Study on Green Controllable Preparation of Coal Gangue-Based 13-X Molecular Sieves and Its CO2 Capture Application
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Methods
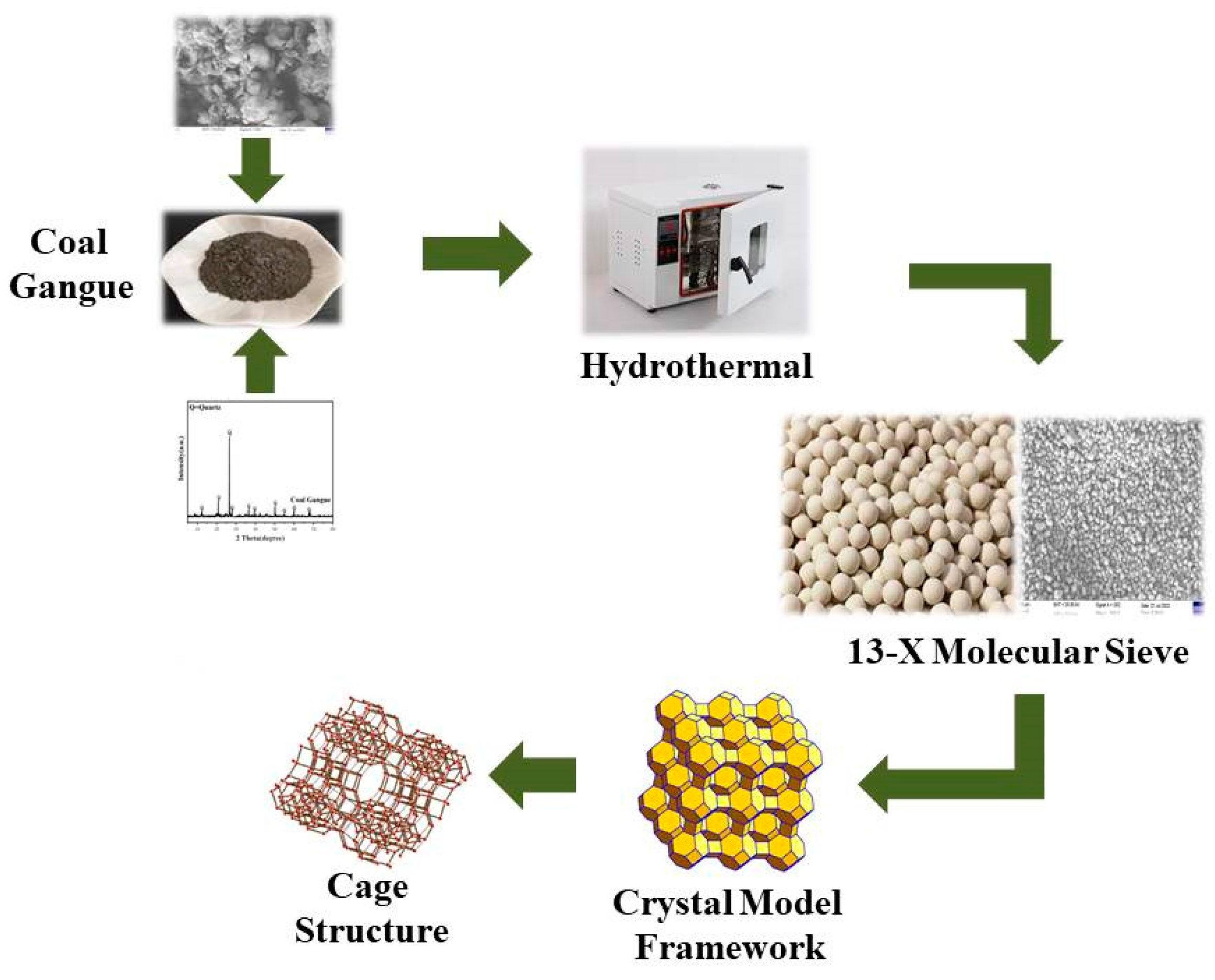
2.2.1. Synthesis of Gangue-Based 13-X Molecular Sieve
2.2.2. Characterization
3. Results and Discussion
3.1. Processing and Analysis of Raw Materials
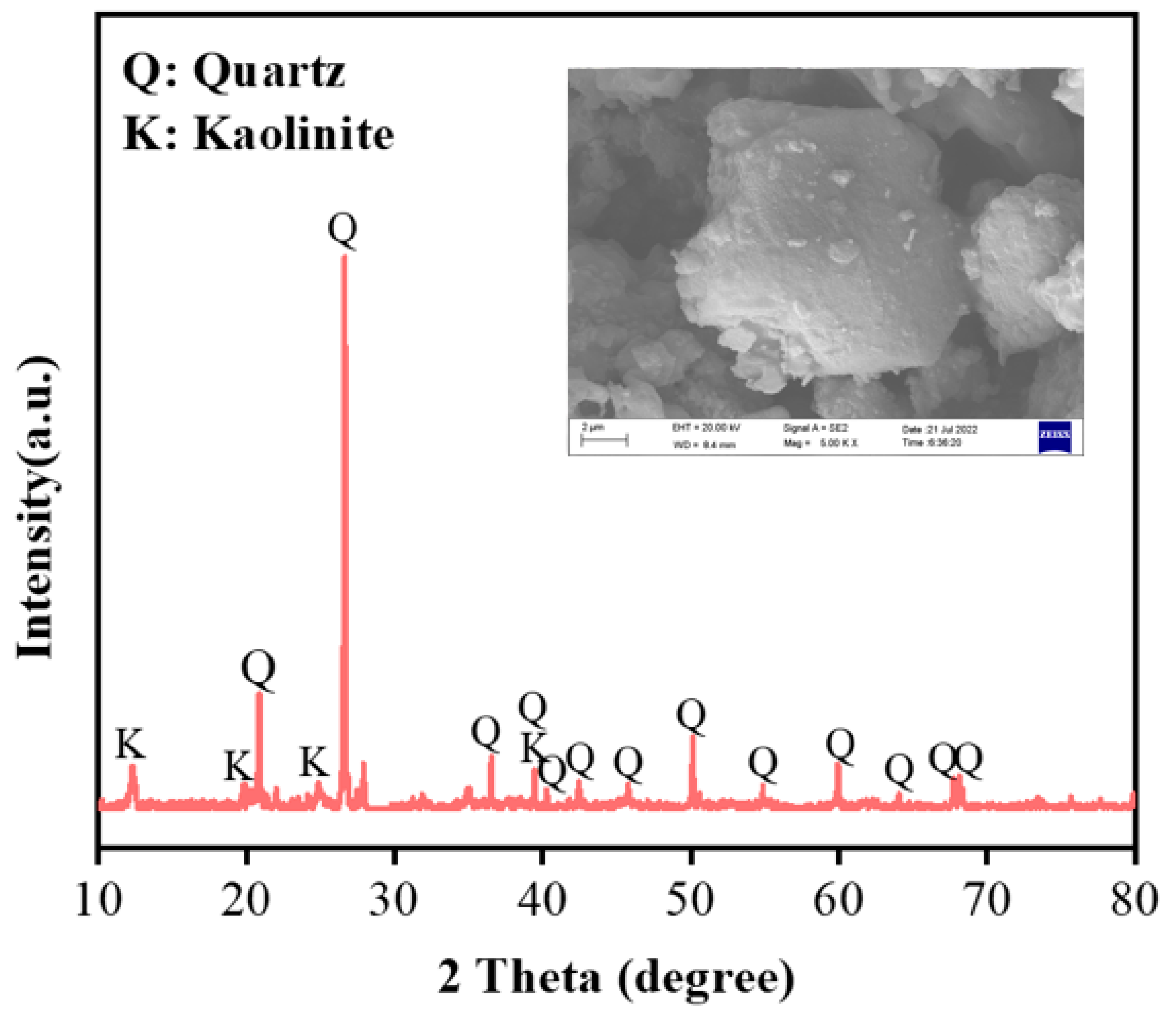
3.1.1. Treatment of Raw Material Gangue
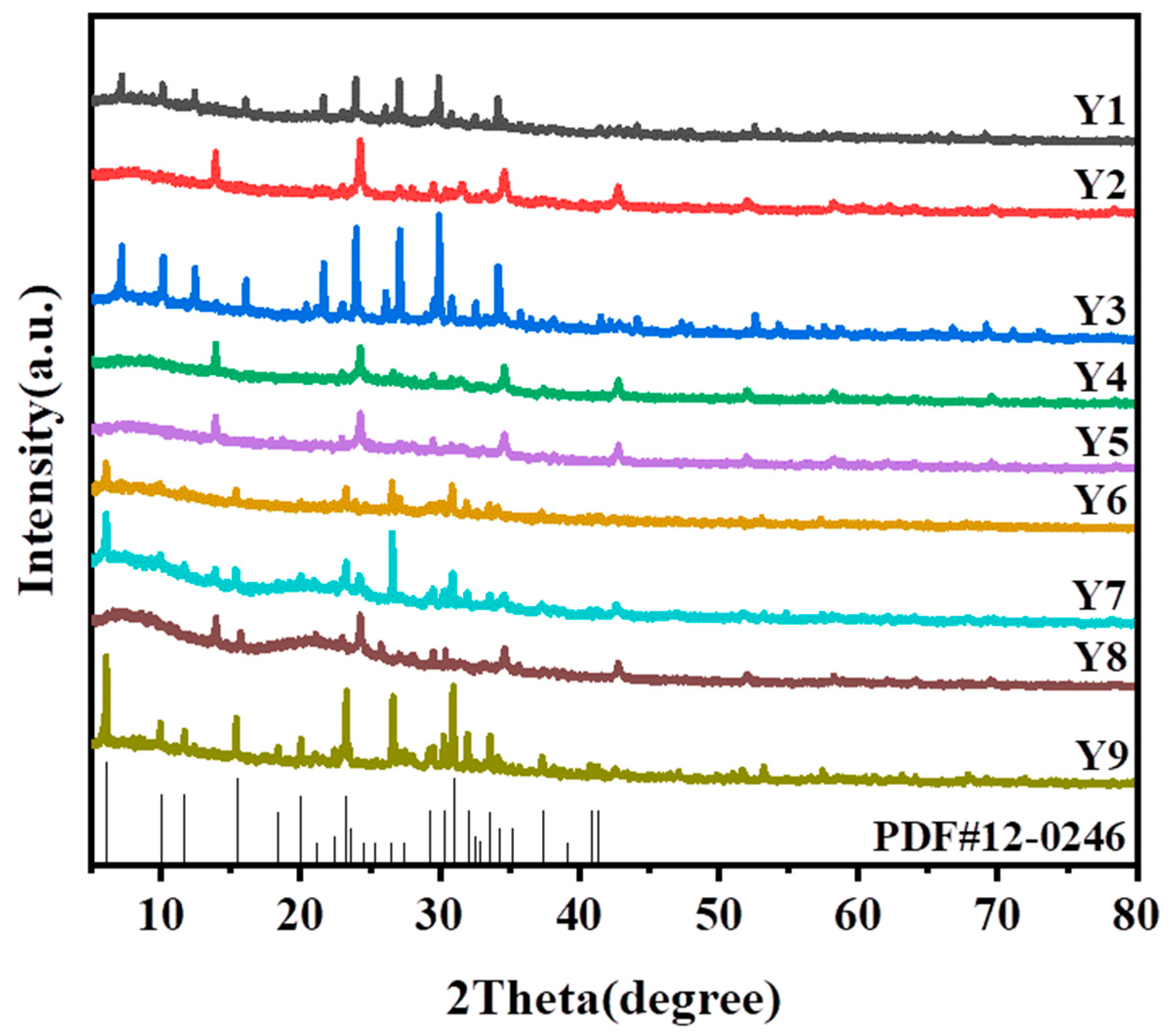
3.1.2. Orthogonal Experimental Design for the Synthesis of 13-X Molecular Sieve
3.2. Evaluation of the Synthesized 13-X Zeolite
3.2.1. X-ray Diffraction Analysis
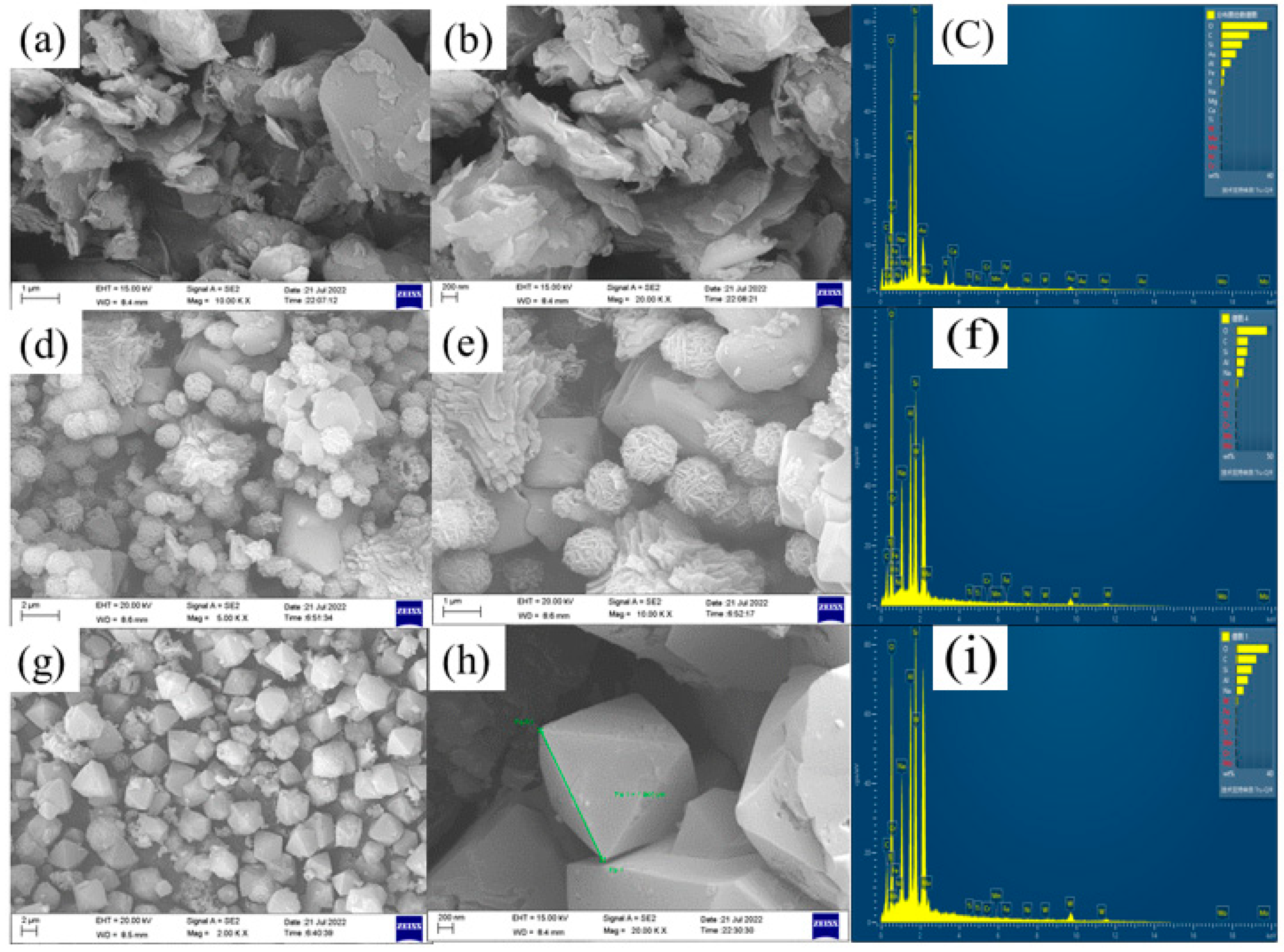
3.2.2. SEM-EDS Analysis
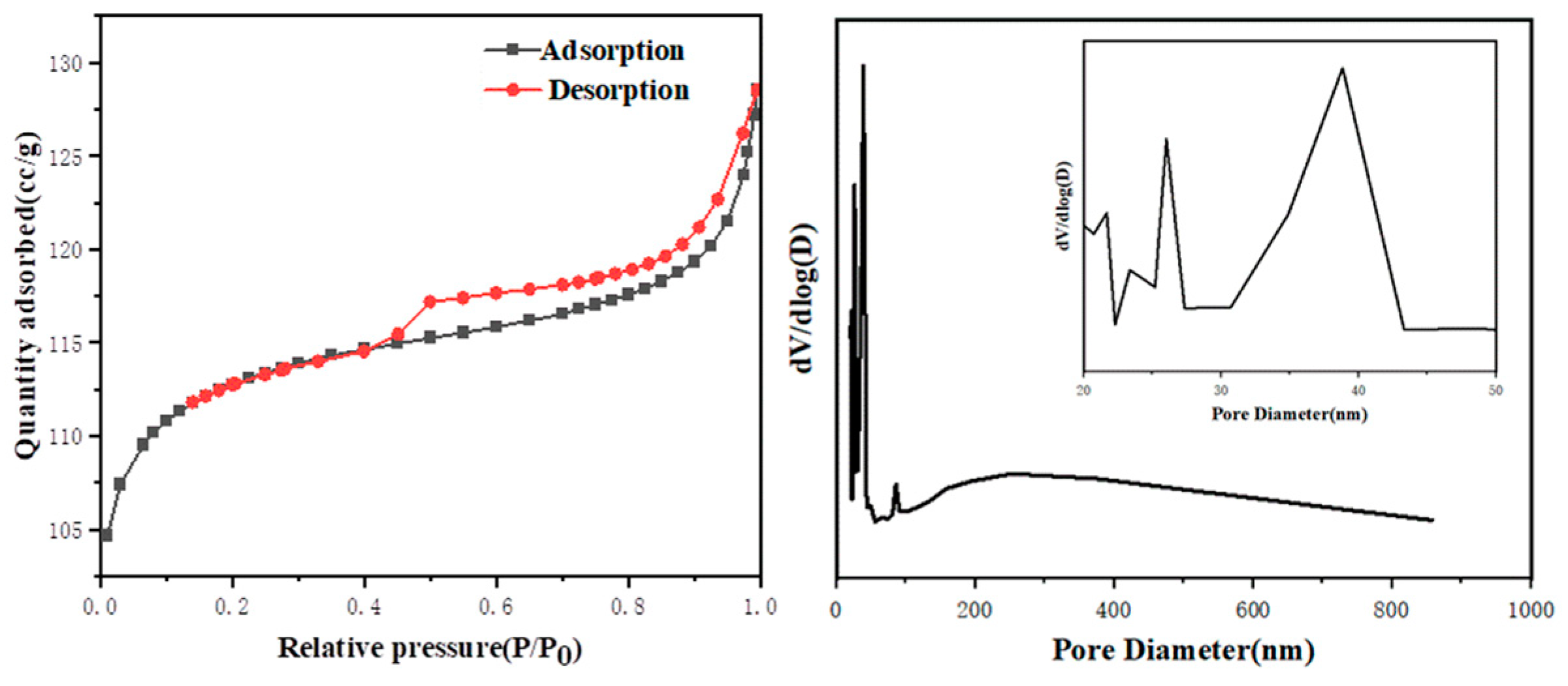
3.2.3. Specific Surface Area Analysis
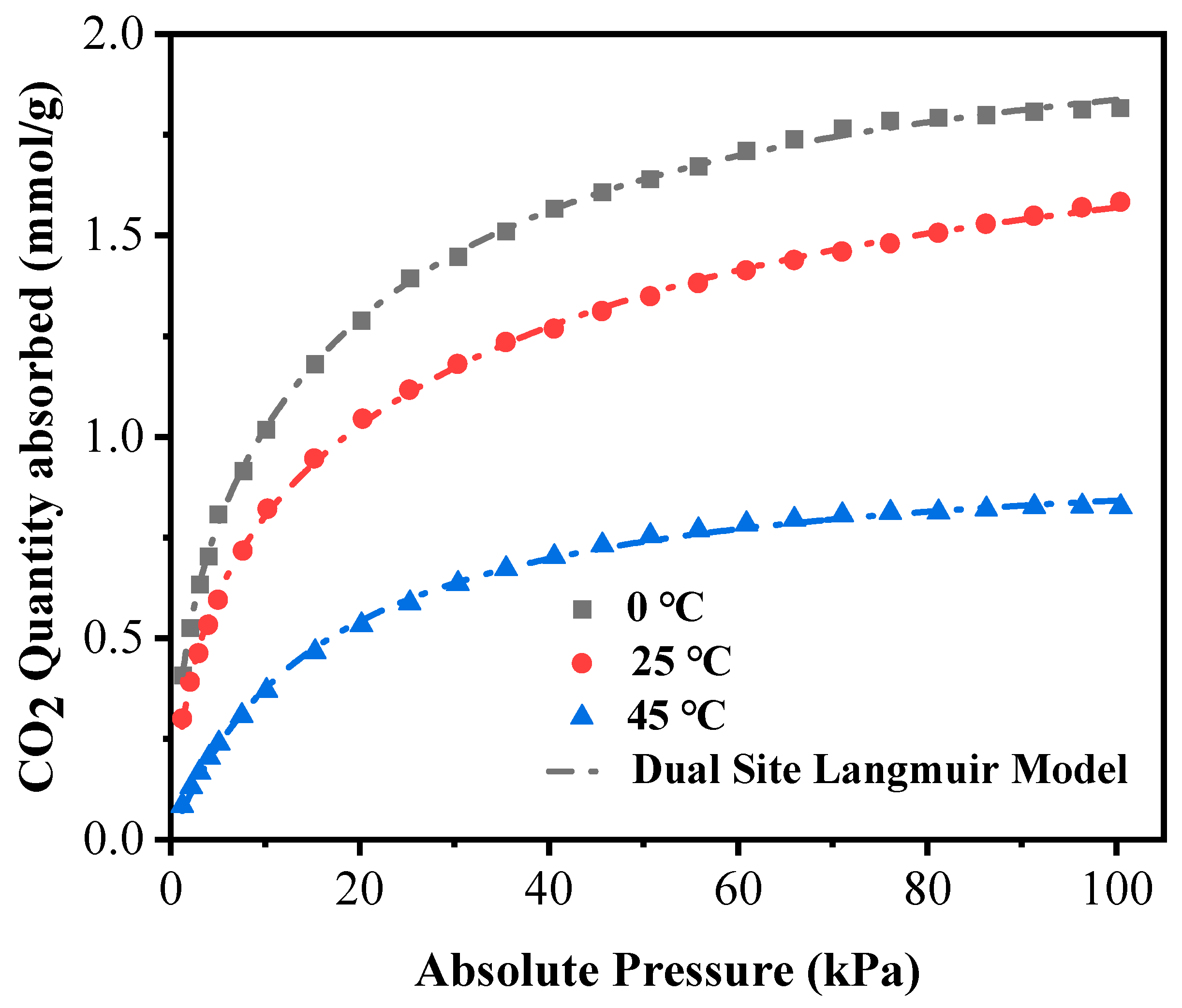
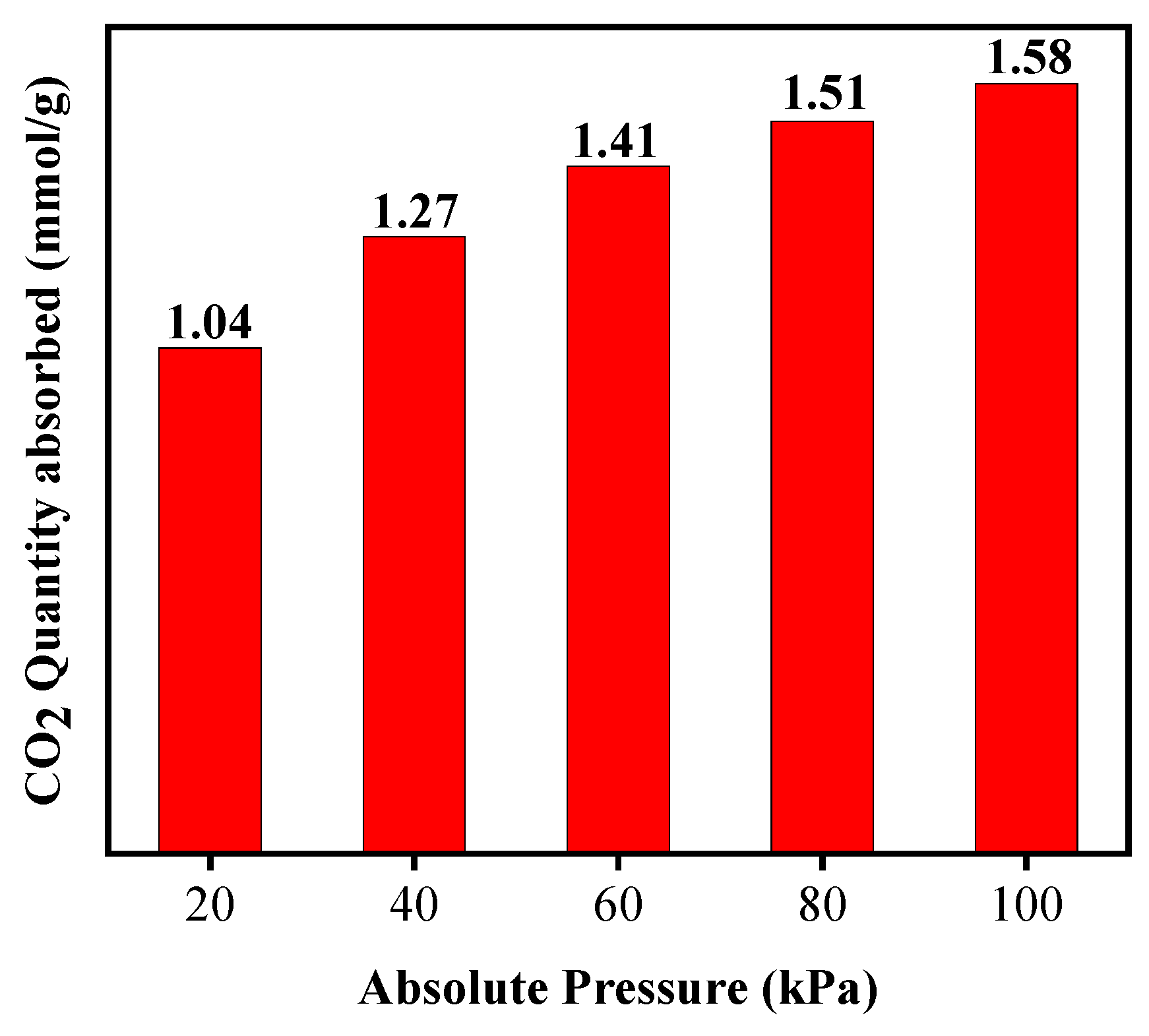
3.3. Measured Characteristics of CO2 Adsorption by Gangue-Based 13-X Molecular Sieve
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegelman, R.L.; Kim, E.J.; Long, J.R. Porous materials for carbon dioxide separations. Nat. Mater. 2021, 20, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, R.; Mahboob, S.; Govindarajan, M.; Al-Ghanim, K.A.; Ahmed, Z.; Al-Mulhm, N.; Vodovnik, M.; Vijayalakshmi, S. A review on biological carbon sequestration: A sustainable solution for a cleaner air environment, less pollution and lower health risks. J. King Saud Univ. Sci. 2021, 33, 101282. [Google Scholar] [CrossRef]
- Qadir, S.; Su, H.; Li, D.; Gu, Y.; Zhao, S.; Wang, S.; Wang, S. Low-cost preferential different amine grafted silica spheres adsorbents for DAC CO2 removal. J. Energy Chem. 2022, 75, 494–503. [Google Scholar] [CrossRef]
