Preparation of Modified Biochar and Its Adsorption of Cr(VI) in Aqueous Solution
Abstract
:1. Introduction
- (1)
- Metal salts or metal oxides are mixed with raw materials and co-pyrolyzed to synthesize modified biochar;
- (2)
- Biochar is prepared by the pyrolysis of raw materials, and biochar is soaked in metal salt or metal oxide solution under certain conditions.
2. Materials and Methods
2.1. Preparation of Corn Stover Biochar
2.1.1. Preparation of Biochar Materials
2.1.2. Determination of Cr(VI) Content in Solution by Native Biochar
2.1.3. Determination of Adsorption Content of Cr(VI) in Solution by Native Biochar at Different Temperatures
2.2. Modification of Biochar and Analysis of Modified Biochar
2.2.1. Preparation of Modified Biochar
2.2.2. Material Characterization Method and Principle
2.2.3. Adsorption Experiments
3. Results and Discussion
3.1. Determination of Pyrolysis Temperature for the Preparation of Native Biomass Carbon
3.2. Material Characterization Method and Principle
3.2.1. XRD Measurement
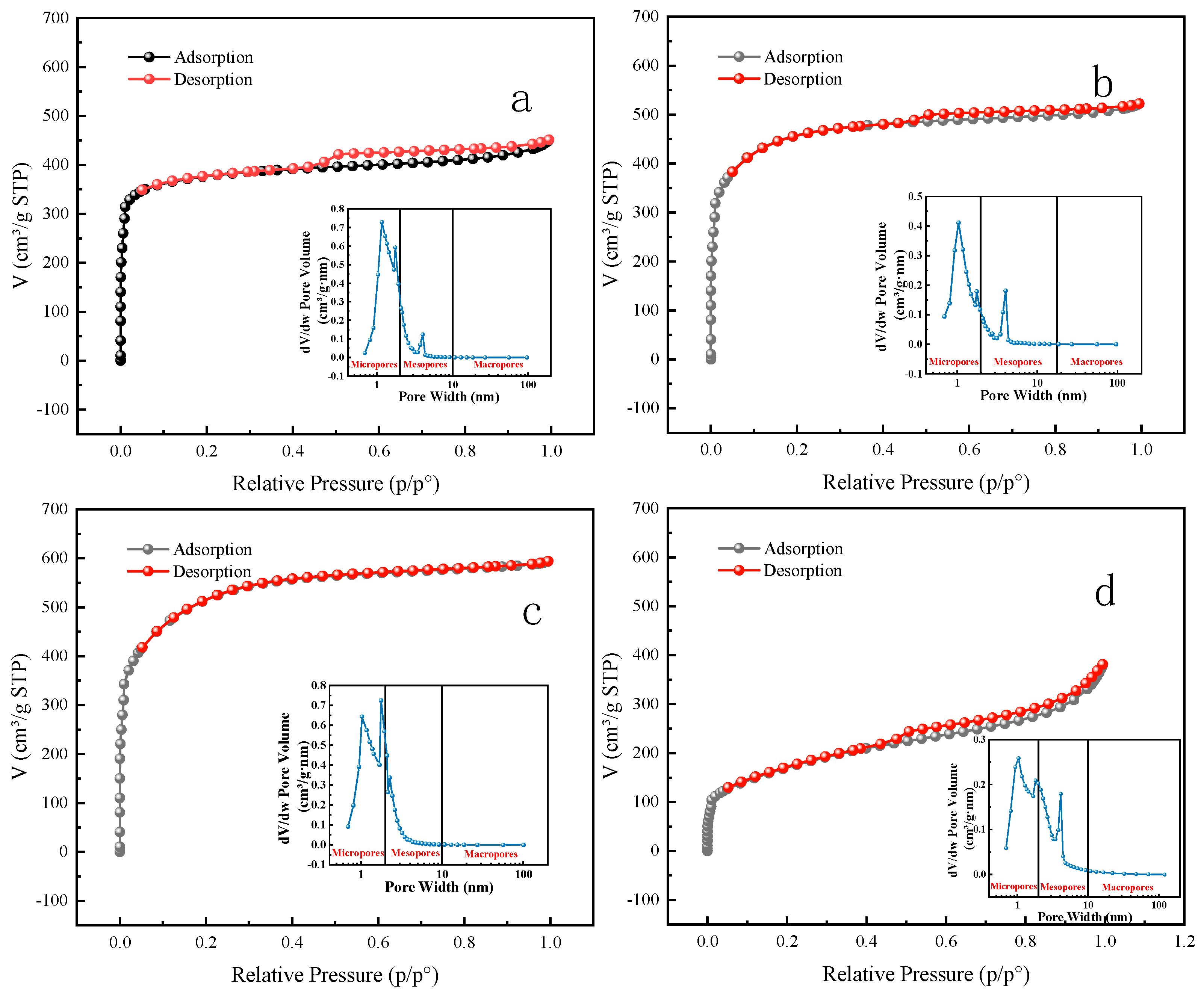
3.2.2. BET and Pore Size Analysis
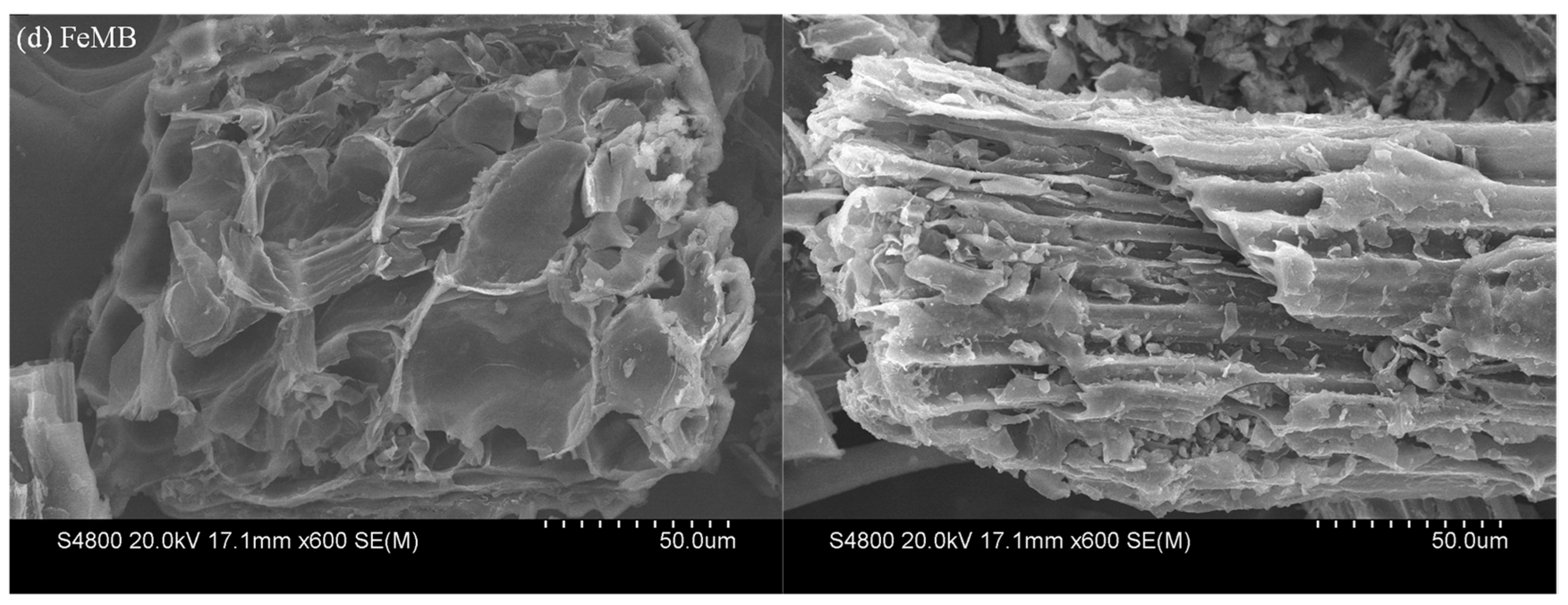
3.2.3. SEM Analysis
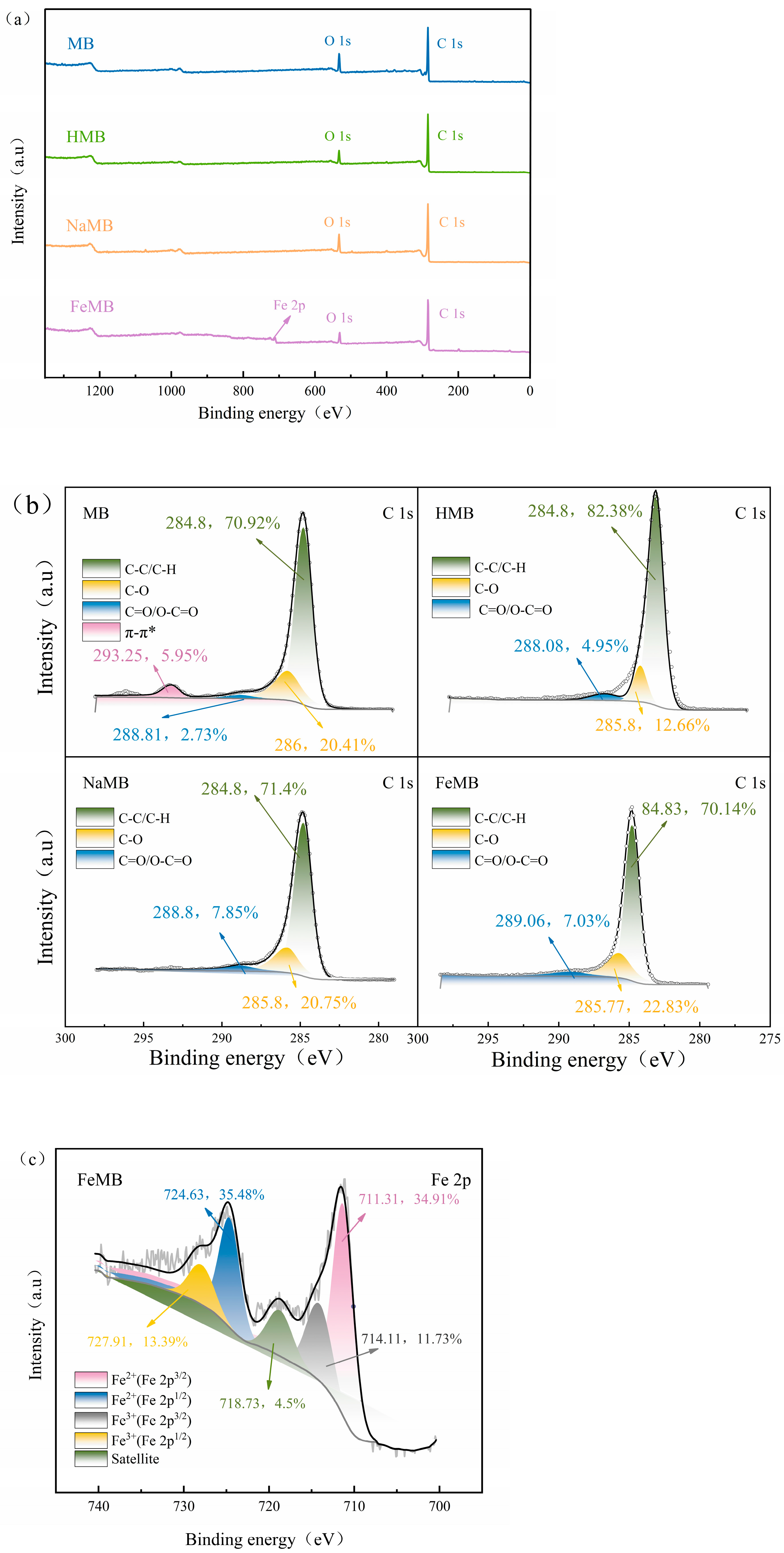
