Abstract
Morphological engineering and semiconductor coupling show significant potential to increase the photocatalytic performance of graphite carbon nitride (g-C3N4). In this work, a unique Z-scheme heterojunction photocatalyst composed of tubular g-C3N4 (TCN) and α-F2O3 was successfully synthesized. Combining the experimental results and characterization, we extensively investigated the charge transfer mechanism of the α-F2O3/tubular g-C3N4 (FO-TCN) heterojunctions and processes in the photocatalytic degradation of tetracycline (TC). The tubular morphology provided a larger specific surface area, enhancing the light absorption area and thus improving the exposure of the active sites. Not only was the light absorption range expanded through the coupling with α-F2O3, but the charge transfer properties of the sample were also strengthened. The synergism between photocatalysis and the Fenton reaction enhanced the photocatalytic performance of the FO-TCN. Due to the previously mentioned beneficial factors, the performance of the FO-TCN photocatalyst was significantly increased; its reaction rate k value in the degradation of TC (0.0482 min−1) was 4.05 times faster than that of single g-C3N4 and it exhibited the best photocatalytic performance (95.02%) for the degradation of TC in 60 min, with an enhancement of 38.41%. Quenching experiments showed that h+ and ·O2− were the major active substances in the photocatalytic degradation process.
1. Introduction
In recent years, with industrial and urban development, a wide range of organic compounds have been introduced into the water environment, posing a serious threat to the ecosystem and to humans. Additionally, contamination with antibiotics has received extensive attention due to its threats to the environment [1,2,3,4]. Tetracycline (TC) is a very common antibiotic used in humans and animals and has a long history of use in the medical and animal feed industries. Typically, it is difficult for TC to be completely metabolized in the body due to its complex molecular structure and high stability, which leads to its widespread occurrence in the environment, inevitably resulting in environmental pollution [5,6]. Thus, there is a need for an advanced and environmentally friendly technology for the degradation of TC. In this context, semiconductor photocatalytic technology has developed into the most modern and environmentally friendly technology for water treatment [7,8,9].
To date, the use of semiconductor photocatalysts has attracted great interest worldwide in the field of the degradation of antibiotics, due to their eco-friendliness and cost-effectiveness [10,11]. In previous studies, the practical applications of conventional photocatalysts (ZnO and CdS) were limited due to their poor solar energy conversion performance and large band gap, especially the fast recombination rate of photogenerated e−/h+ [12,13]. Recently, graphite carbon nitride (g-C3N4) has gained widespread interest for its low production cost, ease of preparation, and suitable band gap [14,15]. However, the application of g-C3N4 is limited by its disadvantages, which include a volume structure with a small specific surface area, insufficient light absorption, and a low photogenerated carrier transfer capacity [16]. Hence, a number of strategies, including morphological adjustment, elemental doping, and combination with semiconductor materials, have been proposed to overcome these inherent disadvantages. Normally, photocatalytic processes take place at the surface of the photocatalyst, and the particular morphology of modified g-C3N4 exhibits excellent photocatalytic performance compared to its bulk counterparts [17,18,19]. Thus far, g-C3N4 photocatalysts in various nanostructured forms (nanosheet, nanotube, flower-like, microsphere, etc.) have been prepared successfully [20,21,22,23,24]. Wang et al. prepared novel, spongy g-C3N4 with a porous structure via protonated melamine annealing under an N2/H2 atmosphere, showing thinner nanosheets and a larger surface area [25]. Yang et al. prepared flower-like P-doped graphitic carbon nitride (g-C3N4) with a large surface area and high porosity via a facile thermal copolymerization method [26]. The larger surface area can provide more active sites directly and is conducive to shortening the diffusion distance of photogenerated carriers as well [27]. Due to the latest experimental research, g-C3N4 nanotubes have attracted great interest for their particular structure, which gives them excellent surface photocatalytic properties, promising electron transfer rates, and excellent optical qualities. However, liquid-phase reactions and template-assisted processes have generally been used to synthesize the previously studied g-C3N4 nanotubes [28]; these synthesis methods are inherently uncontrollable and difficult to perform because of the high-pressure conditions and the complex template residues required. In recent studies, a new, simple, and highly desirable method for the mass synthesis of tubular g-C3N4 was developed [29].
Currently, α-Fe2O3 is widely applied in photocatalytic composite material systems because of its non-toxicity, affordability, and property of absorbing visible light [30,31,32,33,34,35]. However, its widespread application as a highly efficient photocatalyst is restricted by the fast recombination of the photogenerated e−/h+ pairs. Meanwhile, α-Fe2O3 presents a band gap in which the CB and VB edge potentials are close to 0.49 eV and 2.29 eV, respectively, which are lower than the corresponding values for g-C3N4 [36]. Theoretically, g-C3N4 could fit comfortably with Fe2O3, forming II-scheme (or Z-scheme) heterojunctions at their interface [37], and this allows for the energy levels to be staggered and the photogenerated carriers to be transferred [38]. g-C3N4 is combined with α-Fe2O3 to produce a α-Fe2O3/g-C3N4 heterojunction photocatalyst. In this situation, it plays an important role not only as a catalytic component but also as a viable and outstanding iron-based photo-Fenton catalyst [39]. The principle of the photo-Fenton reaction is that H2O2, in the presence of the catalyst Fe2+, generates strong oxidizing power in the form of -OH and triggers the production of additional reactive oxygen species to achieve the degradation of organic matter. The reaction system can convert complexes such as Fe(OH)2+ into Fe2+ under UV light irradiation so that Fe3+/Fe2+ maintains a good cycle, which, in turn, accelerates the production of ·OH through H2O2 [40,41,42]. Thus, it is a feasible solution to construct an α-Fe2O3/g-C3N4 photocatalysis–Fenton system.
