Electrocrystallization and Morphology of Copper Coatings in the Presence of Organic Additives
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jhothiraman, J.; Balachandran, R. Electroplating: Applications in the semiconductor industry. Adv. Chem. Eng. Sci. 2019, 9, 239–261. [Google Scholar] [CrossRef]
- Chang, T.; Leygraf, C.; Wallinder, I.O.; Jin, Y. Understanding the barrier layer formed via adding BTAH in copper film electrodeposition. J. Electrochem. Soc. 2019, 166, D10. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Q.; Zhou, K.; Le, Y.; Liu, W.; Wang, Y.; Wang, F. Effect of cetyl-trimethyl-ammonium-bromide (CTAB) and bis (3-sulfopropyl) disulfide (SPS) on the through-silicon-via (TSV) copper filling. Microelectron. Eng. 2019, 217, 111109. [Google Scholar] [CrossRef]
- Tan, M.; Harb, J.N. Additive Behavior during copper electrodeposition in solutions containing Cl− , PEG, and SPS. J. Electrochem. Soc. 2003, 150, C420. [Google Scholar] [CrossRef]
- Pasquale, M.A.; Gassa, L.M.; Arvia, A.J. Copper electrodeposition from an acidic plating bath containing accelerating and inhibiting organic additives. Electrochim. Acta 2008, 53, 5891–5904. [Google Scholar] [CrossRef]
- Tao, Z.; Long, Z.; Tengxu, L.; Liu, G.; Tao, X. The synergistic effects of additives on the micro vias copper filling. J. Electroanal. Chem. 2022, 918, 116456. [Google Scholar] [CrossRef]
- Dow, W.-P.; Yen, M.-Y.; Liao, S.-Z.; Chiu, Y.-D.; Huang, H.-C. Filling mechanism in microvia metallization by copper electroplating. Electrochim. Acta 2008, 53, 8228–8237. [Google Scholar] [CrossRef]
- Kasach, A.A.; Kurilo, I.I.; Kharitonov, D.S.; Radchenko, S.L.; Zharskii, I.M. Sonochemical electrodeposition of copper coatings. Russ. J. Appl. Chem. 2018, 91, 207–213. [Google Scholar] [CrossRef]
