A Study on a New Type of High-Performance Resin-Coated Sand for Petroleum Fracturing Proppants
Abstract
1. Introduction
2. Experimental
2.1. Raw Materials
2.2. Experimental Procedure
2.3. Tests
2.3.1. Apparent Density Tests
2.3.2. Breakage Ratio Tests
2.3.3. Acid Solubility Tests
2.3.4. Scanning Electron Microscopy (SEM) and Infrared Spectroscopy (IR)
3. Results and Discussion
3.1. Reaction Mechanism of the Modification of Quartz Sand
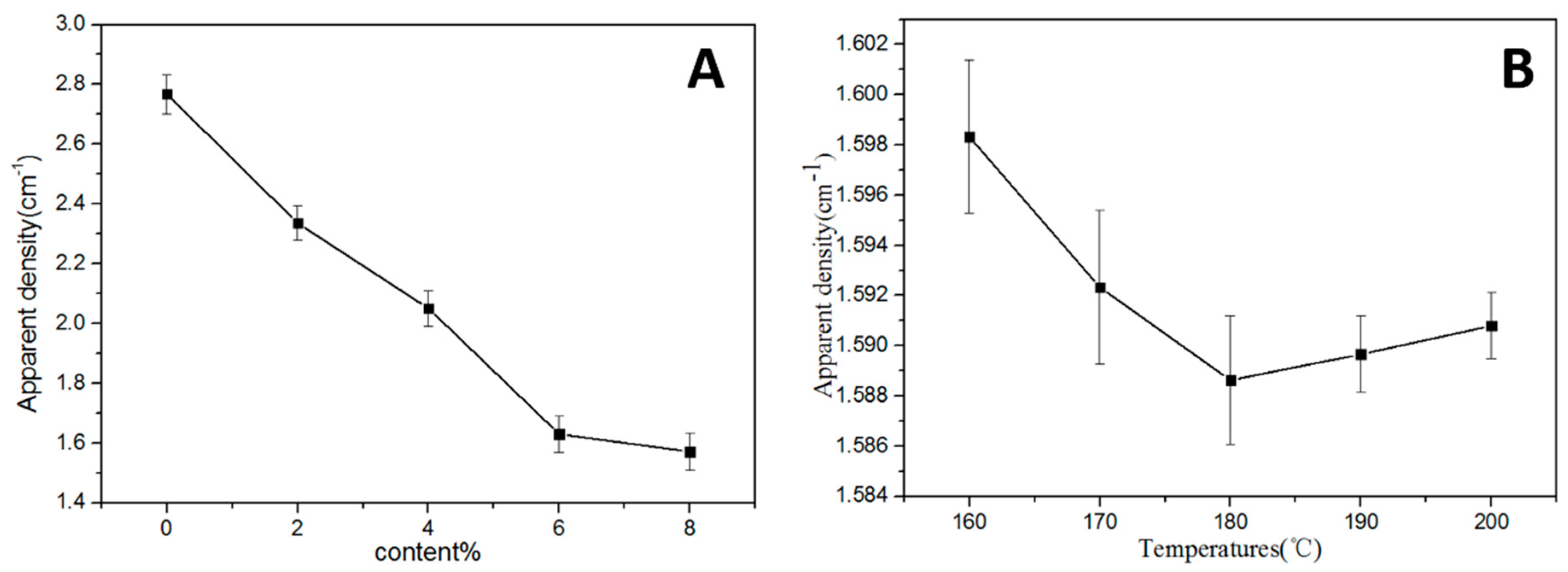
