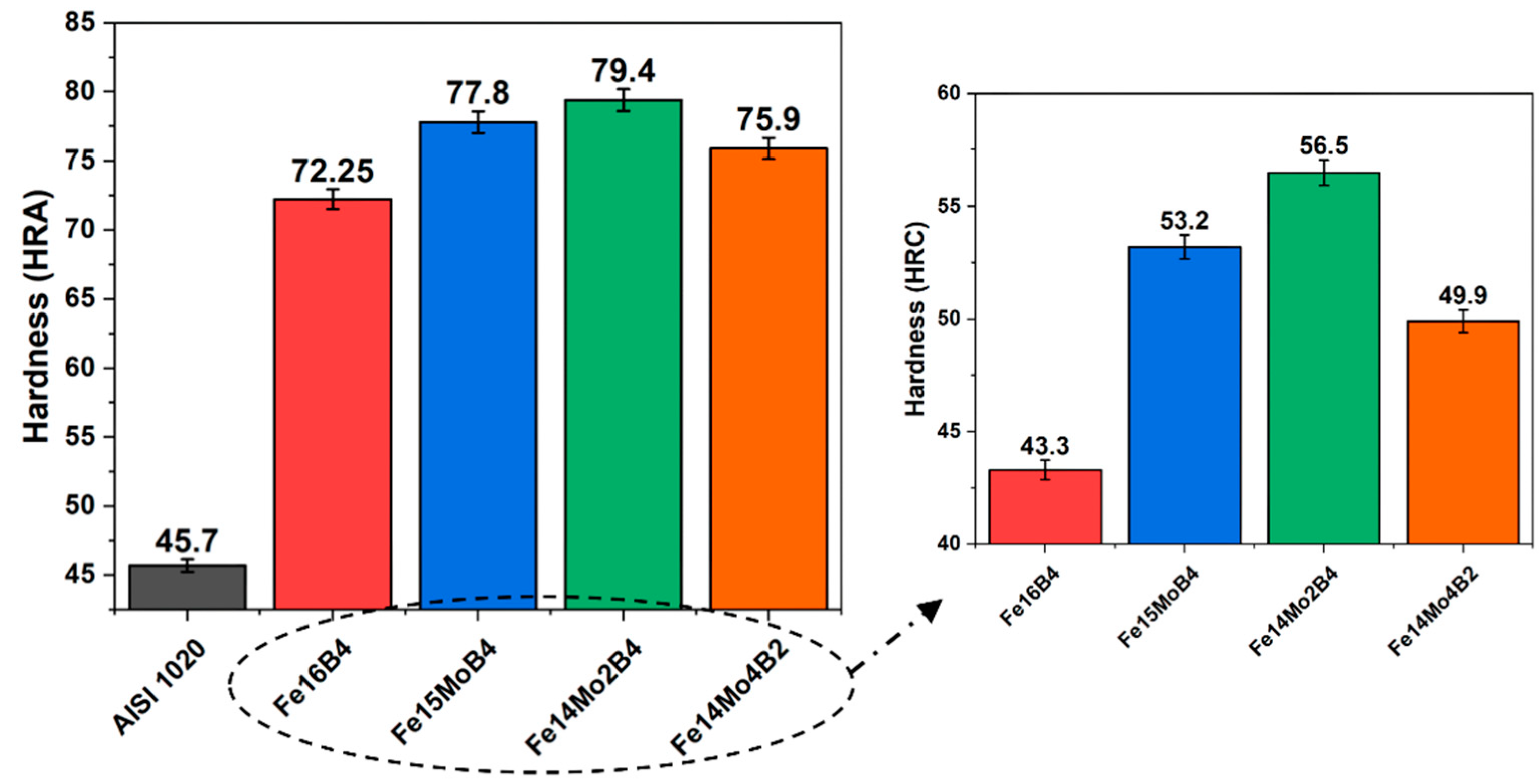
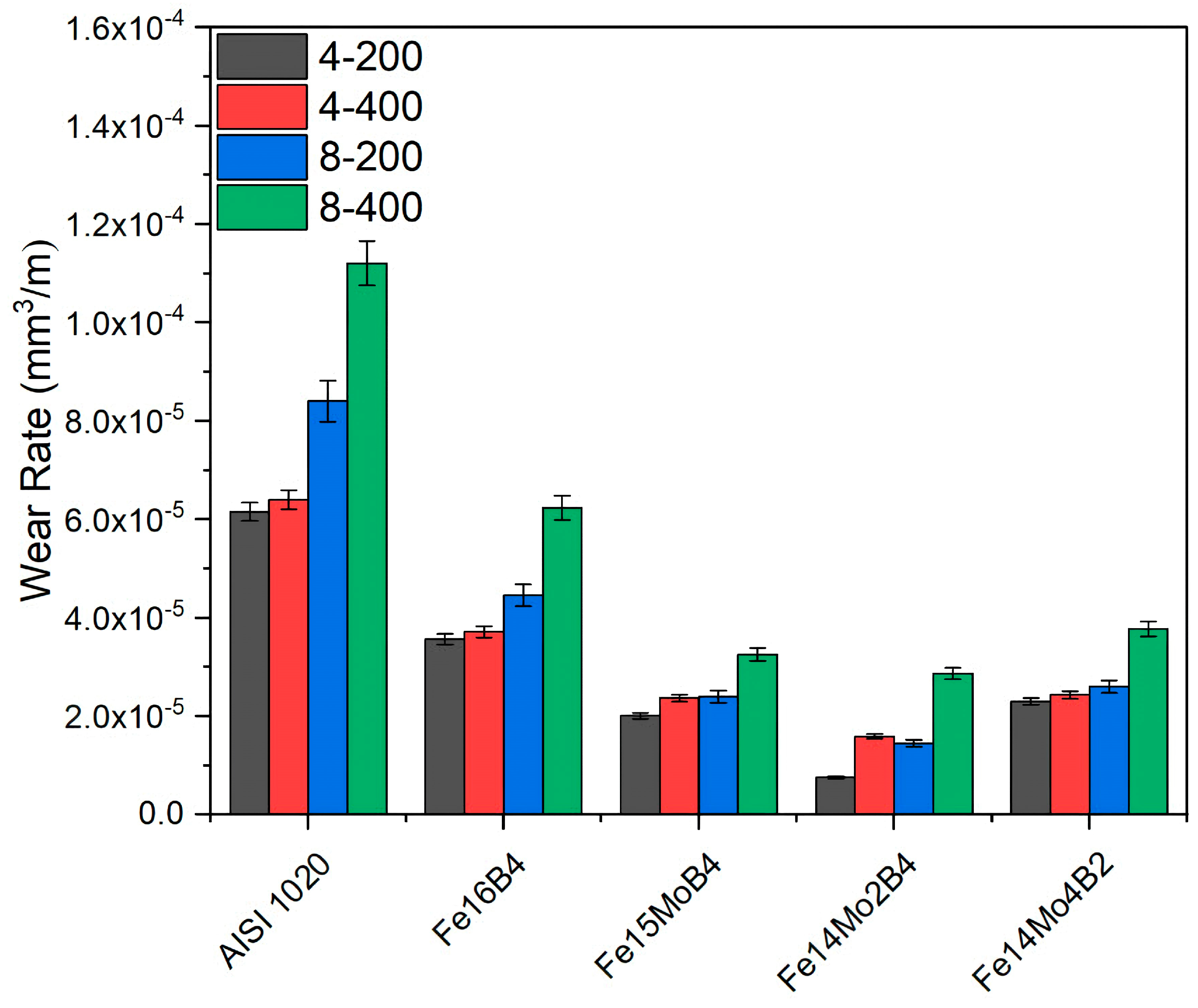
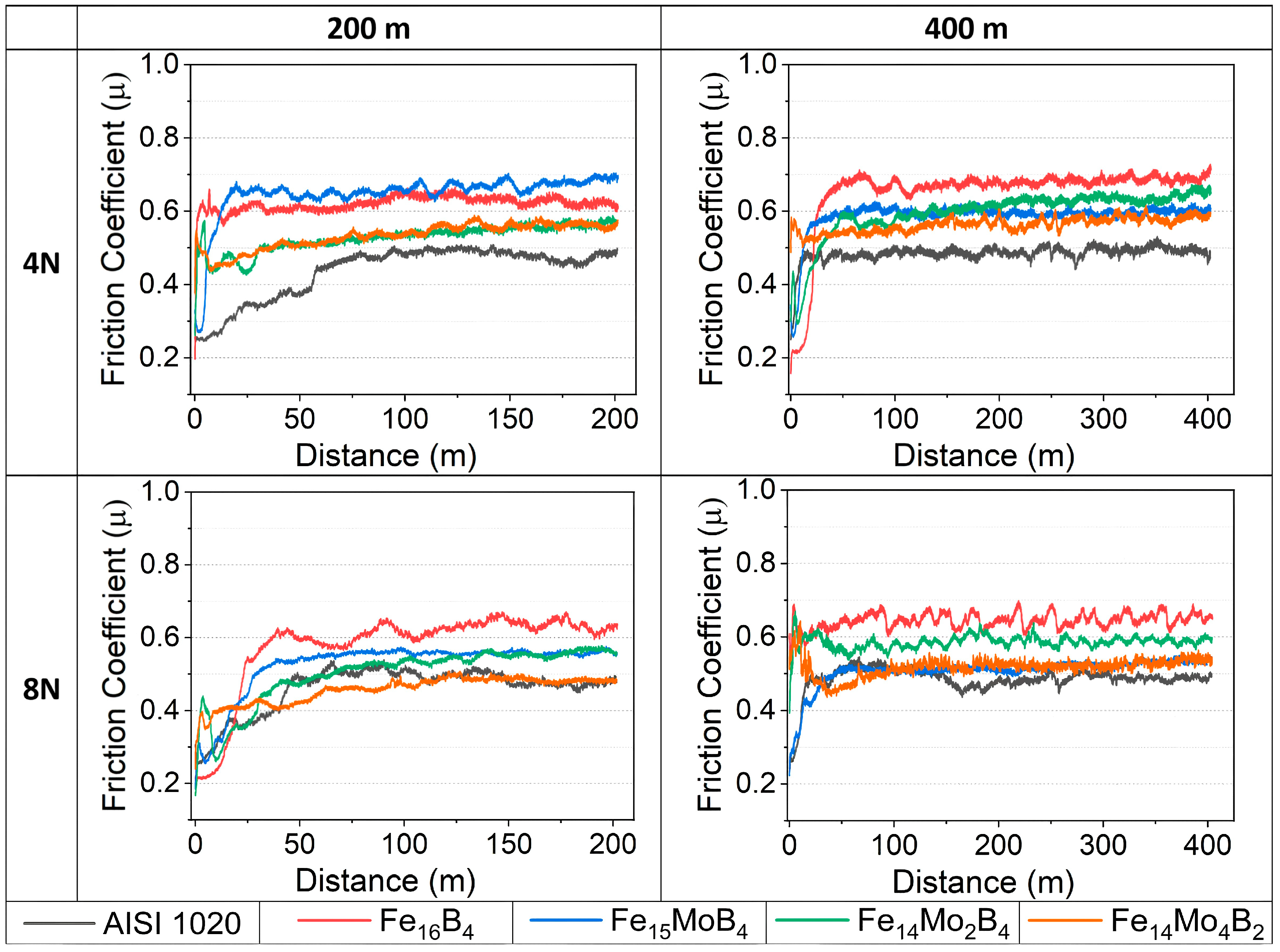
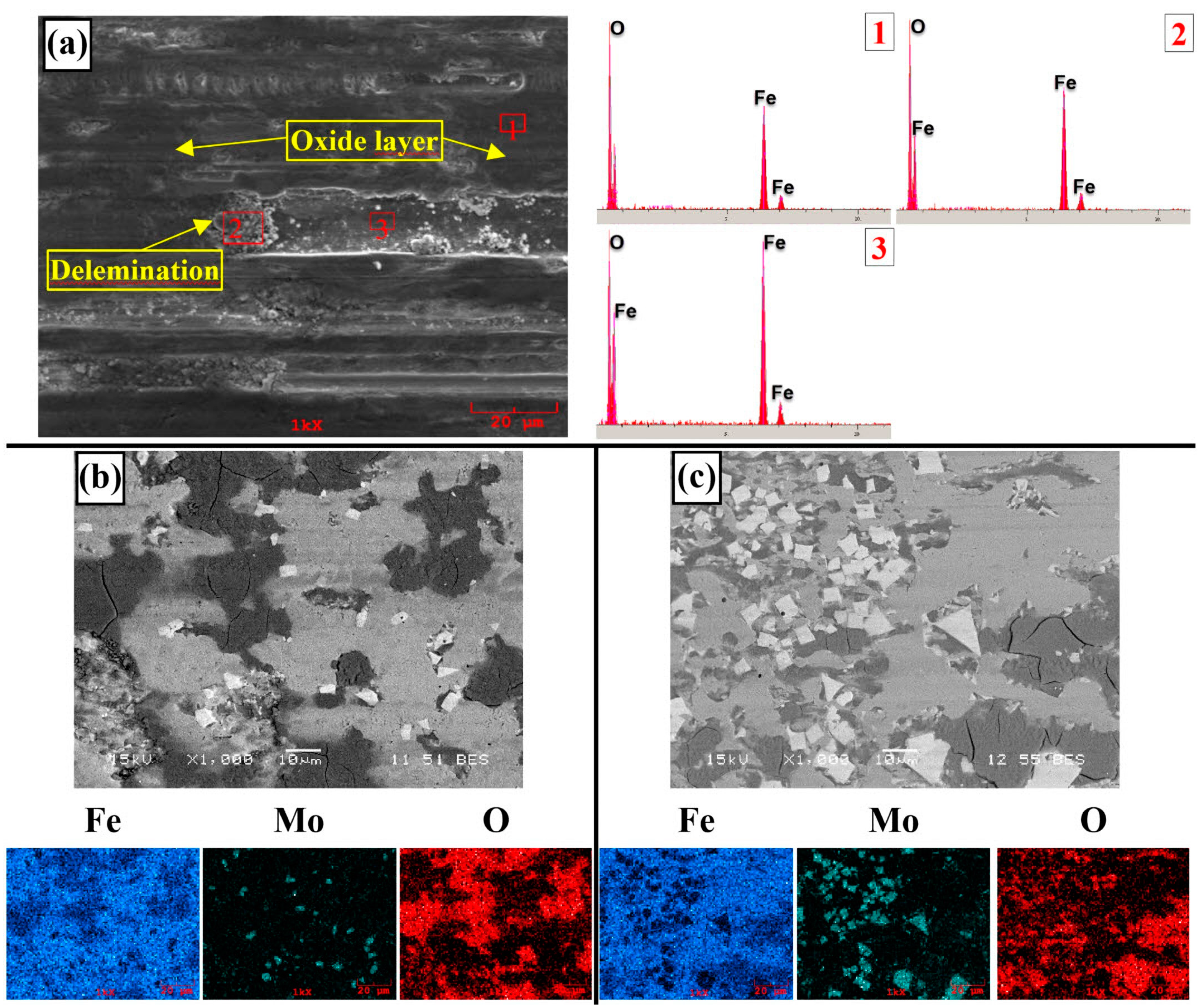
3.2. Hardness and Wear Tests
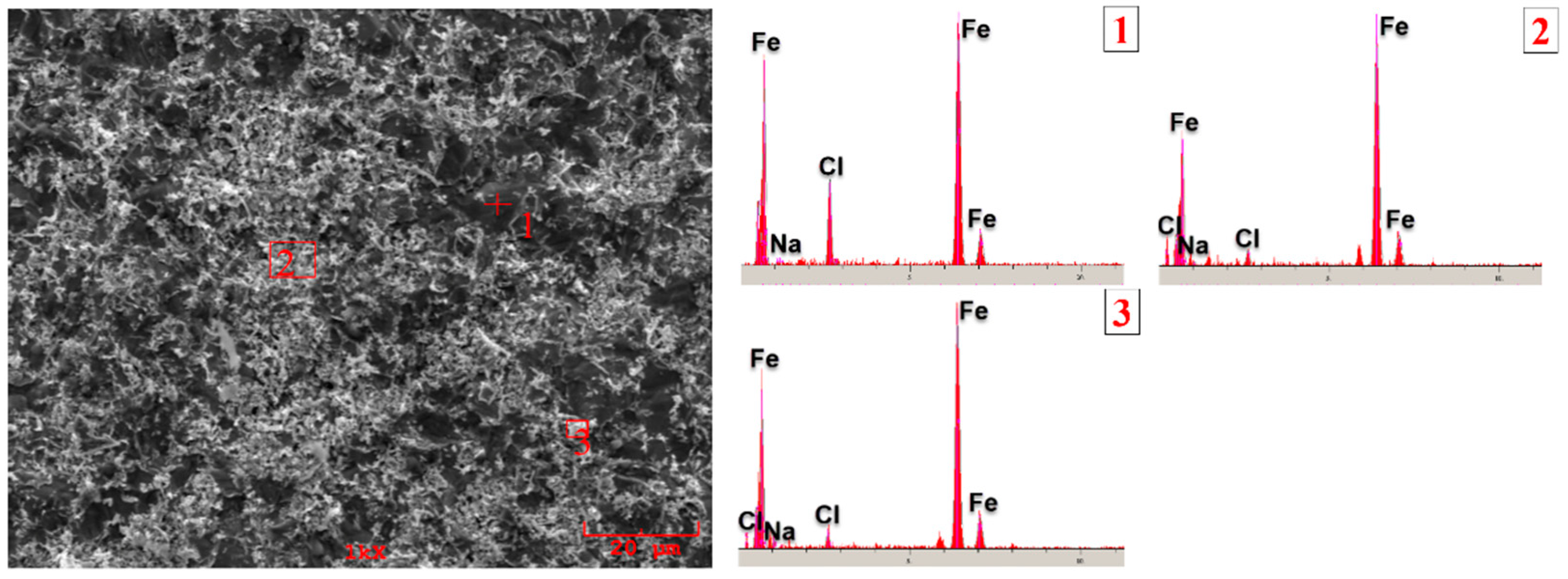
3.3. Corrosion Behavior
4. Conclusions
- It has been determined that changes made in the electrode cover composition change the phases in the microstructure and that even trace changes have a significant morphological effect on some phases. In the study, α-Fe, FeMo2B2, Fe2B, and R-Fe63Mo37 phases were detected as major phases, and Fe3B and Fe7Mo6 phases were detected as minor phases. It has also been determined that molybdenum can dissolve in the α-Fe and Fe2B phase and can affect the morphological and mechanical properties of both these phases and the eutectic structures formed by these phases.
- Neither molybdenum nor boron could provide the effect of both on the hardness and wear resistance of the coating. In addition, this effect reaches its maximum level for mixtures made in certain proportions. For this reason, optimizing the compositions in hardfacing coating works was critical. In the study, the highest hardness value was obtained in the Fe14Mo2B4-based coating as 56.4 HRC. It was observed that the hardness of this coating was ~73% higher than the substrate material and ~30.5% higher than the Fe16B4-based coating. According to microhardness measurements, although the hardness of the phases in the microstructure varies over a wide range, the highest phase hardness was measured as 3228 HV in the FeMo2B2 phase. In the study, the highest wear resistance was obtained in the Fe14Mo2B4-based coating. According to the wear rate values, up to ~8.1 times higher wear resistance was obtained in the Fe14Mo2B4-based coating compared to the AISI 1020 substrate material and up to ~4.7 times higher than the Fe16B4-based coating.
- According to the corrosion test results, it was observed that there was no significant difference between the corrosion potentials of the substrate material and the coated samples. However, a significant difference was detected between the corrosion current density values. The current density of all samples with hardfacing coating is lower than the base material, meaning their corrosion resistance was better. In the study, the lowest current density value was measured as 2.078 µA/cm2 in the Fe16B4-based coating and it was found to be ~13.6 times more resistant to corrosion than the substrate material. Although the corrosion resistance of the Fe16B4-based coating, that is, molybdenum-free, was high, it has been determined that the corrosion resistance increases with the increasing molybdenum amount in molybdenum-containing hardfacing coatings.
