Phase Transformations of Iron Nitrides during Annealing in Nitrogen and Hydrogen Atmosphere
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nitriding and Annealing Materials and Procedures
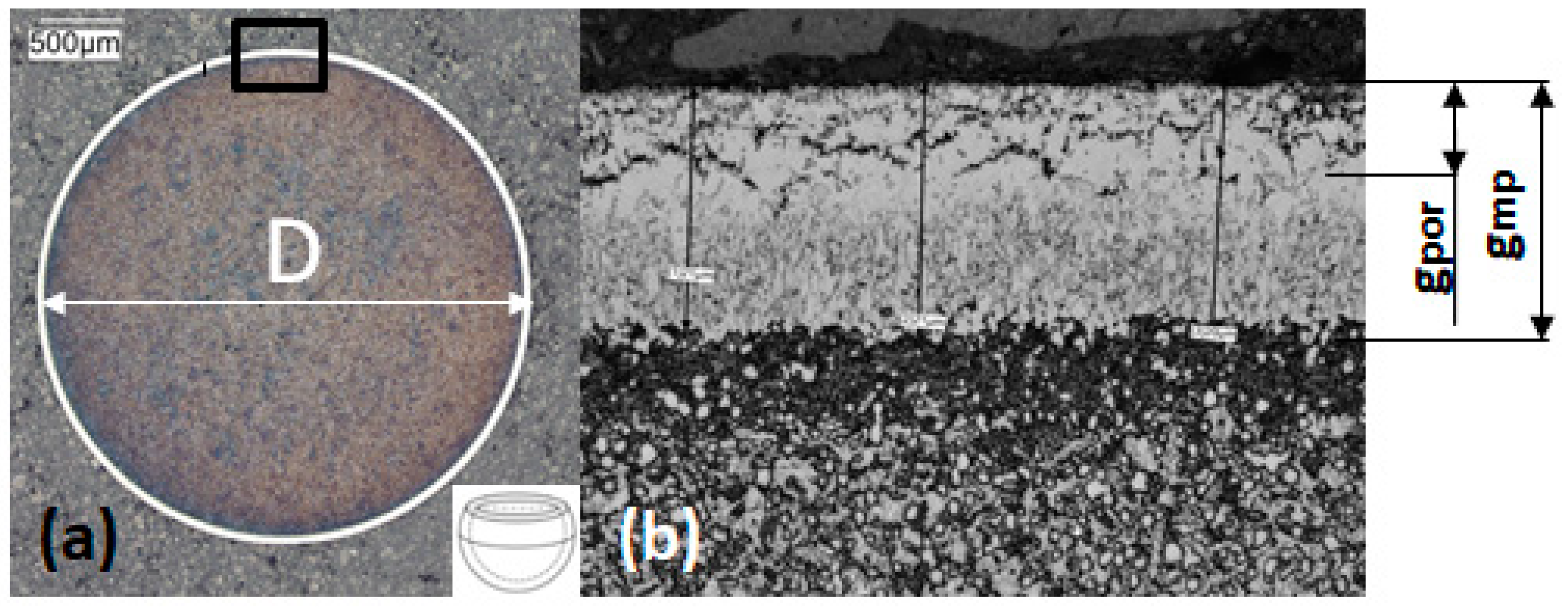
2.2. Metallographic Research
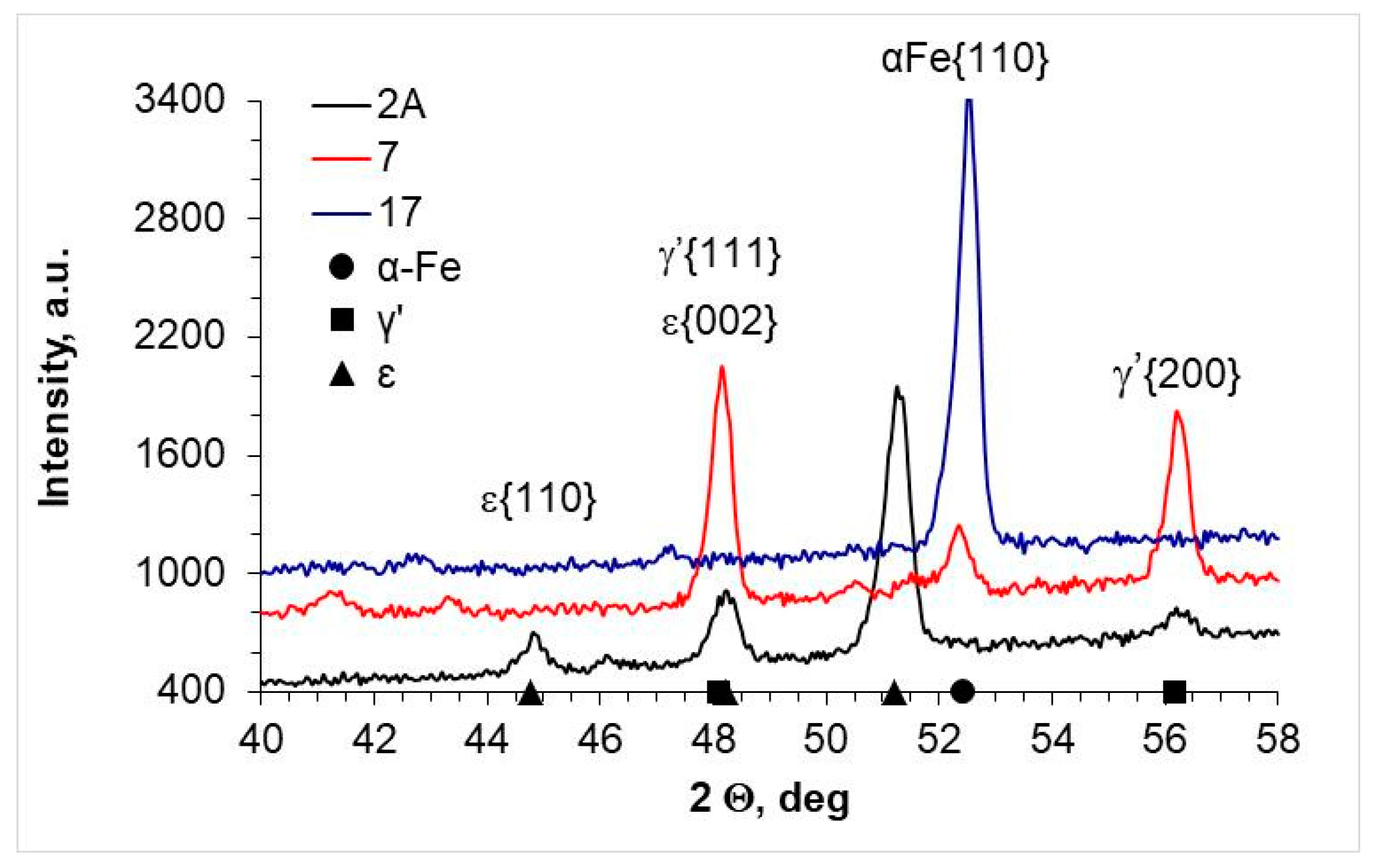
2.3. X-ray Phase Analysis and Chemical Composition Research