3.2. Influence of Polyimide Resin Content on the Performances of the Proppants
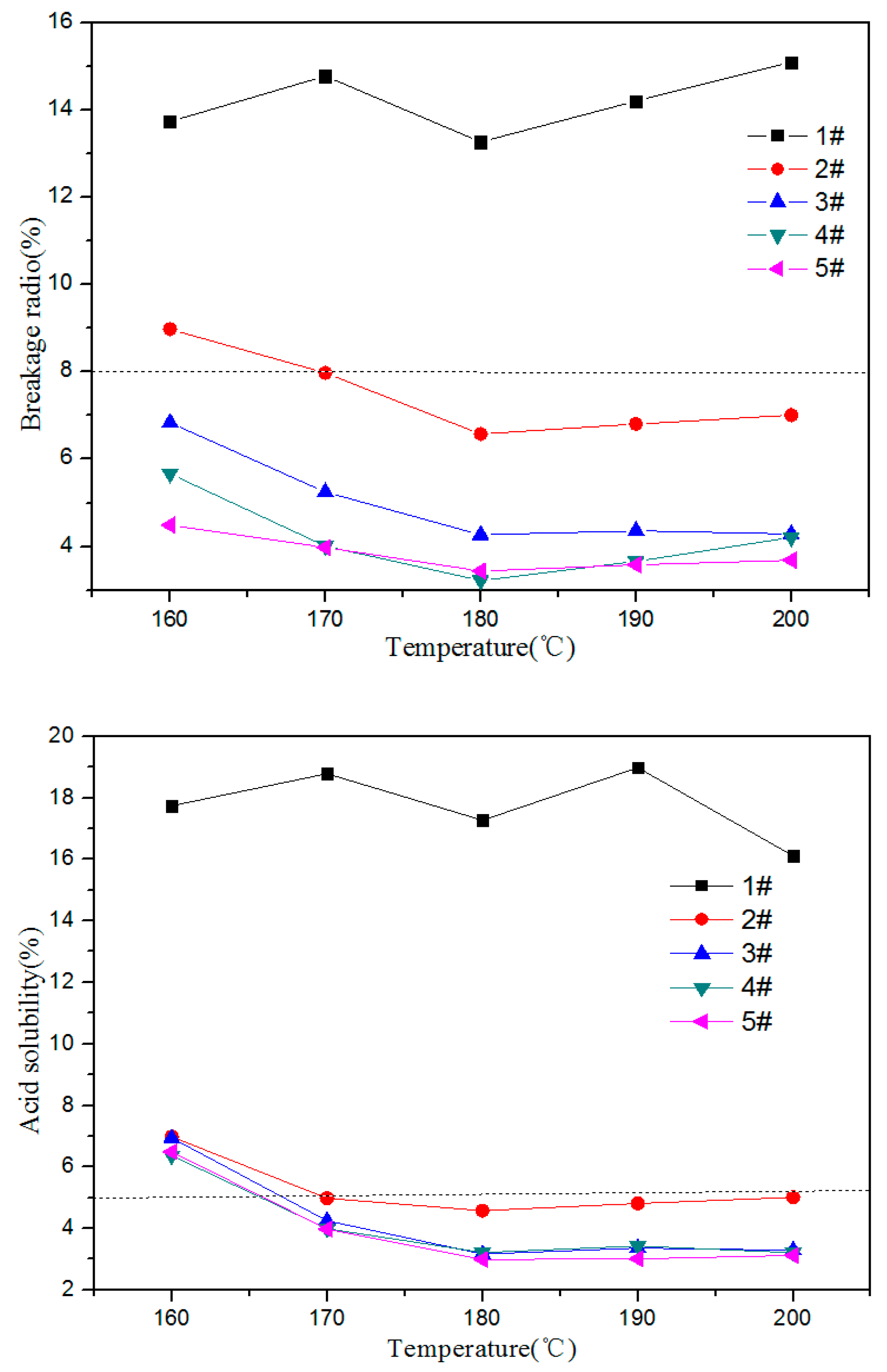
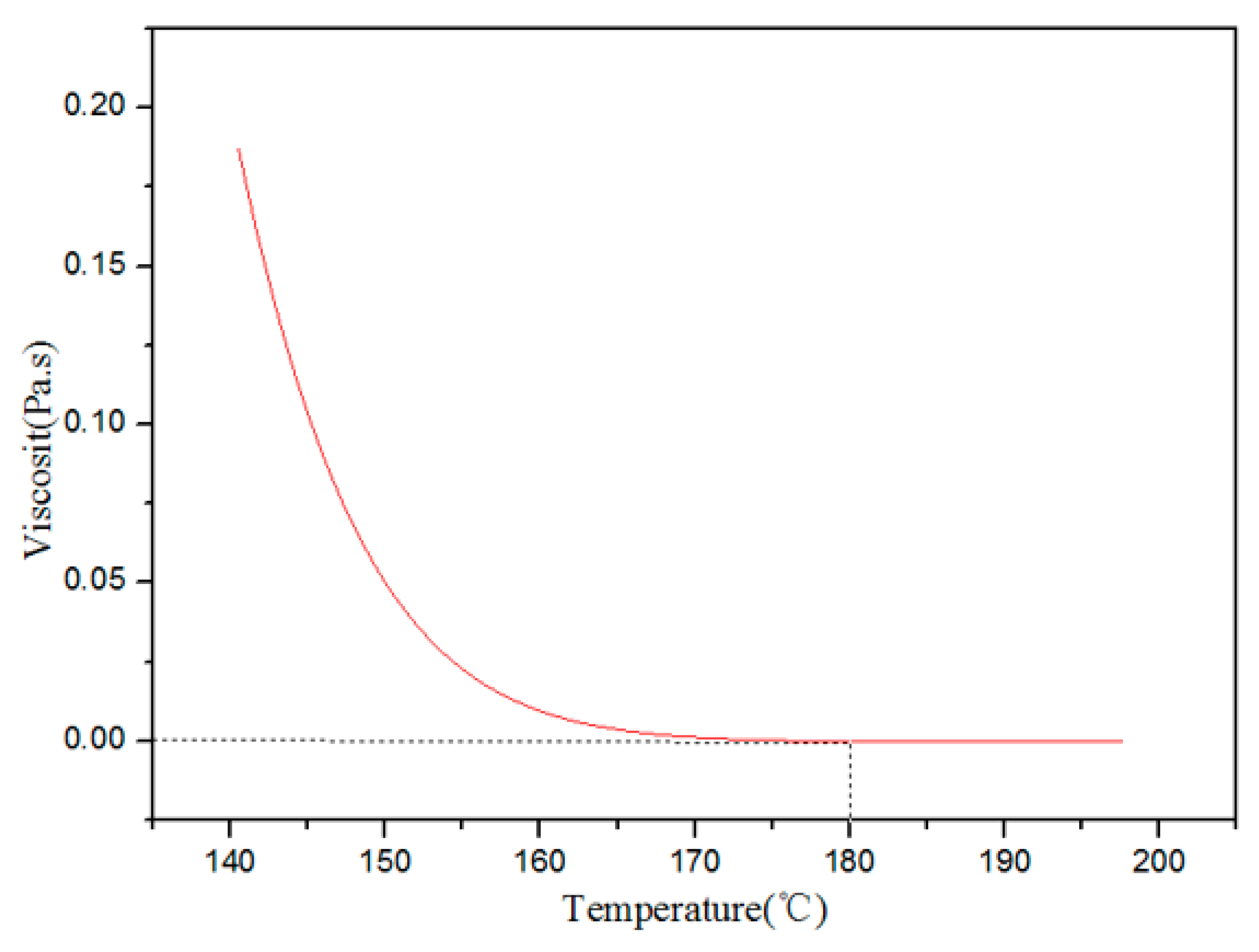
3.3. Influence of the Polyimide Resin Curing Temperature on the Performances of the Proppants
