Abstract
This research explores the electrochemical synthesis of Pd-Se coatings from acidic chloride solutions using cyclic voltammetry to understand the reaction mechanism. The study examines how the applied potential and electrolyte composition affect the coatings’ properties. Energy-dispersive X-ray spectroscopy and X-ray diffraction were used for elemental and phase analyses, respectively, while a scanning-electron microscope assessed the surface morphology. The findings indicate that the deposition potential significantly affected the coatings’ properties, altering the selenium-deposition reaction’s mechanism and the coatings’ elemental and phase composition and morphology. As the potential decreases, the mechanism transforms, influencing the elemental and phase compositions and the coatings’ morphology. The feasibility of co-depositing palladium with selenium in varying stoichiometric ratios and diverse phase compositions was confirmed. The post-heat-treatment-phase analysis highlighted a mix of intermetallic phases, with Pd17Se15 being predominant in the solutions with 1:2 and 1:1 palladium-to-selenium ratios. Electrolysis at lower potentials and from electrolytes with higher palladium-to-selenium ratios results in pure palladium coatings.
1. Introduction
The group of noble-metal chalcogenides has garnered substantial interest within the electronics industry for its diverse range of applications, including optical devices and catalysts [1,2,3,4]. This group includes, for example, palladium selenides. Palladium selenides, a subset of this group, have garnered particular attention due to their versatility. The phase diagram reveals numerous palladium–selenium intermetallic compounds, with a spotlight on four key phases: PdSe2, Pd4Se, Pd7Se4, and Pd17Se15 [5].
The PdSe2, characterized by its pentagonal atomic structure, wide tuneable bandgap, and air stability, belongs to the class of two-dimensional compounds with intriguing optical properties [6,7]. This phase has found utility in the electronics industry as a crucial component in ultra-fast photodetectors and ultrafast photonic devices [6,8,9]. There are works on using this phase as a catalyst in hydrogen-evolution reactions [10]. However, PdSe2 is characterized by a catalytically inert large-scale baseline, which dramatically reduces the use of this phase as a catalyst [11].
In contrast to palladium diselenide, the other aforementioned phases find much more applications as catalysts [12,13,14]. Kukunuri et al. synthesized the Pd4Se, Pd7Se4, and Pd17Se15 phases by using thermolysis and checked their properties for activity in HER and ORR reactions, and as a counter electrode in dye-sensitized solar cells. Out of these three phases, the Pd4Se phase showed the best catalytic activity and stability in hydrogen and oxygen evolution in alkaline solutions [15,16,17]. In turn, Sarma et al.’s article showed that the Pd17Se15 phase synthesized by the solvothermal method exhibits good catalytic properties [18].
While solvothermal and thermolysis methods have contributed significantly to the synthesis of palladium–selenium compounds, they are not the sole approaches available. A diverse array of techniques, including hydrothermal methods, selenization, exfoliation, chemical vapor deposition, and molecular beam epitaxy, further broaden the possibilities for obtaining Pd-Se compounds [6,19,20,21,22,23,24,25].
Considering the multifaceted applications of palladium selenides and the diverse synthesis methods at our disposal, our pursuit of alternative approaches, such as the electrochemical method, becomes increasingly intriguing. Can we develop an innovative synthesis technique that simplifies the process and proves cost-effective while offering precise control over composition and morphology? This question is poised to generate substantial interest in research laboratories and industrial sectors. Among the various options, the electrochemical method stands out as a promising choice, given its established track record, cost-effectiveness, and tunability, making it an attractive option for both researchers and industry professionals.
The first attempts at the electrodeposition of coatings containing palladium and selenium proved successful. The coatings were synthesized from acid-chloride solutions containing the same concentrations of palladium and selenium. Coatings with different elemental and phase compositions were obtained, while, at the same time, intermetallic compounds were detected [26]. It now seems that the critical factor is to understand the mechanism through which electrode reactions occur during the co-depositing of these two elements. Knowledge of the phenomena occurring during electrolysis will enable the complete control of the palladium–selenide synthesis process. It is worth mentioning that while the literature provides information on the deposition mechanism of palladium or selenium alone, there are no studies on the analysis of the co-deposition mechanism of these elements [27,28,29,30,31,32,33].
This article presents the results of systematic research on the electrochemical synthesis of palladium–selenium coatings from acid-chloride solutions. The influence of the concentrations of individual reagents on the mechanism and kinetics of electrode reactions was investigated. Based on the cyclic voltammetry results, the coating-deposition process was carried out using the potentiostatic method. The influence of the applied potential and electrolyte composition on the properties of the deposits was investigated.
2. Materials and Methods
The solutions were prepared by using various concentrations of PdCl2 (99% Aldrich, St. Louis, MO, USA), (Pd dissolved in 0.1 M HCl at T = 70 °C) and SeO2 (99.999% Aldrich). The addition of 0.2 M NaCl (AR) was used to support the electrolyte. All ingredients were dissolved in deionized water. Furthermore, HCl (35%–38% POCH, Gliwice, Poland) was used to acidify the solution to pH = 2.
In order to investigate the mechanism and kinetics of the electrode reactions, cyclic voltammetry was performed using a three-electrode system containing a glassy carbon-disc electrode (GC) with an area of 0.196 cm2 as the working electrode, platinum as the counter electrode, and the Ag/AgCl chloride electrode as the reference electrode. The AUTOLAB 302N (Metrohm, Herisau, Switzerland) potentiostat controlled the process parameters.
The next stage was the electrodeposition process. The system of electrodes in the deposition process was the same as during the cyclic voltammetry tests, except that the working electrode was a copper plate with an area of 2.8 cm2. To remove impurities, the copper sheet was chemically polished in a solution containing H3PO4, HNO3, and CH3COOH acid in the volume ratio 1:1:1 at the temperature of 70 °C for 40 s, rinsed with deionized water, and dried. Next, a gold layer was sputtered onto copper, with a thickness at least 200 nm. The electrode prepared in this way was placed in a Teflon® holder and mounted as a rotating disc electrode in the Pine Research MSR Rotator (Pine Research Instrumentation, Durham, NC, USA). The electrodeposition process was carried out at potentials range of −0.1 ÷ −0.8 V for 300 s, using a rotation rate of 800 rpm at room temperature. The obtained coatings were annealed in a quartz tube at 200 °C for 2 h under argon atmosphere and then cooled to room temperature in the presence of an inert gas.
The samples, both before and after annealing, were analyzed for elemental composition using the EDS method in three places in the sample: at its edges and in the centre. The results were averaged, and a deviation was calculated. The analyses were performed on an area of 0.4 mm2 and accelerating voltages of 10 kV. Phase analysis was performed by XRD using a Rigaku Miniflex II spectrometer (Rigaku, Tokyo, Japan) with the use of a copper tube (λ = 1.54059). Pictures of the morphologies of the obtained coatings were made using the JEOL JCM-6000 NEOSCOPE (Rigaku, Tokyo, Japan) scanning microscope with a magnification of 4000×.