Herein, g-C3N4 is adopted as a supporting system for α-Fe2O3 based on its ease of modification and superior photocatalytic properties. In the present study, the structure and properties of pristine g-C3N4 are modified through morphology engineering, and FO-TCN samples are prepared. The increased specific surface area of the TCN offers more sites to load Fe2O3, and the loaded α-Fe2O3 has the benefits of the Fenton and the photocatalytic reactions. The scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), nitrogen adsorption/desorption, Fourier-transform infrared (FT-IR), X-ray photoelectron spectroscopy (XPS), and ultraviolet–visible diffuse reflection spectroscopy (UV–vis DRS) techniques were used for the characterization of the photocatalysts with FO-TCN. In terms of photocatalytic degradation activity, compared with another sample, the synthesized FO-TCN shows extraordinary photocatalytic performance for the degradation of TC. Furthermore, to investigate the active species’ photocatalytic performance, radical trapping experiments were carried out. TBA, EDTA-2Na, L-tryptophan, and ASC were used as quenchers for (·OH), (h+), 1O2, and (·O2−), respectively. The results show that both h+ and ·O2− play important roles in the degradation of TC.
2. Experimental
2.1. Preparation of Photocatalysts
2.1.1. Synthesis of CN and FO-CN
Melamine (6 g) was heated in a muffle furnace to 550 °C at 2.3 °C·min−1 and calcined for 4 h. The product was then allowed to cool down to room temperature naturally. After obtaining the CN, 0.25 g iron nitrate and 0.3 g ammonium bicarbonate were added to 100 mL ethanol, and the mixture was then ultrasonicated for 30 min at 25 °C and stirred for 4 h. Finally, the samples were calcined using the same procedures described above.
2.1.2. Synthesis of Tubular TCN and FO-TCN
The TCN was synthesized through a precursor-reforming strategy [43]. Firstly, urea (8 g) and melamine (6 g) were placed in 40 mL deionized water, respectively. Then, the melamine and urea solutions were mixed together. Subsequently, the mixture was placed in a 100 mL autoclave lined with Teflon and maintained at 180 °C for 1 d. In the process of hydrothermal treatment, melamine can partly form cyanuric acid at 180 °C. The regular precursors of TCN were eventually produced by the self-assembly of the molecules between melamine and cyanuric acid. The precipitate was washed with 50 mL ethanol and then 50 mL deionized water three times. The precursor was then ground into a powder and calcined at 550 °C to form TCN. Based on the previous synthesis process, FO-TCN was synthesized as follows. After obtaining the precursors, a white sample, 0.25 g iron nitrate with 0.30 g ammonium bicarbonate, was added to 100 mL ethanol; then, the mixture was ultrasonically treated for 30 min and stirred for 4 h. Finally, it was heated following the same procedures as for TCN synthesis to obtain the FO-TCN sample.
2.2. Photocatalyst Characterization
XRD (XRD-7000, Shimadzu, Kyoto, Japan) was used to characterize the as-prepared powder catalysts. FI-TR was performed (Shimadzu, Japan, IRTracer-100) over the range of 400–4000 nm with the KBr pellet method. The morphologies of the as-prepared samples were tested by SEM (Phenom Pharos G1, Eindhoven, The Netherlands). The specific surface area, pore size and pore volume of the samples were obtained from nitrogen adsorption/desorption isotherms on a (KuBo-X1000, Bjbuilder, Beijing, China) machine. XPS (Thermo Escalab 250-Xi, Thermo, Wilmington, NC, USA) was used to analyze the surface composition and chemical state distribution of the samples, and C 1s was typically used as a reference for calibration. The light absorption of the as-prepared samples was analyzed with a UV–vis spectrophotometer (UV-2700i, Shimadzu, Suzhou, China) with the wavelength range of 200–800 nm.
2.3. Photocatalytic Performance Evaluation
The index used to evaluate the photocatalytic performance of the samples was the degradation efficiency of TC. A 300 W xenon lamp (Perfectlight, PLS-SXE300+/UV, Beijing, China) was used to simulate light energy. Typically, 20 mg photocatalyst was added to 100 mL TC (20 mg/L) solution and stirred for 60 min under dark conditions to reach adsorption/desorption equilibrium. Next, 50 μL H2O2 solution (30%) was added to the system. Then, the light energy was introduced into the system, and 3 mL of the suspension was taken at 5 min intervals. The suspension was then filtered by a 0.22 µm Millipore filter and finally evaluated using a spectrophotometer (Techcomp TU-1901, Shanghai, China) to test the concentration of TC at a wavelength of 357 nm. The formula for the calculation of the photocatalytic efficiency of the degradation of TC (η, %) is shown in Equation (1):
where C0 is the initial concentration of TC, while Ct is the concentration of TC after a degradation time of t minutes.
3. Results and Discussion
3.1. Physical and Chemical Properties
Characterization of the crystal structure and chemical molecular structure is necessary for the understanding of photocatalysts. XRD and FT-IR characterization were firstly performed. As shown in Figure 1a, the as-prepared samples had distinct diffraction peaks at 13.0° and 27.7°, corresponding to the respective (100) and (002) crystal planes of CN, which can be attributed to an in-plane arrangement of ordered tri-s-triazine units and the interlayer stacking of the conjugated aromatic system, respectively [44]. The characteristic peak of TCN at the 27.7° location was apparently weaker compared to the CN sample, which was attributed to the size effect originating from the ultra-thin tubular structure, thus causing a reduction in the stacking structure in the layer [45]. It appeared that the XRD spectra of both FO-CN and FO-TCN had two groups of peaks matching Fe2O3 and g-C3N4, respectively. The peaks at 24.2°, 33.2°, 35.8°, 41.0°, 49.6°, 54.2°, 57.7°, 62.5° and 64.1° of α-Fe2O3 could be assigned to the (012), (104), (110), (113), (024), (116), (018), (214) and (300) crystal planes (α-Fe2O3 JCPDS. No.33-0664). Notably, the characteristic peak at 27.7° in FO-TCN was slightly shifted because the Fe2O3 modulated the layer spacing, reflecting the fact that Fe2O3 was not confined to the shallow surface but had strong interfacial interactions with g-C3N4. After loading with the α-Fe2O3, the sample displayed the corresponding characteristic peaks, while the diffraction peaks of g-C3N4 in the samples decreased, indicating that FO-TCN heterostructures had been successfully fabricated.

Figure 1.
(a) XRD patterns and (b) FT-IR spectra of CN, TCN, FO-CN and FO-TCN, respectively.