3. Results
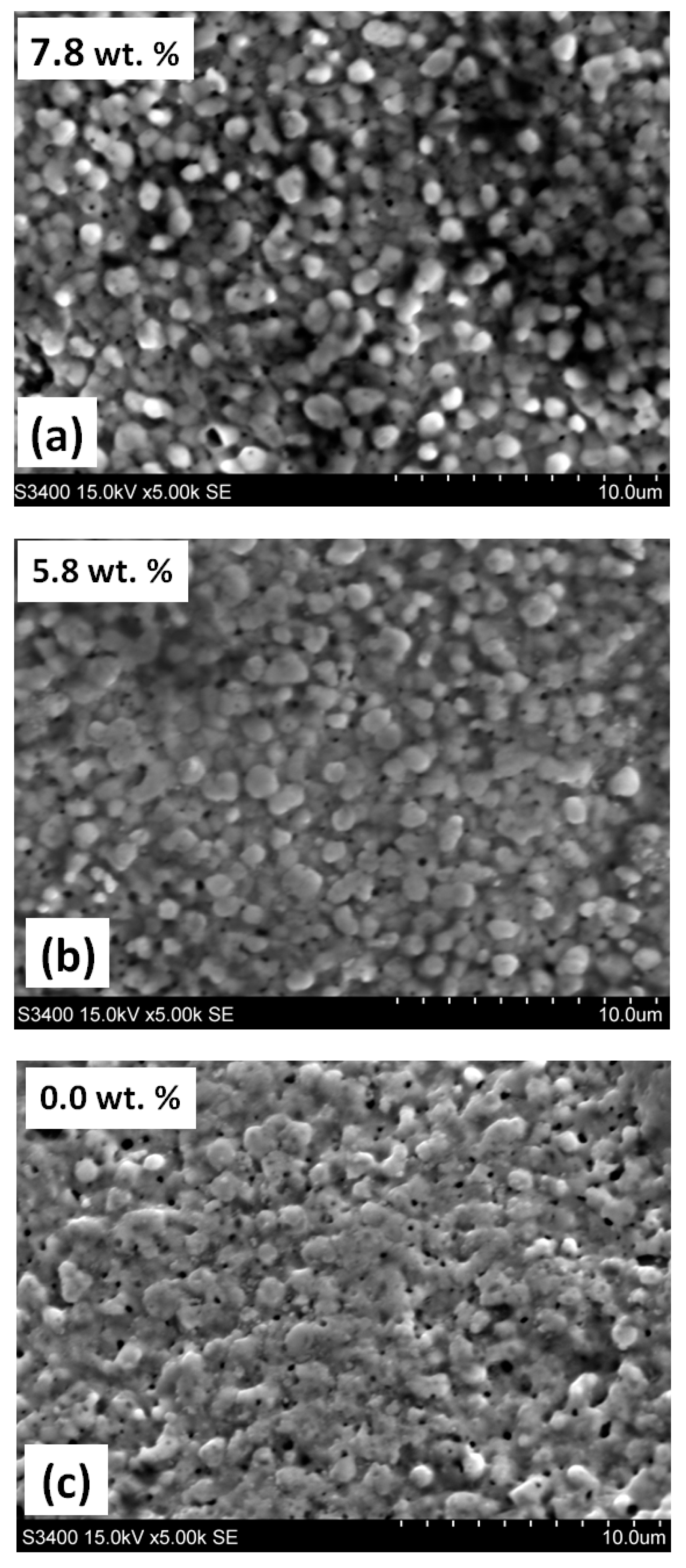
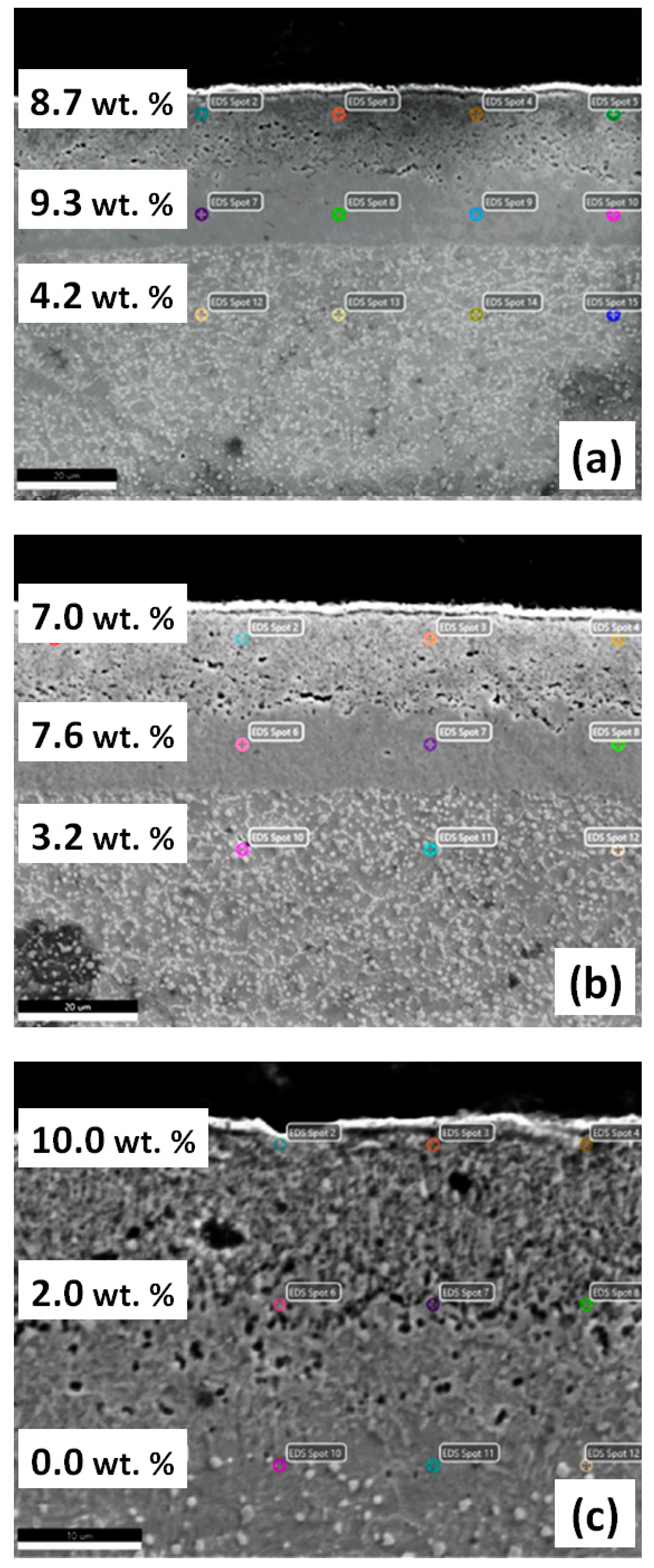
3.1. Annealing in a Nitrogen Atmosphere at 200 Pa
3.2. Annealing in Nitrogen and Hydrogen at Atmospheric Pressure with Recording of Sample Mass Change
4. Discussion of Results
5. Conclusions
- During annealing in nitrogen, iron nitrides undergo thermal reduction.