- Greer, K.; Zeller, D.; Woroniak, J.; Coulter, A.; Winchester, M.; Palomares, M.D.; Pauly, D. Global trends in carbon dioxide (CO2) emissions from fuel combustion in marine fisheries from 1950 to 2016. Mar. Policy 2019, 107, 103382. [Google Scholar] [CrossRef]
- Jacobson, T.A.; Kler, J.S.; Hernke, M.T.; Braun, R.K.; Meyer, K.C.; Funk, W.E. Direct human health risks of increased atmospheric carbon dioxide. Nat. Sustain. 2019, 2, 691–701. [Google Scholar] [CrossRef]
- Hanna, R.; Abdulla, A.; Xu, Y.; Victor, D.G. Emergency deployment of direct air capture as a response to the climate crisis. Nat. Commun. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Djalante, R. Key assessments from the IPCC special report on global warming of 1.5 C and the implications for the Sendai framework for disaster risk reduction. Prog. Disaster Sci. 2019, 1, 100001. [Google Scholar] [CrossRef]
- Wadi, B.; Golmakani, A.; Manovic, V.; Nabavi, S.A. Effect of combined primary and secondary amine loadings on the adsorption mechanism of CO2 and CH4 in biogas. Chem. Eng. J. 2021, 420, 130294. [Google Scholar] [CrossRef]
- Aaron, D.; Tsouris, C. Separation of CO2 from flue gas: A review. Sep. Sci. Technol. 2005, 40, 321–348. [Google Scholar] [CrossRef]
- Luzzi, E.; Aprea, P.; de Luna, M.S.; Caputo, D.; Filippone, G. Mechanically coherent zeolite 13X/Chitosan aerogel beads for effective CO2 capture. ACS Appl. Mater. Interfaces 2021, 13, 20728–20734. [Google Scholar] [CrossRef]
- Drage, T.; Arenillas, A.; Smith, K.; Pevida, C.; Piippo, S.; Snape, C. Preparation of carbon dioxide adsorbents from the chemical activation of urea–formaldehyde and melamine–formaldehyde resins. Fuel 2007, 86, 22–31. [Google Scholar] [CrossRef]