- From this study, it can be concluded that molybdenum is a good alternative that can be added to Fe-B-based hardfacing alloys to improve the properties of the coating. Furthermore, extending this study to reveal the effect of high temperature wear behavior on the coating will be beneficial for potential applications.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, K.; Zheng, Z.; Zhang, L.; Liu, Y.; Chen, S. Method for Dynamic Prediction of Oxygen Demand in Steelmaking Process Based on BOF Technology. Processes 2023, 11, 2404. [Google Scholar] [CrossRef]
- Steel Pipe Coatings Market to Hit $14.0 Billion by 2033. Focus Powder Coat. 2023, 2023, 1–6. [CrossRef]
- Pelser, W.A.; Marais, J.H.; van Laar, J.H.; Mathews, E.H. Development and Application of an Integrated Approach to Reduce Costs in Steel Production Planning. Process. Integr. Optim. Sustain. 2022, 6, 819–836. [Google Scholar] [CrossRef]
- Schubert, E.; Klassen, M.; Zerner, I.; Walz, C.; Sepold, G. Light-Weight Structures Produced by Laser Beam Joining for Future Applications in Automobile and Aerospace İndustry. J. Mater. Process. Technol. 2001, 115, 2–8. [Google Scholar] [CrossRef]
- Dong, Q.; Chen, X.; Gao, Y.; Hu, J.; Chen, X.; Xu, G. Steel BT—Civil Engineering Materials for Transportation Infrastructure; Dong, Q., Chen, X., Gao, Y., Hu, J., Chen, X., Xu, G., Eds.; Springer Nature: Singapore, 2023; pp. 267–296. ISBN 978-981-99-1300-8. [Google Scholar]
- Ren, X.; Fu, H.; Xing, J.; Yi, Y. Research on High-Temperature Dry Sliding Friction Wear Behavior of Ca Ti Modified High Boron High Speed Steel. Tribol. Int. 2019, 132, 165–176. [Google Scholar] [CrossRef]
- Qi, Y.; Luo, H.; Zheng, S.; Chen, C.; Lv, Z.; Xiong, M. Effect of Temperature on the Corrosion Behavior of Carbon Steel in Hydrogen Sulphide Environments. Int. J. Electrochem. Sci. 2014, 9, 2101–2112. [Google Scholar] [CrossRef]
- Liu, D.S.; Liu, R.P.; Wei, Y.H.; Pan, P. Properties of Cobalt Based Hardfacing Deposits with Various Carbon Contents. Surf. Eng. 2013, 29, 627–632. [Google Scholar] [CrossRef]
- Hutchings, I.; Shipway, P. 7—Surface Engineering, 2nd ed.; Hutchings, I., Shipway, P.B.T.-T., Eds.; Butter-worth-Heinemann: Oxford, UK, 2017; pp. 237–281. ISBN 978-0-08-100910-9. [Google Scholar]
- Burkov, A.A.; Kulik, M.A. Wear-Resistant and Anticorrosive Coatings Based on Chrome Carbide Cr7C3 Obtained by Electric Spark Deposition. Prot. Metall. Phys. Chem. Surf. 2020, 56, 1217–1221. [Google Scholar] [CrossRef]