- Pena, E.M.D.; Roy, S. Electrodeposited copper using direct and pulse currents from electrolytes containing low concentration of additives. Surf. Coat. Technol. 2018, 339, 101–110. [Google Scholar] [CrossRef]
- Kondo, K.; Matsumoto, T.; Watanabe, K. Role of additives for copper damascene electrodeposition: Experimental study on inhibition and acceleration effects. J. Electrochem. Soc. 2004, 151, C250. [Google Scholar] [CrossRef]
- Jin, Y.; Sun, M.; Mu, D.; Ren, X.; Wang, Q.; Wen, L. Investigation of PEG adsorption on copper in Cu2+-free solution by SERS and AFM. Electrochim. Acta 2012, 78, 459–465. [Google Scholar] [CrossRef]
- Meudre, C.; Ricq, L.; Hihn, J.-Y.; Moutarlier, V.; Monnin, A.; Heintz, O. Adsorption of gelatin during electrodeposition of copper and tin–copper alloys from acid sulfate electrolyte. Surf. Coat. Technol. 2014, 252, 93–101. [Google Scholar] [CrossRef]
- Chang, T.; Jin, Y.; Wen, L.; Zhang, C.; Leygraf, C.; Odnevall, I.; Zhang, J. Synergistic effects of gelatin and convection on copper foil electrodeposition. Electrochim. Acta 2016, 211, 245–254. [Google Scholar] [CrossRef]
- Tantavichet, N.; Pritzker, M. Copper electrodeposition in sulphate solutions in the presence of benzotriazole. J. Appl. Electrochem. 2006, 36, 49–61. [Google Scholar] [CrossRef]
- Kim, J.J.; Kim, S.-K.; Bae, J.-U. Investigation of copper deposition in the presence of benzotriazole. Thin Solid Film. 2002, 415, 101–107. [Google Scholar] [CrossRef]
- Emekli, U.; West, A.C. Effect of additives and pulse plating on copper nucleation onto Ru. Electrochim. Acta 2009, 54, 1177–1183. [Google Scholar] [CrossRef]
- Portela, A.L.; Lacconi, G.I.; Teijelo, M.L. Nicotinic acid as brightener agent in copper electrodeposition. J. Electroanal. Chem. 2001, 495, 169–172. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, C.; Cai, D.; Huang, Y.; Adi, K.; Hong, Y.; Chen, Y.; Zhou, G.; Armini, S.; De Gendt, S.; et al. Hydroquinone oriented growth control to achieve high-quality copper coating at high rate for electronics interconnection. J. Taiwan Inst. Chem. Eng. 2020, 112, 130–136. [Google Scholar] [CrossRef]