To better illustrate the chemical molecular structure, the FT-IR spectra of the as-prepared samples are shown in Figure 1b. There were a number of main vibrational bands at 3000–3500 cm−1 corresponding to -NH and -OH stretching vibrations. The strong absorption peaks from 1200 to 1700 cm−1 could be attributed to the stretching vibration of the C-N heterocycles. Moreover, the sturdy absorption peak at 810 cm−1 was ascribed to the characteristic stretching vibration of tri-s-triazine units [46]. The typical absorption peaks of g-C3N4 were present in all the as-prepared samples. However, the corresponding peak position of the Fe-O bond in the composite was not obvious in the range of 450–1000 cm−1. This could be attributed to the low loading of α-Fe2O3, which agreed with previously reported research [47,48]. A small shift in the peaks indicated that there were some interfacial interactions among α-Fe2O3 and g-C3N4 in the FO-TCN composite sample, which helped to form heterojunctions.
The morphologies and microstructures of CN, TCN, FO-CN and FO-TCN were examined by SEM. As shown in Figure 2a, CN presented an aggregated and chunky structure, while TCN formed an obvious tubular morphology (Figure 2b). Thus, the specific surface area can be increased and more active sites can be provided with the tubular morphology. It can be seen that α-F2O3 particles are distributed as aggregations on the surface of g-C3N4 in Figure 2c. Most importantly, it was clear that the FO-TCN retained the tubular structure when α-F2O3 was introduced to the TCN system. The close binding of α-F2O3 particles to the tubular surface of g-C3N4 can be observed in Figure 2d, which confirms the formation of a contact interface between the α-F2O3 particles and tubular g-C3N4.
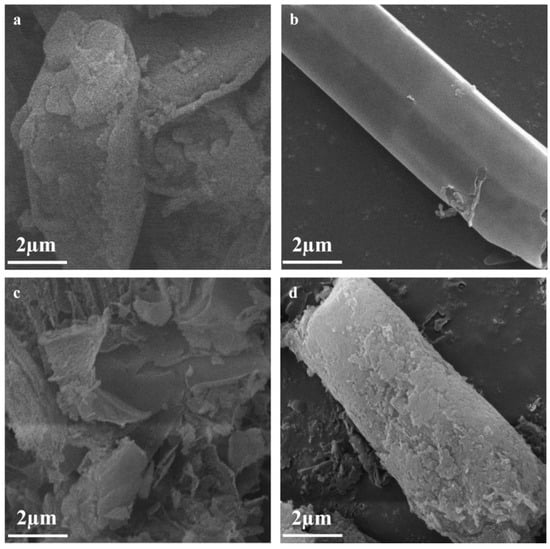
Figure 2.
SEM images of (a) CN, (b) TCN, (c) FO-CN and (d) FO-TCN, respectively.
In addition, to better illustrate the prepared tubular morphologies of the samples, the nitrogen adsorption/desorption isotherms and the Barrett—Joyner–Halenda (BJH) pore size distribution curves are shown in Figure 3a,b. As shown in Figure 3a, CN, TCN, FO-CN and FO-TCN all showed the typical type IV isotherm curves that characterize mesoporous materials. The pore volume of the original CN was smaller because of its bulk structure, whereas the modified TCN had a larger pore volume. The Brunauer–Emmett–Teller (BET) specific surface areas (SBET) of CN, TCN, FO-CN and FO-TCN were calculated to be 21.569 m2·g−1, 50.127 m2·g−1, 16.091 m2·g−1 and 92.053 m2·g−1, respectively. In summary, the g-C3N4 morphology in the present study greatly increased the specific surface area, thereby offering more anchor points for α-F2O3. Notably, the specific surface area of FO-TCN was higher than those of TCN and BCN, suggesting that the introduction of α-F2O3 increased the specific surface area of the composite and thus provided more active sites. This tubular structure resulted in a large specific surface area, providing more active sites for surface reactions and facilitating photogenerated electron transfer over shorter distances, thereby significantly enhancing the charge separation and transfer efficiency.
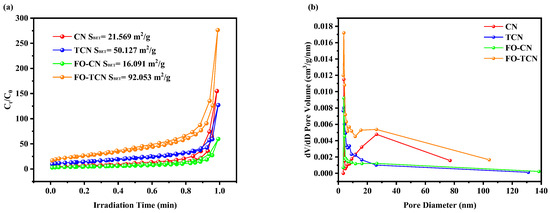
Figure 3.
(a) Nitrogen adsorption/desorption isotherms and (b) pore size distribution curves.
XPS was applied to analyze the chemical state of the elements on the catalyst surfaces. As shown in Figure 4a, the elements of C, N, O and Fe could be determined in the XPS spectra of both FO-CN and FO-TCN. Then, the chemical states of FO-CN and FO-TCN were determined through high-resolution XPS spectroscopy. In the C 1s spectrum exhibited in Figure 4b, the two major peaks located at 284.8 eV and 288.3 eV belong to occasional graphitic carbon atoms (C-C) and sp2 hybridized carbon atoms of the aromatic rings (N-C=N), respectively. In addition, a peak at 286.1 eV corresponding to the sp3-coordinated carbon (C-N) can be determined. In Figure 4c, N 1s of FO-CN shows that the main peak at 398.6 eV was identified as the sp2 hybridized N of C-N=C, the second one at 400.0 eV was ascribed to the N-C3 group and the last one at 400.8 eV was attributed to the terminal C-N=C carbon atom, while the peak of FO-TCN was slightly shifted towards a larger binding energy value. The O 1s of both FO-CN and FO-TCN are shown in Figure 4d. FO-CN presented two peaks at 529.9 and 531.9 eV corresponding to lattice oxygen and surface hydroxyl groups, respectively, while the binding energy of the lattice oxygen of FO-TCN shows a 0.2 eV negative shift. In Figure 4e, the Fe 2p of FO-CN shows two distinct peaks at 711.1 and 724.9 eV, and two oscillating satellite peaks at 717.3 and 732.2 eV, indicating that the Fe ions in FO-CN have a +3 value, while the Fe 2P3/2 of FO-TCN shows a weak positive shift. UV–vis DRS was used to characterize the optical absorption properties of the materials. As shown in Figure 5a, both CN and TCN had absorption edges at around 450 nm, respectively, which agreed with the previously reported absorption edges. Moreover, the introduction of α-F2O3 caused a red shift in the absorption edges of FO-CN and FO-TCN. This may have been caused by the synergistic effect between α-F2O3 and g-C3N4, which can expand the light absorption range of composite materials. Thus, the ability of the material to absorb visible light was enhanced through the introduction of Fe2O3, which is beneficial to the generation of charge carriers. Furthermore, the Kubelka–Munk function was used through the conversion of UV–vis DRS data to obtain the band gap energy of the photocatalyst, and the band gaps of CN, TCN, FO-CN and FO-TCN were 2.69 eV, 2.63 eV, 2.29 eV and 2.40 eV, respectively (Figure 5b). As shown in Figure S1, every average relative deviation of the band gap in the CN, TCN, FO-CN and FO-TCN systems was less than 2%. The band gap of the prepared composite was decreased after the introduction of α-F2O3 with a band gap of 2.01 eV (Figure 5c) into the system, which was beneficial to the photocatalyst for the further absorption of sunlight and the improvement of the photocatalytic performance.