- Bazhin, P.; Titov, N.; Zhidovich, A.; Avdeeva, V.; Kolomeichenko, A.; Stolin, A. Features of the Carbo-Vibroarc Surfacing in the Development of Multicomponent Cermet Wear-Resistant Coatings. Surf. Coat. Technol. 2022, 429, 127952. [Google Scholar] [CrossRef]
- Vasilescu, M.; Dobrescu, M. Hardfacing Corrosion and Wear Resistant Alloys. Adv. Mater. Res. 2015, 1114, 196–205. [Google Scholar] [CrossRef]
- Kocaman, E.; Kılınç, B.; Şen, Ş.; Şen, U. Development of Surface Properties with In Situ TiB2 Intermetallic-Assisted Coating by Fe(18-X)Ti2BX (x = 3,4,5)-Based Electrodes. Arab. J. Sci. Eng. 2021, 47, 8485–8501. [Google Scholar] [CrossRef]
- Trembach, B. Comparative Studies of the Three-Body Abrasion Wear Resistance of Hardfacing Fe-Cr-C-B-Ti Alloy. IOP Conf. Ser. Mater. Sci. Eng. 2023, 1277, 012016. [Google Scholar] [CrossRef]
- Ardigo-Besnard, M.; Tellier, A.; Besnard, A.; Chateau-Cornu, J.-P. Effect of the Microstructure on the Tribological Properties of HIPed and PTA-Welded Fe-Based Hardfacing Alloy. Surf. Coat. Technol. 2021, 425, 127691. [Google Scholar] [CrossRef]
- Kılınç, B.; Kocaman, E.; Şen, Ş.; Şen, U. Effect of Vanadium Content on the Microstructure and Wear Behavior of Fe(13-x)VxB7 (x = 0–5) Based Hard Surface Alloy Layers. Mater. Charact. 2021, 179, 111324. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.; Ge, Y.; Zhuang, M.; Ma, Z. Microstructure and Wear Resistance of High-Chromium Cast Iron with Mul-ticomponent Carbide Coating via Laser Cladding. Coatings 2023, 13, 1474. [Google Scholar] [CrossRef]
- Durmuş, H.; Çömez, N.; Gül, C.; Yuddaşkal, M.; Uzun, R.O. Ferromolibden ve Ferrobor Takviyeli Lazer Kaplamaların Aşınma Karakteristiği ve Mikroyapısı. DÜMF Mühendislik Derg. 2019, 10, 1009–1017. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; Zhang, Y.; Lian, G.; Cao, Q.; Yang, J. Sensitivity Analysis for Process Parameters in Mo2FeB2 Ternary Boride Coating by Laser Cladding. Coatings 2022, 12, 1420. [Google Scholar] [CrossRef]
- Belov, D.S.; Blinkov, I.V.; Sergevnin, V.S.; Chernogor, A.V.; Demirov, A.P.; Polyanskii, A.M. Structure and Phase Formation in Arc PVD Zr–B–Si–C–Ti–(N) Coatings. Inorg. Mater. 2023, 59, 157–163. [Google Scholar] [CrossRef]