3. Results and Discussion
3.1. Cyclic Voltammetry
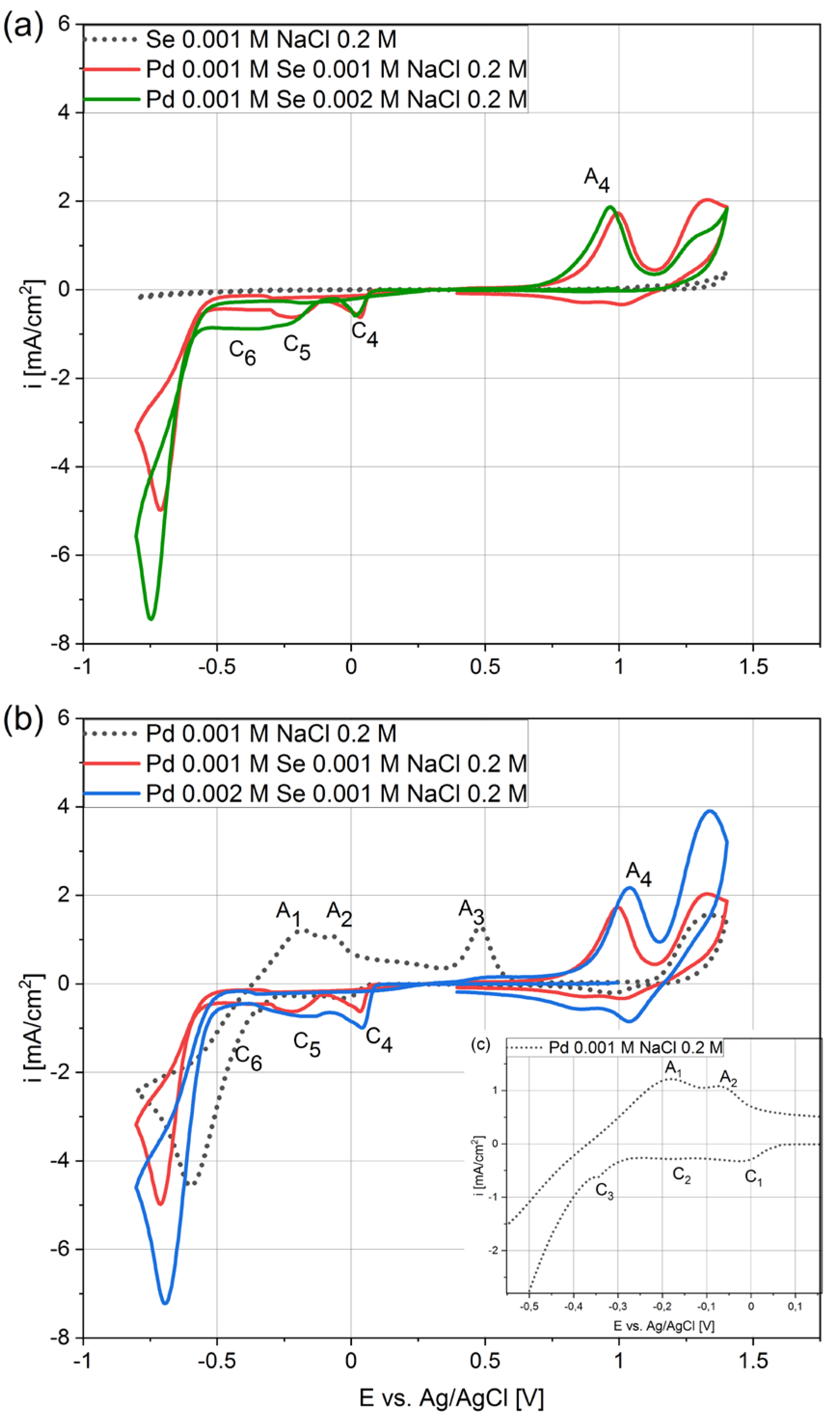
Cyclic voltammetry provides insight into the electrochemical deposition of coatings. The analysis of voltammetric curves allows the determination of the range of potentials in which the electrochemical reactions responsible for the deposition of coating components occur. Moreover, other phenomena may arise during the deposition of two-component coatings that do not occur during the deposition of individual elements, especially for chalcogenide coatings [34]. Comparing the results of voltammetric tests obtained in solutions containing one element with those obtained in solutions containing both allows the detection of the differences that appear and the adequate interpretation of the mechanism of the co-deposition process.
Figure 1 presents graphs with voltammograms for the solutions containing palladium or selenium ions and both elements in different stoichiometric ratios. The different stoichiometric ratios allow us to determine the influence of individual elements’ concentrations in the electrolytes on the kinetics of the process. The analysis of the electrodeposition of palladium and selenium alone is comprehensively described in the literature [27,35]. However, there is no analysis of the mechanism behind the co-deposition of both elements.
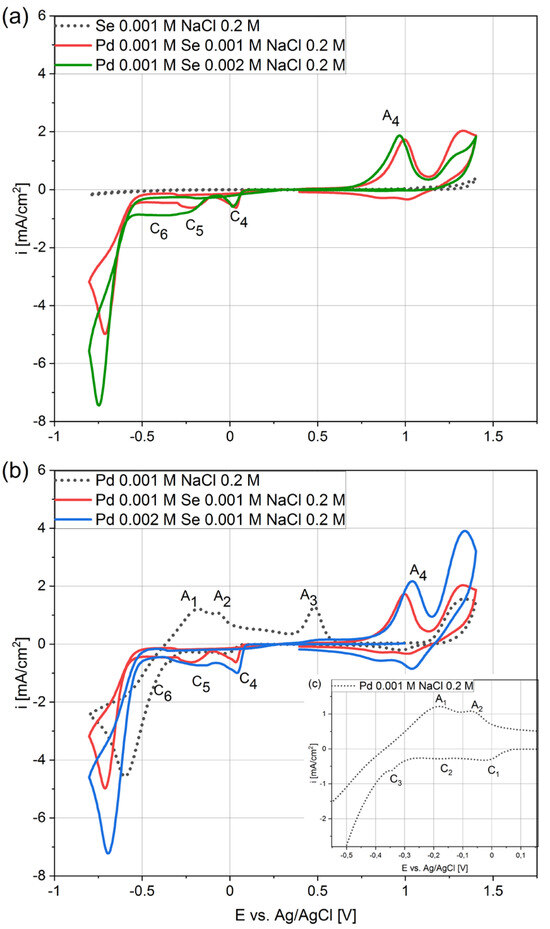
Figure 1.
Cyclic voltammograms for GC at 40 mV/s scan rate in electrolytes with different stoichiometric ratios of palladium and selenium (Ax—anodic peak, Cx—cathodic peak).
As shown in Figure 1a (dotted curve), no peaks appeared on the voltammogram during the selenium analysis. This is related to the high crystallization overpotential, which means that selenium does not deposit or deposits very slowly [34,35]. The absence of any anode peaks when the sweep was carried out in the reverse direction confirms the absence of the deposition process. In turn, the reduction of the palladium ions started at 0.05 V, and the negative currents were much higher (Figure 1b,c, black dotted curve). Hence, the deposition process is much faster than that of selenium. The C1 cathode peak at −0.03 V is related to the palladium-ion reduction that occurred according to reaction (1). Next, two small cathode peaks appeared, namely C2 at −0.18 V and C3 at −0.35 V, as did the corresponding anode peaks A2 and A3. These phenomena have already been described by Mech et al. [27] and Czerwinski et al. [36]. The reduction of hydrogen ions and their adsorption on the electrode surface takes place first. Next, hydrogen absorption inside the palladium takes place (2). The cathodic part of the voltammogram ends with a high increment of the negative current resulting from the hydrogen ions’ reduction on the palladium surface. On the anode side of the plot, peaks A1 and A2 correspond to the oxidation reactions of previously absorbed and adsorbed hydrogen, while peak A3 is related to the oxidation of the palladium.