3.2.4. X-ray Photoelectron Spectroscopy (XPS) Measurement
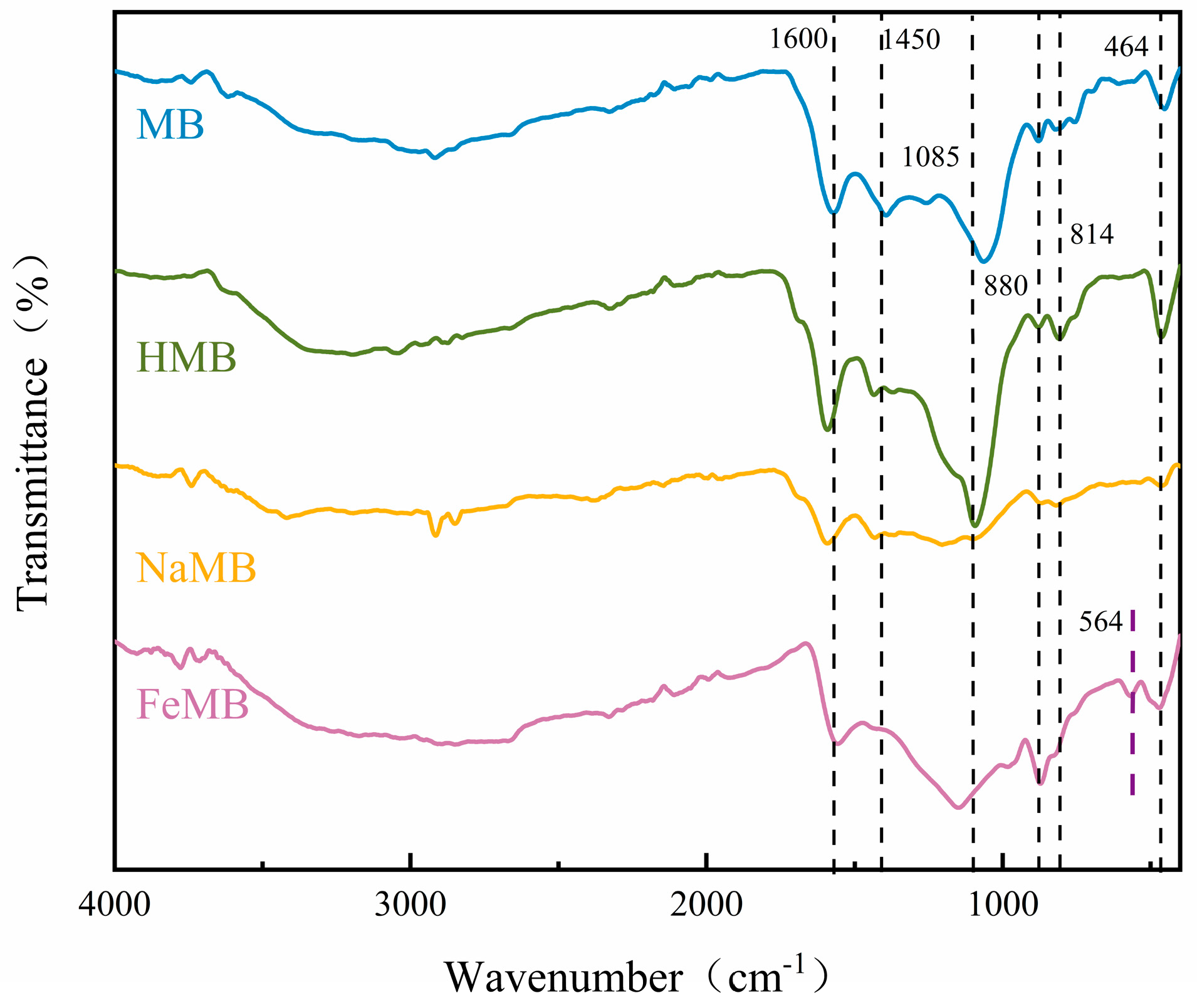
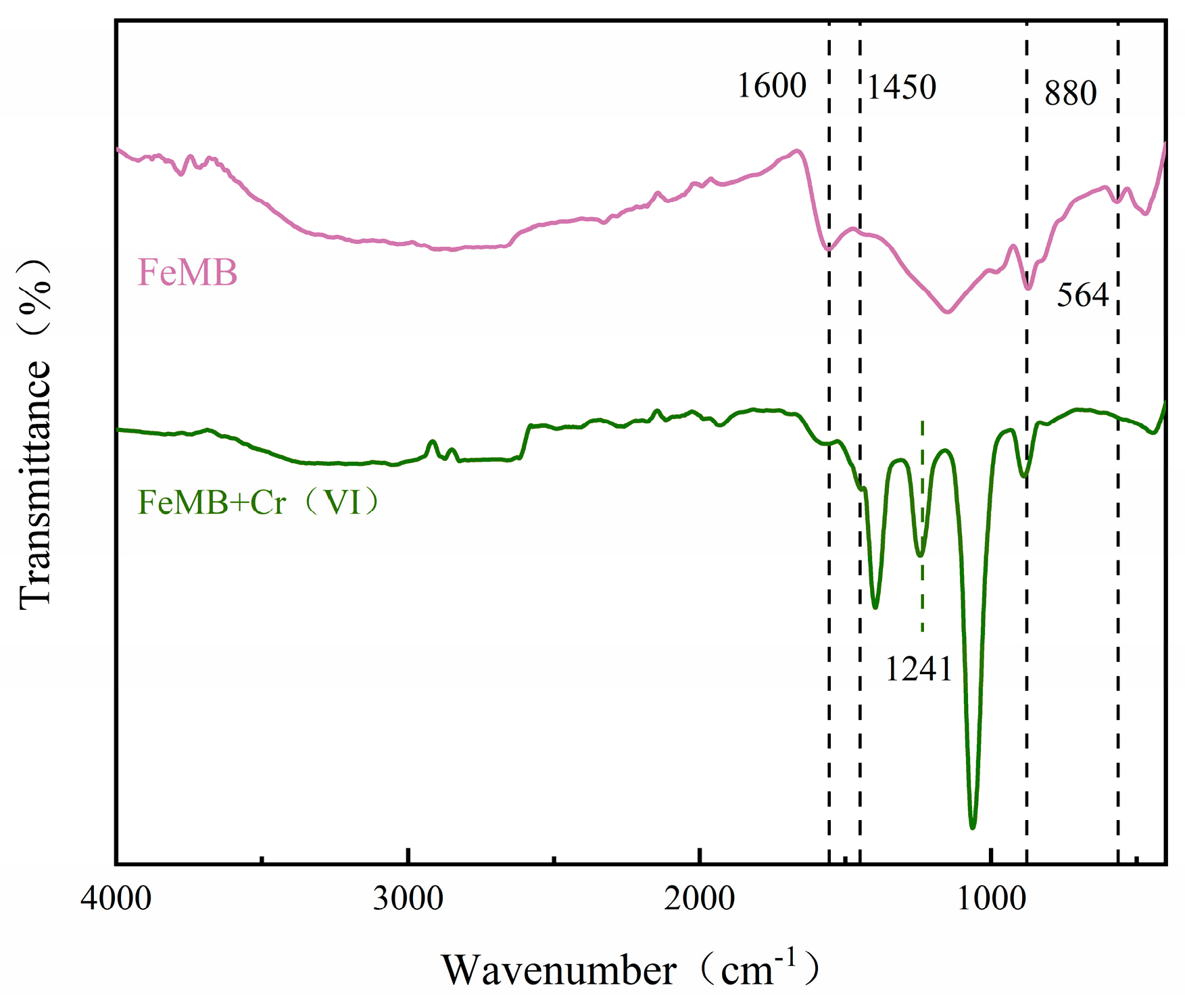
3.2.5. Fourier-Transform Infrared Spectroscopy (FTIR) Determination
3.3. Adsorption Single-Factor Experiment
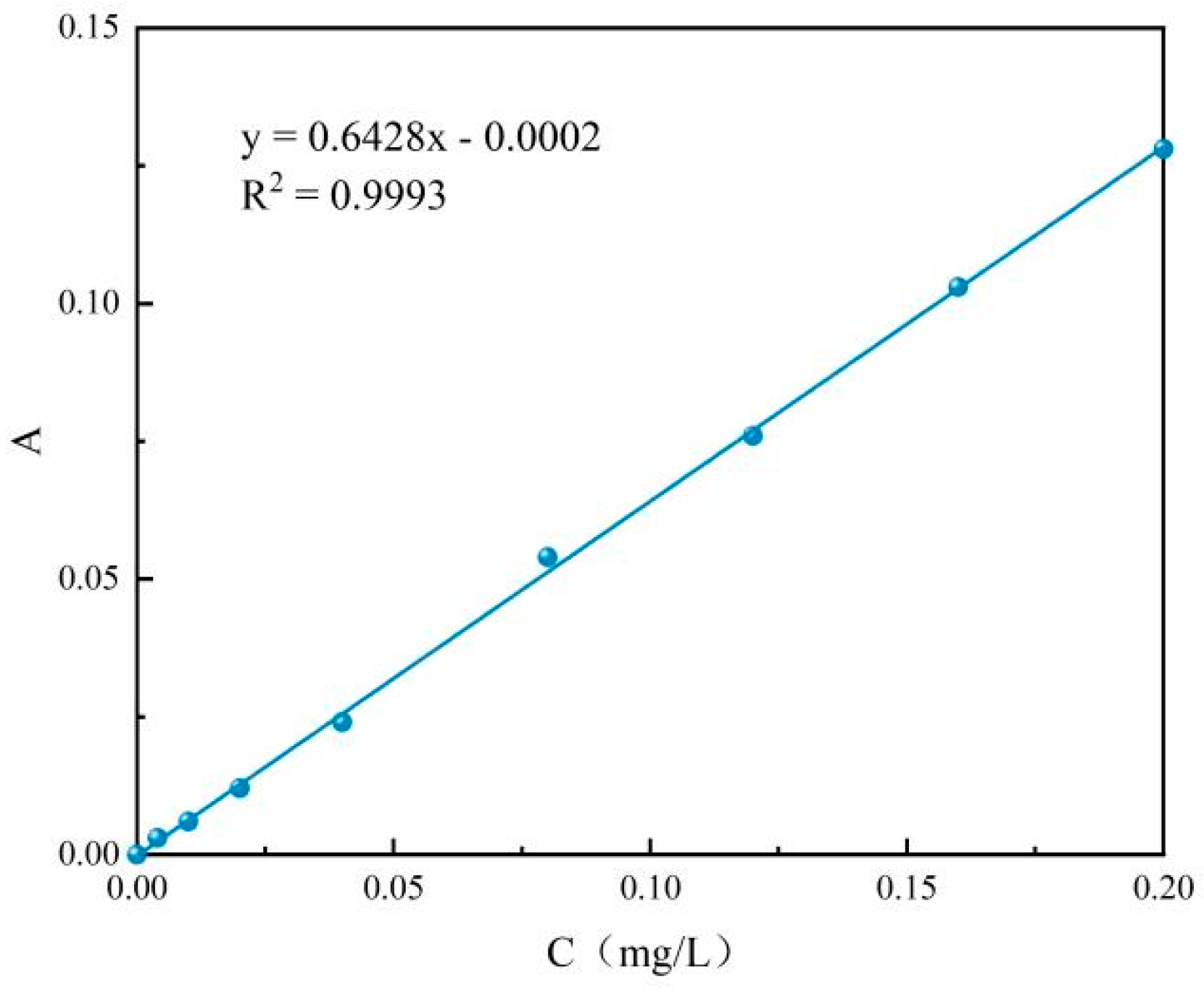
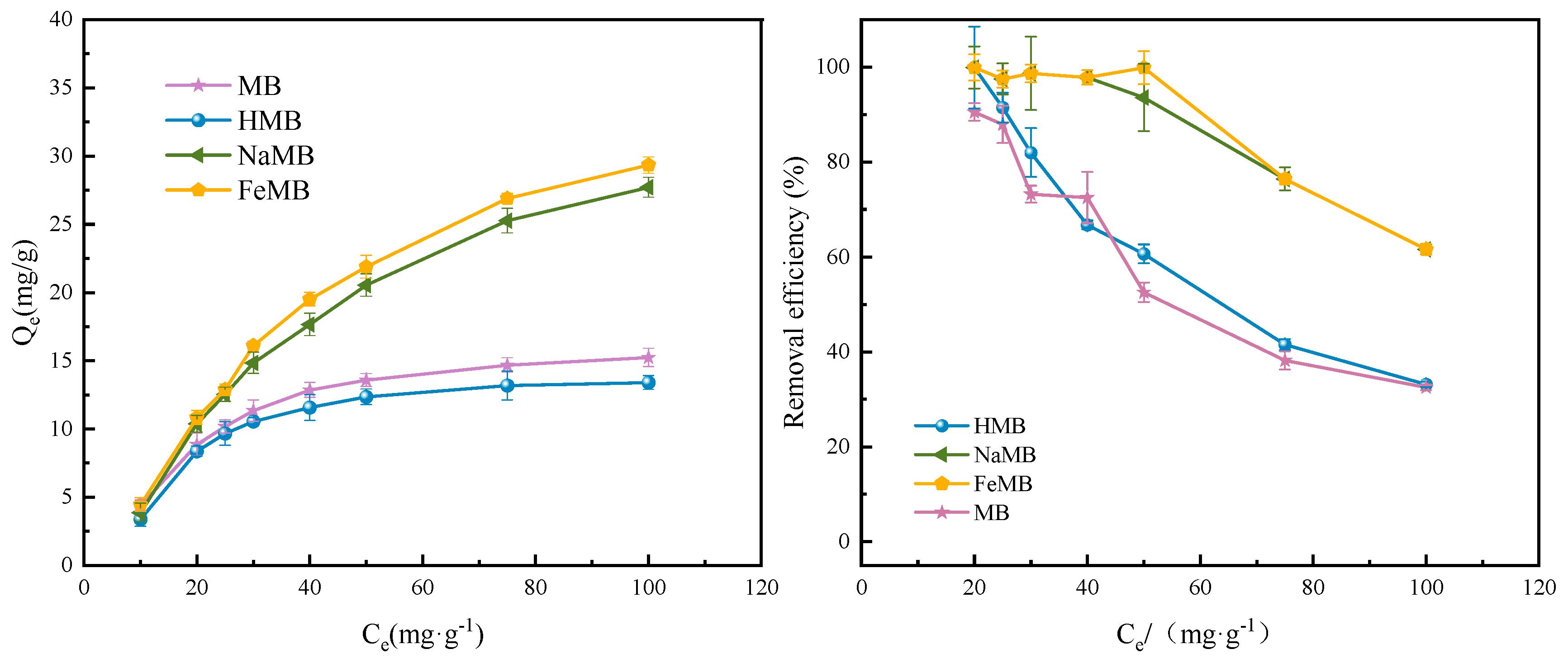
3.3.1. Initial Concentration
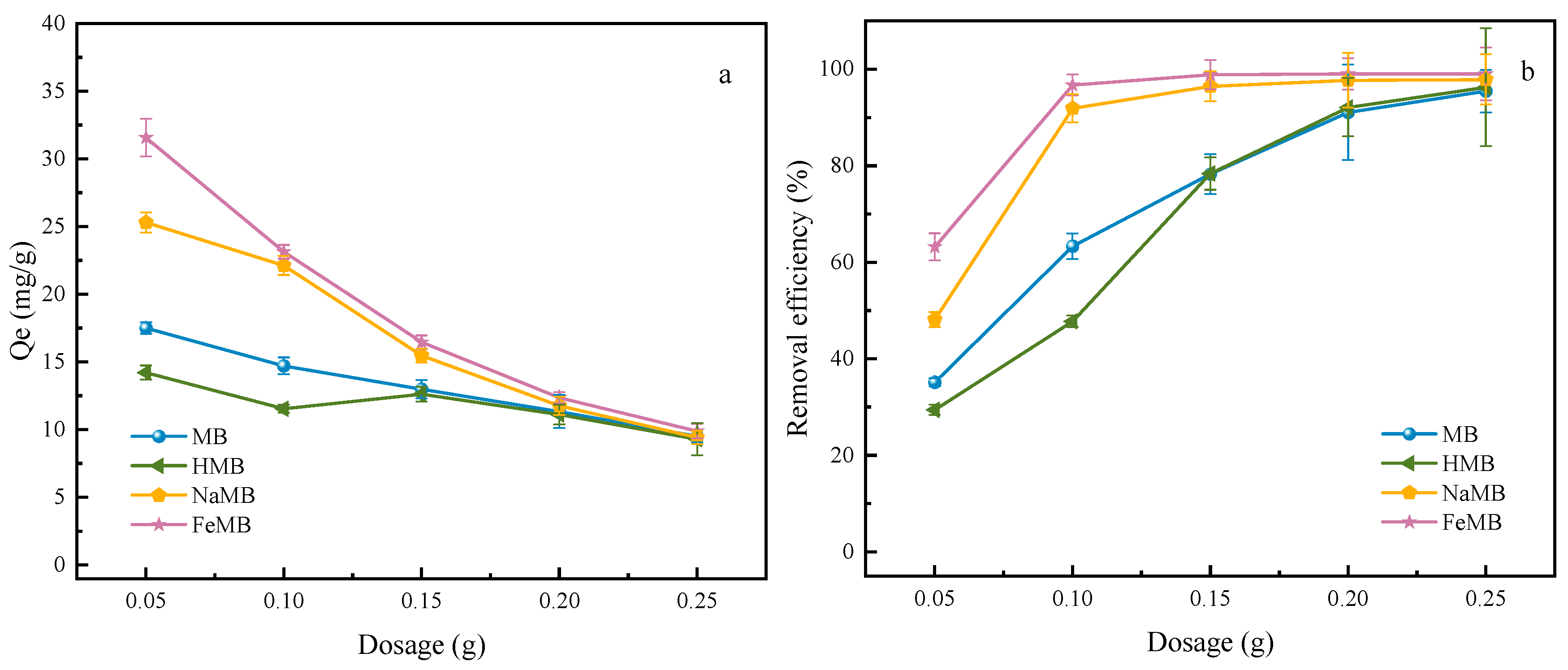
3.3.2. Dosage
3.3.3. pH
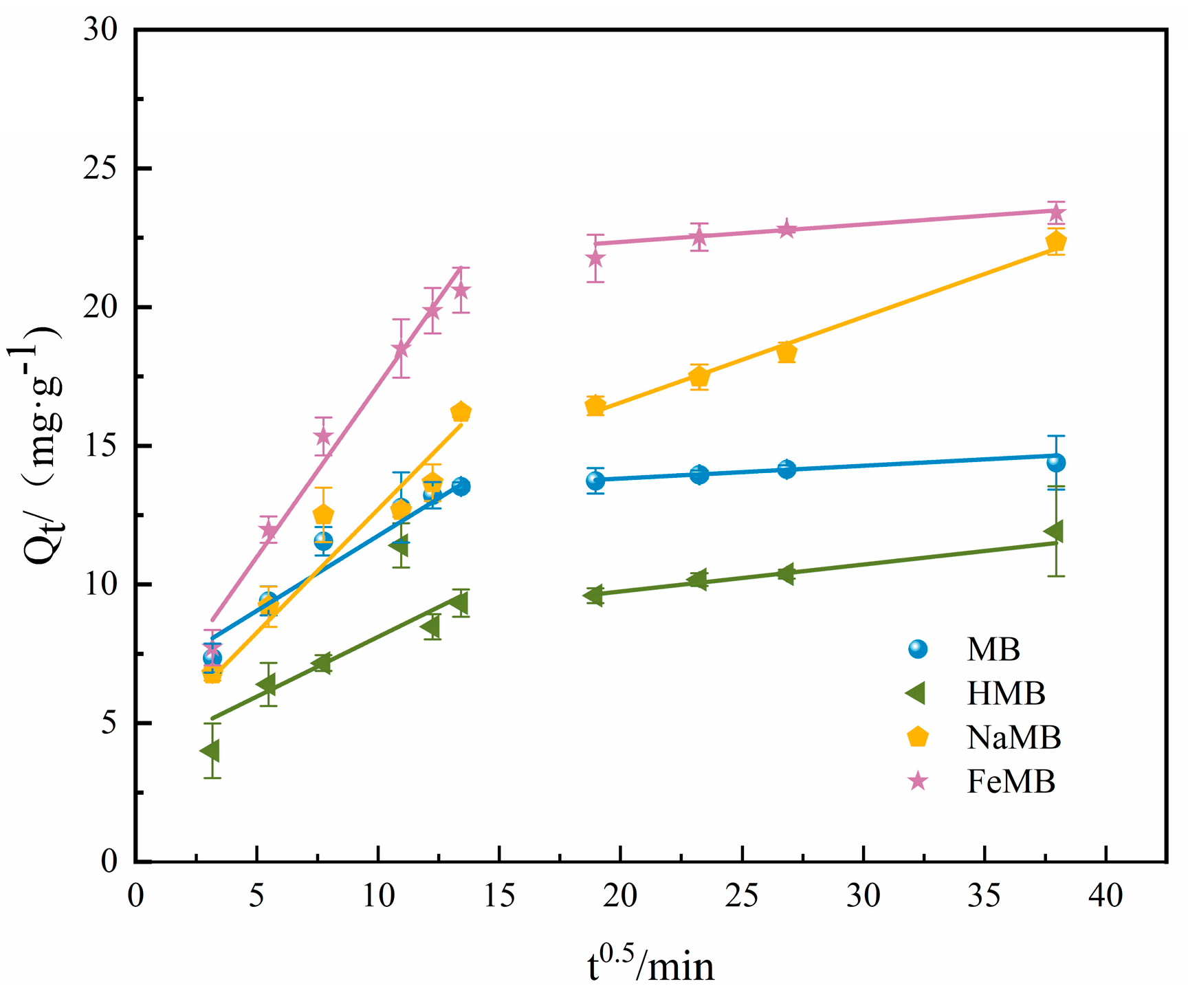
3.4. Adsorption Kinetics Experiment
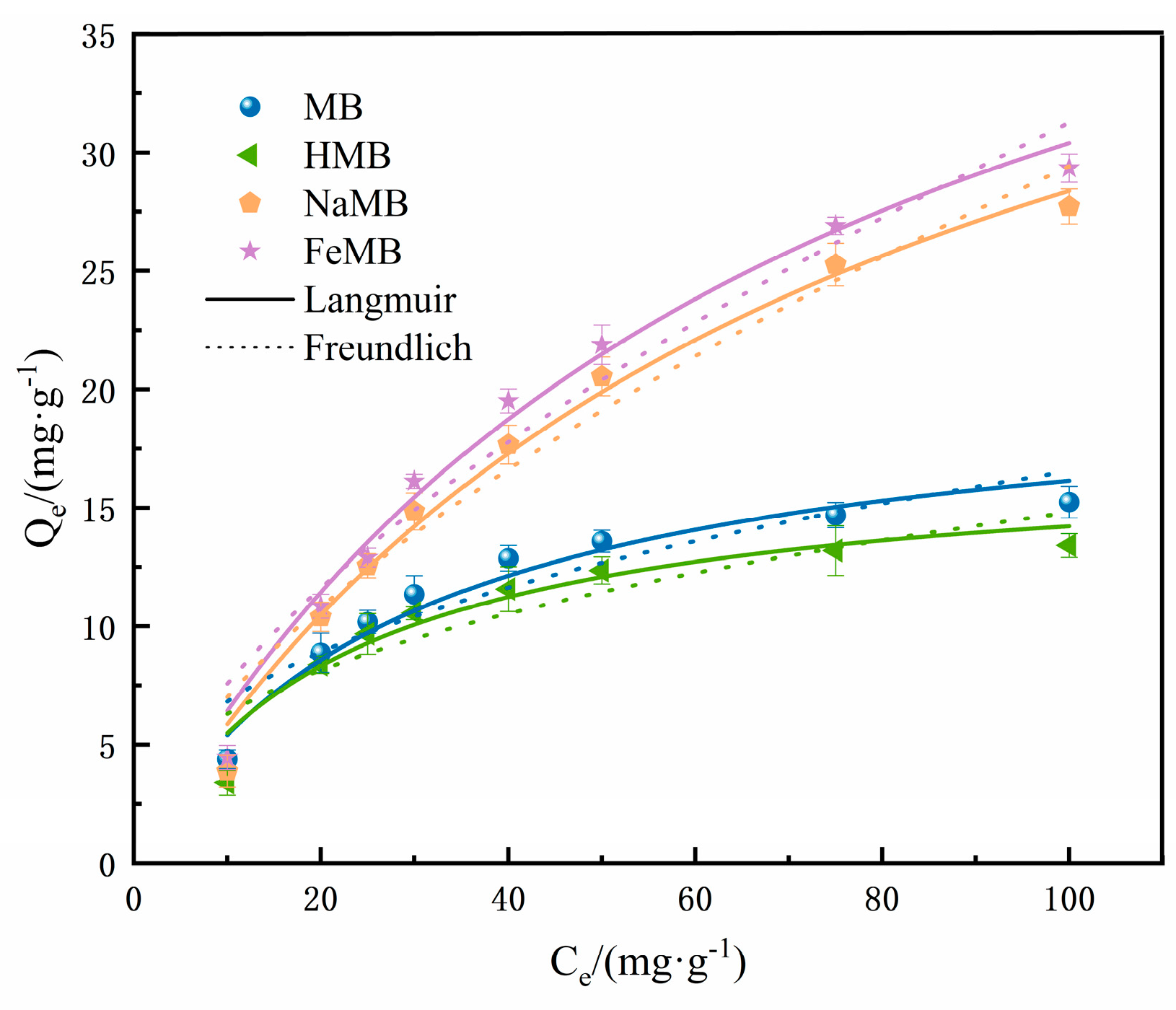
3.5. Isothermal Adsorption Experiments
- Ce—Cr(VI) solution equilibrium concentration, mg/L;