- Prysyazhnyuk, P.; Shlapak, L.; Ivanov, O.; Korniy, S.; Lutsak, L.; Burda, M.; Hnatenko, I.; Yurkiv, V. In Situ Formation of Molybdenum Borides at Hardfacing by Arc Welding with Flux-Cored Wires Containing a Reaction Mixture of B4C/Mo. Eastern-Eur. J. Enterp. Technol. 2020, 4, 46–51. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Yang, Y.; Wang, X.-Y.; Li, W.; Tian, W. Microstructure and Wear Properties of İn-Situ Molybdenum Composite Coatings by Plasma Spraying Mo-B4C and MoO3-Al-B4C Composite Powders. Surf. Coat. Technol. 2023, 469, 129769. [Google Scholar] [CrossRef]
- Ye, F.; Zhang, Y.; Lou, Z.; Wang, Y. Enhanced Tribological Performance of Micro-Beam Plasma-Cladded Ni60 Coatings with Addition of Mo and Ag Lubricants in a Wide Temperature Range. Coatings 2023, 13, 1996. [Google Scholar] [CrossRef]
- Sun, M.; Pang, M. Defect Formation Mechanism and Performance Study of Laser Cladding Ni/Mo Composite Coating. Coatings 2021, 11, 1460. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, G.; Wang, T.; Ren, S.; Bai, Z.; Cao, Q. Investigations on Microstructure and Wear Resistance of Fe-Mo Alloy Coating Fabricated by Plasma Transferred arc Cladding. Surf. Coat. Technol. 2018, 350, 480–487. [Google Scholar] [CrossRef]
- Dilawary, S.A.A.; Motallebzadeh, A.; Paksoy, H.A.; Akhter, R.; Atar, E.; Cimenoglu, H. LSM of Stellite 12 + 10wt % Mo Hardfacing Alloy for Enhancement in Wear Resistance. In Proceedings of the XXXI International Conference on Surface Modification Technologies (SMT31), Mons, Belgium, 5–7 July 2017. [Google Scholar]
- Li, Q.; Wang, Q.; Zhang, L.; Chen, D.X.; Jin, H.; Li, J.D.; Zhang, J.W. Microstructure, Wear and Electrochemical Behaviors of Laser Cladding Fe-Based Coatings with Various Molybdenum Contents. Mater. Res. Express 2022, 9, 026504. [Google Scholar] [CrossRef]
- Jiang, R.S.; Wang, Y.T.; Hu, L.; Xu, G.; De Liu, Z. Effect of Mo Content on the Corrosion Resistance of Fe-Based Amorphous Composite Coating. Mater. Sci. Forum 2016, 849, 636–641. [Google Scholar] [CrossRef]
- Bahmaeia, M.; Pourzarea, R.; Ghezloo, A. The Effect of Cerium Pre-Treatment on the Corrosion Resistance of Steel Sheets. Russ. J. Gen. Chem. 2013, 83, 2386–2391. [Google Scholar] [CrossRef]
- Hazza, M.; El-Dahshan, M. The Effect of Molybdenum on the Corrosion Behaviour of Some Steel Alloys. Desalination 1994, 95, 199–209. [Google Scholar] [CrossRef]
- Rockel, M.B. The Effect of Molybdenum on the Corrosion Behavior of Iron-Chromium Alloys. Corrosion 2013, 29, 393–396. [Google Scholar] [CrossRef]
- Kocaman, E.; Kılınç, B.; Şen, Ş.; Şen, U. In-situ TiB2 and Fe2Ti İntermetallic Assisted Hard Coatings by Fe-Ti-B Based Hardfacing electrodes. J. Alloys Compd. 2022, 900, 163478. [Google Scholar] [CrossRef]