- During annealing of nitrided steel with a layer of nitrides with the structure of ε + γ′, in the first instance, ε undergoes reduction according to the reaction ε → γ′ + N2↑; then after the complete transformation of the ε phase, the γ′ phase undergoes reduction according to the reaction γ′ → Feα + N2↑.
- During annealing in hydrogen, iron nitrides are reduced due to the chemical reaction: Fe3N + 1.5H2 → 3Feα + NH3↑, Fe4N + 1.5H2 → 4Feα + NH3↑
- Reduction of the ε phase to γ′ and reduction of the γ′ phase to iron is accompanied by nitrogen emission into the atmosphere and, consequently, a loss of mass of the annealed samples.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borgioli, F. The Corrosion Behavior in Different Environments of Austenitic Stainless Steels Subjected to Thermochemical Surface Treatments at Low Temperatures: An Overview. Metals 2023, 13, 776. [Google Scholar] [CrossRef]
- Godec, M.; Ruiz-Zepeda, F.; Podgoenik, B.; Donik, C.; Kocijan, A.; Balantic, D.A.S. The influence of the plasma-nitriding temperature on the microstructure evolution and surface properties of additive-manufactured 18Ni300 maraging steel. Surf. Coat. Technol. 2022, 4333, 12808. [Google Scholar] [CrossRef]