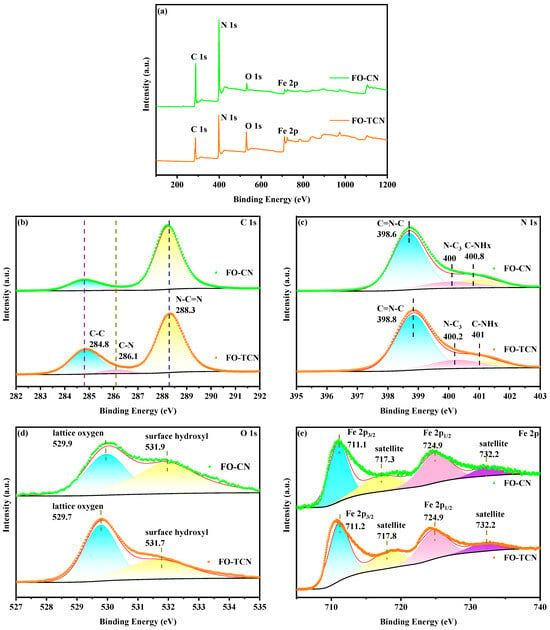
Figure 4.
XPS spectra of FO-CN and FO-TCN: (a) survey, (b) C 1s, (c) N 1s, (d) O 1s and (e) Fe 2p.
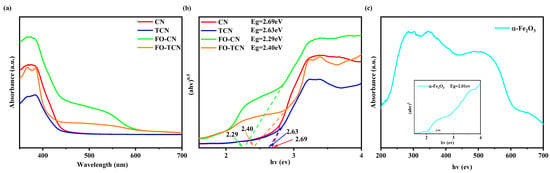
Figure 5.
(a) UV–vis DRS of samples, (b) corresponding band gap energy diagram of CN, TCN, FO-CN, FO-TCN and (c) UV–vis DRS of pristine α-Fe2O3.
The conduction band (CB) and valence band (VB) edge potentials of the photocatalysts were calculated according to the empirical Equations (2) and (3):
X is the absolute potential of the semiconductor, Ee is the electron free energy relative to the hydrogen potential level (4.5 eV) and Eg is the band gap of the semiconductor, which was obtained through UV–vis DRS in our experiment. Thus, it can be deduced that the CB and VB edge potentials of TCN were −1.02 eV and +1.61 eV (vs. NHE), while the CB and VB edge potentials of α-F2O3 were +0.38 eV and +2.39 eV (vs. NHE), respectively.
3.2. Photocatalytic Activity and Mechanisms
In order to investigate the photocatalytic ability of the samples, the photocatalytic degradation of TC was performed. The adsorption/desorption equilibrium of TC with the photocatalyst was reached under the conditions of 60 min dark treatment before light irradiation [49]. Figure 6a shows the variation in the TC concentration (Ct/C0) with light irradiation time over the as-prepared photocatalysts. Previous studies have shown that TC is a stable compound that is poorly metabolized in the body and cannot be decomposed in the absence of photocatalysts under light irradiation [7]. The different photocatalysts showed different photodegradation efficiency in 60 min, which was in the order of CN (56.61%) < TCN (78.77%) < FO-CN (82.28%) < FO-TCN (95.02%). In the 60 min photocatalytic process, the degradation efficiency of CN was the lowest (56.61%). The degradation efficiency of TCN was 22.16% higher than that of CN, which showed that the unique tubular morphology of TCN increased the photocatalytic capacity of TCN. As expected, all composites displayed superior photocatalytic decomposition efficiency compared to both pristine CN and TCN, which proves that the introduction of α-F2O3 to g-C3N4 is valuable. FO-TCN exhibited the best photocatalytic performance (95.02%) for the degradation of TC in 60 min, with an enhancement of 38.41% and 16.25% compared to CN and TCN. The apparent rate constant (k) can be calculated with Equation (4):
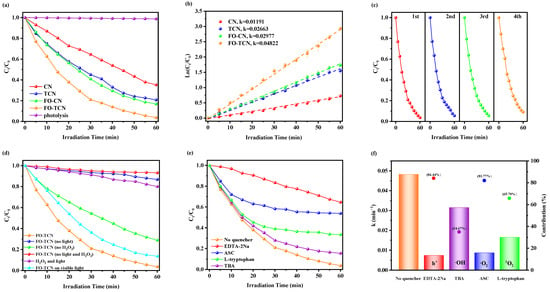
Figure 6.
(a) Photocatalytic degradation curves of TC over different samples, (b) kinetic curves and the corresponding apparent rate constants, (c) the cycle experiment for the degradation of TC in FO-TCN system, (d) photocatalytic degradation experiments with different treatment conditions, (e) degradation of TC with different quenching agents, (f) corresponding kinetic constants and the relative contributions of different quenchers.
As can be seen from the kinetic curves in Figure 6b and Figure S2, the average value of k followed the sequence of CN (0.0119 min−1) < TCN (0.0266 min−1) < FO-CN (0.0298 min−1) < FO-TCN (0.0482 min−1), and every average relative deviation in k in the CN, TCN, FO-CN and FO-TCN systems was less than 1%. FO-TCN indicated the highest k value (0.0482 min−1), which was 4.05 and 1.81 times higher than that of CN and TCN, respectively. The above experimental results show that FO-TCN had the strongest ability for TC degradation. The rate of TC degradation and the value of the rate constant k of FO-TCN were clearly higher than those of the other samples. This result proves that the regulation of the morphology is an important factor in the improvement of the photocatalytic performance. The tubular structure could not only improve the light absorption area but also expand the contact area with TC contaminants. Meanwhile, the addition of α-F2O3 to g-C3N4 was beneficial because the photocatalytic performance of FO-TCN was enhanced, which was attributed to the synergistic effect of enhancing the light response and improving the efficiency of photogenerated carrier separation. As is well known, an important measurement of the practical application of a catalyst is its reusability. Thus, we carried out a cycling experiment. As shown in Figure 6c, FO-TCN achieved degradation efficiency of 90.91% after four cycles, reflecting the good reusability of the sample.