- Kocaman, E.; Kilinç, B.; Şen, S.; Şen, U. Effect of Chromium Content on Fe(18-x)CrxB2(X=3,4,5) Hardfacing Electrode on Microstructure, Abrasion and Corrosion Behavior. J. Fac. Eng. Archit. Gazi Univ. 2020, 36, 177–190. [Google Scholar] [CrossRef]
- Duraisamy, R.; Kumar, S.M.; Kannan, A.R.; Shanmugam, N.S.; Sankaranarayanasamy, K.; Ramesh, M. Tribological Performance of Wire Arc Additive Manufactured 347 Austenitic Stainless Steel under Unlubricated Conditions at Elevated Temperatures. J. Manuf. Process. 2020, 56, 306–321. [Google Scholar] [CrossRef]
- Sunbul, S.E.; Akyol, S.; Onal, S.; Ozturk, S.; Sozeri, H.; Icin, K. Effect of Co, Cu, and Mo Alloying Metals on Electrochemical and Magnetic Properties of Fe-B Alloy. J. Alloys Compd. 2023, 947, 169652. [Google Scholar] [CrossRef]
- Ouyang, X.; Chen, G.; Yin, F.; Liu, Y.; Zhao, M. Effect of Molybdenum on the Microstructures of As-Cast Fe-B Alloys and Their Corrosion Resistance in Molten Zinc. Corrosion 2017, 73, 942–952. [Google Scholar] [CrossRef]
- Beardsley, M.B.; Sebright, J.L. Structurally Integrated Coatings for Wear and Corrosion; Caterpillar Inc.: Peoria, IL, USA, 2018. [Google Scholar]
- Wang, H.Q.; Sun, J.S.; Li, C.N.; Geng, S.N.; Sun, H.G.; Wang, G.L. Microstructure and Mechanical Properties of Molybdenum–İron–Boron–Chromium Cladding Using Argon arc Welding. Mater. Sci. Technol. 2016, 32, 1694–1701. [Google Scholar] [CrossRef]
- Sarasola, M.; Gómez-Acebo, T.; Castro, F. Microstructural Development during Liquid Phase Sintering of Fe and Fe–Mo Alloys Containing Elemental Boron Additions. Powder Metall. 2005, 48, 59–67. [Google Scholar] [CrossRef]
- Guillermet, A.F. The Fe−Mo (Iron−Molybdenum) System. Bull. Alloy. Phase Diagr. 1982, 3, 359–367. [Google Scholar] [CrossRef]
- Cho, I.-S.; Savelyev, K.D.; Golod, V.M. Development of Thermophysical Calculator for Stainless Steel Casting Alloys by Using CALPHAD Approach. China Foundry 2017, 14, 353–358. [Google Scholar] [CrossRef]
- Utkin, S.; Bondar, A.; Kublii, V.; Kapitanchuk, L.; Tikhonova, I. Solidus Surface of the Mo–Fe–B System. Powder Metall. Met. Ceram. 2020, 59, 89–105. [Google Scholar] [CrossRef]
- Chen, K.; Yang, X.; Li, W.; Xia, G.; Wang, S.; Wang, K. Study on the Wear and Corrosion Resistance of Fe–Mo Coatings on 65Mn Steel Ploughshares by Laser Cladding. Appl. Phys. A 2022, 128, 795. [Google Scholar] [CrossRef]
- Huang, Z.; Xing, J.; Guo, C. Improving Fracture Toughness and Hardness of Fe2B in High Boron White Cast İron by Chromium Addition. Mater. Des. 2010, 31, 3084–3089. [Google Scholar] [CrossRef]