- C0—Cr(VI) solution initial concentration, mg/L;
- qm—Cr(VI) the maximum equilibrium adsorption capacity calculated by the adsorption isotherm model, mg/L;
- KL—Langmuir adsorption equilibrium constant;
- RL—Langmuir separation factor.
- KF—Freundlich adsorption equilibrium constant;
- n—the constant of the adsorption trend.
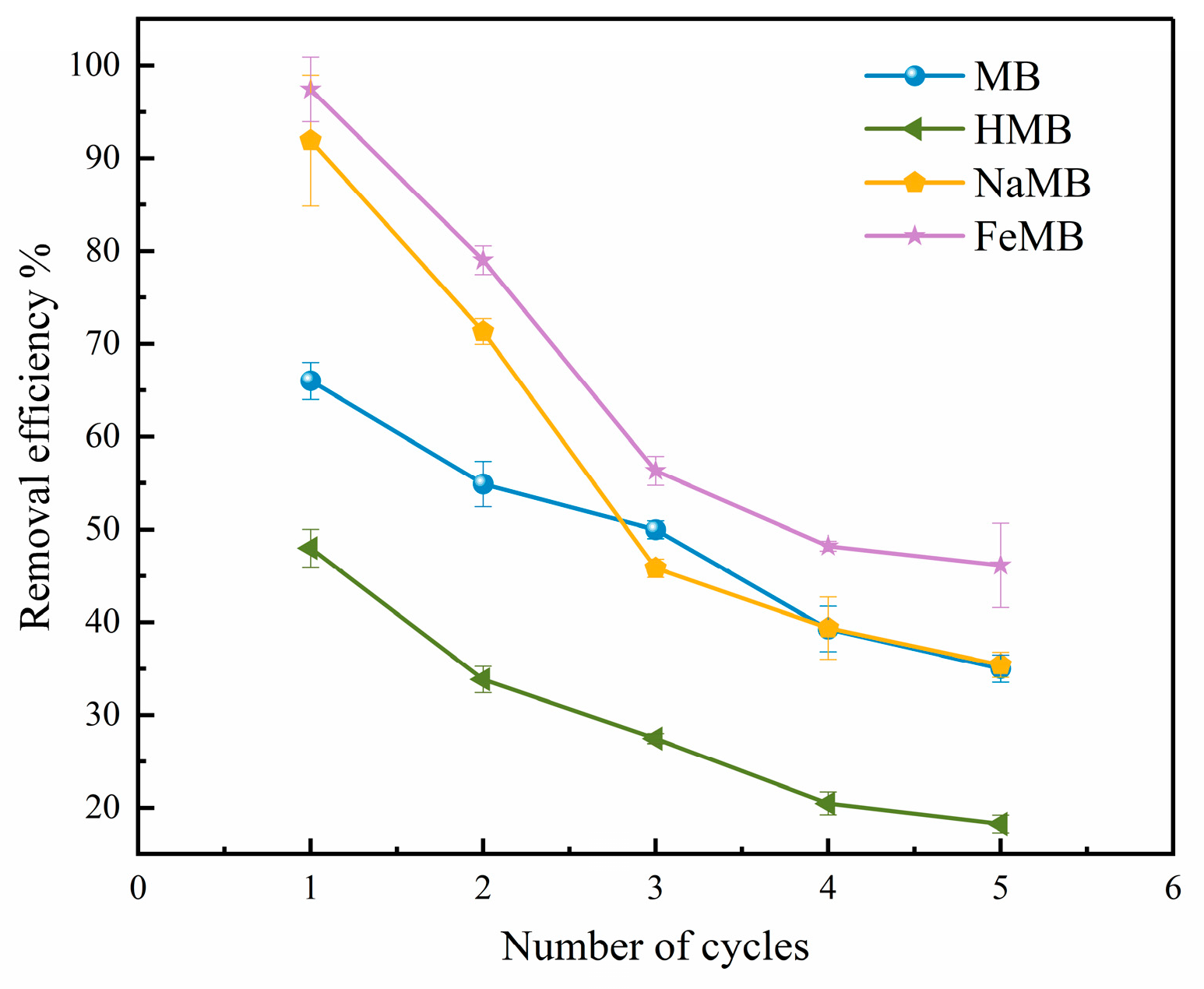
3.6. Adsorption Cycle Regeneration Experiment
3.7. Adsorption Mechanism Exploration
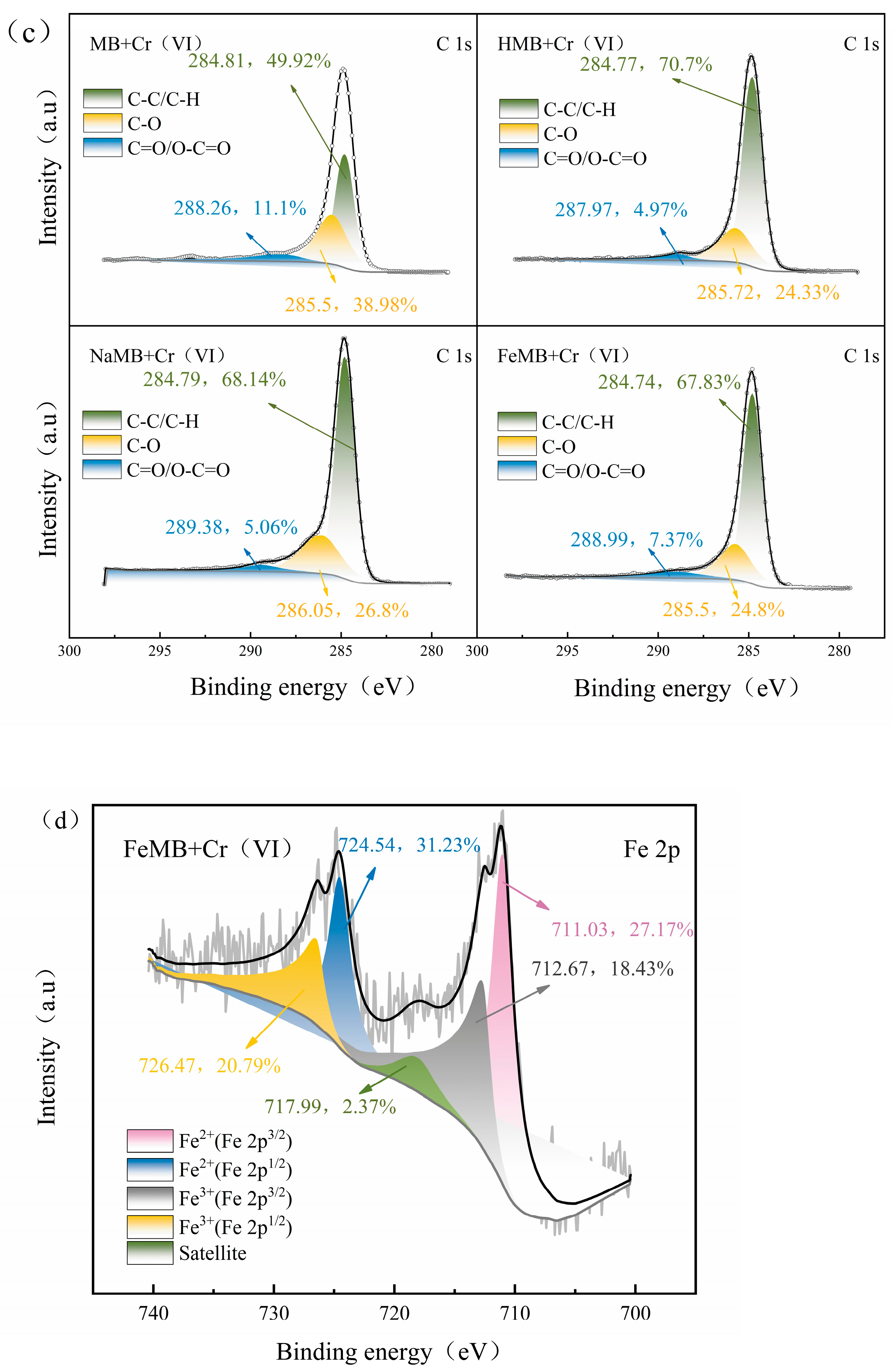
3.7.1. XPS Analysis after Cr(VI) Adsorption
3.7.2. FTIR Analysis after Cr(VI) Adsorption
4. Conclusions