- Lu, C.; Bai, H.; Wu, B.; Su, F.; Hwang, J.F. Comparative study of CO2 capture by carbon nanotubes, activated carbons, and zeolites. Energy Fuels 2008, 22, 3050–3056. [Google Scholar] [CrossRef]
- Shang, S.; Tao, Z.; Yang, C.; Hanif, A.; Li, L.; Tsang, D.C.; Gu, Q.; Shang, J. Facile synthesis of CuBTC and its graphene oxide composites as efficient adsorbents for CO2 capture. Chem. Eng. J. 2020, 393, 124666. [Google Scholar] [CrossRef]
- Sun, H.; La, P.; Yang, R.; Zhu, Z.; Liang, W.; Yang, B.; Li, A.; Deng, W. Innovative nanoporous carbons with ultrahigh uptakes for capture and reversible storage of CO2 and volatile iodine. J. Hazard. Mater. 2017, 321, 210–217. [Google Scholar] [CrossRef]
- Przepiórski, J.; Czyżewski, A.; Pietrzak, R.; Toyoda, M.; Morawski, A.W. Porous carbon material containing CaO for acidic gas capture: Preparation and properties. J. Hazard. Mater. 2013, 263, 353–360. [Google Scholar] [CrossRef]
- Zhao, Y.; Ding, H.; Zhong, Q. Synthesis and characterization of MOF-aminated graphite oxide composites for CO2 capture. Appl. Surf. Sci. 2013, 284, 138–144. [Google Scholar] [CrossRef]
- Jiang, M.; Li, H.; Zhou, L.; Xing, R.; Zhang, J. Hierarchically porous graphene/ZIF-8 hybrid aerogel: Preparation, CO2 uptake capacity, and mechanical property. ACS Appl. Mater. Interfaces 2018, 10, 827–834. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem Chem. Sustain. Energy Mater. 2009, 2, 796–854. [Google Scholar] [CrossRef]
- Silva, J.A.; Schumann, K.; Rodrigues, A.E. Sorption and kinetics of CO2 and CH4 in binderless beads of 13X zeolite. Microporous Mesoporous Mater. 2012, 158, 219–228. [Google Scholar] [CrossRef]