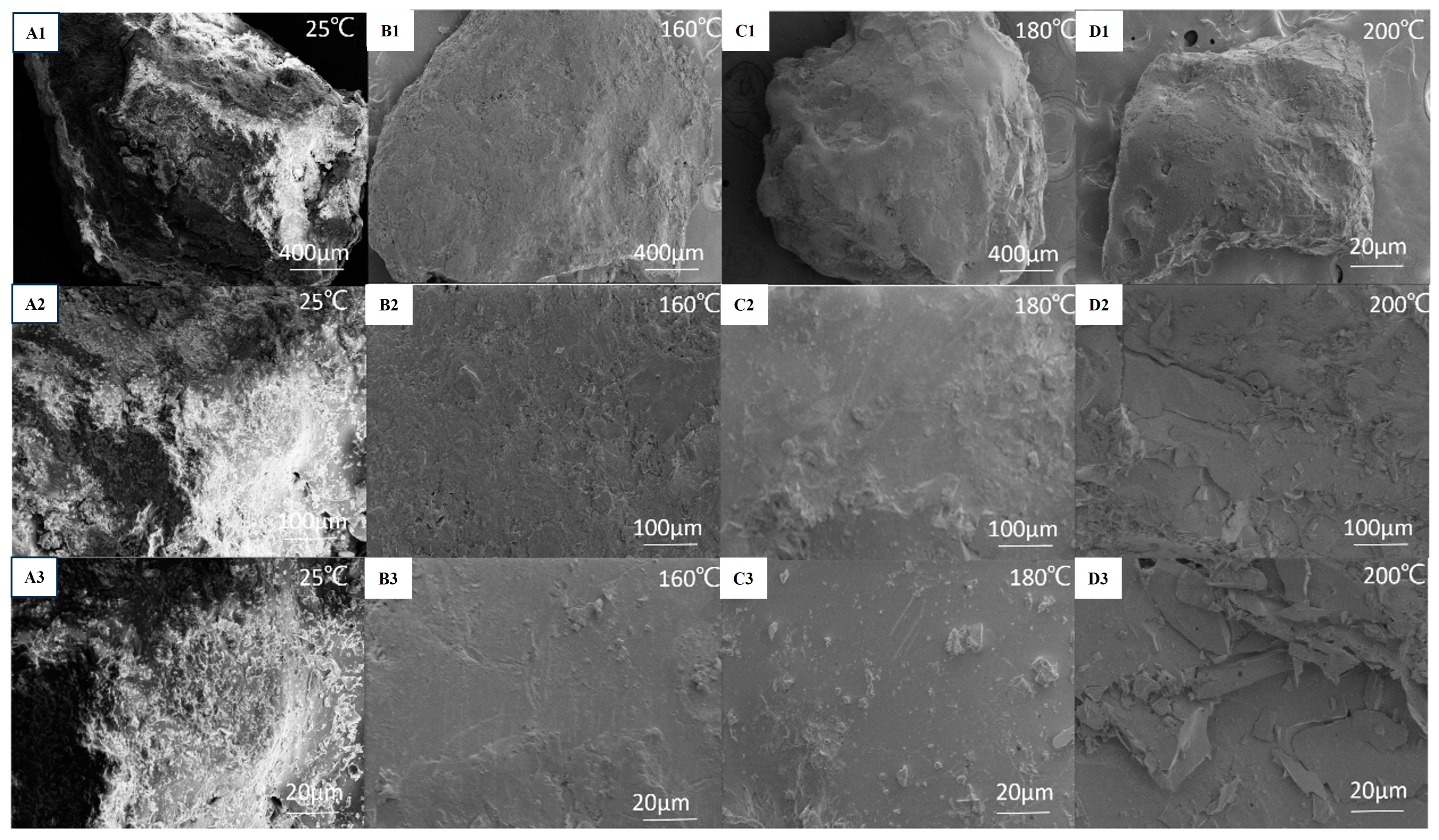
3.4. Influence of the Polyimide Resin Curing Temperature on the Microstructure of the Proppants
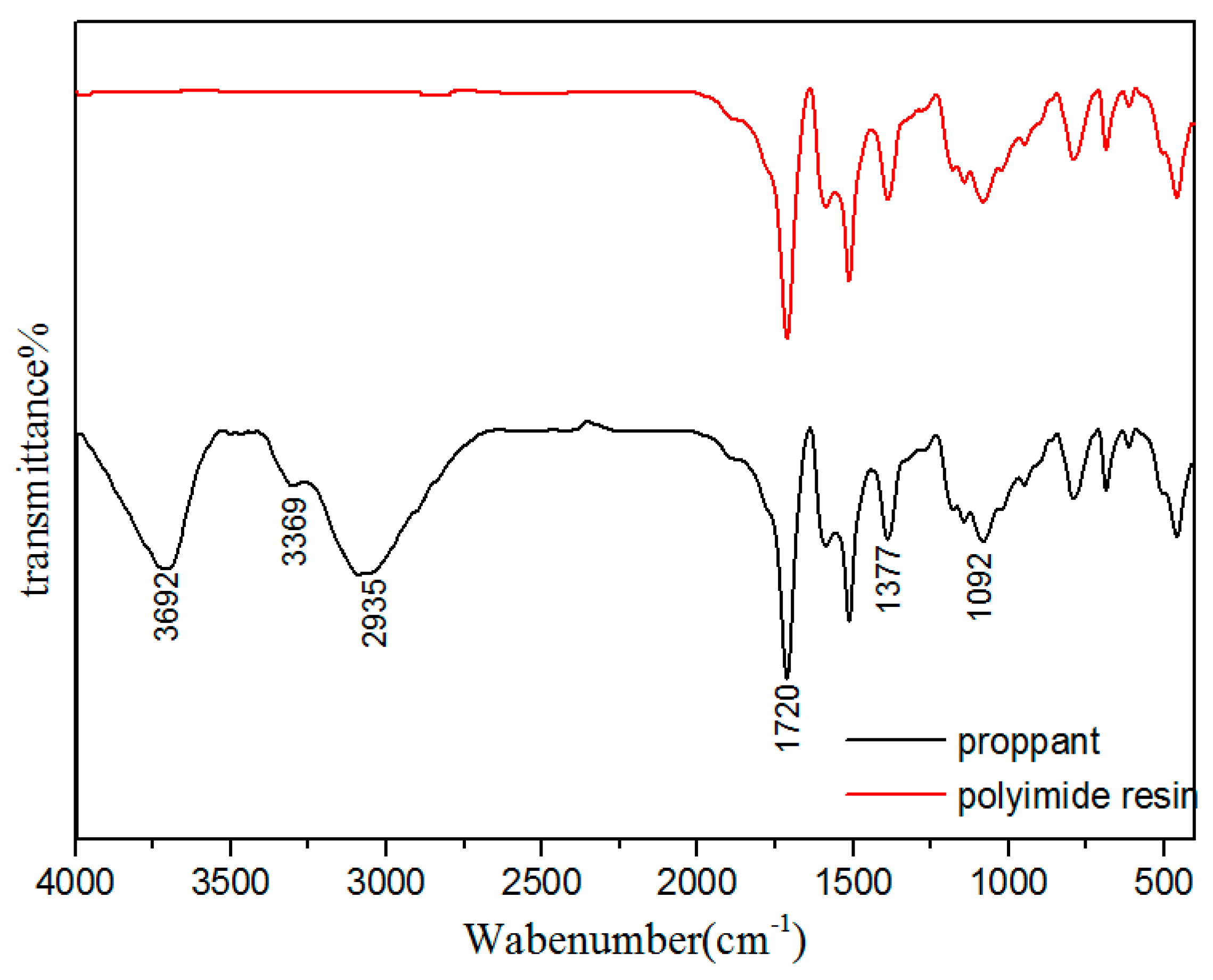
3.5. Chemical Composition Analysis of Proppant Surface
3.6. Influence of the Polyimide Resin Curing Temperature and Content on the Apparent Density of the Proppants
3.7. Comparison of Different Types of Support Agents
4. Conclusions
- There was a superior performance from the proppant prepared from polyimide-coated quartz sand.