As shown in Figure 6d, the effects of the light energy, H2O2 and photocatalyst on TC degradation were studied by conducting degradation experiments in different experimental situations. The experimental results showed that only the FO-TCN photocatalyst was not able to degrade TC without light energy. Meanwhile, in the absence of light energy, the photocatalytic degradation of TC by FO-TCN supported by H2O2 alone in the Fenton system was low, proving that the efficiency of the Fenton reaction was not optimal. In addition, it showed that H2O2 could slightly decompose TC under light energy without the addition of a photocatalyst. This was due to the oxidation of H2O2 itself to generate hydroxyl radicals (·OH) under light energy. Furthermore, the degradation rate of TC over FO-TCN decreased clearly in the absence of H2O2, which demonstrated that TC decomposition was accelerated by the photo-Fenton reaction. In addition, the degradation of TC in the range of visible light was studied comparatively. The slightly decreased efficiency (87.3%) of TC degradation was observed under visible light irradiation. The reason lies in the fact that not only is the full spectrum more energetic, but the g-C3N4 has some light absorption below 420 nm.
In order to study the contributions of the different oxidative species generated to the degradation of TC in the FO-TCN system, the addition of quenchers was used to perform active species trapping tests. Herein, ethylene diamine tetra-acetic acid (EDTA-2Na), L-ascorbic acid (ASC), L-tryptophan and tert-butyl alcohol (TBA) were used to quench h+, ·O2−, 1O2 and ·OH, respectively [50]. The photocatalytic degradation efficiencies of TC over the FO-TCN photocatalyst in the presence of these four quenchers are shown in Figure 6e,f. When ASC and EDTA-2Na were introduced into the photocatalytic system, the degradation efficiency of TC dropped significantly from 95.02% to 46.15% and to 35.38%, respectively. Furthermore, the degradation efficiency of TC was also decreased from 95.02% to 66.56% in the presence of L-tryptophan. These experiments indicated that both ·O2− and h+ had a very important effect on the photocatalytic degradation of TC for the FO-TCN photocatalyst, while 1O2 also played a certain of role in the process of TC degradation. As shown in Figure 6f, the corresponding apparent kinetic constants were 0.0482, 0.0750, 0.0879, 0.0165, 0.0315 min−1, respectively. Furthermore, the relative contribution (R) of different active species to the degradation of TC was calculated through Equations (5)–(8) [44]:
The corresponding contribution rates of h+, ·O2−, 1O2 and ·OH to the TC removal were calculated to be 84.44%, 81.17%, 65.70% and 34.67%, respectively. These results show that both h+ and ·O2− played the dominant role in the FO-TCN photocatalytic system for the degradation of TC, while 1O2 played a subordinate role that also cannot be ignored. The strong electron coupling effect was responsible for the abundance of active species discovered in the FO-TCN heterojunction, which stimulated the photogenerated charge kinetics and provided a large number of reactive centers to accelerate the degradation reaction.
The possible photocatalytic reaction mechanism of the FO-TCN heterojunction is illustrated in Figure 7 according to Equations (9)–(17). Based on the data from the trapping experiment, -O2− was a crucial active substance in the FO-TCN system and had a significant influence on the photocatalytic degradation of TC. According to the previous results derived from Figure 5, the CB and VB edge potentials of TCN were −1.02 eV and +1.61 eV (vs. NHE), while the CB and VB edge potentials of α-F2O3 were +0.38 eV and +2.39 eV (vs. NHE), so they had a staggered energy band arrangement.
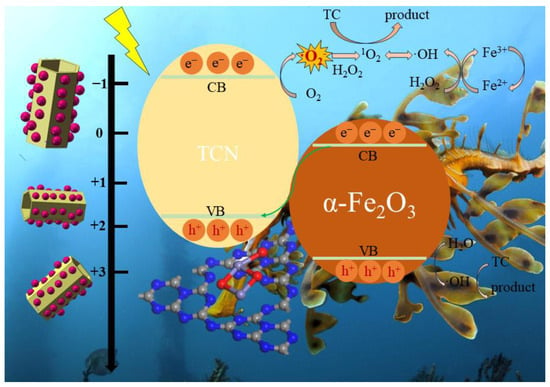
Figure 7.
The proposed mechanism of TC degradation by the FO-TCN system.
In the typical type II heterojunction, FO-TCN is not able to form ·O2−, due to the fact that the redox potential of O2/·O2− is lower compared to the CB of α-F2O3, and the electrons pooled on the CB of α-F2O3 do not have sufficient power to produce the ·O2− free radical, apparently contradicting the conclusions of the free radical scavenging experiments [51].
Meanwhile, the presence of ·O2− was demonstrated in the results of the radical scavenging experiment. Therefore, it is easy to conclude that the carrier transfer pathway between the FO-TCN heterojunction synthesized was in accordance with the charge transfer mechanism of the Z-scheme, which fully maintained the redox performance of the catalyst. Both g-C3N4 and α-F2O3 can be excited to generate photo-induced e− and h+ under light radiation. At the interface between two components, the e− in the CB of α-F2O3 can move to the VB of g-C3N4 via an internal electric field, resulting in strong electrostatic attraction between e− in the CB of α-F2O3 and h+ in the VB of g-C3N4. This process reduces the recombination of e− and h+ pairs by increasing the separation of the charge carriers e− in the CB of g-C3N4 and h+ in the VB of α-F2O3. More importantly, the h+ itself is a highly oxidizing agent and can directly oxidize pollutants to achieve degradation. In addition, compared to the standard redox potential of O2/O2−, the electrons in the CB of g-C3N4 can be trapped with O2 to produce ·O2−, since the CB potential of g-C3N4 is more negative. Then, the ·O2− radical is combined with H2O2 to form (1O2), which is compatible with the Fenton reaction mechanism. Finally, the portion of photogenerated electrons of α-F2O3 could enhance the photo-Fenton catalytic process through participation in the Fe3+/Fe2+ cycle process, and they can activate H2O2 to produce ·OH, while forming a typical Fenton system. The photo-Fenton reaction has higher catalytic activity in the degradation of TC compared to the purely photocatalytic reaction. In addition, the continuous conversion of Fe3+/Fe2+ could be maintained by the introduction of H2O2, which is the critical factor to trigger the photo-Fenton reaction. Only trace amounts of H2O2 are required to promote the photocatalytic performance of FO-TCN, thus eliminating the cost of adding H2O2. Furthermore, since the VB of Fe2O3 has a more positive potential than ·OH/OH− and ·OH/H2O, the vacancies accumulated on the VB may oxidize OH− and H2O to generate ·OH.