- Saberi, A.; Bakhsheshi-Rad, H.R.; Abazari, S.; Ismail, A.F.; Sharif, S.; Ramakrishna, S.; Daroonparvar, M.; Berto, F. A Comprehensive review on surface modifications of biodegradable magnesium-based implant alloy: Polymer coatings opportunities and challenges. Coatings 2021, 11, 747. [Google Scholar] [CrossRef]
- Arratia, R.; Aros, H.; Schrebler, R.; Carlesi, J.C. Use of polyethylene glycol as organic additive in copper electrodeposition over stainless steel cathodes. Lat. Am. Appl. Res. Pesqui. Apl. Lat. Am. 2012, 42, 371–376. [Google Scholar]
- Kelly, J.; West, A. Copper deposition in the presence of polyethylene glycol I. Quartz crystal microbalance study. J. Electrochem. Soc. 1998, 145, 3472–3476. [Google Scholar] [CrossRef]
- Kruft, M.; Wohlmann, B.; Stuhlmann, C.; Wandelt, K. Chloride adsorption on Cu(111) electrodes in dilute HCl solutions. Surf. Sci. 1997, 377–379, 601–604. [Google Scholar] [CrossRef]
- Reid, J.D.; David, A.P. Effects of polyethylene glycol on the electrochemical characteristics of copper cathodes in an acid copper medium. Plat. Surf. Finish. 1987, 74, 66–70. [Google Scholar]
- Dow, W.-P.; Chiu, Y.-D.; Yen, M.-Y. Microvia filling by Cu electroplating over a Au seed layer modified by a disulfide. J. Electrochem. Soc. 2009, 156, D155. [Google Scholar] [CrossRef]
- Farndon, E.; Walsh, F.; Campbell, S. Effect of thiourea, benzotriazole and 4,5-dithiaoctane-1,8-disulphonic acid on the kinetics of copper deposition from dilute acid sulphate solutions. J. Appl. Electrochem. 1995, 25, 574–583. [Google Scholar] [CrossRef]
- Schultz, Z.; Feng, Z.; Biggin, M.; Gewirth, A. Vibrational spectroscopic and mass spectrometric studies of the interaction of bis(3-sulfopropyl)-disulfide with Cu surfaces. J. Electrochem. Soc. 2006, 153, C97. [Google Scholar] [CrossRef]