[PdCl4]2− + 2e− → Pd0 + 4Cl−
Pd + H+ + e− → Pd − Hads → Pd − Habs
Significant modifications of the voltammogram appeared when comparing the palladium–selenium curve (Figure 1b, red) with the palladium curve (Figure 1b, dotted). The sharp C4 peak and the broad C5 peak (red curve) appeared, and they were significantly higher than the C1 and C2 (dotted curve). It should be emphasized that selenium was not deposited in the potential range discussed (Figure 1a, black dotted curve) when there were no palladium ions in the electrolyte. The higher maximum of the peaks assigned as C4 and C5 proves that the kinetics of the reactions are much faster. A long plateau labeled C6 appears after the C5 peak, indicating another electrochemical reaction. Routinely, the cathodic scans ended with an intense current increase associated with the hydrogen-ion-reduction reaction. When the scanning direction was reversed, the anodic peaks A1, A2, and A3 disappeared, and instead, a new anodic peak A4 grew. The observed differences indicate that palladium and selenium interact during the co-deposition process, and that the mechanism is very complex.
In order to understand how the elements interact with each other during co-deposition, we performed a voltammetric analysis in solutions containing twice as much selenium or palladium. Increasing the selenium concentration in the initial part of the voltammogram up to −0.25 V does not cause any changes. The rate of electrode reaction C4 does not depend on the selenous acid concentration. The peak C5 increases and smoothly links with the plateau C6. Current increases were also registered in the potential range from −0.25 to −0.6 V, assigned as C6. Moreover, there are no significant differences in the size of the anodic peak A4 in relation to the selenous acid concentration in the electrolyte. A shift of the C4 peak towards more positive potential and an increase in the peaks’ maxima were observed when the concentration of the palladium in the solution was doubled (Figure 1b, red and blue curves). The peak C5 is already slightly higher due to the higher background current from peak C4. The value of the recorded current in the C6 plateau range does not change due to the increase in the palladium concentration. However, a significant increase in the A4 anode peak can be observed.
The captured differences between the voltammograms obtained in the solutions containing different concentrations of individual components indicate the mechanism behind the co-deposition of palladium with selenium. Peak C4 strongly depends on the palladium concentration and is not affected when selenous acid increases from 0.001 to 0.002 M. However, its maximum increases compared to the peak C1 obtained in the solution containing only palladium specious. We suggest that this is a consequence of the decreasing nucleation overpotential associated with the adsorption of selenous acid on the palladium nucleus. Similar effects were described for the Cu-Se system [37]. Some metals induce the strong adsorption of selenous acid in aqueous solutions (3) [32,38,39]. The adsorbed selenous acid at the palladium sites is then reduced and, subsequently, promotes the deposition of palladium according to reaction (4). The suggested mechanism indicates that a small amount of selenium is deposited with the palladium.
Pd + H2SeO3 → Pd-H2SeO3(ads)
Pd-H2SeO3(ads) + 4H+ + 4e− → Pd-Se + 3H2O
The following peak C5 does not depend on the palladium-ion concentration but increases when the selenous acid concentration is doubled. Similar conduct is observed for the C6 plateau. Additional information about selenium’s electrochemical properties can help to interpret the obtained results. Selenium has many valent states (+VI, +IV, −II), leading to several electrochemical reactions [40]. Thus, the reduction of selenium acid and the co-deposition of selenium with other elements requires more attention, particularly concerning its high reactivity and interaction with the substrate [41]. The reduction of selenous acid can occur according to four-electron (5) and six-electron reactions (6). In addition, deposited selenium can also be reduced to hydrogen selenide according to reaction (7). According to our previous experience with selenium [32,42], the peak C5 is attributed to the bulk deposition of selenium according to reaction (5). Next, there is a change in the mechanism behind the selenous acid reduction from a four-electron reaction to a six-electron reaction (6). However, when the metal ions are in the solutions, both elements are co-deposited as intermetallic compounds (8) [34,37].
H2SeO3 + 4H+ + 4e− → Se0 + 3H2O
H2SeO3 + 6H+ + 6e− → H2Se + 3H2O
Se0 + 2H+ + 2e− → H2Se
[PdCl4]2− + H2SeO3 + 4H+ + 6e− → Pd-Se + 4Cl− + 3H2O
The decisive result obtained during the voltammetry tests is the difference between the voltammetric curves obtained when scanning in the direction of the positive potentials. The peak of A4 is larger than the peak of A3, located in a range of more positive potentials. This difference confirms that this peak is related to the dissolution of the palladium and selenium phases, which confirms the possibility of their co-deposition.
3.2. Electrodeposition
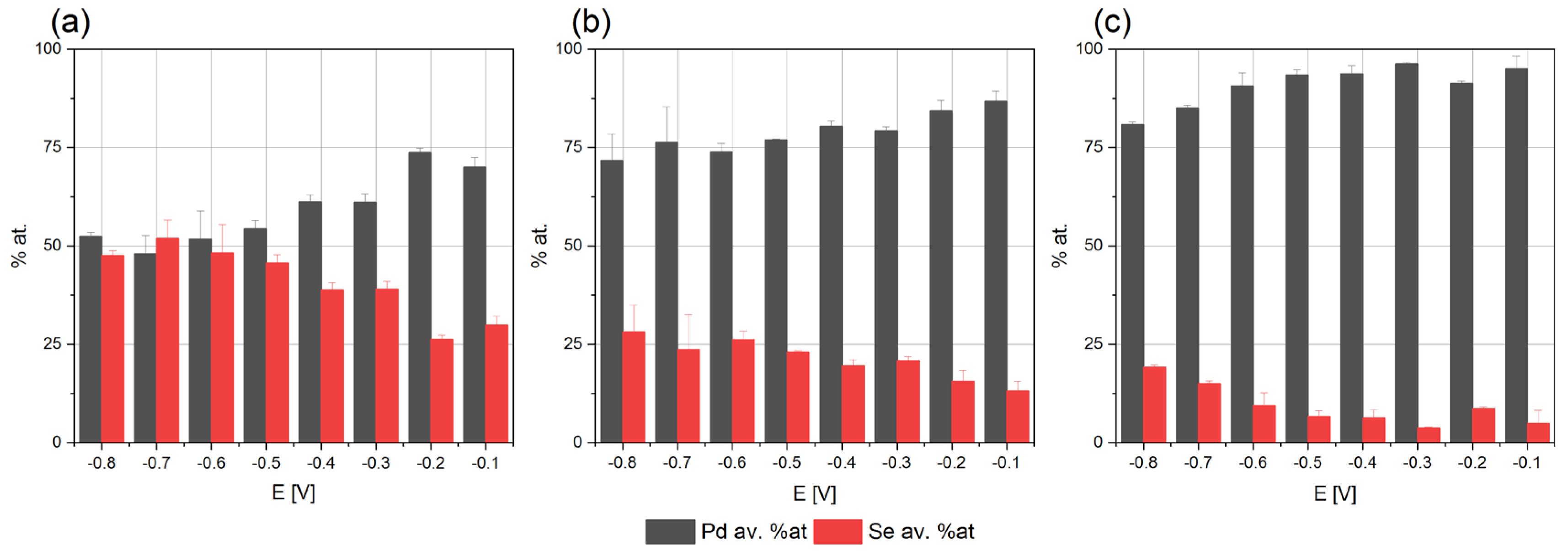
Based on the voltammetry results, the electrodeposition of Pd-Se was conducted from the potential −0.1 up to −0.8 V under potentiostatic conditions. The electrolyte compositions were similar to those used in the voltammetric tests. The deposition process was carried out with electrolytes in which the palladium-to-selenium ratios were 2:1, 1:1 and 1:2, respectively. The concentration of the ingredients was increased to accelerate the deposition process. The obtained results support us in interpreting the voltammetric results and confirm the palladium’s co-deposition with the selenium. Figure 2 shows the elemental compositions of the annealed coatings deposited from the solutions containing palladium and selenium in various stoichiometric ratios. The annealing temperature has a negligible effect on the composition of the coatings in comparison with the data obtained for the samples before the thermal treatment process (Figure S1).