- Under the conditions of 25 °C and pH = 2, the adsorption process of Cr(VI) by MB, HMB, NaMB and FeMB conforms to the Langmuir adsorption isotherm model and the quasi-secondary kinetic model. Among them, FeMB has the best adsorption performance, and the removal rate of Cr(VI) remains above 45% after five cycles.
- The results of SEM (the surface of FeMB is rough, and its surface is loaded with many irregular particles) and XRD (the presence of iron oxide ɑ-Fe2O3 on FeMB) showed that FeCl3-modified biochar successfully loaded Fe on the surface of biochar to form a composite film and made FeMB form a stable crystal structure.
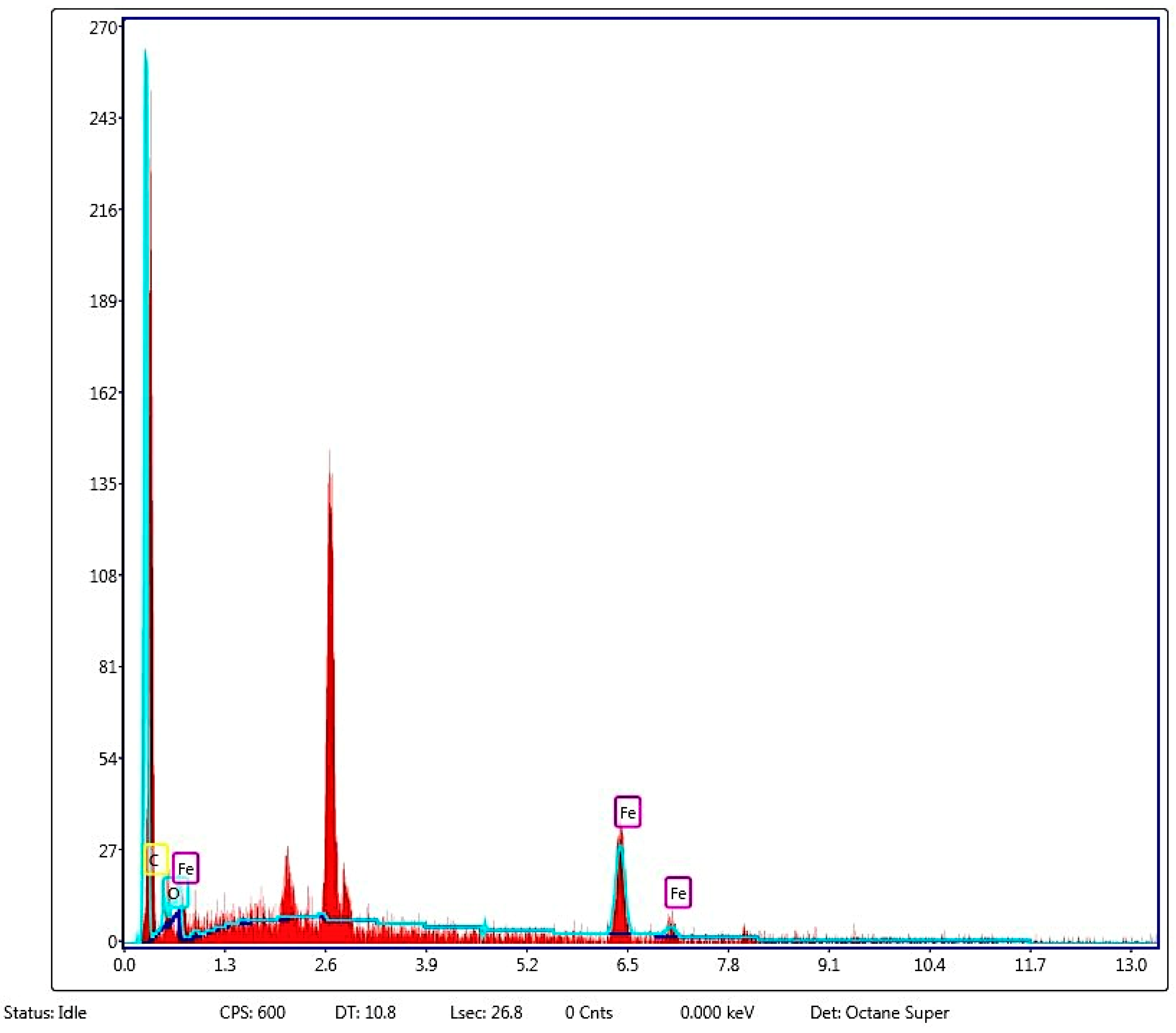
- FTIR and XPS analysis showed that there were FeO and Fe2O3 on the surface of FeMB, which proved that iron oxide particles were also successfully loaded onto the composite film on the surface of FeMB, and iron modification enhanced the aromaticity of biochar, enhanced the role of cations and provided more active sites. According to the BET analysis, the pore structure of FeMB is dominated by slit pore adsorption caused by layered polymers, and most of them are mesoporous, and all the biochars are microporous and mesoporous mixed materials.