- Schmitt, K.; Schmidt, R.; von-Horsten, H.; Vazhenin, G.; Gewirth, A. 3-Mercapto-1-propanesulfonate for Cu electrodeposition studied by in situ shell-isolated nanoparticle-enhanced raman spectroscopy, density functional theory calculations, and cyclic voltammetry. J. Phys. Chem. C 2015, 119, 23453–23462. [Google Scholar] [CrossRef]
- Li, J.; Xu, J.; Wang, X.; Wei, X.; Lv, J.; Wang, L. Novel 2,5-bis(6-(trimethylamonium)hexyl)-3,6-diaryl-1,4-diketopyrrolo[3,4-c] pyrrole pigments as levelers for efficient electroplating applications. Dye. Pigment. 2020, 186, 109064. [Google Scholar] [CrossRef]
- Yaqiang, L.; Ren, P.; Zhang, Y.; Li, R.; Zhang, J.; Yang, P.; Liu, A.; Wang, G.; An, M. Investigation of novel leveler Rhodamine B on copper superconformal electrodeposition of microvias by theoretical and experimental studies. Appl. Surf. Sci. 2022, 615, 156266. [Google Scholar] [CrossRef]
- Varvara, S.; Muresan, L.; Nicoara, A.; Maurin, G.; Popescu, I. Kinetic and morphological investigation of copper electrodeposition from sulfate electrolytes in the presence of an additive based on ethoxyacetic alcohol and triethyl-benzyl-ammonium chloride. Mater. Chem. Phys. 2001, 72, 332–336. [Google Scholar] [CrossRef]
- Nkuna, E.; Popoola, P. Effect of chloride electrolyte additive on the quality of electrorefined copper cathode. Procedia Manuf. 2019, 35, 789–794. [Google Scholar] [CrossRef]
- Varvara, S.; Muresan, L.; Popescu, I.; Maurin, G. Comparative study of copper electrodeposition from sulphate acidic electrolytes in the presence of IT-85 and of its components. J. Appl. Electrochem. 2005, 35, 69–76. [Google Scholar] [CrossRef]
- Zeng, T.-W. Effects of additives in an electrodeposition bath on the surface morphologic evolution of electrodeposited copper. Int. J. Electrochem. Sci. 2021, 16, 210245. [Google Scholar] [CrossRef]