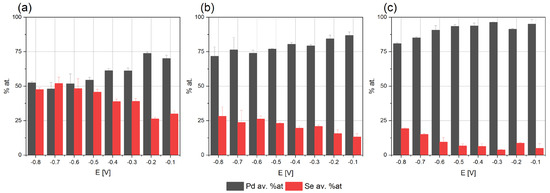
Figure 2.
Elemental compositions of coatings electrodeposited for different potentials from baths: (a) 0.004 M PdCl2, 0.008 M H2SeO3, 0.2 M NaCl, (b) 0.008 M PdCl2, 0.008 M H2SeO3, 0.2 M NaCl, (c) 0.008 M PdCl2, 0.004 M H2SeO3, 0.2 M NaCl, pH = 2 on Cu/Au electrode, 800 rpm after annealing: 200 °C, argon atmosphere.
The co-deposition of palladium with selenium starts at −0.1 V, which is consistent with our conclusions, drawn on the voltammetric tests. The palladium content is higher than that of selenium in all the coatings, irrespective of the deposition potential and electrolyte composition. As the potential decreases, the selenium content increases systematically, and the palladium decreases in solutions containing 0.008 M PdCl2 and 0.008 M H2SeO3, as well as 0.008 M PdCl2 and 0.004 M H2SeO3. We can assume that in the selected concentration ranges, the reaction of the reduction of the palladium ions is diffusion-controlled. The palladium-to-selenium-content ratio decreases as the concentration of selenous acid in the electrolyte increases, which confirms our assumption.
Similarly, when the electrolyte containing 0.004 M PdCl2 and 0.008 M H2SeO3 was used, the selenium content in the coatings obtained in the potential range from −0.1 V to −0.5 V from increased. This dependence may indicate that the reduction of selenous acid is kinetically controlled. However, the composition of the coatings deposited in the potential range from −0.6 to −0.8 V did not change. Referring to the voltammetric tests, an intensive reduction of hydrogen ions begins at such negative potentials. An additional electrochemical reaction may affect the rates of other reactions and make it difficult to interpret the results unequivocally. Moreover, in the discussed range of potentials, the mechanism of the selenous acid’s reduction reaction changes, and the reduction of deposited selenium to hydrogen selenide may also occur (7). Hence, further electrochemical studies are required, confirming our assumptions regarding the interpretation of the mechanism of palladium–selenium co-deposition. In summary, the research on the electrodeposition process indicates the possibility of obtaining coatings with a composition not exceeding 50 at. % of selenium. Moreover, this may result in synthesizing intermetallic compounds with different levels of stoichiometry.
3.3. XRD Results
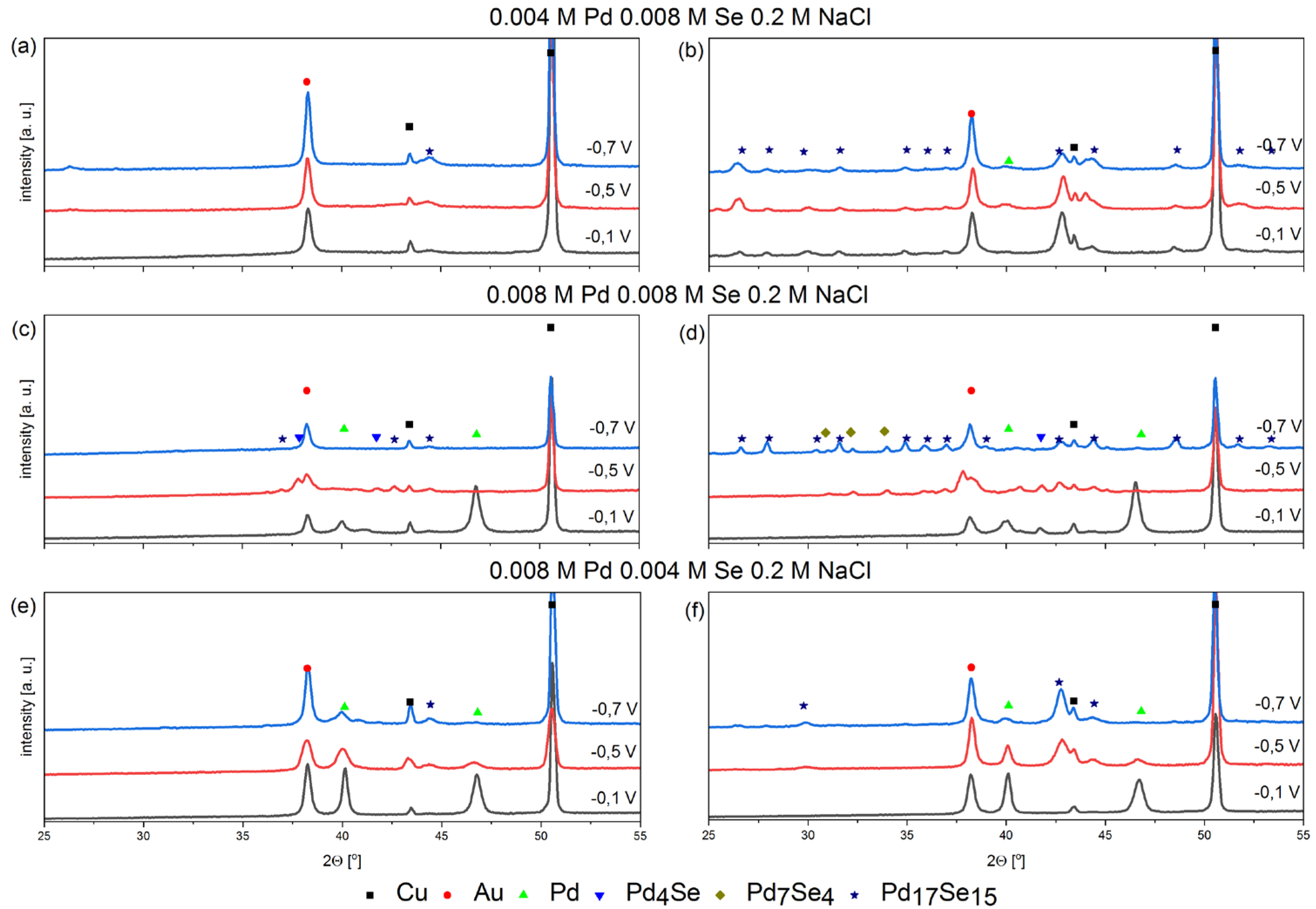
A phase analysis of the deposited coatings was carried out to confirm the possibility of synthesizing Pd-Se intermetallic compounds using the electrochemical method. The coatings were amorphous or very fine-crystalline after deposition, making it difficult to interpret the diffraction patterns. Therefore, the samples were annealed, which helped to reveal the phase composition of the coatings. It should be emphasized that heat treatment does not change the chemical composition of the coatings. However, the possibility of solid-phase reactions cannot be excluded. Based on the obtained results, we attempted to determine the impact of the deposition potentials and the ratios of the palladium and selenium concentrations in the electrolytes on the possibility of the synthesis of intermetallic compounds.