- Dabbawala, A.A.; Ismail, I.; Vaithilingam, B.V.; Polychronopoulou, K.; Singaravel, G.; Morin, S.; Berthod, M.; Al Wahedi, Y. Synthesis of hierarchical porous Zeolite-Y for enhanced CO2 capture. Microporous Mesoporous Mater. 2020, 303, 110261. [Google Scholar] [CrossRef]
- Ma, C.; Du, H.; Liu, J.; Kang, L.; Du, X.; Xi, X.; Ran, H. High-temperature stability of dielectric and energy-storage properties of weakly-coupled relaxor (1-x) BaTiO3-xBi (Y1/3Ti1/2)O3 ceramics. Ceram. Int. 2021, 47, 25029–25036. [Google Scholar] [CrossRef]
- Xu, R.R.; Pang, W.; Yu, J.H.; Huo, Q.S.; Chen, J.S. Chemistry-zeolites and porous materials. In Structural Analysis and Properties of Porous Materials Characterization; Science Press: Beijing, China, 2004; pp. 145–149. [Google Scholar]
- Ran, H.; Du, H.; Ma, C.; Zhao, Y.; Feng, D.; Xu, H. Effects of A/B-site Co-doping on microstructure and dielectric thermal stability of AgNbO3 ceramics. Sci. Adv. Mater. 2021, 13, 741–747. [Google Scholar] [CrossRef]
- Feng, D.; Du, H.; Ran, H.; Lu, T.; Xia, S.; Xu, L.; Wang, Z.; Ma, C. Antiferroelectric stability and energy storage properties of Co-doped AgNbO3 ceramics. J. Solid State Chem. 2022, 310, 123081. [Google Scholar] [CrossRef]
- Serrano, D.P.; Escola, J.M.; Pizarro, P. Synthesis strategies in the search for hierarchical zeolites. Chem. Soc. Rev. 2013, 42, 4004–4035. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, B.; Xiao, Y.; Ma, L.; Wang, C.P.; Lai-Wang, B.; Shu, C.M. Combustion properties of coal gangue using thermogravimetry–Fourier transform infrared spectroscopy. Appl. Therm. Eng. 2017, 116, 244–252. [Google Scholar] [CrossRef]