- Kaczmarek, S.M.; Michalski, J.; Leniec, G.; Fuks, H.; Frączek, T.; Dudek, A. Applying the FMR Technique to Analyzing the Influence of Nitriding on the Magnetic Properties of Steel. Materials 2022, 15, 4080. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, B.; Michalski, J.; Wójcik, G. Mechanical and microstructural aspects of C20-steel blades subjected to gas nitriding. Arch. Civ. Mech. Eng. 2019, 19, 147–156. [Google Scholar] [CrossRef]
- Michalski, J.; Tacikowski, J.; Wach, P.; Lunarska, E.; Baum, H. Formation of single-phase layer of γ’-nitride in controlled gas nitriding. Met. Sci. Heat Treat. 2005, 47, 516–519. [Google Scholar] [CrossRef]
- Sugita, Y.; Takahashi, H.; Komuro, M. Magnetic and Mössbauer studies of single-crystal Fe16N2 and Fe-N martensite films epitaxially grown by molecular beam epitaxy. J. Appl. Phys. 1994, 76, 6637–6641. [Google Scholar] [CrossRef]
- Jutte, R.H.; Kooi, B.J.; Somers, M.A.; Jutte, R.H.; Mittemeijer, E.J. On the oxidation of α-Fe andε-Fe2N1−z II. Residual strains and blisters in the oxide layer. Oxid. Met. 1997, 48, 111–128. [Google Scholar] [CrossRef]
- Peltzer Blanca, E.L.; Desimoni, J.; Christensen, N.E.; Emmerich, H.; Cottenier, S. The magnetization of γ’-Fe4N: Theory versus experiment. Phys. Status Solidi B-Basic Solid State Phys. 2009, 246, 909–928. [Google Scholar] [CrossRef]
- Yamashitaa, S.; Masubuchia, Y.; Nakazawab, Y.; Okayamab, T.; Tsuchiya, M.; Kikkawa, S. Crystal structure and magnetic properties of “α′′-Fe16N2 containing residual α-Fe prepared by low-temperature ammonia nitridation. J. Solid State Chem. 2012, 194, 76–79. [Google Scholar] [CrossRef]