The Pd17Se15-phase peaks appeared in all the coatings, regardless of the electrolyte composition when potentials lower than −0.1 V were applied. In the solution with the highest concentration of selenous acid, the Pd17Se15 phase forms primarily. The composition of this compound is very close to the 1:1 stoichiometry. It should be mentioned that small peaks related to the Pd17Se15 phase already appear for the as-deposited coatings (Figure 3a). Moreover, there are no peaks from pure palladium or other intermetallic compounds. These arguments suggest the direct synthesis of the intermetallic compound according to mechanism (8).
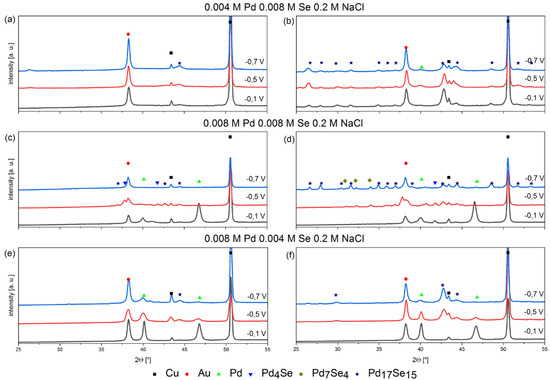
Figure 3.
XRD patterns of electrodeposited coatings at different potentials from bath containing palladium and selenium ions with different stochiometric ratios, pH = 2 on Cu/Au electrode, 800 rpm, before annealing (a,c,e) and after annealing (b,d,f), 200 °C, 2 h, argon atmosphere.
The phases of Pd4Se and Pd7Se4 are additionally formed when the coatings are deposited in the solution containing the same concentrations of palladium and selenium. The appearance of additional intermetallic compounds, the stoichiometry of which indicates a significant advantage for palladium, excludes the possibility of their synthesis according to reaction (8). However, peaks related to the Pd4Se phase appear in the as-deposited coatings. The annealing process increases the number of peaks and the appearance of new peaks, confirming the presence of the Pd4Se phase. At this research stage, we can assume that the synthesis process occurs directly during the co-deposition of two elements. As the voltammetric results described above showed, the phenomenon that is favorable for synthesizing intermetallic compounds is the adsorption of selenous acid on the surface of the deposited palladium. The mechanism described above is conducive to lowering the overpotential of palladium crystallization and may be responsible for synthesizing intermetallic compounds with different levels of stoichiometry. Nevertheless, such a mechanism requires further research to rule out the possibility of synthesizing intermetallic compounds due to heat treatment.
In coatings obtained in a solution containing more palladium than selenium, the peak of pure palladium appears even in coatings obtained at the lowest potential. As mentioned above, the Pd17Se15 phase is also present. The peaks of this intermetallic compound appear even before the coatings are annealed, which confirms the synthesis mechanism according to reaction (8).
Peaks related to pure palladium are noticeable for the coatings obtained in the electrolyte in which the Pd:Se ratios are 2:1 and 1:1. Annealing does not change the shapes of palladium peaks. Pure palladium peaks do not appear in coatings obtained in solutions containing 0.004 M PdCl2 and 0.008 M H2SeO3.
The phase analysis confirmed the possibility of synthesizing various Pd-Se intermetallic compounds. The key parameter that determines the possibility of their synthesis is the selection of the appropriate electrolyte composition and deposition potential. Since palladium deposition is faster than selenium, solutions containing more selenous acid than palladium species should be used to synthesize intermetallic compounds. Moreover, the synthesis of intermetallic compounds is favored at lower potentials, and the use of overly positive potentials promotes the deposition of pure palladium.
3.4. SEM Results
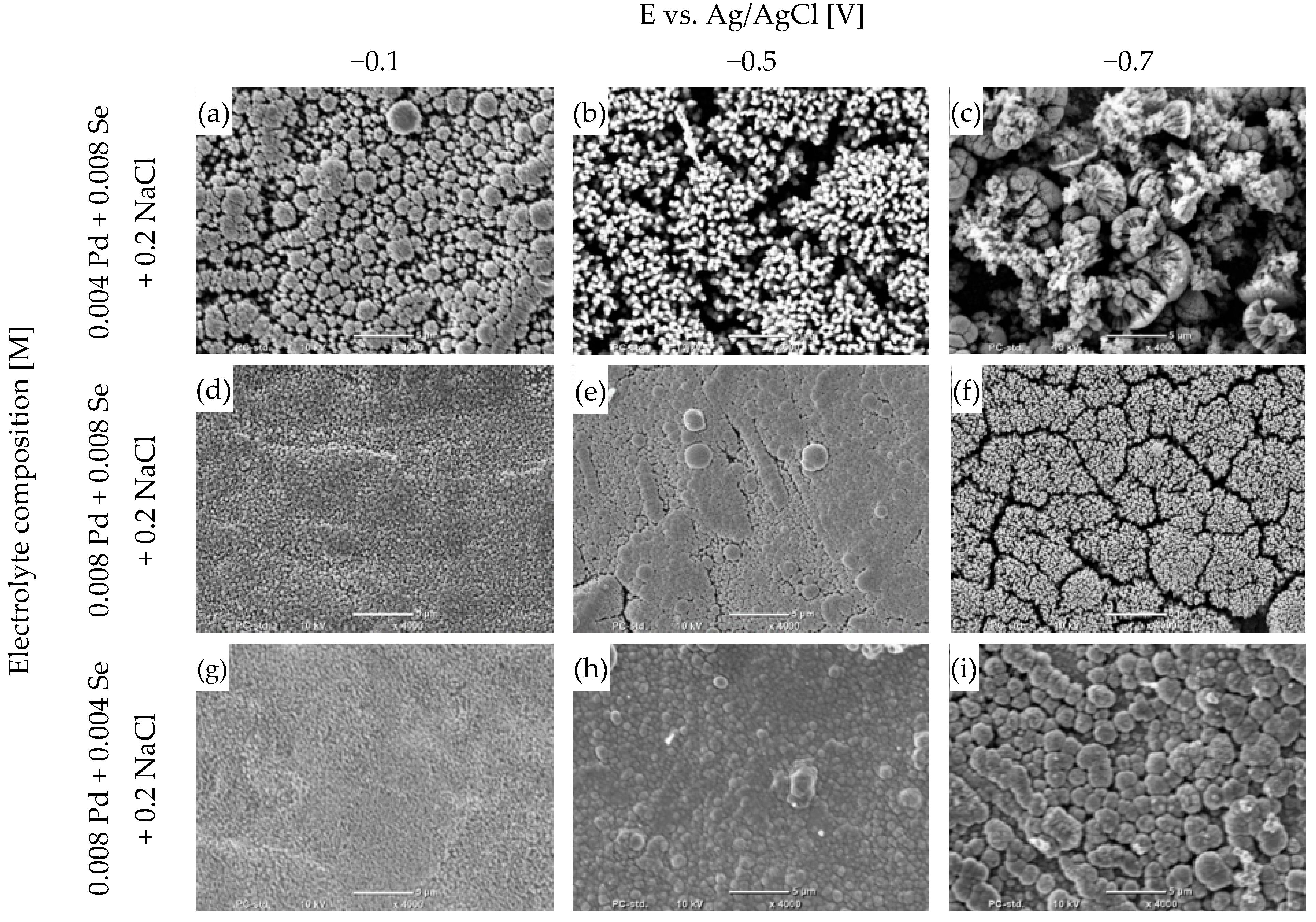
The surface morphology is one of the key features that determine the properties of coatings. Research using scanning-electron microscopy allows us to determine how the parameters of the electrolysis process affect the morphologies of deposited films. According to our previous research, the morphologies of both selenium and metal-selenide coatings significantly depend on the mechanism of selenous acid reduction [41]. Photographs of the deposited coating surfaces after annealing are presented in Figure 4. The annealing process did not cause any changes in the surface structures of the samples (Figure S2), except for the coating deposited at −0.7 V from the electrolyte containing 0.004 M Pd and 0.008 Se. This layer contained the highest amount of selenium out of all the deposited coatings. Selenium has a relatively low melting point and high vapor pressure compared to palladium. Nevertheless, according to the elemental analysis, there was no substantial change in the composition of the coating. Therefore, we can exclude the process of the evaporation of selenium. However, a phase transformation in the coating may have caused structural and morphological changes.