4. Conclusions
A Z-scheme heterojunction of FO-TCN, which showed outstanding catalytic activity in the photocatalytic degradation of TC, was successfully synthesized through a facile precursor-reforming strategy. According to the sample characterization, the tubular morphology offered samples with a large specific surface area, thus providing a larger contact surface for contaminants and increasing the number of active sites. In addition, the particular tubular morphology reduced the transfer pathway distance of photogenerated carriers and enhanced the interaction with the reactant. Under light irradiation, FO-TCN achieved 95.02% efficiency for the degradation of TC in 60 min, which was clearly higher than that of pristine g-C3N4 (56.61%) under the same conditions, and it obtained the highest k value (0.0482 min−1), which was 4.05 times higher than that of pristine g-C3N4. The FO-TCN heterojunction not only shortened the time and path of charge transfer, but also provided sufficient active reaction sites. Furthermore, the major active species ·O2− and h+ were the main active species during the photocatalytic degradation of TC, while the rapid Fe3+/Fe2+ conversion caused by H2O2 facilitated the formation of ·OH, thereby increasing the photocatalytic degradation effect at the same time. Consequently, the present research is very significant in promoting the widespread application of g-C3N4-based heterojunctions in photocatalytic purification treatment, and the technology for the combination of magnetic ferrites with g-C3N4 could also lead to industrial recycling applications in the future. Moreover, the FO-TCN photocatalysts synthesized in this study have excellent properties and practical uses, such as the removal of antibiotics and pharmaceuticals from wastewater.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/coatings13111909/s1, Figure S1. The band gap energy diagram of (a) CN, (b) TCN, (c) FO-CN, and (d) FO-TCN, respectively. Figure S2. Kinetic curve and the corresponding apparent rate constant of (a) CN, (b) TCN, (c) FO-CN, and (d) FO-TCN, respectively.
Author Contributions
Conceptualization, F.X. and X.Q.; methodology, F.X. and J.J.; formal analysis, K.Z., K.L., H.J. and Q.X.; data curation, F.X. and Q.X.; writing—original draft preparation, F.X. and X.Q.; writing—review and editing, F.X., X.Q. and J.J.; supervision, X.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Chongqing (2023NSCQ-MSX1669).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
F.X. acknowledges the support of Chongqing University of Technology’s Action Plan for Quality Development of Postgraduate Education (gzlcx20233170).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, Y.; Li, X.; Zhou, C.; Xiong, W.; Zeng, G.; Huang, D.; Zhang, C.; Wang, W.; Song, B.; Tang, X.; et al. Recent advances in application of graphitic carbon nitride-based catalysts for degrading organic contaminants in water through advanced oxidation processes beyond photocatalysis: A critical review. Water Res. 2020, 184, 116200. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lai, C.; Zeng, G.; Huang, D.; Yang, C.; Wang, Y.; Zhou, Y.; Cheng, M. Efficacy of carbonaceous nanocomposites for sorbing ionizable antibiotic sulfamethazine from aqueous solution. Water Res. 2016, 95, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Saadati, F.; Keramati, N.; Ghazi, M.M. Influence of parameters on the photocatalytic degradation of tetracycline in wastewater: A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 757–782. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Q.; Chen, P.; Zheng, H.; Shi, J.; Shu, H.; Liu, Y. Photocatalytic degradation of tetracycline by using a regenerable (Bi)BiOBr/rGO composite. J. Clean. Prod. 2022, 339, 130771. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, Z.; Zhang, C.; Huang, D.; Zeng, G.; Xiao, R.; Lai, C.; Zhou, C.; Guo, H.; Xue, W.; et al. Construction of iodine vacancy-rich BiOI/Ag@AgI Z-scheme heterojunction photocatalysts for visible-light-driven tetracycline degradation: Transformation pathways and mechanism insight. Chem. Eng. J. 2018, 349, 808–821. [Google Scholar] [CrossRef]
- Calvete, M.J.F.; Piccirillo, G.; Vinagreiro, C.S.; Pereira, M.M. Hybrid materials for heterogeneous photocatalytic degradation of antibiotics. Coord. Chem. Rev. 2019, 395, 63–85. [Google Scholar] [CrossRef]
- He, X.; Kai, T.; Ding, P. Heterojunction photocatalysts for degradation of the tetracycline antibiotic: A review. Environ. Chem. Lett. 2021, 19, 4563–4601. [Google Scholar] [CrossRef]
- Baaloudj, O.; Assadi, I.; Nasrallah, N.; El Jery, A.; Khezami, L.; Assadi, A.A. Simultaneous removal of antibiotics and inactivation of antibiotic-resistant bacteria by photocatalysis: A review. J. Water Process Eng. 2021, 42, 102089. [Google Scholar] [CrossRef]
- Li, D.; Shi, W. Recent developments in visible-light photocatalytic degradation of antibiotics. Chin. J. Catal. 2016, 37, 792–799. [Google Scholar] [CrossRef]