- Lentz, J.; Röttger, A.; Theisen, W. Hardness and Modulus of Fe2B, Fe3(C,B), and Fe23(C,B)6 Borides and Carboborides in the Fe-C-B System. Mater. Charact. 2018, 135, 192–202. [Google Scholar] [CrossRef]
- Azakli, Y.; Cengiz, S.; Tarakci, M.; Gencer, Y. Characterisation of Boride Layer Formed on Fe–Mo Binary Alloys. Surf. Eng. 2016, 32, 589–595. [Google Scholar] [CrossRef]
- Hocaoğlu, R. Fe-Mo-Ti-B-C Esaslı Sert Dolgu Alaşımlı Örtülü Elektrotların Üretimi ve Özelliklerinin Incelenmesi = The Production and Investigation of the Properties of Fe-Mo-Ti-B-C Based Hardfacing Alloy Covered Electrodes; Sakarya University: Serdivan, Turkey, 2021. [Google Scholar]
- Márquez-Herrera, A.; Fernandez-Muñoz, J.; Zapata-Torres, M.; Melendez-Lira, M.; Cruz-Alcantar, P. Fe2B coating on ASTM A-36 Steel Surfaces and İts Evaluation of Hardness and Corrosion Resistance. Surf. Coat. Technol. 2014, 254, 433–439. [Google Scholar] [CrossRef]
- Archard, J.F. Contact and Rubbing of Flat Surfaces. J. Appl. Phys. 1953, 24, 981–988. [Google Scholar] [CrossRef]
- Sedlaček, M.; Podgornik, B.; Vižintin, J. Influence of Surface Preparation on Roughness Parameters, Friction and Wear. Wear 2009, 266, 482–487. [Google Scholar] [CrossRef]
- Chapter 8 Lubrication of Solid Surfaces. In Surface Effects in Adhesion, Fricti0N, Wear, and Lubrication; Buckley, D.H.B.T.-T.S. (Ed.) Elsevier: Amsterdam, The Netherlands, 1981; Volume 5, pp. 511–552. ISBN 0167-8922. [Google Scholar]
- Szeri, A.Z. Tribology, 3rd ed.; Meyers, R.A., Ed.; Academic Press: New York, NY, USA, 2003; pp. 127–152. ISBN 978-0-12-227410-7. [Google Scholar]
- Kalel, S.M.; Patil, S.R. Ceramic Reinforced Metal Matrix Composite ( MMC )—Processing. Int. J. Adv. Res. Sci. Eng. 2018, 7, 1047–1058. [Google Scholar]
- Lee, H.-Y. Effect of Changing Sliding Speed on Wear Behavior of Mild Carbon Steel. Metall. Mater. Int. 2020, 26, 1749–1756. [Google Scholar] [CrossRef]
- Menezes, P.L.; Kishore; Kailas, S.V.; Lovell, M.R. Role of Surface Texture, Roughness, and Hardness on Friction During Unidirectional Sliding. Tribol. Lett. 2011, 41, 1–15. [Google Scholar] [CrossRef]
- Sen, U. Wear Properties of Niobium Carbide Coatings Performed by Pack Method on AISI 1040 Steel. Thin Solid Films 2005, 483, 152–157. [Google Scholar] [CrossRef]
- Sen, U. Friction and Wear Properties of Thermo-Reactive Diffusion Coatings against Titanium Nitride Coated Steels. Mater. Des. 2005, 26, 167–174. [Google Scholar] [CrossRef]
- Sen, S.; Sen, U. Sliding Wear Behavior of Niobium Carbide Coated AISI 1040 Steel. Wear 2008, 264, 219–225. [Google Scholar] [CrossRef]