- The adsorption sites on the surface of the biochar are limited, and increasing the adsorption sites can effectively improve the adsorption efficiency. The modified biochar surface contains more positive charges due to the composite film formed by loading Fe and Fe3+, which provides more adsorption sites for anions HCrO4− and Cr2O7−2. At the same time, due to the loading of trivalent iron, Fe-O groups were introduced into the surface of the biochar, forming coordination adsorption with dichromate ions.
- The adsorption of Cr(VI) by the biochar is from monolayer chemical adsorption. The adsorption mechanism includes electrostatic interaction, surface functional group complexation and reduction, and the adsorption performance is affected by the polarity and hydrophilicity of the biochar.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Kumar, P.S.; Rozbu, M.R.; Chowdhury, A.T.; Nuzhat, S.; Rafa, N.; Mahlia, T.; Ong, H.C.; Mofijur, M. Heavy metal toxicity, sources, and remediation techniques for contaminated water and soil. Environ. Technol. Innov. 2022, 25, 102114. [Google Scholar] [CrossRef]
- Khalef, R.N.; Hassan, A.I.; Saleh, H.M. Heavy Metal’s Environmental Impact; Intech Open: London, UK, 2022. [Google Scholar]
- Hosseini, S.M.; Alibakhshi, H.; Jashni, E.; Parvizian, F.; Shen, J.; Taheri, M.; Ebrahimi, M.; Rafiei, N. A novel layer-by-layer heterogeneous cation exchange membrane for heavy metal ions removal from water. J. Hazard. Mater. 2020, 381, 120884. [Google Scholar] [CrossRef] [PubMed]
- Foong, C.Y.; Wirzal, H.; Bustam, M.A. A review on nanofibers membrane with amino-based ionic liquid for heavy metal removal. J. Mol. Liq. 2020, 297, 111793. [Google Scholar] [CrossRef]
- Wu, H.; Wang, W.; Huang, Y.; Han, G.; Yang, S.; Su, S.; Sana, H.; Peng, W.; Cao, Y.; Liu, J. Comprehensive evaluation on a prospective precipitation-flotation process for metal-ions removal from wastewater simulants. J. Hazard. Mater. 2019, 371, 592–602. [Google Scholar] [CrossRef]
- Allioux, F.; Kapruwan, P.; Milne, N.; Kong, L.; Fattaccioli, J.; Chen, Y.; Dumée, L.F. Electro-capture of heavy metal ions with carbon cloth integrated microfluidic devices. Sep. Purif. Technol. 2018, 194, 26–32. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Marwa, E.; Ahmed, S.E.; Samer, F.; Rooney, D.W.; Osman, A.I. Competitive adsorptive removal of promazine and promethazine from wastewater using olive tree pruning biochar: Operational parameters, kinetics, and equilibrium investigations. Environ. Sci. Pollut. Res. Int. 2023, 30, 82387–82405. [Google Scholar]
- Thangagiri, B.; Sakthivel, A.; Jeyasubramanian, K.; Seenivasan, S.; Raja, J.D. Modeling and optimization of removal of Pb(II) in aqueous solutions by biochar derived from neem leaves using central composite design of response surface methodology. Biomass Convers. Biorefin. 2023. [Google Scholar] [CrossRef]
- Tag, A.T.; Duman, G.; Ucar, S.; Yanik, J. Effects of feedstock type and pyrolysis temperature on potential applications of biochar. J. Anal. Appl. Pyrolysis 2016, 120, 200–206. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Li, Y.; Shan, S.; Yang, R.; Gai, X.; Mao, J.; Huang, N.; Tou, Y.; Meas, A. Preparation of bamboo biochars by low-temperature hydrothermal method and its adsorption of organics. Trans. Chin. Soc. Agric. Eng. 2016, 32, 240–247. [Google Scholar]
- Song, J.; Zhang, S.; Li, G.; Du, Q.; Yang, F. Preparation of montmorillonite modified biochar with various temperatures and their mechanism for Zn ion removal. J. Hazard. Mater. 2020, 391, 121692. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, V.K.; Nagappan, S.; Bhosale, R.R.; Lay, C.-H.; Nguyen, D.D.; Pugazhendhi, A.; Chang, S.W.; Kumar, G. Review on sustainable production of biochar through hydrothermal liquefaction: Physico-chemical properties and applications. Bioresour. Technol. 2020, 310, 123414. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, Z.; Xu, L.; Buyong, F.; Chay, T.C.; Li, Z.; Cai, Y.; Hu, B.; Zhu, Y.; Wang, X. Modified biochar: Synthesis and mechanism for removal of environmental heavy metals. Carbon Res. 2022, 1, 8. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, L.; Li, H.; Westholm, L.J.; Carvalho, L.; Thorin, E.; Yu, Z.; Yu, X.; Skreiberg, Ø. A critical review on production, modification and utilization of biochar. J. Anal. Appl. Pyrolysis 2022, 161, 105405. [Google Scholar] [CrossRef]
- Lü, F.; Lu, X.; Li, S.; Zhang, H.; Shao, L.; He, P. Dozens-fold improvement of biochar redox properties by KOH activation. Chem. Eng. J. 2022, 429, 132203. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, H.; Wu, J.; Chen, W.; Chen, Y.; Xuezhi, G.; Yang, H.; Chen, H. Physicochemical and adsorption properties of biochar from biomass-based pyrolytic polygeneration: Effects of biomass species and temperature. Biochar 2021, 3, 657–670. [Google Scholar]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, H.; Li, W.; Ding, X.; Chu, H. Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. J. Anal. Appl. Pyrolysis 2021, 155, 105081. [Google Scholar] [CrossRef]
- Liu, Y.; Lonappan, L.; Brar, S.K.; Yang, S. Impact of biochar amendment in agricultural soils on the sorption, desorption, and degradation of pesticides: A review. Sci. Total Environ. 2018, 645, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Li, H.; Mannering, J.; Huang, P.; Zheng, X.; Kulak, A.; Baker, D.; Iruretagoyena, D.; Menzel, R. Electrically Heatable Graphene Aerogels as Nanoparticle Supports in Adsorptive Desulfurization and High-Pressure CO2 Capture. Adv. Funct. Mater. 2020, 30, 2002788. [Google Scholar] [CrossRef]
- Jing, F.; Pan, M.; Chen, J. Kinetic and isothermal adsorption-desorption of PAEs on biochars: Effect of biomass feedstock, pyrolysis temperature, and mechanism implication of desorption hysteresis. Environ. Sci. Pollut. Res. 2018, 25, 11493–11504. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhou, B.; Budai, A.; Jeng, A.; Hao, X.; Wei, D.; Rasse, D. Study of biochar properties by scanning electron microscope–energy dispersive X-ray spectroscopy (SEM-EDX). Commun. Soil Sci. Plant Anal. 2016, 47, 593–601. [Google Scholar] [CrossRef]