- Tayal, A.; Gupta, M.; Kumar, D.; Reddy, V.R.; Gupta, A.; Amir, S.M.; Korelis, P.; Stahn, J. Correlation between iron self-diffusion and thermal stability in doped iron nitride thin films. J. Appl. Phys. 2014, 116, 222206. [Google Scholar] [CrossRef]
- Lehrer, E. Über das Eisen—Wasserstoff—Ammoniak Gleichgewicht. Z. Elektrochem. 1930, 36, 383–393. [Google Scholar] [CrossRef]
- Moszyńska, I.; Moszyński, D.; Arabczyk, W. Hysteresis in nitriding and reduction in the nanocrystalline iron-ammonia-hydrogen system. Przem. Chem. 2009, 88, 526–529. [Google Scholar]
- Arabczyk, W.; Pelka, R.; Kocemba, I.; Brzoza-Kos, A.; Wyszkowski, A.; Lendzion-Bieluń, Z. Study of Phase Transformation Processes Occurring in the Nanocrystalline Iron/Ammonia/Hydrogen System by the Magnetic Permeability Measurement Method. J. Phys. Chem. C 2022, 126, 7704–7710. [Google Scholar] [CrossRef]
- Kardonina, N.I.; Yurovskikh, A.S.; Kolpakov, A.S. Transformations in the Fe-N System. Met. Sci. Heat Treat. 2010, 52, 457–467. [Google Scholar] [CrossRef]
- Van Voorthuysen, E.D.; Boerma, D.O.; Chechenin, N.C. Low-temperature extension of the Lehrer diagram and the iron-nitrogen phase diagram. Metall. Mater. Trans. A 2002, 33, 2593–2598. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Sakita, M.; Nakamura, M.; Kobira, T. Synthesis and Characteristics of Fe4N Powders and Thin Films. J. Magn. Magn. Mater. 2000, 215–216, 529–531. [Google Scholar] [CrossRef]