- Zhao, H.; Xie, L.; Xin, C.; Li, N.; Zhao, B.; Li, L. Effect of Molybdenum Content on Corrosion Resistance and Corrosion Behavior of Ti-Mo Titanium Alloy in Hydrochloric Acid. Mater. Today Commun. 2023, 34, 105032. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, C.; Chen, Q.; Yang, Y.; Li, N.; Liu, L. Study of Structure and Corrosion Resistance of Fe-Based Amorphous Coatings Prepared by HVAF and HVOF. Corros. Sci. 2011, 53, 2351–2356. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Liao, H.; Zeng, M.; Ma, S. A Review on Relationship between Morphology of Boride of Fe-B Alloys and the Wear/Corrosion Resistant Properties and Mechanisms. J. Mater. Res. Technol. 2019, 8, 6308–6320. [Google Scholar] [CrossRef]
- Ma, S.; Xing, J.; Fu, H.; Yi, D.; Li, Y.; Zhang, J.; Zhu, B.; Gao, Y. Microstructure and İnterface Characteristics of Fe–B Alloy in Liquid 0.25wt.% Al–Zn at Various Bath Temperatures. Mater. Chem. Phys. 2012, 132, 977–986. [Google Scholar] [CrossRef]
- Ma, S.; Xing, J.; Fu, H.; Yi, D.; Zhang, J.; Li, Y.; Zhang, Z.; Zhu, B.; Ma, S. Interfacial Morphology and Corrosion Resistance of Fe–B Cast Steel Containing Chromium and Nickel in Liquid Zinc. Corros. Sci. 2011, 53, 2826–2834. [Google Scholar] [CrossRef]
- Ma, S.; Xing, J.; Fu, H.; Yi, D.; Zhi, X.; Li, Y. Effects of Boron Concentration on the Corrosion Resistance of Fe–B Alloys İmmersed in 460 °C Molten Zinc Bath. Surf. Coat. Technol. 2010, 204, 2208–2214. [Google Scholar] [CrossRef]
- Abakay, E.; Şen, U. Effect of Morphological and Microstructural Variations on the Properties of Electroless Nickel Boron Coatings. Trans. Indian Inst. Metall. 2023, 76, 657–664. [Google Scholar] [CrossRef]
- Gu, Z.; Mao, P.; Gou, Y.; Chao, Y.; Xi, S. Microstructure and Properties of MgMoNbFeTi2Yx High Entropy Alloy Coatings by Laser Cladding. Surf. Coat. Technol. 2020, 402, 126303. [Google Scholar] [CrossRef]
- Qin, L.-Y.; Lian, J.-S.; Jiang, Q. Effect of Grain Size on Corrosion Behavior of Electrodeposited Bulk Nanocrystalline Ni. Trans. Nonferrous Metall. Soc. China 2010, 20, 82–89. [Google Scholar] [CrossRef]
- Lu, B.; Luo, J.; Chiovelli, S. Corrosion and Wear Resistance of Chrome White İrons—A Correlation to Their Composition and Microstructure. Metall. Mater. Trans. A 2006, 37, 3029–3038. [Google Scholar] [CrossRef]
- Kocaman, E.; Kılınç, B.; Durmaz, M.; Şen, Ş.; Şen, U. The İnfluence of Chromium content on Wear and Corrosion Behavior of Surface Alloyed Steel with Fe(16−x)Crx(B,C)4 Electrode. Eng. Sci. Technol. Int. J. 2021, 24, 533–542. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Z.; Gao, Y.; Wang, X.; Zheng, C. Effect of Cr Content on Corrosion Resistance of Ni-XCr-Mo Laser-Cladding Coatings under H2S-Induced High-Temperature Corrosion Atmosphere. Materials 2022, 15, 1885. [Google Scholar] [CrossRef] [PubMed]