- Li, H.; Li, M.; Zheng, F.; Wang, J.; Chen, L.; Hu, P.; Zhen, Q.; Bashir, S.; Liu, J.L. Efficient removal of water pollutants by hierarchical porous zeolite-activated carbon prepared from coal gangue and bamboo. J. Clean. Prod. 2021, 325, 129322. [Google Scholar] [CrossRef]
- Guo, Y.; Yan, K.; Cui, L.; Cheng, F. Improved extraction of alumina from coal gangue by surface mechanically grinding modification. Powder Technol. 2016, 302, 33–41. [Google Scholar] [CrossRef]
- Lü, Q.; Dong, X.; Zhu, Z.; Dong, Y. Environment-oriented low-cost porous mullite ceramic membrane supports fabricated from coal gangue and bauxite. J. Hazard. Mater. 2014, 273, 136–145. [Google Scholar] [CrossRef]
- Yun, Y.; Gao, R.; Yue, H.; Liu, X.; Li, G.; Sang, N. Polycyclic aromatic hydrocarbon (PAH)-containing soils from coal gangue stacking areas contribute to epithelial to mesenchymal transition (EMT) modulation on cancer cell metastasis. Sci. Total Environ. 2017, 580, 632–640. [Google Scholar] [CrossRef]
- Li, J.; Wang, J. Comprehensive utilization and environmental risks of coal gangue: A review. J. Clean. Prod. 2019, 239, 117946. [Google Scholar] [CrossRef]
- Qiu, G.; Luo, Z.; Shi, Z.; Ni, M. Utilization of coal gangue and copper tailings as clay for cement clinker calcinations. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2011, 26, 1205–1210. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, W.X.; Zhao, H.Q.; Yang, Q.C.; Yang, Z.P. Potential ecological risk assessment and prediction of soil heavy-metal pollution around coal gangue dump. Nat. Hazards Earth Syst. Sci. 2014, 14, 1599–1610. [Google Scholar] [CrossRef]