- Wojciechowski, P.; Lewandowski, M. Iron Nitride Thin Films: Growth, Structure, and Properties. Cryst. Growth Des. 2022, 22, 4618–4639. [Google Scholar] [CrossRef] [PubMed]
- Liapina, T.; Leineweber, A.; Mittemeijer, E.J.; Kockelmann, W. The lattice parameters of ɛ-iron nitrides: Lattice strains due to a varying degree of nitrogen ordering. Acta Mater. 2004, 52, 173–180. [Google Scholar] [CrossRef]
- Gupta, M.; Tayal, A.; Gupta, A.; Gupta, R.; Stahn, J.; Horisberger, M.; Wildes, A. Iron and Nitrogen Self-diffusion in Non-Magnetic Iron Nitrides. J. Appl. Phys. 2011, 110, 123518. [Google Scholar] [CrossRef]
- Lei, X.; Wang, J.; Peng, R.; Wang, W. The controllable magnetic properties of Fe3N nanoparticles synthesized by a simple urea route. Mat. Res. Bul. 2020, 122, 110662. [Google Scholar] [CrossRef]
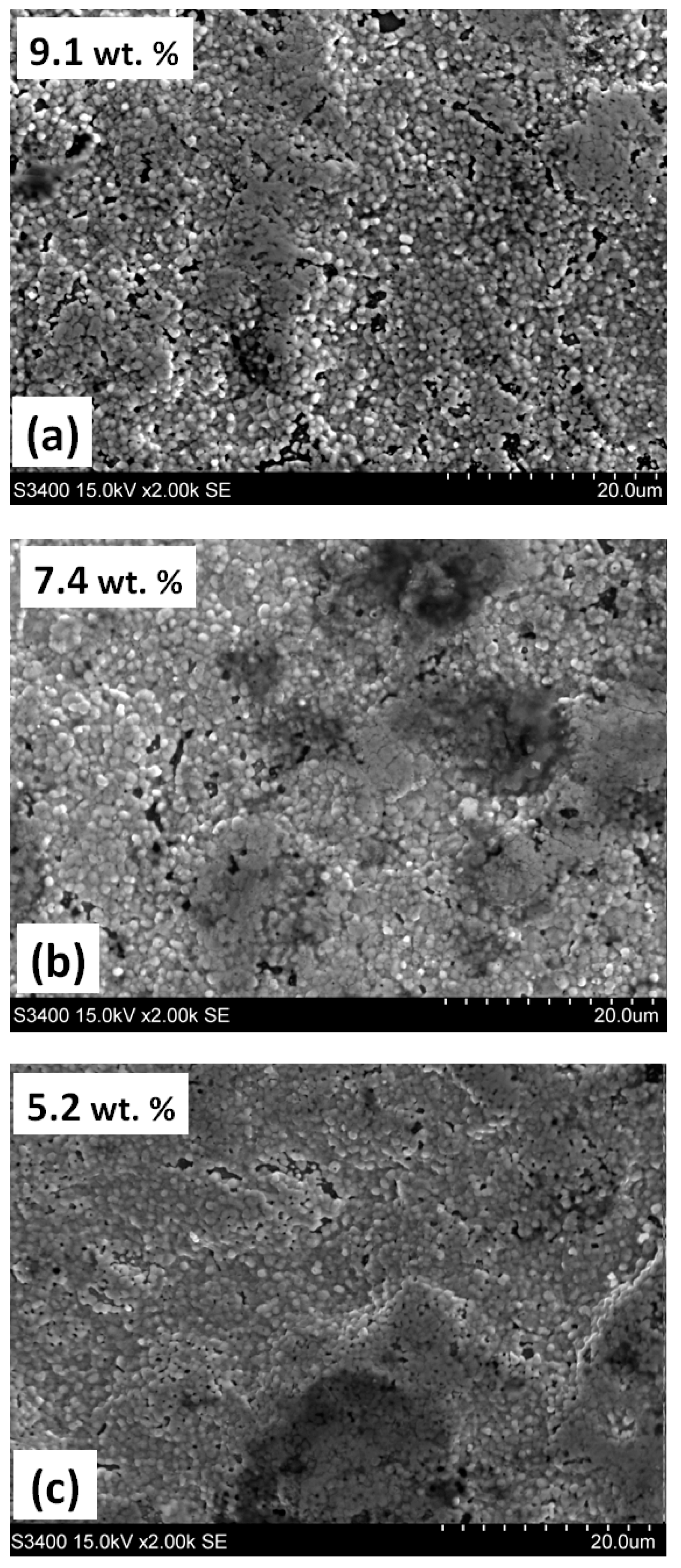
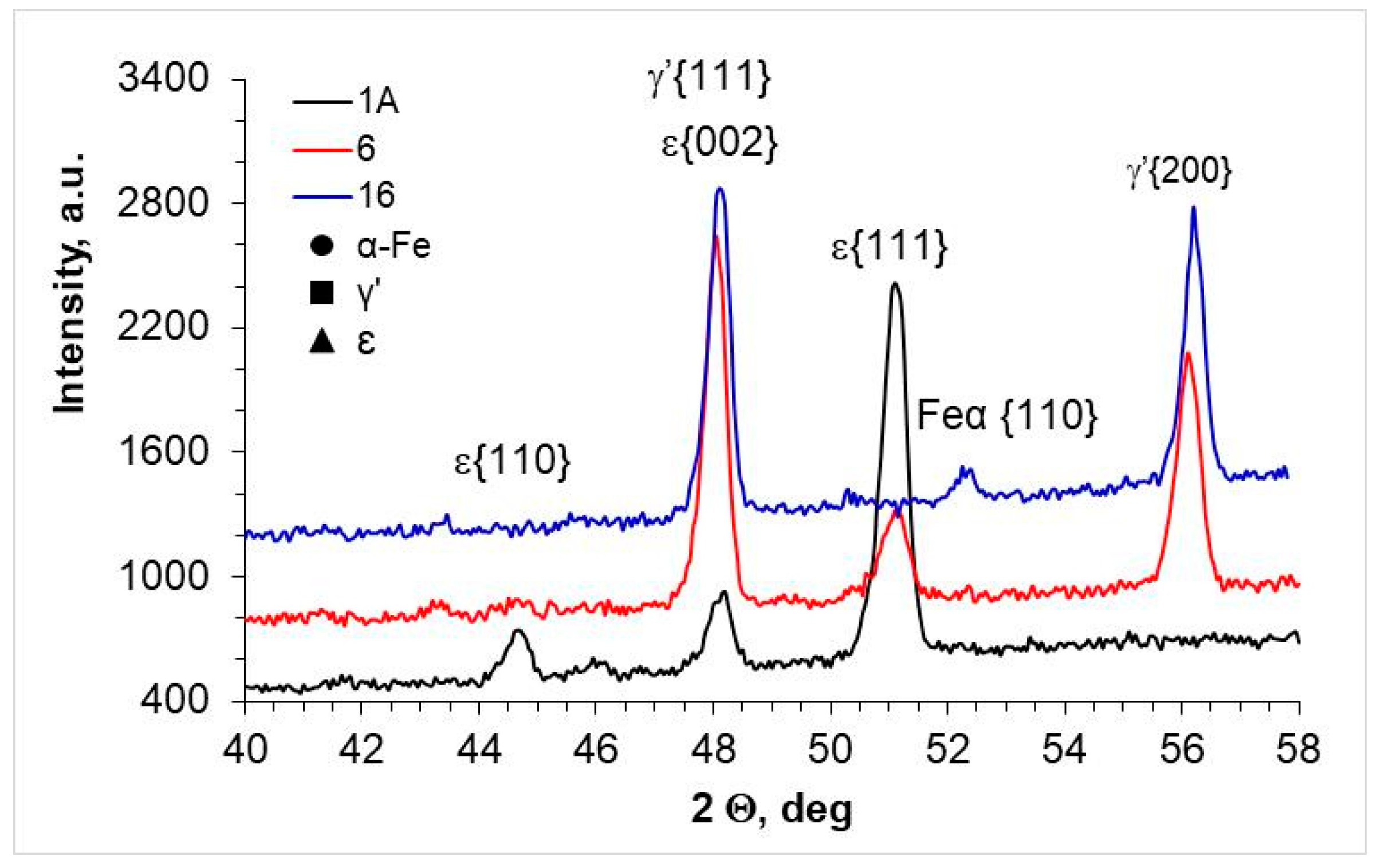
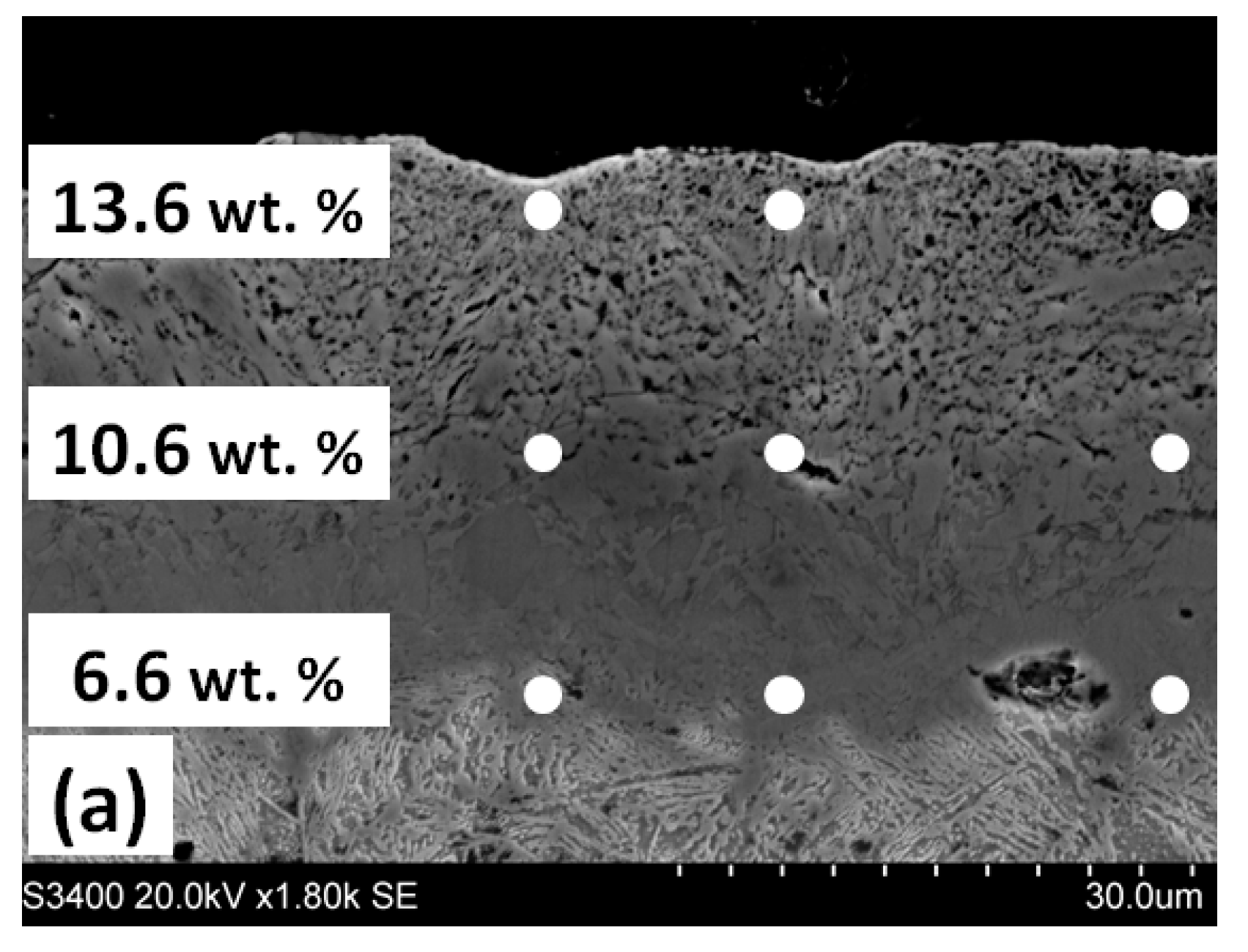
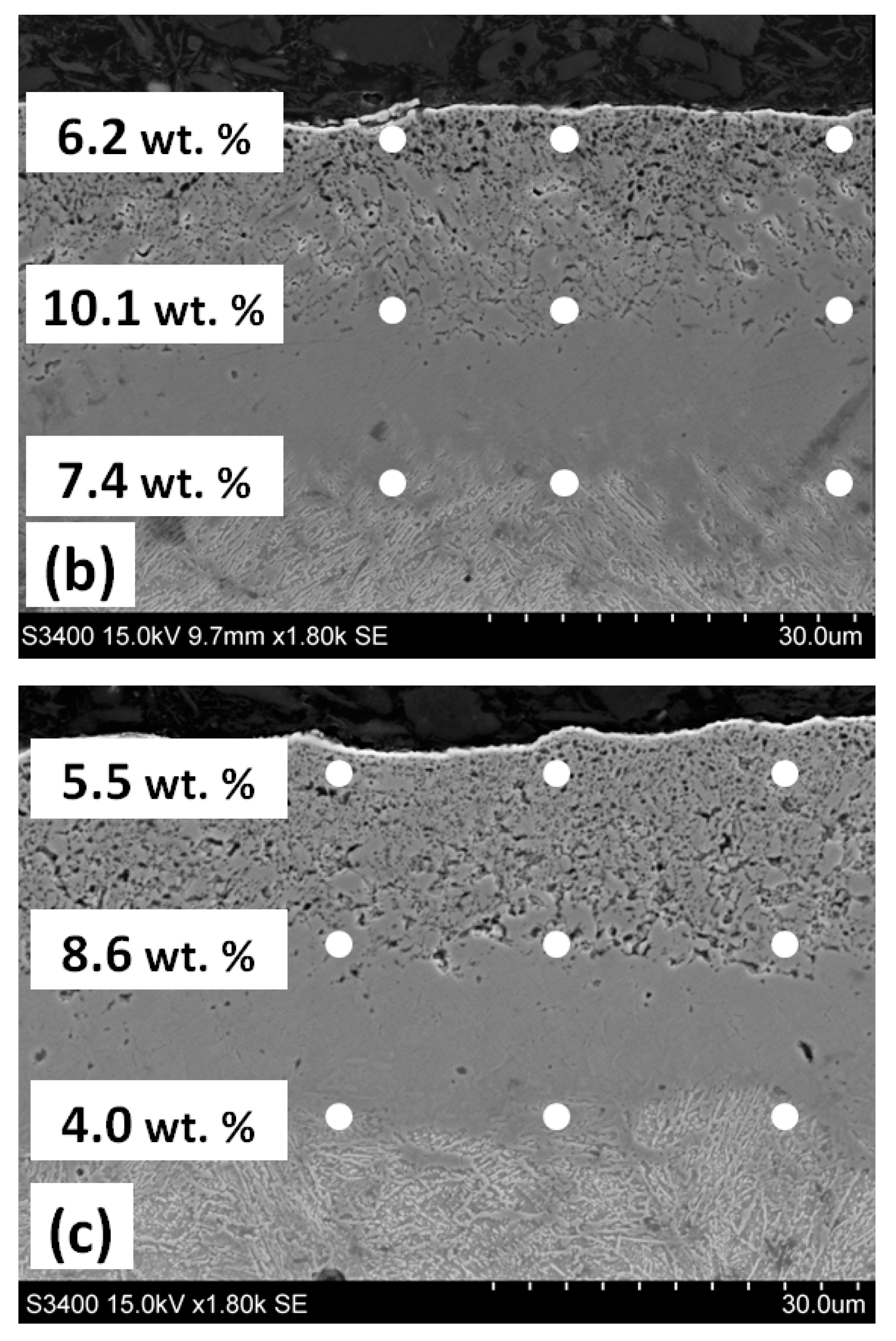

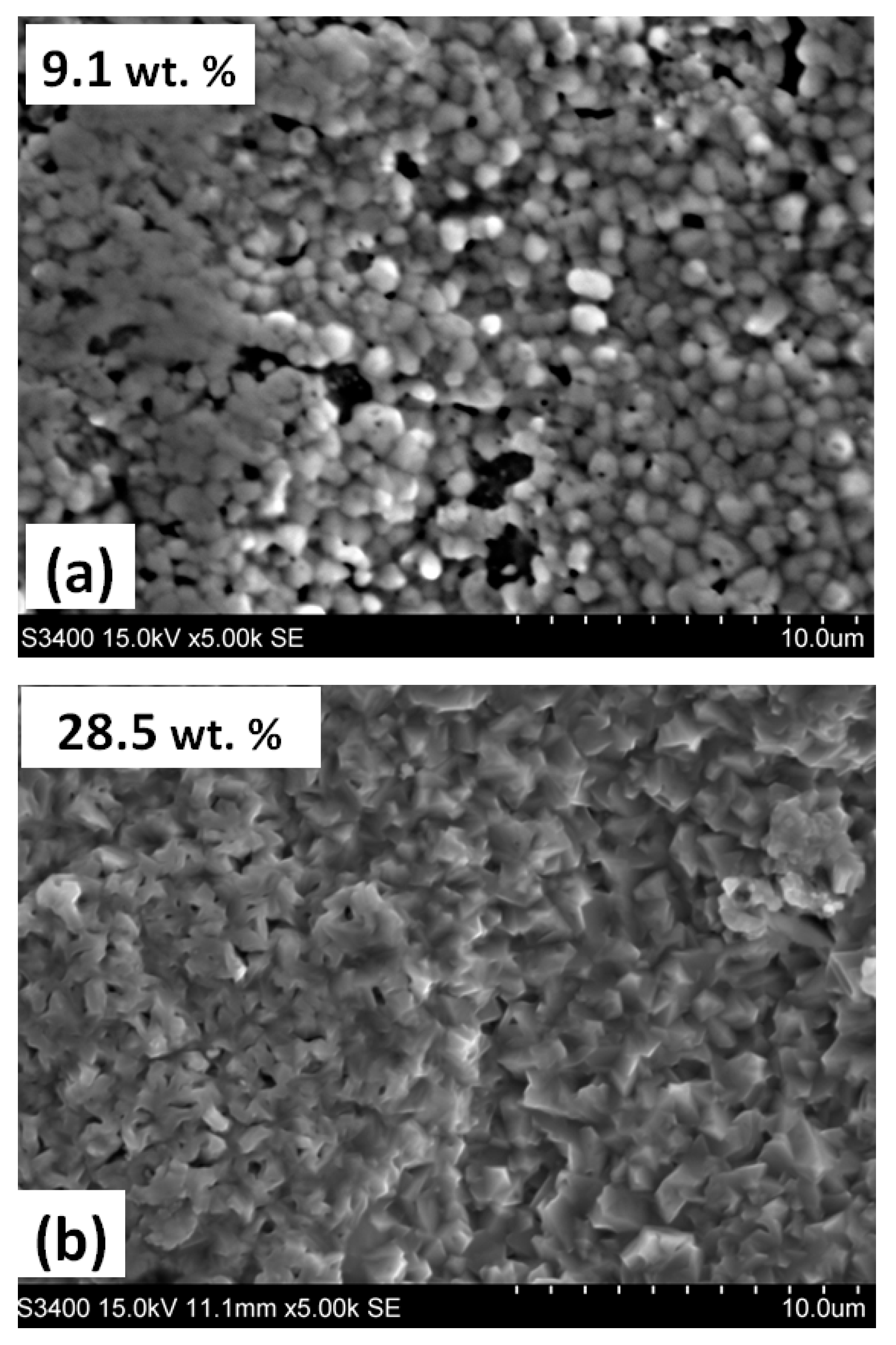

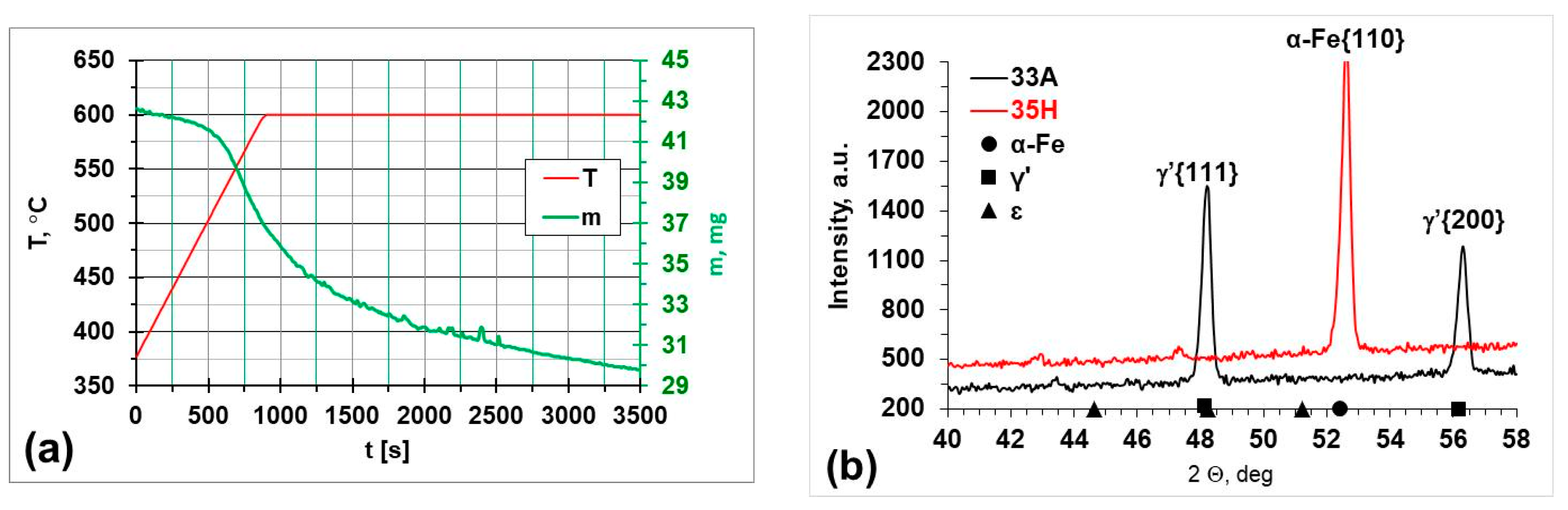
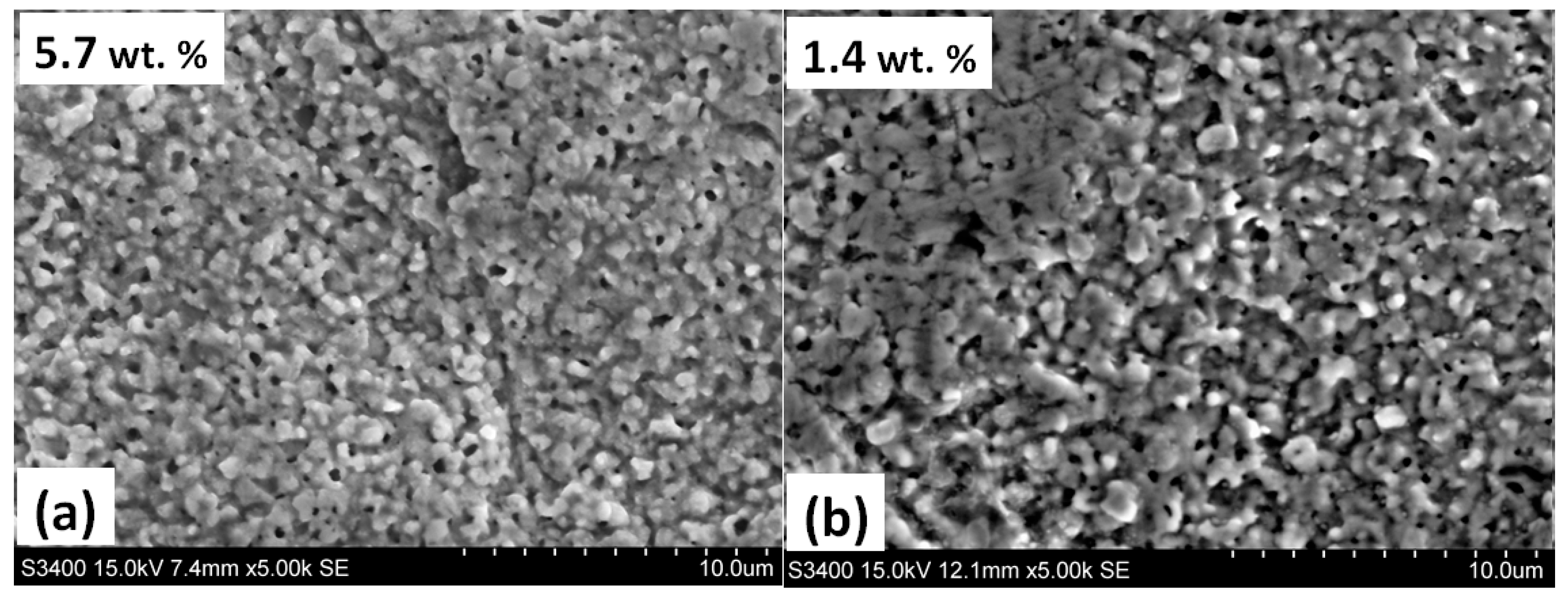
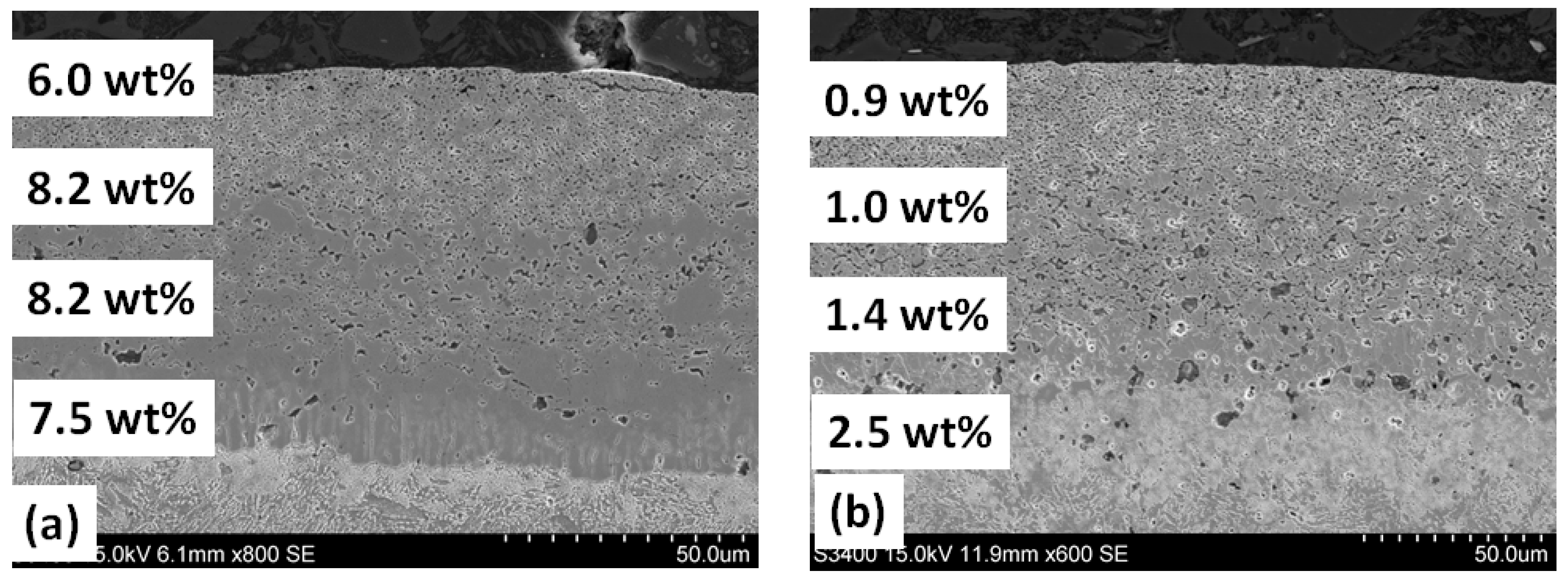
| Steel Grade | Sample No. | D 1 (mm) | Element Content in wt.% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | S | P | Cr | Fe | |||
| AISI 52100 | 2A, 7, 17 | 3.9 | 1.0 | 0.3 | 0.45 | 0.014 | 0.02 | 1.5 | rest |
| AISI 1010 | 1A, 8, 16, 37N, 33A, 35H | 2.5, 3.9 | 0.1 | 0.30 | 0.80 | 0.045 | 0.04 | - | rest |