| C | Cr | Mo | B | Mn | Si | P | S | Fe | |
|---|---|---|---|---|---|---|---|---|---|
| H08A | <0.1 | 0.064 | - | - | 0.35–0.40 | 0.10 | <0.02 | <0.02 | Bal. |
| AISI 1020 | 0.22 | 0.025 | 0.02 | - | 0.52 | 0.17 | 0.023 | 0.019 | Bal. |
| Ferro-Mo | - | - | 60 | - | - | 1.5 | 0.050 | 0.10 | 38.35 |
| Ferro-B | 0.312 | - | 18.58 | 0.39 | 0.029 | 0.003 | 80.602 |
| Compound | B | Mo | Fe |
|---|---|---|---|
| Fe16B4 | 20 | - | Bal. |
| Fe15MoB4 | 20 | 5 | Bal. |
| Fe14Mo2B4 | 20 | 10 | Bal. |
| Fe14Mo4B2 | 10 | 20 | Bal. |
| Sample | Matrix (HV0.01) | Eutectic (α-Fe-M2B) (HV0.01) | Eutectic (α-Fe-FeMo2B2) (HV0.01) | FeMo2B2 (HV0.01) | R-(Fe63Mo37) (HV0.01) |
|---|---|---|---|---|---|
| AISI 1020 | 143–147 | - | - | - | - |
| Fe16B4 | 173–180 | 478–542 | - | - | - |
| Fe15MoB4 | 424–542 | 996–1200 | - | 1953–2973 | - |
| Fe14Mo2B4 | 459–573 | 1053–1242 | 642–956 | 1970–3228 | - |
| Fe14Mo4B2 | 368–379 | - | 520–754 | - | 718–840 |
| Sample | Ecor (mV) | Icor (µA/cm2) | Cr (mpy) |
|---|---|---|---|
| AISI 1020 | −609.315 | 28.331 | 16.291 |
| Fe16B4 | −683.455 | 2.078 | 1.544 |
| Fe15MoB4 | −669.678 | 15.287 | 11.245 |
| FeMo2B4 | −688.666 | 6.601 | 4.808 |
| FeMo4B2 | −632.627 | 5.650 | 3.603 |
| Sample | Rs (Ω) | CPE-1 (µF.cm−2) | Rct-1 (kΩ) |
|---|---|---|---|
| AISI 1020 | 42.78 | 2.2 × 10−4 | 1.29 |
| Fe16B4 | 40.41 | 2.29 × 10−4 | 4.87 |
| Fe15MoB4 | 126.3 | 1.08 × 10−4 | 4.25 |
| Fe14Mo2B4 | 134.4 | 2.43 × 10−4 | 1.4 |
| Fe14Mo4B2 | 129 | 1.11 × 10−4 | 4.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocaman, E. Effect of the Molybdenum Content on Wear and Corrosion Behavior of Fe-B-Based Surface-Alloyed Layer. Coatings 2023, 13, 2050. https://doi.org/10.3390/coatings13122050
Kocaman E. Effect of the Molybdenum Content on Wear and Corrosion Behavior of Fe-B-Based Surface-Alloyed Layer. Coatings. 2023; 13(12):2050. https://doi.org/10.3390/coatings13122050
Chicago/Turabian StyleKocaman, Engin. 2023. "Effect of the Molybdenum Content on Wear and Corrosion Behavior of Fe-B-Based Surface-Alloyed Layer" Coatings 13, no. 12: 2050. https://doi.org/10.3390/coatings13122050
APA StyleKocaman, E. (2023). Effect of the Molybdenum Content on Wear and Corrosion Behavior of Fe-B-Based Surface-Alloyed Layer. Coatings, 13(12), 2050. https://doi.org/10.3390/coatings13122050