- Marchon, D.; Kawashima, S.; Bessaies-Bey, H.; Mantellato, S.; Ng, S. Hydration and rheology control of concrete for digital fabrication: Potential admixtures and cement chemistry. Cem. Concr. Res. 2018, 112, 96–110. [Google Scholar] [CrossRef]
- Zhou, W.; Du, H.; Kang, L.; Du, X.; Shi, Y.; Qiang, X.; Li, H.; Zhao, J. Microstructure evolution and improved permeability of ceramic waste-based bricks. Materials 2022, 15, 1130. [Google Scholar] [CrossRef]
- Valencia, L.; Rosas, W.; Aguilar-Sanchez, A.; Mathew, A.P.; Palmqvist, A.E. Bio-based micro-/meso-/macroporous hybrid foams with ultrahigh zeolite loadings for selective capture of carbon dioxide. ACS Appl. Mater. Interfaces 2019, 11, 40424–40431. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Shen, Y.; Liu, B.; Zhang, D.; Tang, Z.; Li, G.; Fu, B. CO2 capture from dry flue gas by means of VPSA, TSA and TVSA. J. CO2 Util. 2020, 35, 153–168. [Google Scholar] [CrossRef]
- Zhou, C.; Alshameri, A.; Yan, C.; Qiu, X.; Wang, H.; Ma, Y. Characteristics and evaluation of synthetic 13X zeolite from Yunnan’s natural halloysite. J. Porous Mater. 2013, 20, 587. [Google Scholar] [CrossRef]
- Hedin, N.; Chen, L.J.; Laaksonen, A. Sorbents for CO2 capture from flue gas—Aspects from materials and theoretical chemistry. Nanoscale 2010, 2, 1819–1841. [Google Scholar] [CrossRef]
- Wilke, A.; Weber, J. Hierarchical nanoporous melamine resin sponges with tunable porosity—Porosity analysis and CO2 sorption properties. J. Mater. Chem. 2011, 21, 5226–5229. [Google Scholar] [CrossRef]