- When the polyimide resin content is 6% and the curing temperature is 180 °C, the apparent density of the proppant is 1.592 g/cm3, the breaking rate is only 3.22% under 52 MPa, and the acid solubility is 3.08%.
- Polyimide-coated sand has many advantages for producing high-quality proppants, such as a simple preparation and superior performance. This study used quartz sand and polyimide resin as raw materials, which are easy to obtain, and the resulting polyimide-coated sand proppant exhibited an improved performance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, B.; Ding, Y.H.; Shen, H.; Cui, Z.Q.; He, C.M. Hydraulic fracturing technology in oil and gas development. Adv. Mater. Res. 2014, 962–965, 560–563. [Google Scholar] [CrossRef]
- Liao, Z.; Li, X.; Ge, L.; Yang, Z.; Zhu, J.; Xue, Q.; Wang, H. Lightweight proppants in unconventional oil and natural gas development: A review. Sustain. Mater. Technol. 2022, 33, e00484. [Google Scholar] [CrossRef]
- Aslannezhad, M.; Kalantariasl, A.; You, Z.; Iglauer, S.; Keshavarz, A. Micro-proppant placement in hydraulic and natural fracture stimulation in unconventional reservoirs: A review. Energy Rep. 2021, 7, 8997–9022. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, F.; Weijermars, R.; Hu, X.; Wu, M.; Hu, L.; Li, B. Quantifying micro-proppants crushing rate and evaluating propped micro-fractures. Gas Sci. Eng. 2023, 110, 204915. [Google Scholar] [CrossRef]
- Pangilinan, K.D.; Al Christopher, C.; Advincula, R.C. Polymers for proppants used in hydraulic fracturing. J. Pet. Sci. Eng. 2016, 145, 154–160. [Google Scholar] [CrossRef]
- Hou, L.; Sun, B.; Li, Y.; Du, Q.; Yan, L. Impact of unconventional oil and gas exploitation on fracturing equipment and materials development. Nat. Gas Ind. 2013, 33, 105–110. [Google Scholar]
- Osiptsov, A.A. Hydraulic fracture conductivity: Effects of rod-shaped proppant from lattice-boltzmann simulations and lab tests. Adv. Water Resour. 2017, 104, 293–303. [Google Scholar] [CrossRef]
- Chen, T.; Wang, G.Z.; Gao, J.; Yang, Y.D.; Ma, R.; Lei, X.R.; Yan, C.J. Study on resin coated sand proppant used for oil production. Adv. Mater. Res. 2012, 524–527, 1910–1914. [Google Scholar] [CrossRef]
- De Campos, V.P.P.; Sansone, E.C.; Silva, G.F.B.L. Hydraulic fracturing proppants. Cerâmica 2018, 64, 219–229. [Google Scholar] [CrossRef]
- Palisch, T.; Wilson, B.; Duenckel, B. New technology yields ultrahigh-strength proppant. SPE Prod. Oper. 2014, 30, 76–81. [Google Scholar] [CrossRef]
- Rickards, A.R.; Brannon, H.D.; Wood, W.D. High strength, ultralightweight proppant lends new dimensions to hydraulic fracturing applications. SPE Prod. Oper. 2006, 21, 212–221. [Google Scholar] [CrossRef]
- Michael, F.M.; Krishnan, M.R.; Li, W.; Alsharaeh, E.H. A review on polymer-nanofiller composites in developing coated sand proppants for hydraulic fracturing. J. Nat. Gas Sci. Eng. 2020, 83, 103553. [Google Scholar] [CrossRef]
- Gu, M.; Dao, E.; Mohanty, K.K. Investigation of ultra-light weight proppant application in shale fracturing. Fuel 2015, 150, 191–201. [Google Scholar] [CrossRef]
- Gaurav, A.; Dao, E.K.; Mohanty, K.K. Evaluation of ultra-light-weight proppants for shale fracturing. J. Pet. Sci. Eng. 2012, 92–93, 82–88. [Google Scholar] [CrossRef]