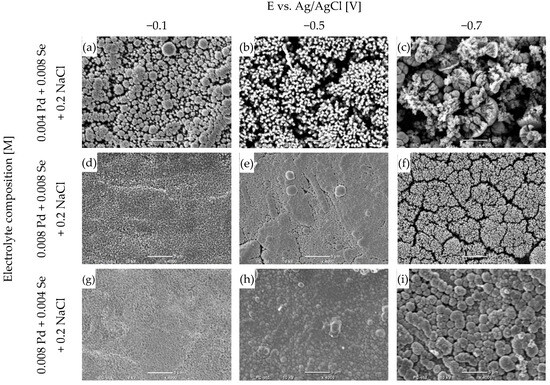
Figure 4.
SEM micrographs of coatings electrodeposited for different potentials from a bath containing palladium and selenium ions with different stoichiometric ratios, pH = 2, on Cu/Au electrode, 800 rpm, after annealing process: 200 °C, 2 h, argon atmosphere. The scale line in the photographs is 5 μm.
The morphologies of the coatings depend significantly on the deposition conditions, but also on the phase composition. The coatings obtained in electrolytes at lower potentials are homogeneous and compact (Figure 4d,g,h), with a fine crystalline structure. Both elemental and phase analyses indicate that pure palladium is the predominant component. The increase in selenium content causes the surface structure to change, and the spherical grains and the gaps between them are visible (Figure 4a,i). The dominant phase in these coatings is Pd17Se15, and the phase diffractograms of these two are almost identical (Figure 3). The coatings are highly textured, as confirmed by one dominant peak verifying the presence of the Pd17Se15 phase. Undoubtedly, it is surprising that these coatings were deposited at significantly different potentials, in which the reaction mechanisms behind the reduction of the palladium ions and selenous acid were different. The above similarity shows that the structure of the deposited material also determines the surface of the coating to a large extent.
Another change in morphology occurred when electrolytes containing higher concentrations of selenous acid were used and the deposition potential was lower (Figure 4b,f). Very dense bunches of needles appeared on the surfaces. Furthermore, the compound Pd17Se15 is the dominant phase in the coatings. However, the diffraction patterns show many low peaks, confirming its presence. The coating does not have a homogeneous texture, as in the (a) and (i) coatings. A radical change in the surface structure results from initiating the palladium-and-selenium-co-deposition process according to reaction (8).
Another change in the appearance of the coatings occurs when the concentration of selenous acid is at its highest and the deposition potential is at its lowest (Figure 4c). The surface of the coating becomes very heterogeneous and porous. The dendrites have different shapes and do not adhere especially tightly to each other, as in the case of the needles obtained in coatings (b) and (f) (Figure 4). The reason for the formation of such coatings is the intense hydrogen evolution accompanying the reduction reactions of palladium ions and selenous acid. In addition, the highest concentration of selenous acid in the electrolyte can cause more selenium to deposit in the coating. The excess selenium can be reduced according to reaction (7). As a result of the dissolution of selenium, characteristic heterogeneous and porous coatings are formed [41].
When comparing the surfaces of the obtained coatings, a strong dependence on the deposition potential and the electrolyte composition is undoubtedly noticeable. The change in potential determines the mechanism underlying the electrode reactions, which affects the contents of the elements in the coating and the phase composition. All these factors determine the appearance of the coatings. An important observation is that the appropriate selection of the proportions of palladium and selenium in the electrolyte makes it possible to obtain coatings with very similar chemical and phase compositions, as well as surface morphologies, despite significantly different deposition potentials.
An important observation is that coatings primarily showing the presence of the Pd17Se15 phase can be obtained from solutions containing the same or higher concentrations of selenium compared to palladium. The Pd17Se15 phase appears when we use lower potentials from −0.5 to −0.7 V. This suggests a selective and controlled deposition process, which is essential for obtaining coatings dominated by the Pd17Se15 phase. According to the literature, the Pd17Se15 is a very promising candidate as a catalyst for the oxygen-reduction reaction [15] or as a counter-dye-sensitized solar cell [17].
In extreme cases, in which the concentration of selenous acid is higher than that of palladium ions and the deposition potential is very low, we obtain very heterogeneous and porous coatings. The above observations are highly significant because the surface structure determines the properties of coatings and their application possibilities.
4. Conclusions
The above research allowed us to determine the influence of the applied potential and the stoichiometric ratio of palladium and selenium in electrolytes on the compositions and morphologies of coatings.
Voltammetric studies indicate that palladium is preferentially deposited at more positive potentials. Decreasing the potential causes the co-deposition of selenium with palladium to begin. However, the reduction of selenous acid and the deposition of selenium are only possible if the palladium is deposited first due to the high overpotential of selenium crystallization. Palladium selenides are formed at low potentials due to the change in the mechanism behind selenous acid reduction from a four-electron to a six-electron process. The initiation of the six-electron reduction reaction of selenous acid leads to the direct synthesis of palladium selenide with the stoichiometry of Pd17Se15.
These results are reflected in the elemental and phase composition, as well as the morphologies, of the deposits. The obtained coatings contain no more than 50 at. % of selenium. With a more negative potential, the selenium content increases, and that of palladium decreases simultaneously. This shows the possibility of obtaining intermetallic compounds with different stoichiometric ratios, which was confirmed by the XRD analysis. The dominant phase is Pd17Se15, which appears regardless of the electrolyte composition. Moreover, in the solution containing the same concentrations of palladium and selenium, the presence of Pd4Se and Pd7Se4 phases was detected. The increased selenium content in the coating results in a porous morphology consisting of bunches of needles. At the potential of −0.1 V, the presence of a pure palladium phase was detected; therefore, to obtain intermetallic compounds, more negative potentials should be used. Coatings in which pure palladium is the predominant component are more homogeneous and fine-grained. When the annealing process was conducted at 200 °C, the composition and morphology of the coatings did not change. However, the heat treatment improved the crystallinity of the coatings deposited at more negative potentials and with greater selenium contents.