- Rabell, G.O.; Cruz, M.R.A.; Juárez-Ramírez, I. Hydrogen production of ZnO and ZnO/Ag films by photocatalysis and photoelectrocatalysis. Mater. Sci. Semicond. Process. 2021, 134, 105985. [Google Scholar] [CrossRef]
- Tang, X.; Xue, Q.; Qi, X.; Cheng, C.; Yang, M.; Yang, T.; Chen, F.; Qiu, F.; Quan, X. DFT and experimental study on visible-light driven photocatalysis of rare-earth-doped TiO2. Vacuum 2022, 200, 110972. [Google Scholar] [CrossRef]
- Guo, T.; Wang, K.; Zhang, G.; Wu, X. A novel α-Fe2O3@g-C3N4 catalyst: Synthesis derived from Fe-based MOF and its superior photo-Fenton performance. Appl. Surf. Sci. 2019, 469, 331–339. [Google Scholar] [CrossRef]
- Papailias, I.; Todorova, N.; Giannakopoulou, T.; Ioannidis, N.; Dallas, P.; Dimotikali, D.; Trapalis, C. Novel torus shaped g-C3N4 photocatalysts. Appl. Catal. B Environ. 2020, 26, 118733. [Google Scholar] [CrossRef]
- Liu, C.; Huang, H.; Cui, W.; Dong, F.; Zhang, Y. Band structure engineering and efficient charge transport in oxygen substituted g-C3N4 for superior photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 230, 115–124. [Google Scholar] [CrossRef]
- Liu, C.; Huang, H.; Ye, L.; Yu, S.; Tian, N.; Du, X.; Zhang, T.; Zhang, Y. Intermediate-mediated strategy to horn-like hollow mesoporous ultrathin g-C3N4 tube with spatial anisotropic charge separation for superior photocatalytic H2 evolution. Nano Energy 2017, 41, 738–748. [Google Scholar] [CrossRef]
- Liang, Q.; Li, Z.; Yu, X.; Huang, Z.-H.; Kang, F.; Yang, Q.-H. Macroscopic 3D Porous Graphitic Carbon Nitride Monolith for Enhanced Photocatalytic Hydrogen Evolution. Adv. Mater. 2015, 27, 4634–4639. [Google Scholar] [CrossRef]
- Nguyen, T.K.A.; Pham, T.-T.; Gendensuren, B.; Oh, E.-S.; Shin, E.W. Defect engineering of water-dispersible g-C3N4 photocatalysts by chemical oxidative etching of bulk g-C3N4 prepared in different calcination atmospheres. J. Mater. Sci. Technol. 2022, 103, 232–243. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Wei, Y.; Xue, J.; Chen, H.; Ding, L.; Caro, J.; Wang, H. Water Transport with Ultralow Friction through Partially Exfoliated g-C3 N4 Nanosheet Membranes with Self-Supporting Spacers. Angew. Chem. Int. Ed. Engl. 2017, 56, 8974–8980. [Google Scholar] [CrossRef]
- Guo, S.; Deng, Z.; Li, M.; Jiang, B.; Tian, C.; Pan, Q.; Fu, H. Phosphorus-Doped Carbon Nitride Tubes with a Layered Micro-nanostructure for Enhanced Visible-Light Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. Engl. 2016, 55, 1830–1834. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lin, Q.; Yin, L.; Yang, Y.; Qiu, Y.; Lu, C.; Yang, H. Biomimetic Design of Hollow Flower-Like g-C3N4@PDA Organic Framework Nanospheres for Realizing an Efficient Photoreactivity. Small 2019, 15, e1900011. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Sun, Z.; Huang, L.; Chen, M.; Wu, L. A “ship-in-a-bottle” strategy to fabricate highly crystallized nanoporous graphitic C3N4 microspheres under pressurized conditions. J. Mater. Chem. A 2019, 7, 8952–8959. [Google Scholar] [CrossRef]
- Patnaik, S.; Sahoo, D.P.; Parida, K. Recent advances in anion doped g-C3N4 photocatalysts: A review. Carbon 2021, 172, 682–711. [Google Scholar] [CrossRef]
- Wang, C.; Han, T.; Xin, C.; Miao, H. Synthesizing the High Surface Area g-C3N4 for Greatly Enhanced Hydrogen Production. Catalysts 2021, 11, 832. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, Y.; Wang, Y.; Hu, S.; Wang, B.; Liao, Q.; Li, H.; Bao, J.; Ge, G.; Jia, S. Three-dimensional flower-like phosphorus-doped g-C3N4 with a high surface area for visible-light photocatalytic hydrogen evolution. J. Mater. Chem. A 2018, 6, 16485–16494. [Google Scholar] [CrossRef]
- Roškarič, M.; Zavašnik, J.; Zámbó, D.; Kotnik, T.; Kovačič, S.; Žerjav, G.; Pintar, A. Optimization Method Based on Simplex for Surface Area Improved Photocatalytic Performance of g-C3N4. ACS Catal. 2023, 13, 13282–13300. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, R.; Shang, L.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Template-free large-scale synthesis of g-C3N4 microtubes for enhanced visible light-driven photocatalytic H2 production. Nano Res. 2018, 11, 3462–3468. [Google Scholar] [CrossRef]
- Wang, W.; Zeng, Z.; Zeng, G.; Zhang, C.; Xiao, R.; Zhou, C.; Xiong, W.; Yang, Y.; Lei, L.; Liu, Y.; et al. Sulfur doped carbon quantum dots loaded hollow tubular g-C3N4 as novel photocatalyst for destruction of Escherichia coli and tetracycline degradation under visible light. Chem. Eng. J. 2019, 378, 122132. [Google Scholar] [CrossRef]
- Pham, V.V.; Truong, T.K.; Hai, L.V.; La, H.P.P.; Nguyen, H.T.; Lam, V.Q.; Tong, H.D.; Nguyen, T.Q.; Sabbah, A.; Chen, K.H.; et al. S-Scheme α-Fe2O3/g-C3N4 Nanocomposites as Heterojunction Photocatalysts for Antibiotic Degradation. ACS Appl. Nano Mater. 2022, 5, 4506–4514. [Google Scholar] [CrossRef]
- Gan, P.; Zhang, Z.; Hu, Y.; Li, Y.; Ye, J.; Tong, M.; Liang, J. Insight into the role of Fe in the synergetic effect of persulfate/sulfite and Fe2O3@g-C3N4 for carbamazepine degradation. Sci. Total Environ. 2022, 819, 152787. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zou, X.; Zhou, Y.; Zeng, Y.; Long, Y.; Yuan, Z.; Wu, Q.; Li, M.; Wang, Y.; Xiang, B. Hydrothermal synthesis of graphene-encapsulated 2D circular nanoplates of α-Fe2O3 towards enhanced electrochemical performance for supercapacitor. J. Alloys Compd. 2019, 775, 63–71. [Google Scholar] [CrossRef]