- Yaqiang, L.; Ren, P.; Zhang, Y.; Wang, S.; Zhang, J.; Yang, P.; Liu, A.; Wang, G.; Chen, Z.; An, M. The influence of leveler brilliant green on copper superconformal electroplating based on electrochemical and theoretical study. J. Ind. Eng. Chem. 2022, 118, 78–90. [Google Scholar] [CrossRef]
- Ren, P.; An, M.; Yang, P.; Zhang, J. Revealing the acceleration effect of SPS and Cl− on copper surface: Instantaneous nucleation and multi-step energy change. Appl. Surf. Sci. 2022, 583, 152523. [Google Scholar] [CrossRef]
- Sekar, R. Synergistic effect of additives on electrodeposition of copper from cyanide-free electrolytes and its structural and morphological characteristics. Trans. Nonferrous Met. Soc. China 2017, 27, 1665–1676. [Google Scholar] [CrossRef]
- Gallaway, J.; Willey, M.; West, A. Acceleration kinetics of PEG, PPG, and a triblock copolymer by SPS during copper electroplating. J. Electrochem. Soc. 2009, 156, D146. [Google Scholar] [CrossRef]
- Kim, S.-K.; Josell, D.; Moffat, T. Electrodeposition of Cu in the PEI-PEG-Cl-SPS additive system reduction of overfill bump formation during superfilling. J. Electrochem. Soc. 2006, 153, C616. [Google Scholar] [CrossRef]
- Diao, S.; Wang, Y.; Jin, H. Electronucleation mechanism of copper in wastewater by controlled electrodeposition analysis. RSC Adv. 2020, 10, 38683–38694. [Google Scholar] [CrossRef]
- Fabbri, L.; Giurlani, W.; Mencherini, G.; De Luca, A.; Passaponti, M.; Piciollo, E.; Fontanesi, C.; Caneschi, A. Optimisation of thiourea concentration in a decorative copper plating acid bath based on methanesulfonic electrolyte. Coatings 2022, 12, 376. [Google Scholar] [CrossRef]
- Gladysz, O.; Łoś, P. The electrochemical nucleation of copper on disc-shaped ultramicroelectrode in industrial electrolyte. Electrochim. Acta 2008, 54, 801–807. [Google Scholar] [CrossRef]