In summary, the electrochemical method, with its inherent flexibility and precision, was proven to be an effective method for synthesizing coatings with a predominant phase of Pd17Se15. By manipulating the electrolyte composition and the deposition potentials, we can achieve the targeted synthesis of the Pd17Se15 phase, demonstrating the method’s ability to produce coatings with a specified elemental and phase composition and morphology for various applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/coatings13121993/s1. Figure S1: Elemental composition of coatings electrodeposited for different potentials from baths: (a). 0.004 M PdCl2, 0.008 M H2SeO3, 0.2 M NaCl, (b). 0.008 M PdCl2, 0.008 M H2SeO3, 0.2 M NaCl, (c). 0.008 M PdCl2, 0.004 M H2SeO3, 0.2 M NaCl, pH = 2 on Cu/Au electrode, 800 rpm; Figure S2: SEM micrographs of coatings electrodeposited for different potentials from bath containing palladium and selenium ions with different stochiometric ratios, pH = 2, on Cu/Au electrode, 800 rpm. Figure S3. Standard patterns of (a) Pd4Se (JCPDF 01-073-1386), (b) Pd4Se (JCPDF 03-065-3721), (c)Pd4Se (JCPDF 00-011-0508).
Author Contributions
Conceptualization, M.Ś. and R.K.; data curation, M.Ś., M.S., and R.K.; formal analysis, M.Ś. and R.K.; funding acquisition R.K.; investigation, M.Ś.; methodology, M.Ś., A.J., M.S., D.K., and R.K.; project administration R.K.; resources, M.Ś., A.J., and M.S.; software, M.S.; supervision, R.K.; validation, M.S., D.K., and R.K.; visualization, M.Ś.; writing—original draft; M.Ś.; writing—review and editing, R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Polish National Center of Science under grant 2016/21/B/ST8/00431.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Chen, E.; Xu, W.; Chen, J.; Warner, J.H. 2D Layered Noble Metal Dichalcogenides (Pt, Pd, Se, S) for Electronics and Energy Applications. Mater. Today Adv. 2020, 7, 100076. [Google Scholar] [CrossRef]
- Chen, R.; Xu, D.; Guo, G.; Gui, L. Preparation of Ag2Se and Ag2Se1−xTex Nanowires by Electrodeposition from DMSO Baths. Electrochem. Commun. 2003, 5, 579–583. [Google Scholar] [CrossRef]
- Bron, M.; Bogdanoff, P.; Fiechter, S.; Dorbandt, I.; Hilgendorff, M.; Schulenburg, H.; Tributsch, H. Influence of Selenium on the Catalytic Properties of Ruthenium-Based Cluster Catalysts for Oxygen Reduction. J. Electroanal. Chem. 2001, 500, 510–517. [Google Scholar] [CrossRef]
- Xu, X.; Robertson, J.; Li, H. Semiconducting Few-Layer PdSe2 and Pd2Se3: Native Pointdefects and Contacts with Native Metallic Pd17Se15. Phys. Chem. Chem. Phys. 2020, 22, 7365–7373. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H. The Pd-Se (Palladium-Selenium) System. J. Phase Equilibria 1992, 13, 69–72. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, M.; Zhang, W.; Wang, G.; Fu, S. Palladium Selenide as a Broadband Saturable Absorber for Ultra-Fast Photonics. Nanophotonics 2020, 9, 2557–2567. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, Q.; Binru, Z.; Dai, X.; Wei, S.; Yaqiang, M. Superlattices and Microstructures Electronic and Optical Properties of PdSe2 from Monolayer to Trilayer. Superlattices Microstruct. 2020, 142, 106514. [Google Scholar] [CrossRef]
- Venkatesan, A.; Rathi, S.; Kim, Y.; Kim, H.; Whang, D.; Yun, S.J.; Kim, G.-H. Few-Layer PdSe2-Based Field-Effect Transistor for Photodetector Applications. Mater. Sci. Semicond. Process. 2020, 115, 105102. [Google Scholar] [CrossRef]
- Li, G.; Yin, S.; Tan, C.; Chen, L.; Yu, M.; Li, L.; Feng, Y. Fast Photothermoelectric Response in CVD-Grown PdSe2 Photodetectors with In-Plane Anisotropy. Adv. Funct. Mater. 2021, 31, 2104787. [Google Scholar] [CrossRef]
- Wu, Z.; Lu, L.; Lu, L.; Liang, X.; Dun, C.; Yan, S.; Mu, E.; Liu, Y.; Hu, Z. Formation of Hexagonal PdSe2 for Electronics and Catalysis. J. Phys. Chem. C 2020, 124, 10935–10940. [Google Scholar] [CrossRef]
- Long, C.; Liang, Y.; Jin, H.; Huang, B.; Dai, Y. PdSe2: Flexible Two-Dimensional Transition Metal Dichalcogenides Monolayer for Water Splitting Photocatalyst with Extremely Low Recombination Rate. ACS Appl. Energy Mater. 2019, 2, 513–520. [Google Scholar] [CrossRef]
- Duan, Y.; Tang, Q.; He, B.; Yu, L. Transparent Counter Electrode from Palladium Selenide for Bifacial Dye-Sensitized Solar Cell. Mater. Lett. 2015, 160, 511–514. [Google Scholar] [CrossRef]
- Sarma, S.C.; Vemuri, V.; Mishra, V.; Peter, S.C. “Sacrificial Protection in Action!”: Ultra-High Stability of Palladesite Mineral towards the Oxygen Reduction Reaction. J. Mater. Chem. A 2019, 7, 979–984. [Google Scholar] [CrossRef]
- Singh, P.; Singh, A.K. Palladium(II) Complexes of N,N-Diphenylacetamide Based Thio/Selenoethers and Flower Shaped Pd16S7 and Prismatic Pd17Se15 Nano-Particles Tailored as Catalysts for C-C and C-O Coupling. Dalt. Trans. 2017, 46, 10037–10049. [Google Scholar] [CrossRef] [PubMed]
- Kukunuri, S.; Naik, K.; Sampath, S. Effects of Composition and Nanostructuring of Palladium Selenide Phases, Pd4Se, Pd7Se4 and Pd17Se15, on ORR Activity and Their Use in Mg–Air Batteries. J. Mater. Chem. A 2017, 5, 4660–4670. [Google Scholar] [CrossRef]
- Kukunuri, S.; Austeria, P.M.; Sampath, S. Electrically Conducting Palladium Selenide (Pd4Se, Pd17Se15, Pd7Se4) Phases: Synthesis and Activity towards Hydrogen Evolution Reaction. Chem. Commun. 2016, 52, 206–209. [Google Scholar] [CrossRef]
- Kukunuri, S.; Karthick, S.N.; Sampath, S. Robust, Metallic Pd17Se15 and Pd7Se4 Phases from Single Source Precursor and Their Use as Counter Electrodes in Dye Sensitized Solar Cells. J. Mater. Chem. A 2015, 3, 17144–17153. [Google Scholar] [CrossRef]