| Sample No. | Nitriding Parameters | Annealing Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stage I | Stage II | Inlet Atmosphere | T (°C) | t (min) | Inlet Atmosphere/ Pressure | |||||
| T (°C) | t (h) | Np (atm−0.5) | T (°C) | t (h) | 1 Np (atm−0.5) | |||||
| 2A, 1A | 570 | 5 | 2.5 | - | - | - | - | - | - | - |
| 7, 6 | 570 | 5 | 2.5 | - | - | - | - | 520 | 120 | N2/200 Pa |
| 17, 16 | 570 | 5 | 2.5 | - | - | - | - | 520 | 300 | N2/200 Pa |
| 37N | 570 | 5 | 2.5 | - | - | - | - | 520 | 90 | N2/1033 Pa |
| 33A | 580 | 5 | 3.5 | 600 | 11 | 0.5 | 2 NH3/NH3zd | - | - | - |
| 35H | 580 | 5 | 3.5 | 600 | 11 | 0.5 | NH3/NH3zd | 600 | 45 | H2/1033 Pa |
| Steel Grade | Sample No. | D (mm) | 1 gmp (µm) | 2 gpor (µm) |
|---|---|---|---|---|
| AISI 52100 | 2A | 3.9 | 29–30 | 14–15 |
| AISI 52100 | 7 | 3.9 | 29–30 | 14–15 |
| AISI 52100 | 17 | 3.9 | - | - |
| AISI 1010 | 1A | 3.9 | 33–34 | 14–15 |
| AISI 1010 | 6 | 3.9 | 33–34 | 14–15 |
| AISI 1010 | 16 | 3.9 | 33–34 | 14–15 |
| AISI 1010 | 37N | 3.9 | 32–33 | 14–15 |
| AISI 1010 | 33A | 2.5 | 78–80 | 50–60 |
| AISI 1010 | 35H | 2.5 | - | - |
| Steel Grade | Sample No. | 1 PC WL | PC WL (%) | |
|---|---|---|---|---|
| γ′ | ε | |||
| AISI 52100 | 2A | Fe4N-γ′; Fe2-3N-ε | 22 ± 2 | 78 ± 2 |
| 7 | Fe4N-γ′ | 97 ± 2 | Below 5 | |
| 17 | - | Below 5 | Below 5 | |
| Steel Grade | Sample No. | PC WL | PC (%) | |