- Quinet, M.; Lallemand, F.; Ricq, L.; Hihn, J.-Y.; Delobelle, P.; Arnould, C.; Mekhalif, Z. Influence of organic additives on the initial stages of copper electrodeposition on polycrystalline platinum. Electrochim. Acta 2009, 54, 1529–1536. [Google Scholar] [CrossRef]
- Michailova, E.; Vitanova, I.; Stoychev, D.; Milchev, A. Initial stages of copper electrodeposition in the presence of organic additives. Electrochim. Acta 1993, 38, 2455–2458. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, Y.; Li, M. Structural effect of inhibitors on adsorption and desorption behaviors during copper electroplating for through-silicon vias. Electrochim. Acta 2021, 372, 137907. [Google Scholar] [CrossRef]
- Moffat, T.; Josell, D. Extreme bottom-up superfilling of through-silicon-vias by damascene processing: Suppressor disruption, positive feedback and turing patterns. J. Electrochem. Soc. 2012, 159, D208. [Google Scholar] [CrossRef]
- Menk, L.; Baca, E.; Blain, M.; McClain, J.; Dominguez, J.; Smith, A.; Hollowell, A. Galvanostatic plating with a single additive electrolyte for bottom-up filling of copper in mesoscale TSVs. J. Electrochem. Soc. 2019, 166, D3226–D3231. [Google Scholar] [CrossRef]
- Josell, D.; Moffat, T. Superconformal copper deposition in through silicon vias by suppression-breakdown. J. Electrochem. Soc. 2018, 165, D23–D30. [Google Scholar] [CrossRef]
- Hayase, M.; Otsubo, K. Copper deep via filling with selective accelerator deactivation by a reverse pulse. J. Electrochem. Soc. 2010, 157, D628–D632. [Google Scholar] [CrossRef]
- Hayashi, T.; Kondo, K.; Saito, T.; Takeuchi, M. High-speed through silicon via (TSV) filling using diallylamine additive. J. Electrochem. Soc. 2011, 158, D715. [Google Scholar] [CrossRef]
- Tulkova, A.; Bobrova, J.; Smirnova, O. Effect of organic additives on the filling of blind vias in the manufacture of electronic devices. Electroplat. Surf. Treat. 2016, 24, 61–67. [Google Scholar] [CrossRef]