- Herman, M.F.; Freed, K.F.; Yeager, D.L. Shake-up peak positions and intensities by many-body theory methods. Chem. Phys. 1978, 32, 437–449. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, C.; Xu, L.; Li, J.; Zhai, M. Adsorption of Cr(Ⅵ) ions using the amphiphilic gels based on 2-(dimethylamino)ethyl methacrylate modified with 1-bromoalkanes. Chem. Eng. J. 2011, 173, 42–48. [Google Scholar] [CrossRef]
- Hou, S.; Su, S.; Kasner, M.L.; Shah, P.; Patel, K.; Madarang, C.J. Formation of highly stable dispersions of silane-functionalized reduced graphene oxide. Chem. Phys. Lett. 2010, 501, 68–74. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Varnoosfaderani, S.; Hebard, A.; Yao, Y.; Inyang, M. Preparation and characterization of a novel magnetic biochar for arsenic removal. Bioresour. Technol. 2013, 130, 457–462. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Wysokinski, A.; Kalembasa, S.; Kuziemska, B. The content of chromium and copper in plants and soil fertilized with sewage sludge with addition of various amounts of CaO and lignite ash. Soil Sci. Annu. 2016, 67, 117. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, X.; Xu, Y.; Chen, B. Environmental Effects of Silicon within Biochar (Sichar) and Carbon-Silicon Coupling Mechanisms: A Critical Review. Environ. Sci. Technol. 2019, 53, 13570–13582. [Google Scholar] [CrossRef] [PubMed]
- Van Quy, D.; Hieu, N.M.; Tra, P.T.; Nam, N.H.; Hai, N.H.; Son, N.T.; Nghia, P.T.; Van Anh, N.T.; Hong, T.T.; Luong, N.H. Synthesis of silica-coated magneticnanoparticles and application in the detection of pathogenic viruses. J. Nanomater. 2013, 2013, 603940. [Google Scholar]
- Liu, L.; Yang, X.; Ahmad, S.; Li, X.; Ri, C.; Tang, J.; Ellam, R.M.; Song, Z. Silicon (Si) modification of biochars from different Si-bearing precursors improves cadmium remediation. Chem. Eng. J. 2023, 457, 141194. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Liu, L.; Ju, M.; Zheng, K. Adsorption behavior of selective recognition functionalized biochar to Cd(II) in wastewater. Materials 2018, 11, 299. [Google Scholar] [CrossRef]
- Hf, A.; Xm, B.; Sz, A.; Ju, M.; Zheng, K. Highly efficient removal of heavy metal ions by carboxymethyl cellulose-immobilized Fe3O4 nanoparticles prepared via high-gravity technology. Carbohydr. Polym. 2019, 213, 39–49. [Google Scholar]
- Solomons, T.W.G.; Fryhle, C. Organic Chemistry; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Guo, N.; Lv, X.; Yang, Q.; Xu, X.; Song, H. Effective removal of hexavalent chromium from aqueous solution by ZnCl2 modified biochar: Effects and response sequence of the functional groups. J. Mol. Liq. 2021, 334, 116149. [Google Scholar] [CrossRef]
- Pan, R.; Bu, J.; Ren, G.; Zhang, Z.; Li, K.; Ding, A. Mechanism of removal of hexavalent chromium from aqueous solution by Fe-modified biochar and its application. Appl. Sci. 2022, 12, 1238. [Google Scholar] [CrossRef]
- Bashir, S.; Zhu, J.; Fu, Q.; Hu, H. Comparing the adsorption mechanism of Cd by rice straw pristine and KOH-modified biochar. Environ. Sci. Pollut. Res. 2018, 25, 11875–11883. [Google Scholar] [CrossRef]







| Adsorbent | Performance Characteristics |
|---|---|
| Biochar | Developed pore structure, Simple process, Renewable recycling use |
| Kieselguhr | Non-toxic, Chemically stable, Water absorption, Strong permeability |
| Bentonite | Porous structure, Adsorptive, Expansibility |
| Silica gel | High activity, Non-toxic, Chemically stable |
| Cellulose | Firmness, Biodegradable, Mechanicalness, Thermal stability |
| Graphene oxide | Rich functional groups, Hydrophilicity |
| Sample | BET Surface Area (m2·g−1) | Vmicropore (cm3·g−1) | Vmesoporous (cm3·g−1) | Average Pore Size (nm) |
|---|---|---|---|---|
| MB | 1215.62 | 0.25 | 0.69 | 2.27 |
| HMB | 1505.92 | 0.46 | 0.659 | 2.14 |
| NaMB | 1716.76 | 0.48 | 0.59 | 2.13 |
| FeMB | 600.36 | 0.21 | 0.52 | 3.82 |
| Biochar | Qe/ (mg·g−1) | Quasi First Order Dynamics | Quasi Second Order Dynamics | ||||
|---|---|---|---|---|---|---|---|
| Qt/ (mg·g−1) | k1/ min−1 | R2 | Qt/ (mg·g−1) | k1/ (g·mg−1·min−1) | R2 | ||
| MB | 14.39 | 13.48 | 0.051 | 0.95 | 14.23 | 0.0060 | 0.99 |
| HMB | 11.92 | 10.12 | 0.038 | 0.90 | 10.88 | 0.0044 | 0.93 |
| NaMB | 22.36 | 17.51 | 0.019 | 0.84 | 19.32 | 0.0014 | 0.92 |
| FeMB | 23.40 | 21.71 | 0.024 | 0.96 | 23.50 | 0.0016 | 0.99 |
| Biochar | Rd12 | kid1/ (mg∙g−1∙h−0.5) | C′1 (mg·g−1) | Rd22 | kid2/ (mg∙g−1∙h−0.5) | C′2 (mg·g−1) |
|---|---|---|---|---|---|---|
| MB | 0.96 | 0.54 | 6.36 | 0.93 | 0.046 | 12.90 |
| HMB | 0.70 | 0.43 | 3.81 | 0.94 | 0.097 | 7.81 |
| NaMB | 0.97 | 0.89 | 3.83 | 0.99 | 0.300 | 10.38 |
| FeMB | 0.97 | 1.24 | 4.81 | 0.889 | 0.063 | 21.09 |
| Biochar | Langmuir Model | Freundlich Model | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Qm/ (mg·g−1) | KL/ (L·mg−1) | RL | R2 | Chi Square | KF/ (L·mg−1) | R2 | Chi Square | ||
| MB | 20.71 | 0.036 | 0.21–0.87 | 0.97 | 0.50 | 2.82 | 0.38 | 0.88 | 1.85 |
| HMB | 17.29 | 0.046 | 0.18–0.69 | 0.93 | 0.92 | 2.68 | 0.37 | 0.81 | 2.37 |
| NaMB | 49.52 | 0.013 | 0.47–0.90 | 0.99 | 0.91 | 1.67 | 0.62 | 0.96 | 2.90 |
| FeMB | 51.94 | 0.014 | 0.45–0.89 | 0.99 | 1.14 | 1.84 | 0.62 | 0.96 | 3.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, J.; Li, W.; Xia, Z.; Zhao, T.; Tan, C.; Wang, Y.; Li, J. Preparation of Modified Biochar and Its Adsorption of Cr(VI) in Aqueous Solution. Coatings 2023, 13, 1884. https://doi.org/10.3390/coatings13111884
Zuo J, Li W, Xia Z, Zhao T, Tan C, Wang Y, Li J. Preparation of Modified Biochar and Its Adsorption of Cr(VI) in Aqueous Solution. Coatings. 2023; 13(11):1884. https://doi.org/10.3390/coatings13111884
Chicago/Turabian StyleZuo, Jinlong, Wenjing Li, Zhi Xia, Tingting Zhao, Chong Tan, Yuyang Wang, and Junsheng Li. 2023. "Preparation of Modified Biochar and Its Adsorption of Cr(VI) in Aqueous Solution" Coatings 13, no. 11: 1884. https://doi.org/10.3390/coatings13111884
APA StyleZuo, J., Li, W., Xia, Z., Zhao, T., Tan, C., Wang, Y., & Li, J. (2023). Preparation of Modified Biochar and Its Adsorption of Cr(VI) in Aqueous Solution. Coatings, 13(11), 1884. https://doi.org/10.3390/coatings13111884