- Xu, Q.-Q.; Huo, W.; Li, S.-S.; Fang, J.-H.; Li, L.; Zhang, B.-Y.; Zhang, F.; Zhang, Y.-X.; Li, S.-W. Crystal phase determined Fe active sites on Fe2O3 (γ- and α-Fe2O3) yolk-shell microspheres and their phase dependent electrocatalytic oxygen evolution reaction. Appl. Surf. Sci. 2020, 533, 147368. [Google Scholar] [CrossRef]
- Liu, S.; Wang, S.; Jiang, Y.; Zhao, Z.; Jiang, G.; Sun, Z. Synthesis of Fe2O3 loaded porous g-C3N4 photocatalyst for photocatalytic reduction of dinitrogen to ammonia. Chem. Eng. J. 2019, 373, 572–579. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, Z.; Hu, Y.; Gong, L.; Li, D.; Li, Z. A novel immobilized Z-scheme P3HT/α-Fe2O3 photocatalyst array: Study on the excellent photocatalytic performance and photocatalytic mechanism. J. Hazard. Mater. 2020, 389, 122119. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Li, X.; Wu, M.; Ding, H.; Li, X. A type II heterojunction alpha-Fe2O3/g-C3N4 for the heterogeneous photo-Fenton degradation of phenol. RSC Adv. 2022, 12, 8300–8309. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Z-Scheme 2D/2D α-Fe2O3/g-C3N4 heterojunction for photocatalytic oxidation of nitric oxide. Appl. Catal. B Environ. 2021, 280, 119409. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, X.; Gong, M.; Zheng, Y. Z-scheme Fe2O3/g-C3N4 heterojunction with excellent photocatalytic property. J. Photonics Energy 2020, 10, 023509. [Google Scholar] [CrossRef]
- Wang, Y.; Song, H.; Chen, J.; Chai, S.; Shi, L.; Chen, C.; Wang, Y.; He, C. A novel solar photo-Fenton system with self-synthesizing H2O2: Enhanced photo-induced catalytic performances and mechanism insights. Appl. Surf. Sci. 2020, 512, 154650. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, W.; Huang, H.; Chen, R.; Shi, H. A 2D/2D S-scheme photo-Fenton catalyst based on ultrathin Bi2MoO6 and Fe2O3 hexagonal nanosheets for efficient tetracycline degradation. Catal. Sci. Technol. 2021, 11, 2948–2956. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, W.; Zhang, H.; Dou, X.; Shi, H. 2D/2D step-scheme α-Fe2O3/Bi2WO6 photocatalyst with efficient charge transfer for enhanced photo-Fenton catalytic activity. Chin. J. Catal. 2021, 42, 97–106. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, S.; Bi, W.; Zhao, X.; Liu, G. Z-scheme Fe2O3-doped Cu2O as an efficient photo-Fenton-like catalyst for degradation of phenol. Mater. Lett. 2019, 234, 13–16. [Google Scholar] [CrossRef]
- Zhang, C.; Qin, D.; Zhou, Y.; Qin, F.; Wang, H.; Wang, W.; Yang, Y.; Zeng, G. Dual optimization approach to Mo single atom dispersed g-C3N4 photocatalyst: Morphology and defect evolution. Appl. Catal. B Environ. 2022, 303, 120904. [Google Scholar] [CrossRef]
- Bai, J.; Xu, H.; Chen, G.; Lv, W.; Ni, Z.; Wang, Z.; Yang, J.; Qin, H.; Zheng, Z.; Li, X. Facile fabrication of α-Fe2O3/porous g-C3N4 heterojunction hybrids with enhanced visible-light photocatalytic activity. Mater. Chem. Phys. 2019, 234, 75–80. [Google Scholar] [CrossRef]
- Mo, Z.; Zhu, X.; Jiang, Z.; Song, Y.; Liu, D.; Li, H.; Yang, X.; She, Y.; Lei, Y.; Yuan, S.; et al. Porous nitrogen-rich g-C3N4 nanotubes for efficient photocatalytic CO2 reduction. Appl. Catal. B Environ. 2019, 256, 117854. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Ni, T.; Gao, R.; Ge, J.; Zhang, F.; Wu, W.; Li, J.; Zhao, Q. 3D interconnected porous g-C3N4 hybridized with Fe2O3 quantum dots for enhanced photo-Fenton performance. Appl. Surf. Sci. 2021, 555, 149657. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Li, X.; He, T.; Wang, R.; Zhao, Y.; Song, H.; Wang, H. 3D/2D Fe2O3/g-C3N4 Z-scheme heterojunction catalysts for fast, effective and stable photo Fenton degradation of azo dyes. J. Environ. Chem. Eng. 2021, 9, 105907. [Google Scholar] [CrossRef]
- Cui, Y.; Briscoe, J.; Wang, Y.; Tarakina, N.V.; Dunn, S. Enhanced Photocatalytic Activity of Heterostructured Ferroelectric BaTiO3/alpha-Fe2O3 and the Significance of Interface Morphology Control. ACS Appl. Mater. Interfaces 2017, 9, 24518–24526. [Google Scholar] [CrossRef]
- Cao, J.; Yang, Z.-H.; Xiong, W.-P.; Zhou, Y.-Y.; Peng, Y.-R.; Li, X.; Zhou, C.-Y.; Xu, R.; Zhang, Y.-R. One-step synthesis of Co-doped UiO-66 nanoparticle with enhanced removal efficiency of tetracycline: Simultaneous adsorption and photocatalysis. Chem. Eng. J. 2018, 353, 126–137. [Google Scholar] [CrossRef]
- Zhou, K.; Liu, Y.; Hao, J. One-pot hydrothermal synthesis of dual Z-scheme BiOBr/g-C3N4/Bi2WO6 and photocatalytic degradation of tetracycline under visible light. Mater. Lett. 2020, 281, 128463. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, H.; Chen, Y.; Shi, H. Efficient Degradation of Tetracycline via Coupling of Photocatalysis and Photo-Fenton Processes over a 2D/2D α-Fe2O3/g-C3N4 S-scheme Heterojunction Catalyst. Acta Phys. Chim. Sin. 2022, 38, 220100. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).