- Gu, M.; Li, Q.; Fu, B.-H.; Xian, X.-H. Role of SPS in chloride ions and PEG additive system for copper electrocrystallisation. Trans. Inst. Met. Finish. 2010, 88, 144–148. [Google Scholar] [CrossRef]
- Aribou, Z.; Khemmou, N.; Belakhmima, R.; Chaouki, I.; Touhami, M.; Touir, R.; Bakkali, S. Effect of polymer additive on structural and morphological properties of Cu-electrodeposition from an acid sulfate electrolyte: Experimental and theoretical studies. J. Electroanal. Chem. 2023, 946, 117722. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, X.; Hua, Y.; Xue, W. The effect of quaternary ammonium-based ionic liquids on copper electrodeposition from acidic sulfate electrolyte. J. Appl. Electrochem. 2015, 45, 79–86. [Google Scholar] [CrossRef]
- Scharifker, B.; Hills, G. Theoretical and experimental studies of multiple nucleation. Electrochim. Acta 1983, 28, 879–889. [Google Scholar] [CrossRef]
- Garfias Garcia, E.; Romero-Romo, M.; Ramírez-Silva, M.T.; Palomar-Pardavé, M. Overpotential nucleation and growth of copper onto polycrystalline and single crystal gold electrodes. Int. J. Electrochem. Sci. 2012, 7, 3102–3114. [Google Scholar] [CrossRef]
- Marro, J.; Okoro, C.; Obeng, Y.; Richardson, K. The Impact of Organic Additives on Copper Trench Microstructure. J. Electrochem. Soc. 2017, 164, D543–D550. [Google Scholar] [CrossRef]
- Scharifker, B.; Mostany, J. Nucleation and growth of new phases on electrode surfaces. In Developments in Electrochemistry: Science Inspired by Martin Fleischmann Chapter: 4; Pletcher, D., Tian, Z.-Q., Williams, D.E., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
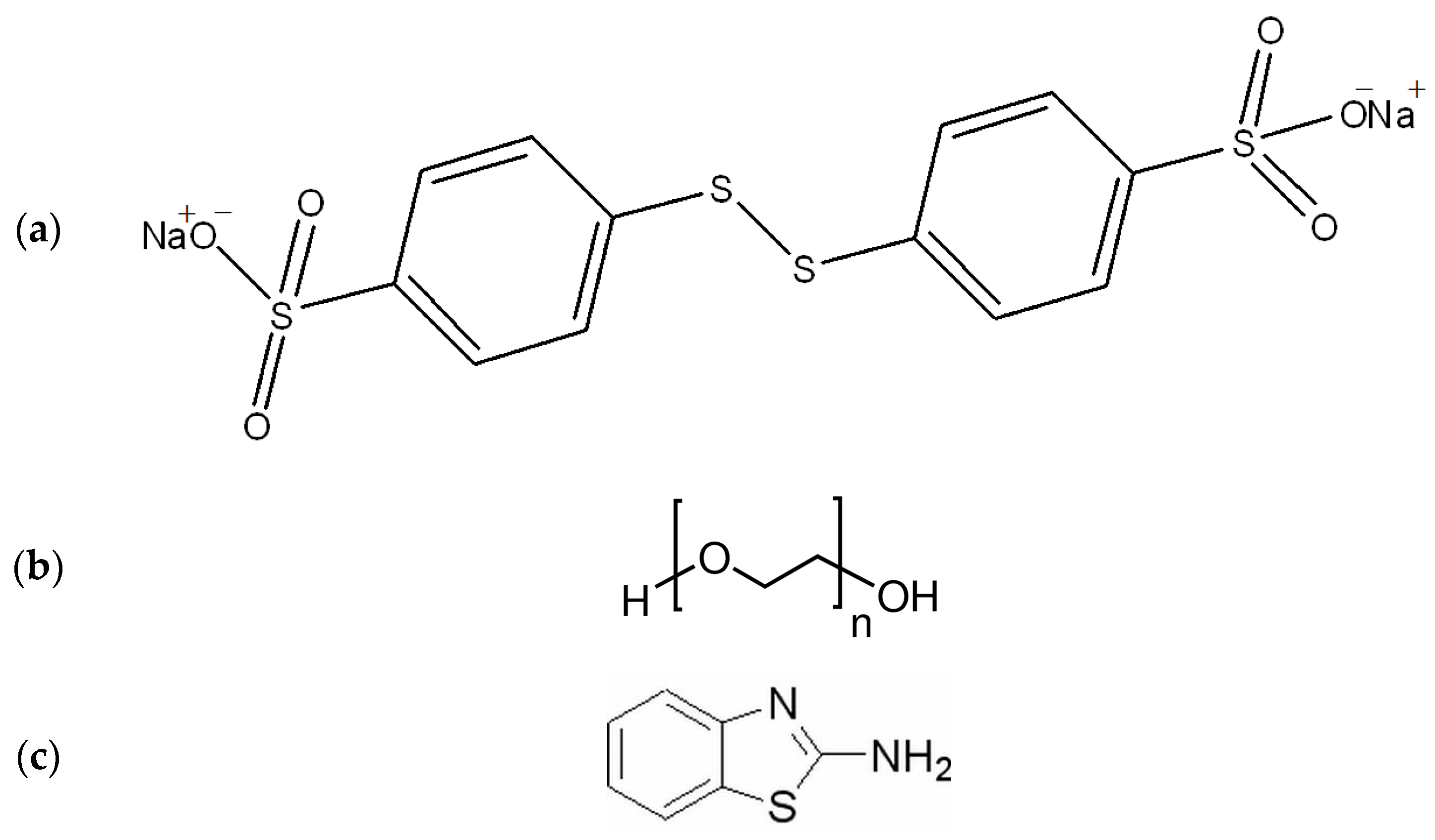
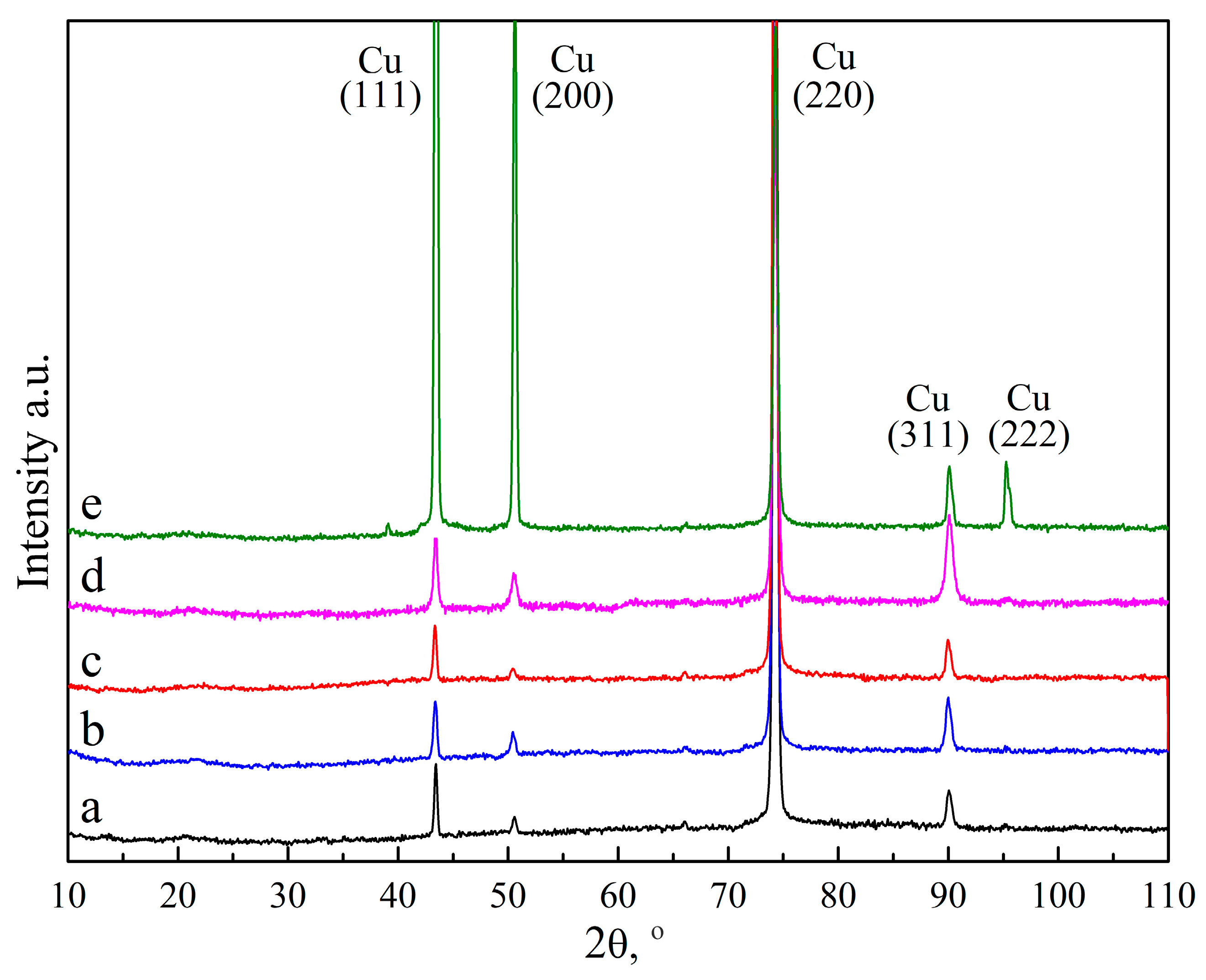

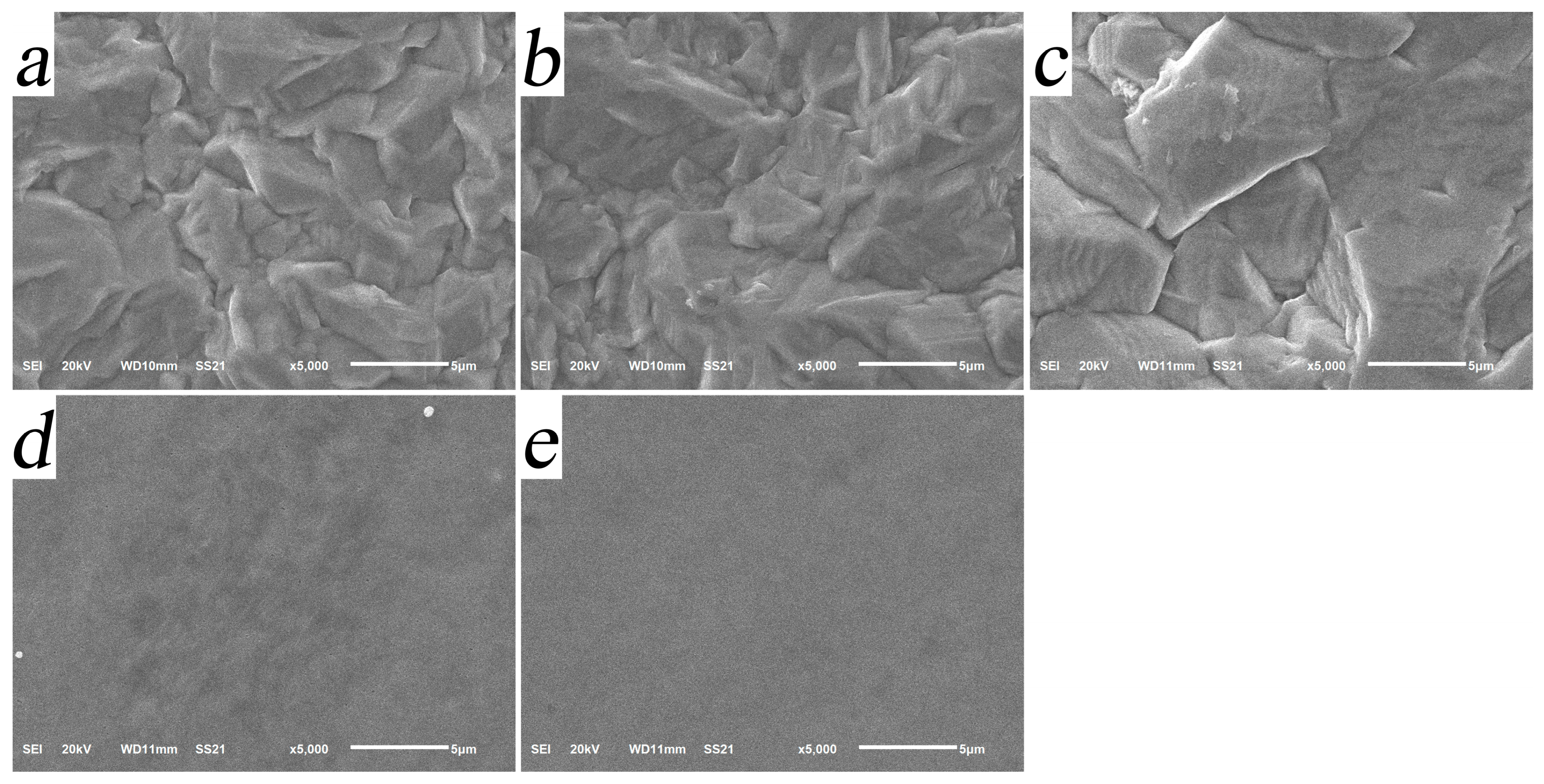

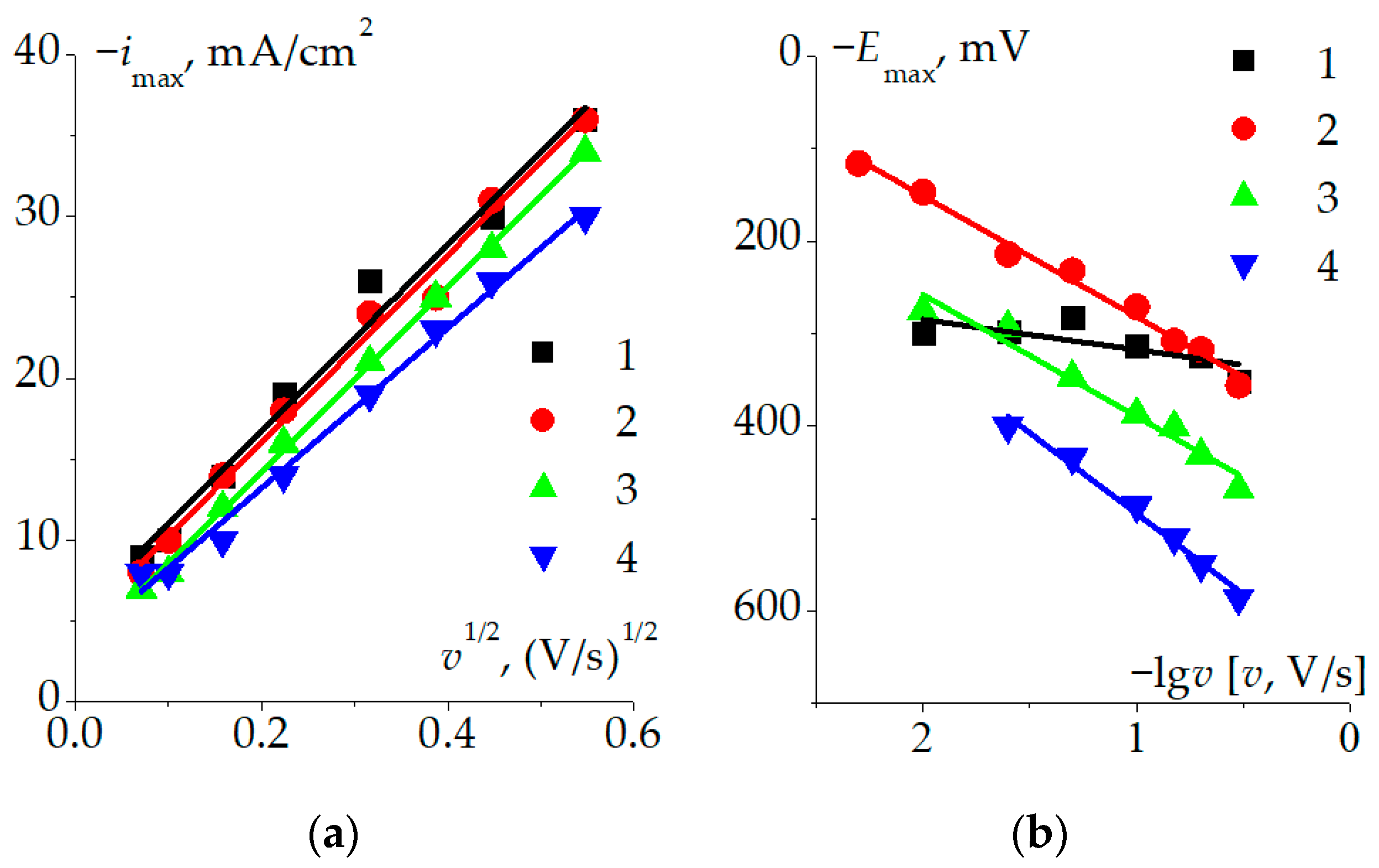


| Additives and Their Concentrations | Electrolyte Code |