- Han, Y.; Hwang, G.; Kim, H.; Haznedaroglu, B.Z.; Lee, B. Amine-impregnated millimeter-sized spherical silica foams with hierarchical mesoporous–macroporous structure for CO2 capture. Chem. Eng. J. 2015, 259, 653–662. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.J.; He, P.Y.; Li, C.J. Synthesis, characterization and modification of monolithic ZSM-5 from geopolymer for CO2 capture: Experiments and DFT calculations. Energy 2019, 179, 422–430. [Google Scholar] [CrossRef]
- Liu, Q.; He, P.; Qian, X.; Fei, Z.; Zhang, Z.; Chen, X.; Tang, J.; Cui, M.; Qiao, X.; Shi, Y. Enhanced CO2 adsorption performance on hierarchical porous ZSM-5 zeolite. Energy Fuels 2017, 31, 13933–13941. [Google Scholar] [CrossRef]
| Oxide | CaO | MgO | SiO2 | Al2O3 | Fe2O3 | TiO2 | K2O | Na2O | Loss |
|---|---|---|---|---|---|---|---|---|---|
| wt% | 0.82 | 1.57 | 61.39 | 23.76 | 4.14 | 0.79 | 2.82 | 1.62 | 2.11 |
| Serial Number | Influence Factor | |||
|---|---|---|---|---|
| Si/Al (PCG/NaAlO2 Mass Ratio) | Alkalinity (CNaOH(aq))/mol·L−1 | Crystallization Temperature/°C | Hydrothermal Time/h | |
| Y1 | 4 | 0.3 | 100 | 8 |
| Y2 | 4 | 0.4 | 120 | 10 |
| Y3 | 4 | 0.5 | 80 | 12 |
| Y4 | 5 | 0.5 | 100 | 10 |
| Y5 | 5 | 0.3 | 120 | 12 |
| Y6 | 5 | 0.4 | 80 | 8 |
| Y7 | 6 | 0.4 | 100 | 12 |
| Y8 | 6 | 0.5 | 120 | 8 |
| Y9 | 6 | 0.3 | 80 | 10 |
| Sample | BET-Specific Surface Area (m2/g) | Average Pore Size (Å) | Pore Volume (m3/g) |
|---|---|---|---|
| Coal Gangue | 2.04 | — | — |
| Y9 | 377.02 | 20.87 | 0.20 |
| Commercial 13-X molecular sieve | 523.88 | 18.62 | — |
| Temperature (°C) | Langmuir Model | Dual-Site Langmuir Model | ||||||
|---|---|---|---|---|---|---|---|---|
| qA (mmol/g) | bA (kPa−1) | R2 | qA (mmol/g) | bA (kPa−1) | qB (mmol/g) | bB (kPa−1) | R2 | |