|---|---|---|---|---|
| γ′ | ε | |||
| AISI 1010 | 1A | Fe4N-γ′; Fe2-3N-ε | 5 ± 2 | 96 ± 2 |
| 6 | Fe4N-γ′ | 35 ± 2 | 65 ± 2 | |
| 16 | Fe4N-γ′ | 98 ± 2 | Below 5 | |
| Steel Grade | Sample No. | PC WL | PC (%) | |
|---|---|---|---|---|
| γ′ | ε | |||
| AISI 1010 | 1A | Fe4N-γ′; Fe2-3N-ε | Below 5 | 98 ± 2 |
| 37N | Fe4N-γ′; Fe2-3N-ε | Above 5 | 96 ± 2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frączek, T.; Michalski, J.; Dudek, A.; Kowalewska-Groszkowska, M.; Owczarek, J. Phase Transformations of Iron Nitrides during Annealing in Nitrogen and Hydrogen Atmosphere. Coatings 2023, 13, 1882. https://doi.org/10.3390/coatings13111882
Frączek T, Michalski J, Dudek A, Kowalewska-Groszkowska M, Owczarek J. Phase Transformations of Iron Nitrides during Annealing in Nitrogen and Hydrogen Atmosphere. Coatings. 2023; 13(11):1882. https://doi.org/10.3390/coatings13111882
Chicago/Turabian StyleFrączek, Tadeusz, Jerzy Michalski, Agata Dudek, Magdalena Kowalewska-Groszkowska, and Justyna Owczarek. 2023. "Phase Transformations of Iron Nitrides during Annealing in Nitrogen and Hydrogen Atmosphere" Coatings 13, no. 11: 1882. https://doi.org/10.3390/coatings13111882
APA StyleFrączek, T., Michalski, J., Dudek, A., Kowalewska-Groszkowska, M., & Owczarek, J. (2023). Phase Transformations of Iron Nitrides during Annealing in Nitrogen and Hydrogen Atmosphere. Coatings, 13(11), 1882. https://doi.org/10.3390/coatings13111882