- Zhang, W.; Mao, J.; Yang, X.; Zhang, H.; Zhang, Z.; Yang, B.; Zhang, Y.; Zhao, J. Study of a novel gemini viscoelastic surfactant with high performance in clean fracturing fluid application. Polymers 2018, 10, 1215. [Google Scholar] [CrossRef]
- Wang, K.; Wang, H.; Zhou, Y.; Li, G.; Wu, Y.; Hao, J.; Tian, Y. Preparation and characterization of low-cost high-performance mullite-quartz ceramic proppants for coal bed methane wells. Sci. Eng. Compos. Mater. 2018, 25, 957–961. [Google Scholar] [CrossRef]
- Szymanska, J.; Wisniewski, P.; Malek, M.; Mizera, J.; Kurzydlowski, K.J. Investigation of key parameters influence on properties of the green pellets and lightweight ceramic proppants obtained by mechanical granulation method. J. Therm. Anal. Calorim. 2016, 125, 1411–1423. [Google Scholar] [CrossRef][Green Version]
- Liang, F.; Sayed, M.; Al-Muntasheri, G.A.; Chang, F.F.; Li, L. A comprehensive review on proppant technologies. Petroleum 2016, 2, 26–39. [Google Scholar] [CrossRef]
- Zoveidavianpoor, M.; Gharibi, A. Application of polymers for coating of proppant in hydraulic fracturing of subterraneous formations: A comprehensive review. J. Nat. Gas Sci. Eng. 2015, 24, 197–209. [Google Scholar] [CrossRef]
- Wei, G.; Huang, H.; Babadagli, T.; Hou, L.; Li, H. Determination of the effect of resin-coating on ceramic proppant settlement for optimal hydraulic fracturing applications. Powder Technol. 2020, 373, 109–117. [Google Scholar] [CrossRef]
- Dong, B.; Cai, J.; Li, S.; Ni, X.; Tu, Z. Development of a new low density high strength hydraulic fracturing proppant. Drill. Fluid Complet. Fluid 2017, 34, 117–120+125. [Google Scholar]
- Liu, L.X.; Niu, R.D.; Jin, H.Y.; Lv, B.Q.; Liu, S. Research of Low Cost Fracturing Proppant. Mater. Sci. Forum 2012, 724, 69–72. [Google Scholar] [CrossRef]
- Hong, L.; Cheng, W. Experimental study on high strength composite ceramsite using fly ash and waste glass. New Build. Mater. 2013, 357–360, 1337–1342. [Google Scholar] [CrossRef]
- Farkas, K.; Varsani, A.; Pang, L. Adsorption of rotavirus, ms2 bacteriophage and surface-modified silica nanoparticles to hydrophobic matter. Food Environ. Virol. 2014, 7, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Niu, S.; Miao, Y.; Gao, X.; Cheng, L.; Gao, F. Preparation and properties of resin coated ceramic proppants with ultra light weight and high strength from coal-series kaolin. Appl. Clay Sci. 2019, 183, 105364. [Google Scholar] [CrossRef]
- Chen, T.; Gao, J.; Zhao, Y.; Liang, T.; Hu, G.; Han, X. Progress of Polymer Application in Coated Proppant and Ultra-Low Density Proppant. Polymers 2022, 14, 5534. [Google Scholar] [CrossRef]
- Sun, H.; He, B.C.; Xu, H.X.; Zhou, F.J.; Zhang, M.A.; Li, H.; Yin, G.Y.; Chen, S.; Xu, X.J.; Li, B. Experimental Investigation on the Fracture Conductivity Behavior of Quartz Sand and Ceramic Mixed Proppants. ACS Omega 2022, 7, 10243–10254. [Google Scholar] [CrossRef]
- Liu, P.; Huang, Q.; Li, J.; Du, J.; Lu, X.; Liu, J.; Liu, C.; Lan, X. Review and Perspectives of Coated Proppant Technology. Energy Fuels 2023, 37, 3355–3370. [Google Scholar] [CrossRef]
- Wu, X.; Huo, Z.; Ren, Q.; Li, H.; Lin, F.; Wei, T. Preparation and characterization of ceramic proppants with low density and high strength using fly ash. J. Alloys Compd. 2017, 702, 442–448. [Google Scholar] [CrossRef]
- ISO 13503-2; Measurement of Properties of Proppants Used in Hydraulic Fracturing and Gravel-Packing Operations. International Organization for Standardization: Beijing, China, 2006.