|---|---|
| No additive | 0 |
| DBDA (0.06 g/L) | I |
| DBDA (0.06 g/L) + NaCl (0.05 g/L) | II |
| DBDA (0.06 g/L) + NaCl (0.05 g/L) + PEG (0.5 g/L) | III |
| DBDA (0.06 g/L) + NaCl (0.05 g/L) + PEG (0.5 g/L) + ABT (0.5 g/L) | IV |
| Edep, mV | Solution | ||||
|---|---|---|---|---|---|
| 0 | I | II | III | IV | |
| −500 | 0.79 | 0.75 | 0.81 | 0.83 | 0.62 |
| −400 | 0.70 | 0.78 | 0.80 | 0.60 | 1.03 |
| −300 | 0.97 | 0.56 | 0.49 | 0.18 | 0.24 |
| Edep, mV | Solution | ||||
|---|---|---|---|---|---|
| 0 | I | II | III | IV | |
| −500 | 5.8 | 6.4 | 8.1 | 4.1 | 1.1 |
| −400 | 2.1 | 3.3 | 5.7 | 1.9 | 0.5 |
| −300 | 1.7 | 7.2 | 5.3 | 2.7 | 1.0 |
| Edep, mV | Solution | ||||
|---|---|---|---|---|---|
| 0 | I | II | III | IV | |
| −500 | 23.8 | 9.8 | 5.8 | 3.4 | 2.6 |
| −400 | 17.3 | 9.2 | 3.8 | 3.3 | 8.8 |
| −300 | 1.5 | 1.5 | 1.6 | 1.8 | 3.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozaderov, O.; Sotskaya, N.; Yudenkova, L.; Buylov, N.; Ilina, E. Electrocrystallization and Morphology of Copper Coatings in the Presence of Organic Additives. Coatings 2023, 13, 1896. https://doi.org/10.3390/coatings13111896
Kozaderov O, Sotskaya N, Yudenkova L, Buylov N, Ilina E. Electrocrystallization and Morphology of Copper Coatings in the Presence of Organic Additives. Coatings. 2023; 13(11):1896. https://doi.org/10.3390/coatings13111896
Chicago/Turabian StyleKozaderov, Oleg, Nadezhda Sotskaya, Ludmila Yudenkova, Nikita Buylov, and Evgeniia Ilina. 2023. "Electrocrystallization and Morphology of Copper Coatings in the Presence of Organic Additives" Coatings 13, no. 11: 1896. https://doi.org/10.3390/coatings13111896
APA StyleKozaderov, O., Sotskaya, N., Yudenkova, L., Buylov, N., & Ilina, E. (2023). Electrocrystallization and Morphology of Copper Coatings in the Presence of Organic Additives. Coatings, 13(11), 1896. https://doi.org/10.3390/coatings13111896