| 0 | 1.91 | 0.13 | 0.9799 | 1.48 | 0.04 | 0.64 | 0.84 | 0.9995 |
| 25 | 1.65 | 0.10 | 0.9796 | 1.29 | 0.03 | 0.65 | 0.48 | 0.9994 |
| 45 | 0.98 | 0.06 | 0.9988 | 0.49 | 0.06 | 0.49 | 0.06 | 0.9988 |
| Sample | Adsorption Temperature (°C) | Adsorption Capacity (mmol/g) | References |
|---|---|---|---|
| zeolite 13-X | 0 | 1.8 | Present work |
| Hierarchical melamine resin sponges | 0 | 1.7 | [40] |
| β-zeolite | 30 | 1.8 | [39] |
| Monolithic Ni/ZSM-5 | 35 | 2.4 | [42] |
| Amine-impregnated Silica foam | 25 | 1.48 | [41] |
| ZSM-5 | 0 | 2.37 | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, D.; Du, H.; Li, Y.; Gao, Y.; Liu, S.; Xu, B.; Huang, H.; Kang, L. Study on Green Controllable Preparation of Coal Gangue-Based 13-X Molecular Sieves and Its CO2 Capture Application. Coatings 2023, 13, 1886. https://doi.org/10.3390/coatings13111886
Yi D, Du H, Li Y, Gao Y, Liu S, Xu B, Huang H, Kang L. Study on Green Controllable Preparation of Coal Gangue-Based 13-X Molecular Sieves and Its CO2 Capture Application. Coatings. 2023; 13(11):1886. https://doi.org/10.3390/coatings13111886
Chicago/Turabian StyleYi, Dawei, Huiling Du, Yefei Li, Yimin Gao, Sifan Liu, Boyang Xu, Haoqi Huang, and Le Kang. 2023. "Study on Green Controllable Preparation of Coal Gangue-Based 13-X Molecular Sieves and Its CO2 Capture Application" Coatings 13, no. 11: 1886. https://doi.org/10.3390/coatings13111886
APA StyleYi, D., Du, H., Li, Y., Gao, Y., Liu, S., Xu, B., Huang, H., & Kang, L. (2023). Study on Green Controllable Preparation of Coal Gangue-Based 13-X Molecular Sieves and Its CO2 Capture Application. Coatings, 13(11), 1886. https://doi.org/10.3390/coatings13111886