- Sarma, S.C.; Kaja, S.M.; Ann Mary, K.A.; Peter, S.C. Reversing the Activity Center in Doped Pd17Se15 to Achieve High Stability Toward the Electrochemical Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2020, 3, 4051–4056. [Google Scholar] [CrossRef]
- Zeng, L.H.; Wu, D.; Lin, S.H.; Xie, C.; Yuan, H.Y.; Lu, W.; Lau, S.P.; Chai, Y.; Luo, L.B.; Li, Z.J.; et al. Controlled Synthesis of 2D Palladium Diselenide for Sensitive Photodetector Applications. Adv. Funct. Mater. 2019, 29, 1806878. [Google Scholar] [CrossRef]
- Mak, C.H.; Lin, S.; Rog, L.; Lau, S.P. Photoresponse of Wafer-Scale Palladium Diselenide Films Prepared by Selenization Method. J. Phys. D. Appl. Phys. 2020, 53, 065102. [Google Scholar] [CrossRef]
- Oyedele, A.D.; Yang, S.; Liang, L.; Puretzky, A.A.; Wang, K.; Zhang, J.; Yu, P.; Pudasaini, P.R.; Ghosh, A.W.; Liu, Z.; et al. PdSe2: Pentagonal Two-Dimensional Layers with High Air Stability for Electronics. J. Am. Chem. Soc. 2017, 139, 14090–14097. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Jiang, J.; Ma, H.; Zhang, Z.; Li, J.; Zhao, B.; Wu, R.; Yang, X.; Zhang, H.; Li, B.; et al. Vapor Phase Growth of Two-Dimensional PdSe2 Nanosheets for High-Photoresponsivity near-Infrared Photodetectors. Nano Res. 2020, 13, 2091–2097. [Google Scholar] [CrossRef]
- Hoffman, A.N.; Gu, Y.; Liang, L.; Fowlkes, J.D.; Xiao, K.; Rack, P.D. Exploring the Air Stability of PdSe2 via Electrical Transport Measurements and Defect Calculations. NPJ 2D Mater. Appl. 2019, 3, 50. [Google Scholar] [CrossRef]
- Jiang, S.; Xie, C.; Gu, Y.; Zhang, Q.; Wu, X.; Sun, Y.; Li, W.; Shi, Y.; Zhao, L.; Pan, S.; et al. Anisotropic Growth and Scanning Tunneling Microscopy Identification of Ultrathin Even-Layered PdSe2 Ribbons. Small 2019, 15, 1902789. [Google Scholar] [CrossRef]
- Li, E.; Wang, D.; Fan, P.; Zhang, R.; Zhang, Y.; Li, G.; Mao, J.; Wang, Y. Construction of Bilayer PdSe2 on Epitaxial Graphene. Nano Res. 2018, 11, 5858–5865. [Google Scholar] [CrossRef]
- Wojtysiak, M.; Jędraczka, A.; Stępień, M.; Kutyła, D.; Kowalik, R. Electrodeposition of Pd–Se Thin Films. Electrochem. Commun. 2021, 127, 107053. [Google Scholar] [CrossRef]
- Mech, K.; Żabiński, P.; Kowalik, R.; Fitzner, K. Kinetics and Mechanism of [PdClx(H2O)4−x]2−x(x = 3,4) Complexes Electro-Reduction. J. Electrochem. Soc. 2013, 160, H770–H774. [Google Scholar] [CrossRef]
- Mech, K.; Zabiński, P.; Kowalik, R.; Fitzner, K. Voltammetric Study of Electro-Reduction of Tetraamminepalladium(II) onto Gold Electrode. J. Electroanal. Chem. 2012, 685, 15–20. [Google Scholar] [CrossRef]
- Quayum, M.E.; Ye, S.; Uosaki, K. Mechanism for Nucleation and Growth of Electrochemical Palladium Deposition on an Au(111) Electrode. J. Electroanal. Chem. 2002, 520, 126–132. [Google Scholar] [CrossRef]
- Kublanovsky, V.S.; Nikitenko, V.N. Mechanism of the Electrodeposition of Palladium Coatings from Glycinate Electrolytes. J. Electroanal. Chem. 2013, 699, 14–20. [Google Scholar] [CrossRef]
- Rezaei, M.; Tabaian, S.H.; Haghshenas, D.F. A Kinetic Description of Pd Electrodeposition under Mixed Control of Charge Transfer and Diffusion. J. Electroanal. Chem. 2012, 687, 95–101. [Google Scholar] [CrossRef]
- Kowalik, R. The Voltammetric Analysis of Selenium Electrodeposition from H2SeO3 Solution on Gold Electrode. Arch. Metall. Mater. 2015, 60, 57–63. [Google Scholar] [CrossRef]
- Maranowski, B.; Strawski, M.; Osowiecki, W.; Szklarczyk, M. Study of Selenium Electrodeposition at Gold Electrode by Voltammetric and Rotating Disc Electrode Techniques. J. Electroanal. Chem. 2015, 752, 54–59. [Google Scholar] [CrossRef]
- Mishra, K.K.; Rajeshwar, K. A Re-Examination of the Mechanisms of Electrodeposition of CdX and ZnX (X = Se, Te) Semiconductors by the Cyclic Photovoltammetric Technique. J. Electroanal. Chem. Interfacial Electrochem. 1989, 273, 169–182. [Google Scholar] [CrossRef]
- Lister, T.E.; Stickney, J.L. Atomic Level Studies of Selenium Electrodeposition on Gold(111) and Gold(110). J. Phys. Chem. 1996, 100, 19568–19576. [Google Scholar] [CrossRef]
- Czerwiński, A.; Łukaszewski, M.; Grdeń, M.; Siwek, H. The Effect of Carbon Oxides on the Absorption of Hydrogen in Palladium Alloys. Przem. Chem. 2004, 83, 508–512. [Google Scholar]
- Thouin, L.; Rouquette-Sanchez, S.; Vedel, J. Electrodeposition of Copper-Selenium Binaries in a Citric Acid Medium. Electrochim. Acta 1993, 38, 2387–2394. [Google Scholar] [CrossRef]
- Andrews, R.W.; Johnson, D.C. Voltammetric Deposition and Stripping of Selenium(IV) at a Rotating Gold-Disk Electrode in 0.1 M Perchloric Acid. Anal. Chem. 1975, 47, 294–299. [Google Scholar] [CrossRef]
- Cabral, M.F.; Pedrosa, V.A.; Machado, S.A.S. Deposition of Selenium Thin Layers on Gold Surfaces from Sulphuric Acid Media: Studies Using Electrochemical Quartz Crystal Microbalance, Cyclic Voltammetry and AFM. Electrochim. Acta 2010, 55, 1184–1192. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; National Association of Corrosion Engineers: Houston, TX, USA, 1974. [Google Scholar]
- Kołczyk, K.; Kowalik, R.; Mech, K.; Żabiński, P. Electrochemical Deposition of Selenium on Copper. Key Eng. Mater. 2016, 682, 189–196. [Google Scholar] [CrossRef]
- Kowalik, R. Microgravimetric Studies of Selenium Electrodeposition onto Different Substrates. Arch. Metall. Mater. 2014, 59, 871–877. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).