- Bienvenu, R.L., Jr. Lightweight Proppants and Their Use in Hydraulic Fracturing. U.S. Patent 5531274A, 2 July 1996. [Google Scholar]
- SY/T5180-2014; Measurement of Properties of Proppants Used in Hydraulic Fracturing and Gravel-Packing Operations. Standardization Technical Committee of Petroleum Industry: Beijing, China, 2014.
- Kondoh, E. Influence of Plasma Surface Treatment of Polyimide on the Microstructure of Aluminum Thin Films. Coatings 2022, 12, 334. [Google Scholar] [CrossRef]
- Akamatsu, K.; Ikeda, S.; Nawafune, H.; Deki, S. Surface modification-based synthesis and microstructural tuning of nanocomposite layers: Monodispersed copper nanoparticles in polyimide resins. Chem. Mater. 2003, 15, 2488–2491. [Google Scholar] [CrossRef]

| Sample | Curing Temperatures (°C) | Quartz Sand | Polyimide Resin |
|---|---|---|---|
| 1# | 180 | 100 | 0 |
| 2# | 180 | 98 | 2 |
| 3# | 180 | 96 | 4 |
| 4# | 180 | 94 | 6 |
| 5# | 180 | 92 | 8 |
| Performance | Quartz Sand | Grain Support Agent | Coating Agent |
|---|---|---|---|
| Crushing rate/% | 22.5 | 5.5 | 2.7 |
| Volume density/g.cm−3 | 1.45 | 1.55 | 1.31 |
| Visual density/g.cm−3 | 2.73 | 2.81 | 2.31 |
| Acid solubility/% | 6.45 | 4.5 | 2.2 |
| Golfness | 0.7 | 0.9 | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Wang, Y.; Yang, T.; Song, Y. A Study on a New Type of High-Performance Resin-Coated Sand for Petroleum Fracturing Proppants. Coatings 2023, 13, 1841. https://doi.org/10.3390/coatings13111841
Wei X, Wang Y, Yang T, Song Y. A Study on a New Type of High-Performance Resin-Coated Sand for Petroleum Fracturing Proppants. Coatings. 2023; 13(11):1841. https://doi.org/10.3390/coatings13111841
Chicago/Turabian StyleWei, Xiaohong, Yuting Wang, Tian Yang, and Yaru Song. 2023. "A Study on a New Type of High-Performance Resin-Coated Sand for Petroleum Fracturing Proppants" Coatings 13, no. 11: 1841. https://doi.org/10.3390/coatings13111841
APA StyleWei, X., Wang, Y., Yang, T., & Song, Y. (2023). A Study on a New Type of High-Performance Resin-Coated Sand for Petroleum Fracturing Proppants. Coatings, 13(11), 1841. https://doi.org/10.3390/coatings13111841





